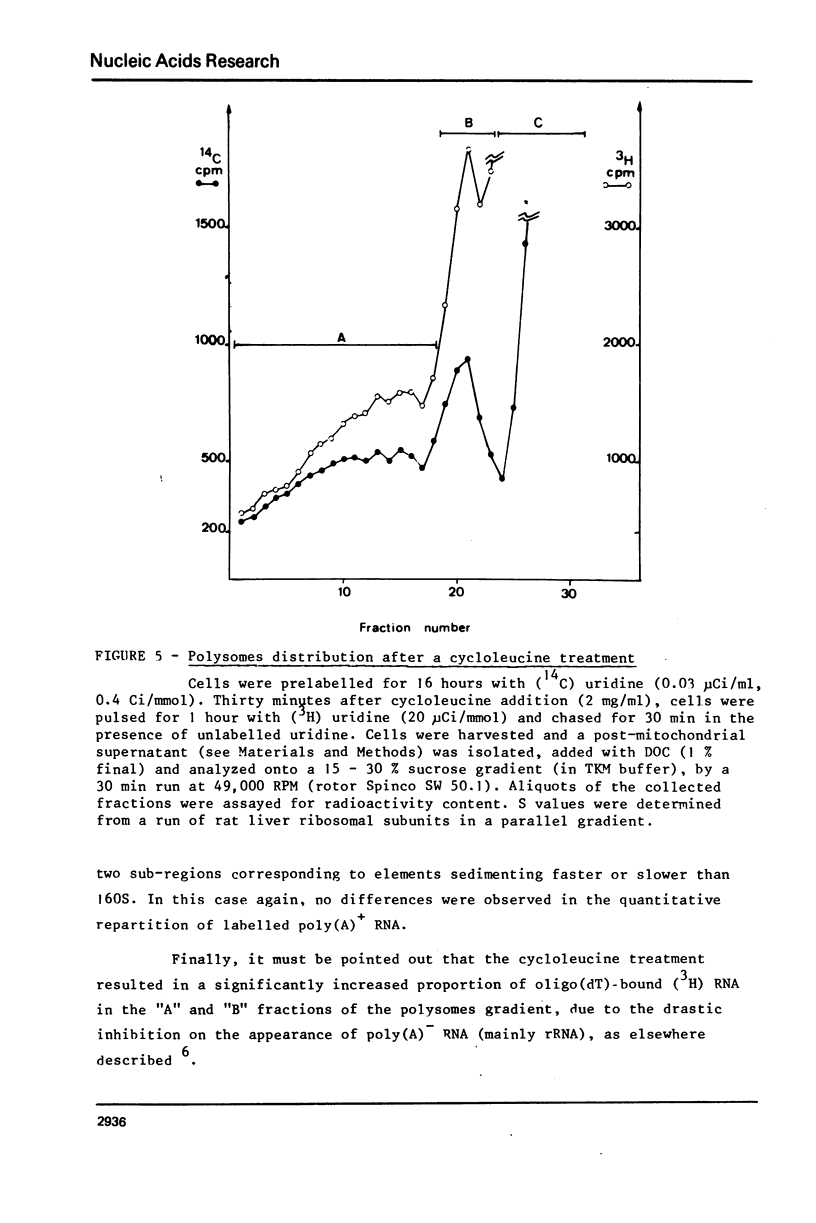
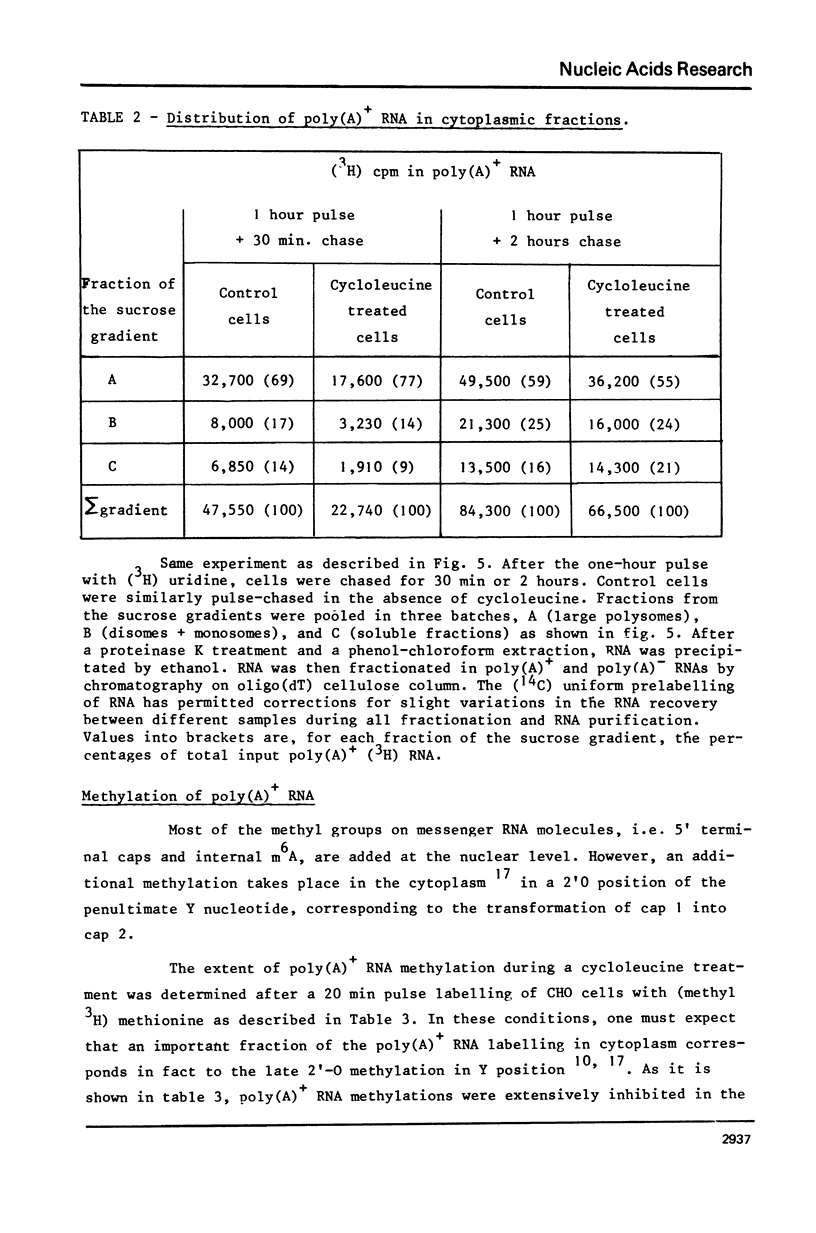
Abstract
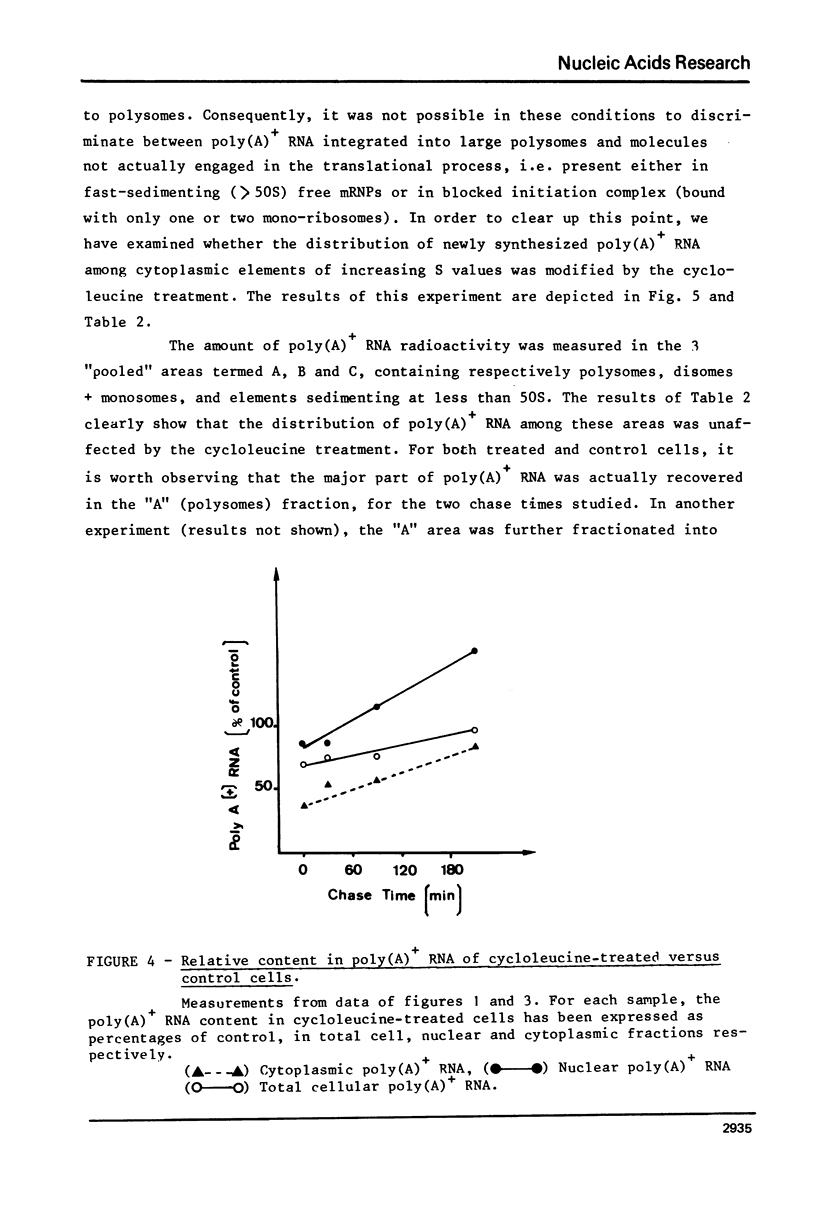
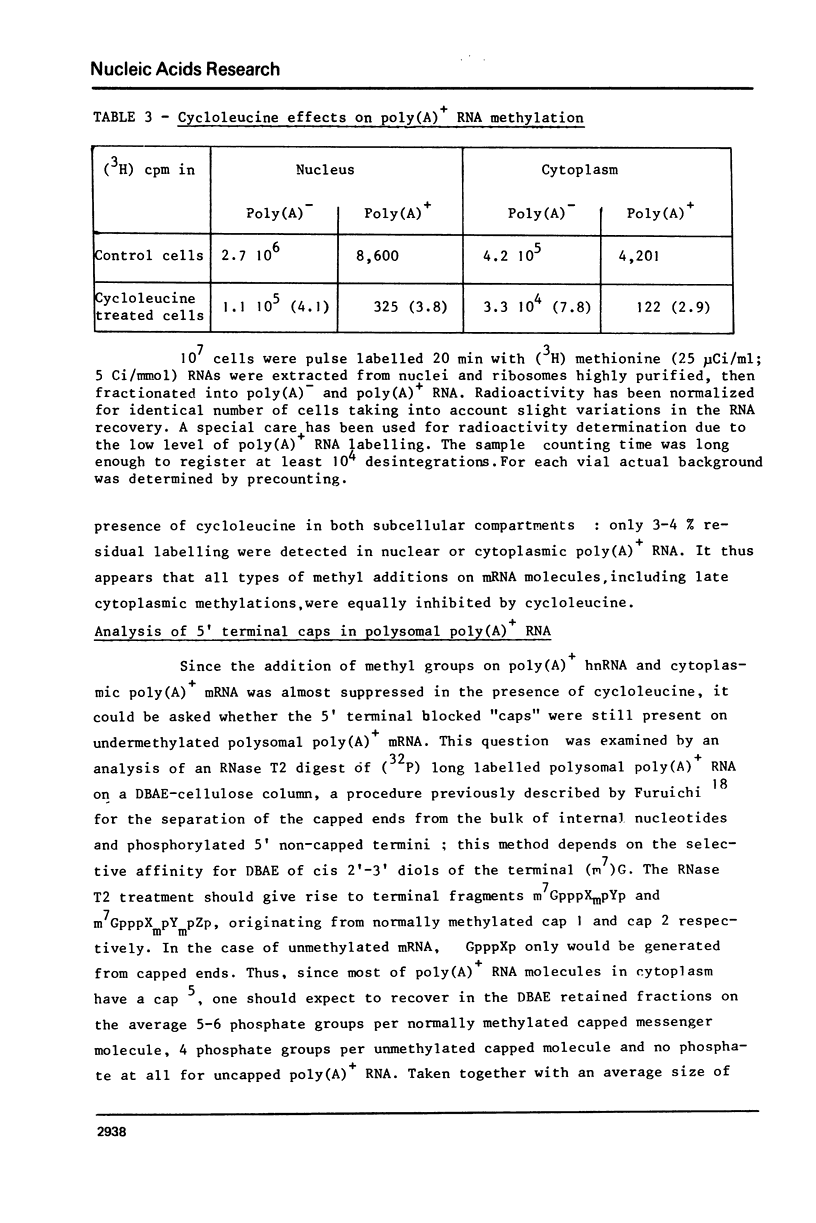
The role of RNA methylations in the control of mRNA maturation and incorporation into polysomes has been investigated through a study of the effects in vivo of cycloleucine, a specific inhibitor of S-adenosyl-methionine mediated methylation. During the cycloleucine treatment, the rate of biosynthesis of hnRNA and its subsequent polyadenylation were only slightly reduced as compared with untreated cells. However a significant lag-time in the cytoplasmic appearance of poly(A)+ undermethylated molecules was observed, in parallel with a transient shift in the average size of hnRNA towards higher molecular weight. Nevertheless, the total amount of pulse-labelled poly(A)+ mRNA transferred to cytoplasm after a long chase time (3 h.) was approximately the same for both cycloleucine-treated and control cells. Extensively undermethylated poly(A)+ cytoplasmic RNAs, possessing a 5' terminal cap were incorporated into polysomes in proportions very similar to control messenger molecules. These results suggest that a normal level of methylation is not stringently required for the production of the functional mRNA molecules although it appears to be of importance for the kinetics of the maturational process.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amalric F., Bachellerie J. P., Caboche M. RNA methylation and control of eukaryotic RNA biosynthesis: processing and utilization of undermethylated tRNAs in CHO cells. Nucleic Acids Res. 1977 Dec;4(12):4357–4370. doi: 10.1093/nar/4.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Banerjee A. K., Shatkin A. J. Methylation-dependent translation of viral messenger RNAs in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1189–1193. doi: 10.1073/pnas.72.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caboche M., Bachellerie J. P. RNA methylation and control of eukaryotic RNA biosynthesis. Effects of cycloleucine, a specific inhibitor of methylation, on ribosomal RNA maturation. Eur J Biochem. 1977 Mar 15;74(1):19–29. doi: 10.1111/j.1432-1033.1977.tb11362.x. [DOI] [PubMed] [Google Scholar]
- Chang C. D., Coward J. K. Effect of S-adenosylhomocysteine and S-tubercidinylhomocysteine on transfer ribonucleic acid methylation in phytohemagglutinin-stimulated lymphocytes. Mol Pharmacol. 1975 Nov;11(6):701–707. [PubMed] [Google Scholar]
- Filipowicz W., Furuichi Y., Sierra J. M., Muthukrishnan S., Shatkin A. J., Ochoa S. A protein binding the methylated 5'-terminal sequence, m7GpppN, of eukaryotic messenger RNA. Proc Natl Acad Sci U S A. 1976 May;73(5):1559–1563. doi: 10.1073/pnas.73.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Shatkin A. J., Stavnezer E., Bishop J. M. Blocked, methylated 5'-terminal sequence in avian sarcoma virus RNA. Nature. 1975 Oct 16;257(5527):618–620. doi: 10.1038/257618a0. [DOI] [PubMed] [Google Scholar]
- Kaehler M., Coward J., Rottman F. In vivo inhibition of Novikoff cytoplasmic messenger RNA methylation by S-tubercidinylhomocysteine. Biochemistry. 1977 Dec 27;16(26):5770–5775. doi: 10.1021/bi00645a019. [DOI] [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Identification of features in 5' terminal fragments from reovirus mRNA which are important for ribosome binding. Cell. 1978 Jan;13(1):201–212. doi: 10.1016/0092-8674(78)90150-2. [DOI] [PubMed] [Google Scholar]
- Levis R., Penman S. The metabolism of poly (A)+ and poly(A)-hnRNA in cultured Drosophila cells studied with a rapid uridine pulse-chase. Cell. 1977 May;11(1):105–113. doi: 10.1016/0092-8674(77)90321-x. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Rose J. K. Relative importance of 7-methylguanosine in ribosome binding and translation of vesicular stomatitis virus mRNA in wheat germ and reticulocyte cell-free systems. J Biol Chem. 1977 Feb 25;252(4):1181–1188. [PubMed] [Google Scholar]
- Maden B. E., Salim M. The methylated nucleotide sequences in HELA cell ribosomal RNA and its precursors. J Mol Biol. 1974 Sep 5;88(1):133–152. doi: 10.1016/0022-2836(74)90299-x. [DOI] [PubMed] [Google Scholar]
- Nau F. The methylation of tRNA. Biochimie. 1976;58(6):629–645. doi: 10.1016/s0300-9084(76)80387-2. [DOI] [PubMed] [Google Scholar]
- Ojala D., Attardi G. Expression of the mitochondrial genome in HeLa cells. XIX. Occurrence in mitochondria of polyadenylic acid sequences, "free" and covalently linked to mitochondrial DNA-coded RNA. J Mol Biol. 1974 Jan 15;82(2):151–174. doi: 10.1016/0022-2836(74)90338-6. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Kinetics of formation of 5' terminal caps in mRNA. Cell. 1976 Jul;8(3):433–442. doi: 10.1016/0092-8674(76)90156-2. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., LaTorre J. Synthesis and turnover of nuclear and cytoplasmic polyadenylic acid in mouse L cells. J Mol Biol. 1974 Jan 25;82(3):315–331. doi: 10.1016/0022-2836(74)90593-2. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Methylated constituents of heterogeneous nuclear RNA: presence in blocked 5' terminal structures. Cell. 1975 Sep;6(1):13–19. doi: 10.1016/0092-8674(75)90068-9. [DOI] [PubMed] [Google Scholar]
- Perry R. P. Processing of RNA. Annu Rev Biochem. 1976;45:605–629. doi: 10.1146/annurev.bi.45.070176.003133. [DOI] [PubMed] [Google Scholar]
- Rottman F. M., Desrosiers R. C., Friderici K. Nucleotide methylation patterns in eukaryotic mRNA. Prog Nucleic Acid Res Mol Biol. 1976;19:21–38. doi: 10.1016/s0079-6603(08)60906-x. [DOI] [PubMed] [Google Scholar]
- Salditt-Georgieff M., Jelinek W., Darnell J. E., Furuichi Y., Morgan M., Shatkin A. Methyl labeling of HeLa cell hnRNA: a comparison with mRNA. Cell. 1976 Feb;7(2):227–237. doi: 10.1016/0092-8674(76)90022-2. [DOI] [PubMed] [Google Scholar]
- Schibler U., Perry R. P. Characterization of the 5' termini of hn RNA in mouse L cells: implications for processing and cap formation. Cell. 1976 Sep;9(1):121–130. doi: 10.1016/0092-8674(76)90058-1. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Tilghman S. M., Curtis P. J., Tiemeier D. C., Leder P., Weissmann C. The intervening sequence of a mouse beta-globin gene is transcribed within the 15S beta-globin mRNA precursor. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1309–1313. doi: 10.1073/pnas.75.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalta J., Zalta J. P., Simard R. Isolation of nucleoli. A method that combines high yield, structural integrity, and biochemical preservation. J Cell Biol. 1971 Nov;51(21):563–568. doi: 10.1083/jcb.51.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]


