Abstract
Efforts toward routine islet cell transplantation as a means for reversing type 1 diabetes have been hampered by islet availability as well as allograft rejection. In vitro transdifferentiation of mouse bone marrow (BM)-derived stem (mBMDS) cells into insulin-producing cells could provide an abundant source of autologous cells for this procedure. For this study, we isolated and characterized single cell-derived stem cell lines obtained from mouse BM. In vitro differentiation of these mBMDS cells resulted in populations meeting a number of criteria set forth to define functional insulin-producing cells. Specifically, the mBMDS cells expressed multiple genes related to pancreatic β-cell development and function (insulin I and II, Glut2, glucose kinase, islet amyloid polypeptide, nestin, pancreatic duodenal homeobox-1 [PDX-1], and Pax6). Insulin and C-peptide production was identified by immunocytochemistry and confirmed by electron microscopy. In vitro studies involving glucose stimulation identified glucose-stimulated insulin release. Finally, these mB-MDS cells transplanted into streptozotocin-induced diabetic mice imparted reversal of hyperglycemia and improved metabolic profiles in response to intraperitoneal glucose tolerance testing. These results indicate that mouse BM harbors cells capable of in vitro trans-differentiating into functional insulin-producing cells and support efforts to derive such cells in humans as a means to alleviate limitations surrounding islet cell transplantation.
Type 1 diabetes is an insulin-dependent, autoimmune disorder characterized by the destruction of insulin-producing β-cells (1). Hence, a reversal of type 1 diabetes could be afforded by replacement of functional β-cells. Unfortunately, islet transplantation has historically been hampered by immune rejection and/or a recurrent attack by underlying autoimmunity against islets, as well as the scarcity of donor islets (2,3). One theoretical alternative for islet transplantation would involve the use of a renewable source of stem cells capable of self-renewal and differentiation, as well as that of insulin production. Indeed, the development of a simple, reliable procedure to obtain autologous stem cells having the ability to differentiate into functional insulin-producing cells would provide a potentially unlimited source of islet cells for transplantation and alleviate the major limitations of availability and allogeneic rejection.
Recent studies have shown that bone marrow (BM)-derived stem (BMDS) cells have the ability to differentiate into a number of neuroectodermal, endothelial, mesenchymal, epithelial, and endodermal cell types (4–10). The ability of hepatic stem cells, such as oval cells and functional hepatocytes, to derive from BM cells has also been suggested in several in vivo (11–13) and in vitro (10) studies. The observation that human BMDS cells can differentiate into mature hepatocytes (14,15) confirms the close interrelationship of BMDS cells and hepatocytes. Previously, we demonstrated (16) that highly purified rat hepatic oval cells can be induced to differentiate into functional insulin-producing cells when cultured long term in a high-glucose environment, that these differentiated oval cells express insulin, glucagon, and pancreatic polypeptide, and that they respond (i.e., produce insulin) to a high-glucose challenge. A key question that remained following those studies was whether BMDS cells could be induced to become functional insulin-producing cells. Because the pancreas and liver have common precursor cells during embryogenesis (17), stem cells of these two organs may have the same origin, that being from BM.
In this present study, we isolated murine BMDS (mBMDS) cells and obtained single-cell–derived cell clones that were subsequently induced to transdifferentiate into insulin-producing cells under culture conditions containing high concentrations of glucose and the addition of β-cell–stimulating factors. The functionality of these cells was confirmed by insulin production and release in a glucose-responsive manner and by their reversal of hyperglycemia after being transplanted into mice rendered diabetic by treatment with streptozotocin (STZ). Taken together, our results indicate that under suitable conditions, mBMDS cells can be induced in vitro to differentiate into functional insulin-producing cells capable of normalizing hyperglycemia in a diabetic animal model. This study provides support for continuing efforts aimed at utilizing adult stem cells as a steady and renewable source of autologous insulin-producing cells for transplantation in patients with type 1 diabetes.
RESEARCH DESIGN AND METHODS
BM isolation and derivation of single-cell–derived stem cell clones
Balb/c mice were purchased from the mouse production facility in the Department of Pathology, University of Florida. All procedures were performed under protocols approved by the Institutional Animal Care and Use Committee at the University of Florida. BM was obtained from the femurs and tibias (long bones) of 10 male Balb/c mice. The bones were sterilized by immersion in 70% ethanol, followed by removal of the remaining skin and muscles. BM was exposed by cutting the ends of the bones and extruded by inserting a needle and forcing cell culture medium with 10% FCS (HyClone, Logan, UT) through the bone shaft. Gentle pipetting resulted in the generation of a single-cell suspension. In such efforts, one mouse would routinely yield ~5 × 106 total BM cells. BM cells (2 × 106/ml) were cultured (37°C, 5% CO2) in 12-well plates with RPMI 1640 medium containing 10% FCS, 20 mmol/l HEPES, 1× penicillin, and streptomycin (Life Technology, Grand Island, PA). One week later, total media in the culture was changed and included removal of floating cells. Three weeks later, adherent cells gaining 80% confluence were passaged by pipetting with or without the use of trypsin. Following three to four passages, the cells become morphologically homogeneous, with a slim-spindle appearance. At this stage, single-cell–derived BMDS cell clones were derived using a cloning cylinder (Fisher Scientific, Pittsburgh, PA), with the selected cells being expanded and used for characterization of stem cell properties and for studies involving in vitro differentiation.
Antibodies
Rabbit anti-insulin polyclonal IgG (Santa Cruz Biotechnology, Santa Cruz, CA), guinea pig anti-insulin (Dako, Carpinteria, CA), rabbit anti-rat C-peptide antibody (Linco Research, St. Charles, MO), anti-rabbit IgG, guinea pig serum, and Cy3-coupled anti–guinea pig IgG (RDI, Research Diagnostics, Flanders, NJ) were obtained and utilized as indicated and in accordance with the manufacturer’s recommendations. Antibodies directed against CD34, CD45, C-kit, and Sca-1 (BD Pharmingen, San Diego, CA) were used for the flow cytometric analysis.
Flow cytometric analysis
The mBMDS cells at three to four passages were released by trypsinization. The cells were incubated with anti-mouse fluorescent dye–labeled hematopoietic antibodies, with 10,000 events acquired for analysis of fluorescence intensity, as previously described (18). Isotype-matched mouse immunoglobulins served as controls for autofluorescence.
In vitro differentiation cultures
To induce the mBMDS cells to undergo pancreatic endocrine cell differentiation, the cloned cells were cultured (37°C, 5% CO2) in basic medium composed of RPMI 1640 medium (10% FCS) for 2–4 months in the presence of low (5.5 mmol/l) or high (23 mmol/l) concentrations of glucose. Cellular differentiation was monitored by observation of three-dimensional, islet-like cell cluster formation and by the expression of genes related to pancreatic β-cell development and insulin production. To promote cellular maturation, the cells were cultured (37°C, 5% CO2) for 7 days in RPMI 1640 medium containing 5.5 mmol/l glucose, 5% FCS, and 10 mmol/l nicotin-amide (Sigma, St. Louis, MO). The cells were then cultured for an additional 5–7 days in the presence of 10 nmol/l exendin 4 (Sigma).
Cell line culture
The rat INS-1 cell line (clone 832/13), a cell line capable of insulin release in response of glucose stimulation, was a generous gift from Dr. Christopher B. Newgard (Duke University, Durham, NC). This cell line was derived from stable transfection of a plasmid containing the human proinsulin gene driven by a cytomegalovirus promoter and has the capacity to express and process both rat and human insulin. The cells were maintained in RPMI 1640 medium with 11.1 mmol/l D-glucose supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, 10 mmol/l HEPES, 2 mmol/l L-glutamine, 1 mmol/l sodium pyruvate, and 50 μmol/l β-mercaptoethanol at 37°C/5% CO2 in a humidified atmosphere (19). This cell line was used as a positive control for studies of insulin content and insulin release.
RT-PCR
Total RNA was prepared from BMDS cell cultures maintained in low- or high-glucose culture for 4 months using TRIzol reagent. To eliminate genomic DNA contamination, mRNAs were purified using oligo-dT cellulose (Micro-FastTrack 2.0 Kit; Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Transcriptional gene expression related to pancreatic endocrine development and function as well as other lineage markers (neuronal, intestine, and liver) from these cultures was determined by RT-PCR according to a published protocol (16) with minor modifications. The forward and reverse primers of each PCR set were designed to be located in different exons based on sequences obtained from GenBank to distinguish the PCR products from DNA contamination. Key PCR products of genes related to pancreatic development were confirmed by sequence analysis. The name and sequences of the primers, the sizes of PCR products, cycles, and annealing temperature for each pair are listed in Table 1.
TABLE 1.
List of mouse gene–specific primers in RT-PCR analysis of cells
| Genes | Forward primer | Reverse primer | Size of PCR product (bp) | GenBank accession no. | Annealing temperature (°C) | No. of cycles |
|---|---|---|---|---|---|---|
| Insulin I | TGG GGG TCG GGA ATC ACT GGT | TGG GCC TTA GTT GCA GTA GTT | 396 | X04725 | 60 | 32 |
| Insulin II | CTG GCC CTG CTC TTC CTC TGG | CTG AAG GTC ACC TGC TCC CGG | 204 | NM0008387 | 58 | 32 |
| Glut-2 | CAT TCT TTG GTG GGT GGC | CCT GAG TGT GTT TGG AGC G | 221 | X16986 | 55 | 35 |
| Glucokinase | GCA GAT CCT GGC AGA GTT CCA | GGA AGG AGA AGG TGA AGC CCA | 408 | BC0011139 | 66 | 32 |
| IAPP | CCT CAT CCT CTC TGT GGC AC | CAC GTT GGT TGG TGG GAG | 175 | M25389 | 55 | 32 |
| Nestin | GGA GAG TCG CTT AGA GGT GC | GTC AGG AAA GCC AAG AGA AG | 327 | NM016701 | 58 | 35 |
| Pdx-1 | TGG ATA AGG GAA TTG CTT AAC CT | TTG GAA CGC TCA A GT TTG TA | 249 | NM008814 | 62 | 32 |
| Pax6 | GCA CAC GCC CTG GTT GGT | CAC TGT ACG TGT TGG TGA G | 512 | NM013627 | 60 | 32 |
| Pax4 | GGA CTC TTT GTG AAT GGC CGG | TTT AGC TGG GCA ATT CGA GCC | 236 | XM133023 | 64 | 35 |
| NeuroD | ATG ACC AAG GCG CGC CTA GA | ACA GGA CAG TCA CTG TAC GCA C | 425 | U28068 | 55 | 35 |
| Oct-4 | GGC GTT CTC TTT GGA AAG GTG TTC | CTC GAA CCA CAT CCT T CT CT | 312 | XM285447 | 56 | 35 |
| Isl-1 | TTT CCC TGT GTG TTG GTT G | GTC TTC TCG GGC TGT TTG T | 501 | NM021459 | 56 | 35 |
| GLP-1R | GAA TAC CGG CGG CAG TGC CA | CTG TGC AAG TGT CTG AAG CCA | 402 | NM_021332 | 56 | 35 |
| Albumin | ATG CTC ATA CGA TGA GCA TGC | ATG GTG GCA GGC TGG GGT TG | 245 | BC024643 | 56 | 35 |
| TTR | TCG CTG GAC TGG TAT TTG TG | GTT GGC TGT GAA AAC CAC ATC C | 323 | BC024702 | 56 | 35 |
| AFP | CGT GAC GGA GAA GAA TGT GC | TCT TAA TTC CTTT GCA ATG GA | 515 | BC066206 | 56 | 35 |
| GFAP | CTA AGA TGA AGT TAT GGG ATG | ACA TTT AAG TGT ATG GCA GT | 389 | NM_010275 | 56 | 35 |
| Tubulin | TCT GGG AGG TCA TCA GCG AT | TCA CGC ACC TTG CTG TGA GCA | 412 | NM_023279 | 56 | 35 |
| NFM | TAT GCT CAG CTC GGC CGA GAG | GCA CTT GAG CCT TCT CGT GGT | 311 | NM_008691 | 56 | 35 |
| Muc2 | GGC ATT GTG TGC CAA CCA AAG | CCT TGG GCA CAC AGG AAT AAA CTG | 235 | XM_133960 | 56 | 35 |
| Sucrase | ACG ATA ATA GCT ATC GCT CT | TAA AGA TTG GCC ATG TTT TCC | 693 | XM_143332 | 56 | 35 |
| Villin | GGC TAT GCA GAT GGT ACC TGT | AGT CGC TGG ACA TCA CAG GA | 341 | NM_009509 | 56 | 35 |
AFP, α fetal protein; GFAP, glial fibrillary acidic protein; IAPP, islet amyloid polypeptide; MUC2, mucin 2.
DNA-PCR for detection of microsatellite polymorphism
Genomic DNA was isolated from tissues and cell lines with phenol-chloroform followed by ethanol precipitation. Extracted DNA was resuspended in TE buffer (10 mmol/l Tris-Cl, pH 7.5, 1 mmol/l EDTA). Five highly polymorphic markers unique for mice, including D2Mit30, D3Mit15, D6Mit15, D11Mit4, and D2Nds3, were selected from the Mouse Genome Informatics database. The sequences for the microsatellite markers can be obtained from The Jackson Laboratories website (http://www.informatics.jax.org). DNA (500 ng) was used as a template in PCRs. The sizes of the DNA products obtained with the five markers (D2Mit30, D3Mit15, D6Mit15, D11Mit4, and D2Nds3) in Balb/c mice are 136, 143, 195, 242, and 400 bp, respectively.
Immunocytochemistry and immunofluorescence
Cytospin slides from differentiated mBMDS (D-mBMDS) cells were made for insulin and C-peptide protein expression. The cells were fixed with 4% formaldehyde for 30 min at room temperature, and immunocytochemistry performed with polyclonal guinea pig anti-insulin (1:500) (Dako) and guinea pig anti-rat C-peptide antibody (1:100) (Linco Research) for 1 h. After washing, the cells were incubated with Cy3-coupled anti-guinea pig (1:1,000) secondary antibodies (RDI) for 30 min. Guinea pig serum was used as a negative control. Cells were then examined by fluorescence microscopy (Olympus BX51).
Deconvolution microscopy
Cells were stained with Cy3-conjugated secondary antibodies after they were incubated with antibodies specific for insulin or C-peptide. The nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and imaged by deconvolution microscopy using an Olympus OMT inverted fluorescent microscope system equipped with Delta Vision deconvolution analysis software. The images depict three-dimensional projections of 25 optical slices (each 0.2-μm thick) through the cell, with the center focused on the DAPI-stained chromatin in the nuclei. All images used in this report were scale adjusted, including images of staining with nonspecific isotype antibody conjugates as a negative control.
Mouse insulin enzyme-linked immunosorbent assay
D-mBMDS cells were cultured (37°C, 5% CO2) in the presence or absence of exendin-4 for 7 days after 1 week of 10 mmol/l nicotinamide treatment in RPMI 1640 containing 5% fetal bovine serum and 5.5-mmol/l glucose. Following this, the cells were confirmed to express insulin genes by RT-PCR. In a parallel experiment, the cells were cultured in the presence of exendin 9–39 for 7 days. The cells were switched to serum-free medium containing 0.5% BSA for 12 h, washed twice with PBS, then stimulated by the addition of 23 mmol/l glucose for 2 h. The culture media was collected and frozen at −70°C until assay for insulin release. Importantly, serum-free culture medium containing 0.5% BSA was used as a control for secreted insulin measurements. Insulin release was detected by using an ultrasensitive mouse insulin enzyme-linked immunosorbent assay (ELISA) kit (Alpco Diagnostics, Windham, NH) following the manufacturer’s protocols. According to the manufacturer’s instructions, this assay does not detect proinsulin.
Electron microscopy with immunogold labeling
For immunogold localization of insulin, the cells were embedded in Lowicryl K4M resin (EM Sciences, Fort Washington, PA). Ultrathin sections were blocked with 5% BSA/5% normal goat serum in PBS and then incubated overnight at 4°C in rabbit anti-insulin antibody (Santa Cruz Biotechnology) diluted 1:50 in PBS containing 0.2% BSA and 10 mmol/l NaN3. After washing, the samples were incubated for 1.5 h at room temperature with the secondary goat anti-rabbit IgG antibody conjugated to 0.8-nm colloidal gold particles (Aurion EM Grade Ultra Small, EM Sciences), washed, treated with 1.25% glutaraldehyde in PBS, and washed again. The gold particles were silver enhanced for 45 min at room temperature (Aurion R-Gent SE; EM Sciences). The samples were counter-stained using uranyl acetate and lead citrate, then viewed using a Zeiss EM-10A transmission electron microscope.
Transplantation studies in mice
Balb/c male mice received two intraperitoneal injections of STZ at 250 and 50 μg/g body wt, 3 days apart, according to published procedures (20,21) with minor modification. Blood glucose levels were monitored using an AccuChek glucose detector (Roche Diagnostics, Indianapolis, IN). Within 12 days of the first injection, all Balb/c mice became hyperglycemic, with blood glucose levels >350 mg/dl. The D-mBMDS cells (5 × 106/mouse) were transplanted into the left renal capsule and the distal tip of the spleen of six diabetic mice. Five diabetic mice received sham surgery without implants as a control. The nonfasting blood glucose levels were monitored at 1600 every 2 days following transplantation. Most of the diabetic mice with sham surgery died between 15 and 20 days because they did not receive insulin treatment. The diabetic mice with D-mBMDS cell transplants were killed 26 days after transplantation. The pancreas tissue was harvested for morphologic analysis. For the intraperitoneal glucose tolerance (IPGT) test, normal nondiabetic Balb/c male mice (n = 5) and diabetic mice (n = 3) with normalized glucose levels following the D-mBMDS cell transplantation received intraperitoneal injections of glucose (2 mg/g body wt) according to published procedures (20). Blood glucose levels were monitored at 0, 30, 60, 90, 120, and 150 min for each mouse.
Measurement of apoptosis
The mBMDS cells were cultured in medium containing 23 mmol/l glucose for various times including 1 week, 2 weeks, 1 month, and 2 months. Cells were passaged when they reached 80–90% confluency. Cultured mBMDS cells in the medium containing a 5.5-mmol/l glucose concentration served as a baseline control for no treatment. Cells were released from culture dishes by trypsinization and incubated in the same medium for an additional 2 h in suspension at 37°C. The cells them were then subjected to testing for apoptosis using an Annexin V-PE apoptosis detection kit I (BD Biosciences Pharmingen) following the manufacturer’s protocol.
Statistics
Evidence of statistical significance was determined by Fisher’s exact testing. A P value of <0.05 was deemed significant.
RESULTS
Derivation and characterization of BMDS cells from mice
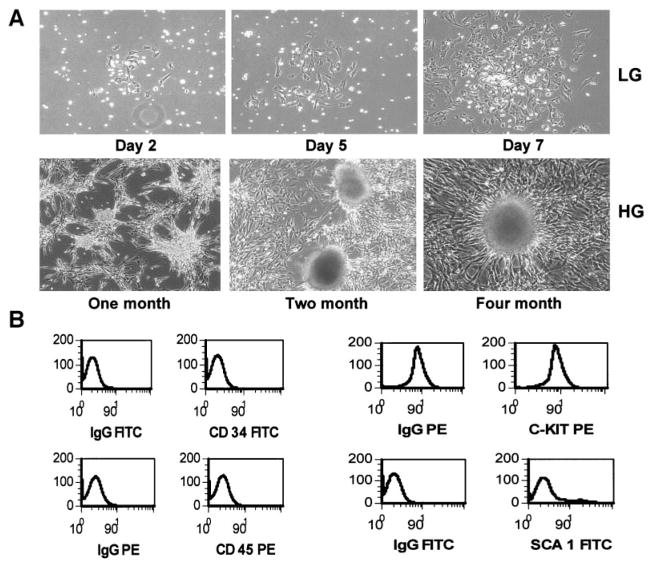
BM cells from 10 Balb/c male mice were used to obtain BMDS cells. Adherent BMDS cells were derived from cultures of unsorted BM cells. The unattached cells were removed following 2–7 days of culture, with spindle-shaped adherent cells (Fig. 1A, upper panel) cultured for an additional 2–3 weeks, until the spindle-shaped adherent cells reached 70–80% confluence. The cells were then released from the substrate surface with trypsin-EDTA or pipetting and were replated under the same culture conditions for several passages. A single-cell–derived cell clone was obtained by trypsinization of a single-cell–derived cell cluster using a cloning cylinder. The single-cell–derived mBMDS cells, as well as the mixed mBMDS cells, were then characterized for their stem cell properties and their phenotypes. Specifically, the resulting cell lines were assessed by flow cytometric analysis of surface markers and by differentiation potential under selective culture conditions. The phenotype of these mBMDS cells was predominantly negative for CD45, CD34, C-kit, and Sca-1, whereas a small subpopulation of these cells was positive for Sca-1 (Fig. 1B). Thus, the immunophenotype appeared similar but not identical to that of multipotent adult progenitor (MAP) cells reported by Jiang et al. (22). We observed that these mBMDS cells could be induced to differentiate into neural or endothelial cells (data not shown), but induction to hepatocytes using the protocols of Schwartz et al. (10) was not successful. These findings are consistent with the notion that mBMDS cells possess stem cell properties. Therefore, we believe that the BMDS cells most likely represent pluripotent mesenchymal stem/precursor cells.
FIG. 1.
Isolation, derivation, and characterization of clonal mBMDS cells. A: BM cells (2 × 106 cells/ml) from Balb/c mice were plated and cultured for 2–7 days to obtain the adherent mBMDS cells (top). Cloned mBMDS cells were used for the in vitro differentiation protocol by culturing cells in the presence of a 23-mmol/l glucose concentration for various times. Many cell clusters at various stages of cluster formation during the course of induction of cell differentiation were observed, with a representative shown in the bottom panel. HG, high glucose; LG, low glucose. B: A representative phenotype of the mBMDS cells. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
In vitro differentiation of mBMDS cells into insulin-producing cells
To induce cell differentiation, six single-cell–derived clones of the mBMDS cells were switched into RPMI 1640 medium containing 10% FCS and a high-glucose concentration (23 mmol/l). After 2–4 months of in vitro induction, four of the six single-cell–derived clonal cultures began to form three-dimensional clusters similar to that shown in our previous study (16) (Fig. 1, lower panel). To promote further differentiation, the cells were switched to medium containing 5% FCS with 10 mmol/l nicotinamide, 10-nmol/l exendin 4, and 5.5 mmol/l glucose. These culture conditions promoted further cluster formation, in which the cell clusters increased in both number and mass, as well as increasing their glucose responsiveness (data not shown). Cellular differentiation was then monitored by RT-PCR for ascertainment of gene expression (see below).
Gene expression of mBMDS and D-mBMDS cells
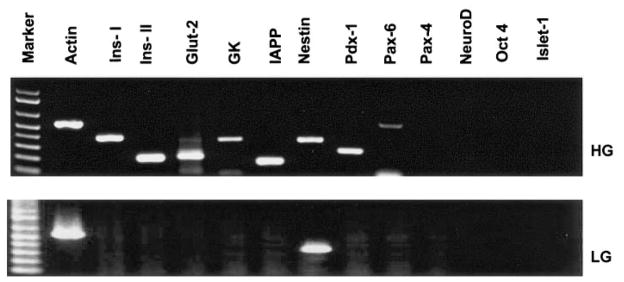
To determine whether the mBMDS cells had undergone pancreatic differentiation, gene expression profiles for pancreatic β-cell differentiation markers and hormones were assessed using RT-PCR. Four of the six mBMDS clones underwent pancreatic endocrine differentiation as evidenced by expression of Pdx-1 and insulin genes. As illustrated in Fig. 2, cells cultured under high-glucose concentrations (23 mmol/l) for 4 months expressed multiple genes characteristic of endocrine β-cell development, including insulin I and II, Glut-2, glucose kinase, islet amyloid polypeptide, nestin, Pdx-1, and Pax6. However, gene expression of Pax4, NeuroD, and islet-1 (Fig. 2, upper panel) was not detected. The Oct-4 gene, typical for pluripotent embryonic stem cells, was not detected in mBMDS or D-mBMDS cells. In contrast, mBMDS cells cultured under a low-glucose concentration (5.5 mmol/l) for 4 months expressed no detectable levels of the aforementioned genes with the exception of nestin (Fig. 2, lower panel). Further differentiated mBMDS cells (late stage), similar to the mouse β-cell line (β-TC) expressed the glucagon-like peptide (GLP)-1 receptor gene (Fig. 3D), yet this gene expression was not observed in undifferentiated mBMDS cells or in early stages of the D-mBMDS cells (data not shown).
FIG. 2.
Gene expression patterns in D-mBMDS cells (high glucose, HG) and undifferentiated mBMDS cells (low glucose, LG). Total RNA was isolated from cultures of undifferentiated mBMDS (grown in LG medium for 4 months) and D-mBMDS (grown in HG medium for 4 months) cell cultures. RT-PCR was performed to detect genes related to β-cell development and insulin production. All PCR products were verified by DNA sequence analysis. The results were repeated at least three times.
FIG. 3.
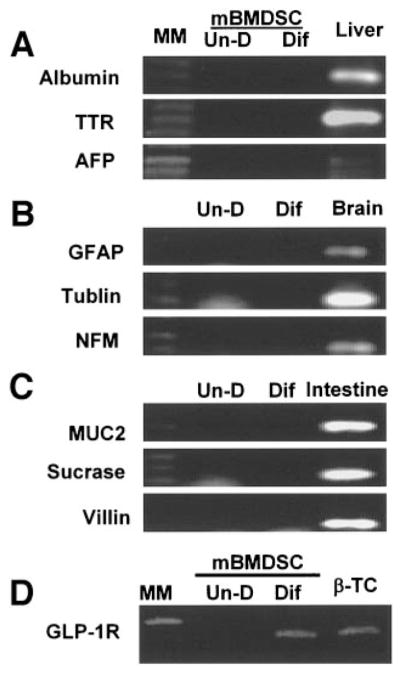
Gene expression of nonpancreatic genes. Total RNA was isolated from cultures of undifferentiated (Un-D) and D-mBMDS (Dif) cell cultures. RT-PCR was performed to detect genes related to hepatic (A), neuronal (B), and intestinal (C) differentiation. GLP-1 receptor gene expression among cells (D) is presented. AFP, α fetal protein; GFAP, glial fibrillary acidic protein; MM, molecular marker; MUC2, mucin 2; NFM, neurofilament protein.
To determine whether any non–β-cell markers were expressed in these cells, several markers representing hepatic, intestinal, and neuronal differentiation were analyzed by RT-PCR to compare gene expression between the undifferentiated and D-mBMDS cells. Mouse liver, brain, and intestinal tissue served as a positive control. As indicated in Fig. 3, no detectable expression of the hepatic (Fig. 3A), intestinal (Fig. 3B), and neuronal (Fig. 3C) genes was observed in either differentiated or undifferentiated cells, with the exception of nestin. These results suggest that culture of mBMDS cells under a long-term, high-glucose condition favors their pancreatic endocrine differentiation.
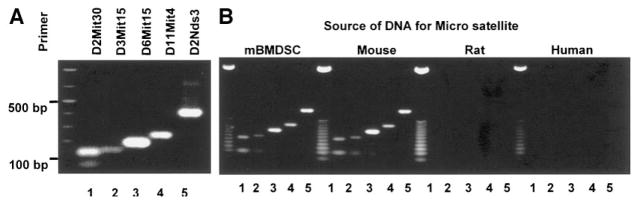
To confirm that the D-mBMDS cells are indeed derived from Balb/c mice and that there is no cross-contamination from different cell lines or species, we analyzed cellular DNA for five mouse microsatellite molecular markers: D2Mit30, D3Mit15, D6Mit15, D11Mit4, and D2Nds3. Tissues from Balb/c mice as well as rat and human were obtained for these analyses. Genomic DNA from Balb/c mice served as a positive control in these analyses. These mouse microsatellite markers have the ability to distinguish tissues among different species (mouse, rat, and human), but in addition, can also be used to distinguish different strains within the same mouse species (i.e., NOD/MrKTac, Balb/cJ, C57BL/6J CAST, etc.) due to polymorphisms at a particular site of the host chromosome. The PCR results assigning the specific polymorphism (Fig. 4A) showed that the sizes of the PCR products of the five markers (D2Mit30, D3Mit15, D6Mit15, D11Mit4, and D2Nds3) were precisely located at 136, 143, 195, 242, and 400 bp, respectively, and that the pattern was identical to that of a Balb/c mouse (Fig. 4B, left panels). There was no cross-contamination among species present (Fig. 4B).
FIG. 4.
Confirmation of the mBMDS cell origin by microsatellite. Five mouse-specific probes were selected for studies of polymorphism to distinguish among species and among mouse strains. The sizes of the PCR products of the five markers (D2Mit30, D3Mit15, D6Mit15, D11Mit4, and D2Nds3) in Balb/c mice are 136, 143, 195, 242, and 400 bp, respectively.
Insulin and C-peptide synthesis by D-mBMDS cells
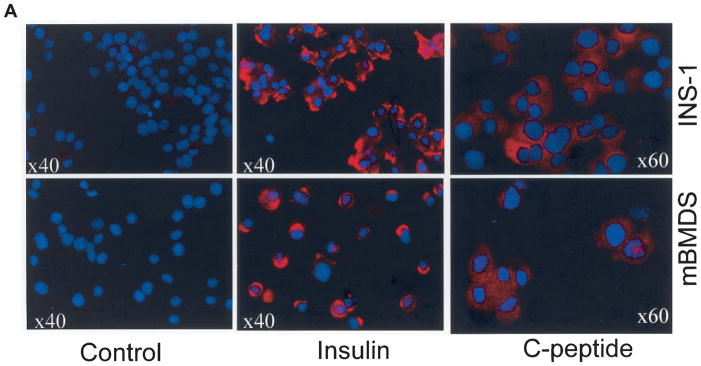
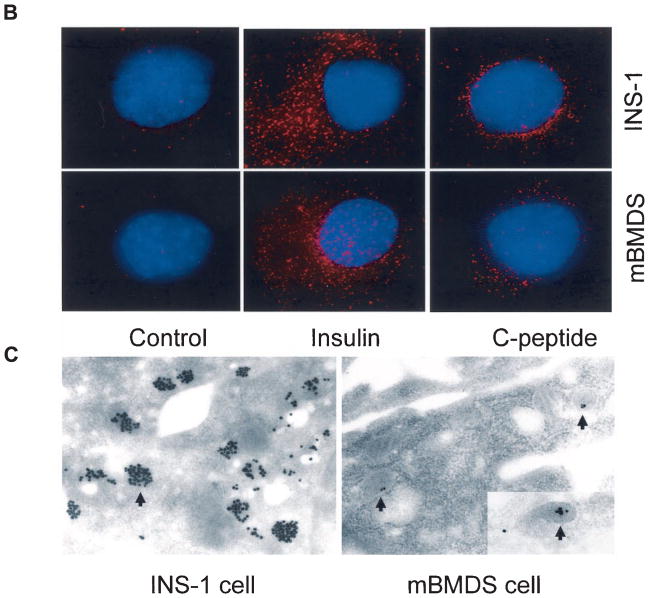
To determine whether the D-mBMDS cells actually synthesize insulin protein and release C-peptide, the differentiated cells after further culture (1 week) under conditions including the presence of nicotinamide and exendin 4, were stained with anti-insulin and anti–C-peptide antibodies and visualized by fluorescence microscopy (Fig. 5A). Figure 5A shows that ~10–20% of the D-mBMDS cells (counted in various microscopic fields) stained strongly with the anti-insulin antibody (bottom middle panel). Strong C-peptide cytoplasmic staining was also detected in 10–20% of the examined cells (Fig. 5A, bottom right panel). The staining pattern and intensity for both insulin and C-peptide was similar to that observed in the positive control INS-1 cells (Fig. 5A, top panels). Matched isotype control antibodies served as negative controls (Fig. 5A, left). These results indicated that the D-mBMDS cells are indeed synthesizing and processing insulin as indicated by the presence of C-peptide, a byproduct of de novo insulin release and one observed following further in vitro differentiation into more mature insulin-producing cells.
FIG. 5.
A: Insulin and C-peptide production by D-mBMDS cells. Cytospin slides made of cultured D-mBMDS and INS-1 cells were stained with anti-insulin (middle) and anti–C-peptide (right) antibodies and visualized under fluorescence microscopy. INS-1 cells were used as a positive control for insulin and C-peptide. A negative control utilizing isotype-matched antibodies is shown (left). B: Analysis of insulin granules by deconvolution microscopy. The distribution of insulin and C-peptide granules in both INS-1 and D-mBMDS cells visualized by deconvolution microscopy following insulin and C-peptide immunostaining. Insulin and C-peptide molecules were stained in red color, and the nuclei were stained with DAPI (blue color). C: Analysis of insulin granules by electron microscopy. Immunogold labeling of insulin in INS-1 (left panel) and in D-mBMDS (right panel) cells is shown. Arrows indicate immunogold-labeled insulin granules.
Analysis of insulin granules by deconvolution and electron microscopy with gold labeling
To confirm and identify the distribution of insulin granules in single cells, we used immunohistochemical staining with anti-insulin antibodies visualized by deconvolution and electron microscopy to compare the D-mBMDS cells with INS-1 cells in terms of their granular distribution and ultrastructure. Figure 5B demonstrates a similar distribution of insulin granules in the D-mBMDS cells to that observed in the INS-1 cells. Figure 5C confirms that the globular structures observed in both INS-1 (Fig. 5C, left) and D-mBMDS (Fig. 5C, right) cells contain insulin (high electron-dense core in the granules are indicated by arrow), but in the case of mBMDS cells, at levels far less than that observed in INS-1 control cells.
Insulin content and release in response to glucose stimulation
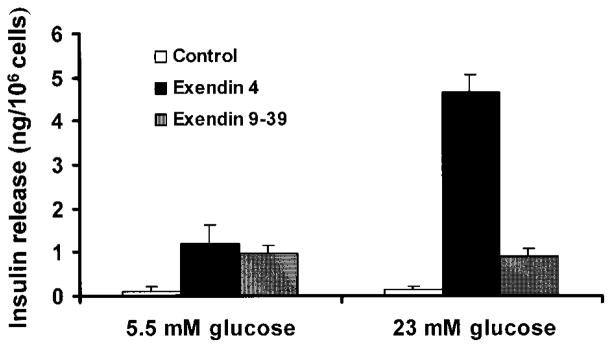
To determine whether the D-mBMDS cells were responsive to a glucose challenge, insulin release from undifferentiated and D-mBMDS cells was measured using an ultrasensitive mouse insulin ELISA. In order to enhance the sensitivity of these cells to high-glucose challenge, the differentiated cells were switched to low-serum, low-glucose medium plus nicotinamide for 1 week. Since the GLP-1R gene was expressed in the latter stages of D-mBMDS cells, as shown in Fig. 3D, we then cultured cells in the presence of either exendin 4, or its antagonist, exendin 9–39, for 7 days before analysis. The cells were then washed twice with PBS and switched to serum-free low-glucose medium containing 0.5% BSA (overnight incubation), then stimulated by the addition of 23 mmol/l glucose for 2 h. The cell culture media was then collected for the analysis. All studies were performed in triplicate. The results shown in Fig. 6 indicate that approximately fourfold more insulin was released in exendin 4–treated cells in comparison to the cells treated with exendin 9–39 under long-term high-glucose conditions. In contrast, control mBMDS cells cultured in low concentrations of glucose (5.5 mmol/l) showed no significant release of insulin in the presence or absence of glucose challenge, even in the presence of exendin 4. These data suggest that high-glucose culture plays an indispensable role in the transdifferentiation of BMDS cells into insulin-producing cells and that differentiated BMDS cells were responsive to glucose challenge. Moreover, the results also indicated that the D-mBMDS cells might represent a precursor to β-like cells and that further induction might be needed to reach a high degree of differentiation and maturation, as would be observed in the in vivo hyperglycemic environment of diabetic animals.
FIG. 6.
Insulin release of mBMDS cells (□), D-mBMDS cells treated with exendin 4 (■), and D-mBMDS cells treated with exendin 9–39 (
 ) upon glucose stimulation. Cells were cultured in RPMI 1640 medium containing 5.5 mmol/l glucose and 5% FCS, plus nicotinamide for 1 week and for an additional 1 week in the presence of either exendin 4 or exendin 9–39. The cells were then switched to serum-free medium containing 0.5% BSA for 12 h and then stimulated with 23 mmol/l glucose for 2 h. The cell culture medium was then collected for assay of insulin release. Released insulin in the media was detected by an ultrasensitive ELISA kit. Results shown here represent those of three separate experiments.
) upon glucose stimulation. Cells were cultured in RPMI 1640 medium containing 5.5 mmol/l glucose and 5% FCS, plus nicotinamide for 1 week and for an additional 1 week in the presence of either exendin 4 or exendin 9–39. The cells were then switched to serum-free medium containing 0.5% BSA for 12 h and then stimulated with 23 mmol/l glucose for 2 h. The cell culture medium was then collected for assay of insulin release. Released insulin in the media was detected by an ultrasensitive ELISA kit. Results shown here represent those of three separate experiments.
As an additional assessment, we quantitatively evaluated the content of insulin in both mBMDS-derived insulin-producing cells as well as in the INS-1 cell line (clone 832/13) (Table 2). The ratio of insulin content to insulin release did not vary dramatically between the D-mBMDS cells (8.7) and INS-1 cells (11.5), although the amount of insulin content and release in INS-1 cells was significantly higher. Because the rat insulinoma INS-1 cell line (clone 832/13) was derived from stable transfection of a plasmid containing the human proinsulin gene driven by a cytomegalovirus promoter, it must be remembered that the content and release of insulin in INS-1 cells does not represent the physiological levels seen in native pancreatic β-cells.
TABLE 2.
Comparison of insulin release and content between mBMDS and INS-1 cells
| Cell type | Insulin release (ng/ml) | Insulin content (ng/ml) | Content/release ratio |
|---|---|---|---|
| INS-1 | 98.3 ± 18.31 | 1,130.3 ± 204.23 | 11.5 |
| D-mBMDS | 1.5 ± 0.17 | 13.3 ± 1.69 | 8.7 |
Data are means ± SD.
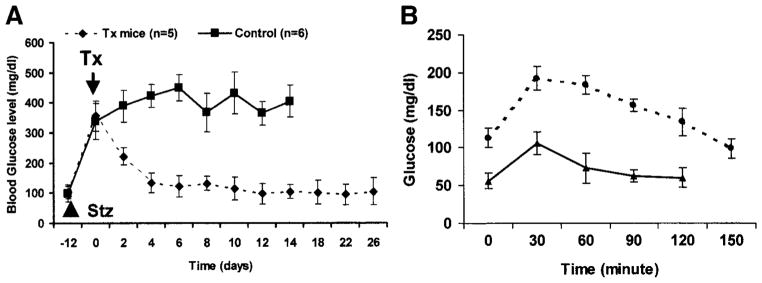
Reversal of hyperglycemia in STZ-induced diabetic mice
To determine whether the D-mBMDS cells possessed the capacity to correct hyperglycemia in diabetic mice, Balb/c mice were STZ induced to become diabetic before transplantation with D-mBMDS cells (5 × 106/mouse). Five STZ-induced diabetic mice received sham surgery without cellular implantation as a control. As demonstrated in Fig. 7A, glucose levels in the mBMDS cell–implanted mice decreased and normalized within 1 week following transplantation. In contrast, blood glucose levels in the diabetic control mice remained elevated (P < 0.01; diabetes frequency at day 14). The diabetic control mice lost weight persistently (data not shown), with many dying between 15 and 20 days after diabetes onset. These results suggested that the D-mBMDS cells are functional in vivo and capable of reversing hyperglycemia in diabetic mice. To further evaluate the function of the implanted mBMDS cells, we performed an IPGT test on control (nondiabetic) Balb/c mice (n = 5) and transplanted Balb/c mice (n = 3) after 14 days of normalized glucose levels following the transplant. These studies involved glucose administration (2 mg/g) provided under fasting conditions. As illustrated in Fig. 7B, blood glucose levels in normal control mice rose rapidly, with peak values obtained at 30 min, followed by a return to the normal range between 60 and 90 min. Blood glucose levels in the implanted mice were generally higher, but likewise displayed a peak at 30 min and returned to the normal range at 150 min. This result indicated that the implanted mBMDS cells were indeed responsive to a glucose challenge in vivo, but were not as effective as native pancreatic β-cells in terms of restoring normoglycemia.
FIG. 7.
A: Cell transplantation using D-mBMDS cells. Balb/c mice became diabetic within 12 days after two intraperitoneal injections of 250 and 50 μg/g body wt STZ over 3 days apart. Glucose levels were monitored by tapped tail-vein blood at 1600 under nonfasting conditions. ■, blood glucose levels in sham surgery diabetic mice (n = 5); ◆, mBMDS cell–implanted mice after their glucose levels had reached >350 mg/dl (n = 6). The arrow indicates the day of implantation (shown as day 0). Values are means ± SD. B: Glucose responses during the IPGT test. Glucose tolerance was tested following an intraperitoneal injection of glucose (2 mg/g body wt) in overnight-fasted control (▲) (n = 5) and implanted (●) (n = 3) mice 14 days after transplantation. The venous blood was collected from the tail vein at 0, 30, 60, 90, 120, and 150 min after the injection. Values are means ± SD.
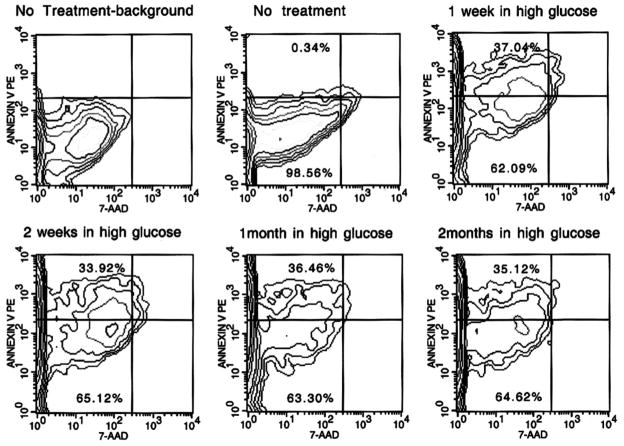
Effects of high-glucose culture on mBMDS cells
These results demonstrated that long-term culturing of adult stem cells under high-glucose conditions promoted their pancreatic endocrine differentiation. However, the potential apoptotic effect of high-glucose cultures on the mBMDS cell was unknown. To evaluate this possibility, the mBMDS cells were cultured under normal-glucose (5.0 mmol/l) or high-glucose (23 mmol/l) conditions for various time periods for up to 2 months, and then the cells were subjected to apoptosis analysis as described in RESEARCH DESIGN AND METHODS. As shown in Fig. 8, the high-glucose cultures indeed induced apoptosis in mBMDS cells, even though overt apoptotic changes were not visible by light microscopy. However, the percentages of the cells undergoing apoptosis remained relatively constant, between 34 and 37% in all culture conditions. Indeed, there were no statistical differences between short-term and long-term high-glucose cultures in that parameter. However, there was a marked difference in the increased number of apoptotic cells present in the high-glucose cultures when compared with their normal culture controls. Interestingly, at least 60% of cells in all high-glucose culture conditions did not show signs of either apoptosis or necrosis.
FIG. 8.
Flow cytometric analysis of apoptotic cells. Cells were cultured under high-glucose conditions for the indicated time periods, then collected for flow analysis. Intact cells were stained with Annexin-PE to detect outer-leaf phosphoserine, which is indicative of bilayer flipping and membrane blebbing in early apoptosis, and counterstained with the intercalating dye, 7AAD, to detect membrane permeability and chromatin degradation. Cells that stained with Annexin V but did not take up the 7AAD (upper left quadrant of each contour plot) were considered “apoptotic.” Cells that had background levels of Annexin V staining (set on untreated cell cultures, upper left panel) and no uptake of 7AAD were considered “viable and nonapoptotic.” The percentage of total cells (out of 10,000 detected events) in the “apoptotic” (upper left) quadrant and the “viable” (lower left) quadrant are given. PE, phycoerythrin
DISCUSSION
Recent studies have demonstrated the feasibility of generating insulin-producing cells obtained from progenitor cells of various cellular sources, including the pancreas (23,24), liver (16), and intestinal epithelium (25), as well as the pluripotent embryonic stem cells of mouse (20,26) and human (27) origin. However, even with the conceptual advances offered by these findings, some obstacles, such as immune rejection and autoimmunity against newly formed β-cells derived from pancreatic stem cells, still remain. Despite their promising potential, it may also prove difficult to obtain enough autologous adult stem cells from these organs.
To overcome these limitations, we explored the possibility of using human and mouse BMDS cells as sources for transdifferentiation into insulin-producing cells under specific in vitro culture conditions. BM has been known for years to represent a safe and abundant source for large quantities of adult stem cells. In the present study, we isolated, cloned, and characterized mBMDS cells. We also generated functional insulin-producing cells from the mB-MDS cells under an in vitro differentiation procedure and confirmed the presence of insulin production by RT-PCR, immunofluorescence, and electron microscopy combined with gold anti-insulin labeling. Furthermore, we tested the functionality of the in vitro–generated insulin-producing cells from mouse BM by measuring insulin release in response to a glucose challenge and by demonstrating a reversal of diabetes upon subsequent implantation of these cells into diabetic mice. In addition to these studies, we have derived islet-like, functional insulin-producing cells from human BMDS cells (D.-Q.T., B.R.B., L.-Z.C., S.A.L., M.A.A., L.-J.Y., unpublished data). Taken collectively, these studies provide direct evidence that the BM contains pluripotent cells capable of being reprogrammed in vitro to become functional insulin-producing cells.
Several in vivo studies (28–30) demonstrate that BM cells contribute to pancreatic β-cell regeneration at a low frequency, ranging from 1 to 2.7%. Ianus et al. (29) provided in vivo evidence of adult mouse BM harboring cells that could transdifferentiate into glucose-competent pancreatic endocrine cells using a cAMP response element–LoxP system, as assessed in cross-sex BM transplant experiments. The above findings indicate that this in vivo process is likely due to transdifferentiation of BM-derived cells into insulin-producing β-cells, rather cell fusion, as the main source of BM-derived hepatocytes repopulating the liver of the mice, as was the case with fumarylaceto-acetate hydrolase deficiency, suggested by other studies (15,31). In our current study, the homogeneous mBMDS cells were used to induce in vitro differentiation into insulin-producing cells; hence, cell fusion is likely not the answer for the presence of competent insulin-producing cells. Other studies confirm that allogeneic BM transplantation with as low as 1% chimerism in pancreatic islets can reverse the diabetogenic process in pre-diabetic mice (28). Hess et al. (30) have provided a theory based on their observations in a recent study to explain the possible mechanism of reversing hyperglycemia in diabetic mice after BM transplantation. They suggest that pancreatic engraftment of donor BM–derived cells expressing endothelial markers after BM transplantation initiate endogenous β-cell regeneration, whereas donor BM–derived insulin-positive β-cells represent a rare event. Kojima et al. (32) recently found extrapancreatic proinsulin-producing cells present in the liver, BM, spleen, adipose tissue, and thymus in hyperglycemic animals and that the majority of these proinsulin-producing cells were derived from the donor BM, as evidenced by BM transplantation experiments. These studies support our observation that BM contains stem cells capable of differentiation into insulin-producing cells.
One of the key questions to address is which cell type in the BM is responsible for pancreatic endocrine transdifferentiation. Unfortunately, it is difficult to extrapolate the cell phenotype from currently available studies using an in vivo approach. Our results from both humans and mice suggest that CD45-negative adherent pluripotent mesenchymal stem cells are capable of transdifferentiation into insulin-producing cells in vitro under high-glucose culture conditions. The common phenotype of the BMDS cells between humans and mice is CD45 negative, CD34 negative, and C-kit negative, indicating that they are unlikely to be hematopoietic stem cells. However, the possibility of circulating pancreatic stem cells cannot be completely excluded. A recent study published by Kodama et al. (33) indicated that injection of splenocytes into pre-diabetic NOD mice reversed diabetes and promoted pancreatic β-cell regeneration. Their results further indicated that CD45-negative splenocytes (presumably mesenchymal precursor cells) were responsible for islet β-cell regeneration. This result indirectly supports our conclusion that the BM-derived stem cells capable of generating islet precursor cells have an immunophenotype and biologic characteristics similar to those of BM mesenchymal cells.
There are two key steps in our cell culture conditions that appear important for inducing the differentiation of BMDS cells into insulin-producing islet-like cells. First, the mBMDS cells initially require culture in medium containing a high-glucose concentration (23 mmol/l) for various durations of time until certain genes, such as Pdx-1, insulin I and II, Glut-2, and islet amyloid polypeptide, become detectable. Second, in order for the D-mBMDS cells to become glucose responsive, further differentiation and maturation are required through either in vitro culture with β-cell–promoting factors, such as nicotinamide and exendin 4, or transplantation of the cells into diabetic animals. In this study, we demonstrated that mBMDS cells cultured under low-glucose conditions did not express the aforementioned genes, and in addition, they did not secrete insulin upon glucose stimulation, even in the 7-day presence of the β-cell–stimulating factors exendin 4 and nicotinamide. Our data indicate that long-term culture in a high-glucose medium reprogrammed these cells toward a pathway of pancreatic endocrine cell differentiation. At a certain period of time (2–4 months) and via a still unclear mechanism, this switch occurred.
It is well known that glucose is a growth factor for β-cells (34). It promotes β-cell replication in vitro and in vivo at a 20- to 30-mmol/l concentration (35) and increases insulin content in cell lines derived from embryonic stem cells (20) at a 5-mmol/l concentration. The effect of chronic hyperglycemia on pancreatic β-cells, however, remains controversial. In an in vivo study, Jonas et al. (36) showed that the expression of several genes important for glucose-stimulated insulin secretion (glucose metabolism enzymes and ion channels/pumps) was gradually decreased with increasing levels of blood glucose. They also suggested a link between stimulation of β-cell growth and a reduced state of differentiation in hyperglycemic animals. However, these observations were primarily focused on pancreatic β-cells. The effects of long-term high-glucose culture on stem cells (adult or embryonic) were unclear. In a previous study, we demonstrated that a long-term culture of purified hepatic oval stem cells in high-glucose (23 mmol/l) medium promoted the oval cells to transdifferentiate into functional insulin-secreting cells (16). In addition, we have observed that overexpression of Pdx-1 in a hepatic stem cell line (WB cells) only results in the generation of pancreatic precursor cells (unpublished observations). These cells did not respond to a glucose challenge in vitro by releasing insulin. Rather, these precursor cells became fully functional under two conditions: one involving long-term culture in high-glucose medium and the other being the transplantation of these cells into diabetic (i.e., hyperglycemic) animals.
The notion that in vitro high-glucose culture (or in vivo hyperglycemia) represents a critical factor for adult stem cell transdifferentiation into insulin-producing cells has been supported by recent two publications. Zalzman et al. (37) demonstrated that culture of immortalized human fetal Pdx-1–expressing hepatocytes in media containing 25 mmol/l glucose activated multiple β-cell genes, produced and stored considerable amounts of insulin, and released insulin in a regulated manner. In another work, Kojima et al. (32) showed that it was hyperglycemia produced by a 25% glucose injection into nondiabetic mice as well as in three other types of hyperglycemic animal models that led to the appearance of proinsulin-positive cells within 3 days in the liver, fat, spleen, BM, and thymus, as well as insulin-positive cells within 15 days in those organs. These studies support our observation that both liver and BM-containing stem cells can be induced under high-glucose conditions to differentiate into insulin-producing cells, and that insulin-producing cells can be derived from liver and BM cells. A sharp difference between our in vitro observations (in months) and those of the in vivo works of Kojima et al. (in days) (32) most likely resides in the required duration of exposure in terms of the need for high-glucose conditions to generate insulin-producing cells. Possible explanations may include that 1) in vivo three-dimensional structure and cell-cell contact and interaction may play a vital role in promoting cell differentiation, 2) other soluble factors in addition to high glucose in vivo may also play a role in accelerating cell differentiation, and 3) it takes a long time for a single-cell–derived cell clone to form three-dimensional cell clusters under high-glucose culture conditions. The above theory is supported by our observations involving our detection of an increase in insulin in culture medium taken from 3-week cultures of whole-marrow adherent cells under high-glucose conditions.
One possible explanation of the constant viable cells in the high-glucose cultures is that cycle events occur between immature stem-like cells and D-mBMDS cells. The immature stem-like cells, which may be more resistant to high-glucose culture conditions, give rise to the D-mBMDS cells, which may be more susceptible to high-glucose-induced apoptosis. These early apoptotic cells were removed following either medium change or cell splits. This assumption helps us explain the strange behavior of the cells we have repeatedly observed, namely the loss of pancreatic endocrine gene expression and insulin production when these cells were quickly expanded to a large quantity for cell transplantation experiments. It has been proposed that rapid growth will reduce cell differentiation. In our system, rapid proliferation may be only a part of the story for the loss of cell differentiation, the other facet being related to the high-glucose–induced cell apoptosis of the D-mBMDS cells and the subsequent loss of the differentiated cells. The latter may explain the variations between experiments and the loss in expression of genes related to pancreatic endocrine differentiation. Second, our experience with transdifferentiation of the human BMDS cells also indicated the requirement for subsequent culture with maturation factors, such as exendin-4 and nicotinamide, in a medium containing low FCS and low glucose to promote cell maturation and to restore the sensitivity to a glucose challenge (D.-Q.T., L.-Z.C., S.A.L., M.A.A., L.-J.Y., unpublished results). GLP-1 is an incretin hormone capable of restoring normal glucose tolerance in aging glucose-intolerant Wistar rats and inducing differentiation of islet Pdx-1–positive ductal cells into insulin-secreting cells (38). GLP-1 stimulates insulin secretion and augments β-cell mass via activation of β-cell proliferation and islet neogenesis (13). A recent study by Suzuki et al. (25) demonstrated that GLP-1 converts intestinal epithelial cells into functional insulin-producing cells. Exendin-4 is a potent GLP-1 agonist that has previously been shown to stimulate both β-cell replication and neogenesis from ductal progenitor cells (39). We have shown that the late stage of D-mBMDS cells expressed the GLP-1 receptor gene and that this expression may correlate with glucose-responsive insulin release.
Nicotinamide is a poly(ADP-ribose) synthetase inhibitor known to differentiate and increase cell mass in cultured human fetal pancreatic cells (40) and to protect cells from desensitization induced by prolonged exposure to large amounts of glucose. Sjoholm, Korsgren, and Andersson (41) demonstrated that nicotinamide promoted formation of fetal porcine islet-like cell clusters and increased the rates of proinsulin biosynthesis in these clusters. They concluded that the stimulatory effects of nicotinamide on insulin production and content by fetal porcine islet-like cell clusters result from neoformation of β-cells through differentiation. Finally, a report by Ramiya et al. (24) described how nicotinamide-treated islets derived from the pancreatic progenitor cell had more insulin and secreted significantly more insulin than cultures treated with glucose alone. Our previously published study (16) showed that nicotinamide promotes in vitro transdifferentiation and maturation of the liver stem cells into insulin-producing cells. Taken together, a combination of exendin 4 and nicotinamide effectively promotes further D-mBMDS cell differentiation in our experimental system.
Our present study demonstrates the potential for cell-based therapy of diabetes involving the generation of autologous insulin-producing cells in vitro from BMDS cells. These in vitro–generated insulin-producing cells could, in theory, provide a potentially unlimited source of islet-like cells without the limitation of immune rejection based on alloimmunity. However, because there are multifactorial influences in the transdifferentiation of BM-derived stem cells into competent insulin-producing cells, there are many questions left unanswered and unresolved issues remain. Among those are answers to the questions of what are the decisive steps (e.g., addition of the glucose, exogenous factors, and timing of factor addition) for the transdifferentiation process to take place? In our experience, these differentiated cells are unlike β-cell–derived cell lines, such as β-TC and INS-1 cells, in terms of their gene expression profiles, cell maturity, and capacity to release insulin in response of glucose stimulation (data not shown). Hence, one can also question whether these cells can really be pushed to the level of maturity like true β-cells by changing the in vitro culture conditions. Another relevant clinical question involves the issue of autoimmunity. Will the immune response to β-cell antigens recognize and destroy the newly generated insulin-producing cells obtained from BMDS cells? Obviously, further research is required to address these important questions. Yet we believe the results demonstrated in this study provide direct evidence supporting the notion that transdifferentiation of adult stem cells to insulin-producing cells may represent a viable therapeutic option for type 1 diabetes.
Acknowledgments
This work was supported by grants R21-DK063270 (to L.-J.Y.) and K08-DK064054 (to L.-J.Y.) from the National Institutes of Health and by the Juvenile Diabetes Research Foundation.
We thank Dr. Jill Verlander Reed and Kim Ahren for technical assistance.
Glossary
- BM
bone marrow
- DAPI
4′,6-diamidino-2-phenylindole
- D-mBMDS
differentiated murine BM-derived stem cells
- ELISA
enzyme-linked immunosorbent assay
- GLP
glucagon-like peptide
- IPGT
intraperitoneal glucose tolerance
- mBMDS
murine BM-derived stem cells
- Pdx
pancreatic duodenal homeobox
- STZ
streptozotocin
References
- 1.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 3.Gunnarsson R, Klintmalm G, Lundgren G, Wilczek H, Ostman J, Groth CG. Deterioration in glucose metabolism in pancreatic transplant recipients given cyclosporin. Lancet. 1983;2:571–572. doi: 10.1016/s0140-6736(83)90598-6. [DOI] [PubMed] [Google Scholar]
- 4.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asahara T, Murohara T, Sullivan A, Silver M, van der ZR, Li T, Witzen-bichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 6.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 8.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 9.Pereira RF, Halford KW, O’Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O, Prockop DJ. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A. 1995;92:4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 12.Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235–240. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ. Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem. 2003;278:471–478. doi: 10.1074/jbc.M209423200. [DOI] [PubMed] [Google Scholar]
- 14.Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000;32:11–16. doi: 10.1053/jhep.2000.9124. [DOI] [PubMed] [Google Scholar]
- 15.Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, Li S, Hatch H, Ahrens K, Cornelius JG, Petersen BE, Peck AB. In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. Proc Natl Acad Sci U S A. 2002;99:8078–8083. doi: 10.1073/pnas.122210699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohgawara H, Kawamura M, Honda M, Karibe S, Iwasaki N, Tasaka Y, Omori Y. Reversal of glucose insensitivity of pancreatic B-cells due to prolonged exposure to high glucose in culture: effect of nicotinamide on pancreatic B-cells. Tohoku J Exp Med. 1993;169:159–166. doi: 10.1620/tjem.169.159. [DOI] [PubMed] [Google Scholar]
- 18.Li SW, Tang D, Ahrens KP, She JX, Braylan RC, Yang L. All-transretinoic acid induces CD52 expression in acute promyelocytic leukemia. Blood. 2003;101:1977–1980. doi: 10.1182/blood-2002-05-1426. [DOI] [PubMed] [Google Scholar]
- 19.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1–derived cell lines with robust ATP-sensitive K+ channel–dependent and –independent glucose-stimulated insulin secretion. Diabetes. 2000;49:424–430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- 20.Soria B, Roche E, Berna G, Leon-Quinto T, Reig JA, Martin F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes. 2000;49:157–162. doi: 10.2337/diabetes.49.2.157. [DOI] [PubMed] [Google Scholar]
- 21.Elliott JI, Dewchand H, Altmann DM. Streptozotocin-induced diabetes in mice lacking alphabeta T cells. Clin Exp Immunol. 1997;109:116–120. doi: 10.1046/j.1365-2249.1997.4241319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 23.Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O’Neil JJ. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci U S A. 2000;97:7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med. 2000;6:278–282. doi: 10.1038/73128. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki A, Nakauchi H, Taniguchi H. Glucagon-like peptide 1 (1–37) converts intestinal epithelial cells into insulin-producing cells. Proc Natl Acad Sci U S A. 2003;100:5034–5039. doi: 10.1073/pnas.0936260100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 27.Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, Tzukerman M. Insulin production by human embryonic stem cells. Diabetes. 2001;50:1691–1697. doi: 10.2337/diabetes.50.8.1691. [DOI] [PubMed] [Google Scholar]
- 28.Zorina TD, Subbotin VM, Bertera S, Alexander AM, Haluszczak C, Gambrell B, Bottino R, Styche AJ, Trucco M. Recovery of the endogenous beta cell function in the NOD model of autoimmune diabetes. Stem Cells. 2003;21:377–388. doi: 10.1634/stemcells.21-4-377. [DOI] [PubMed] [Google Scholar]
- 29.Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. 2003;111:843–850. doi: 10.1172/JCI16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, Thyssen S, Gray DA, Bhatia M. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003;21:763–770. doi: 10.1038/nbt841. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 32.Kojima H, Fujimiya M, Matsumura K, Nakahara T, Hara M, Chan L. Extrapancreatic insulin-producing cells in multiple organs in diabetes. Proc Natl Acad Sci U S A. 2004;101:2458–2463. doi: 10.1073/pnas.0308690100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kodama S, Kuhtreiber W, Fujimura S, Dale EA, Faustman DL. Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science. 2003;302:1223–1227. doi: 10.1126/science.1088949. [DOI] [PubMed] [Google Scholar]
- 34.Soria B. In-vitro differentiation of pancreatic beta-cells. Differentiation. 2001;68:205–219. doi: 10.1046/j.1432-0436.2001.680408.x. [DOI] [PubMed] [Google Scholar]
- 35.Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic β-cells in adult rats after short-term glucose infusion. Diabetes. 1989;38:49–53. doi: 10.2337/diab.38.1.49. [DOI] [PubMed] [Google Scholar]
- 36.Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patane G, Laybutt R, Bonner-Weir S, Weir GC. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem. 1999;274:14112–14121. doi: 10.1074/jbc.274.20.14112. [DOI] [PubMed] [Google Scholar]
- 37.Zalzman M, Gupta S, Giri RK, Berkovich I, Sappal BS, Karnieli O, Zern MA, Fleischer N, Efrat S. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proc Natl Acad Sci U S A. 2003;100:7253–7258. doi: 10.1073/pnas.1136854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hui H, Wright C, Perfetti R. Glucagon-like peptide 1 induces differentiation of islet duodenal homeobox-1–positive pancreatic ductal cells into insulin-secreting cells. Diabetes. 2001;50:785–796. doi: 10.2337/diabetes.50.4.785. [DOI] [PubMed] [Google Scholar]
- 39.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both β-cell replication and neogenesis, resulting in increased β-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 40.Otonkoski T, Beattie GM, Mally MI, Ricordi C, Hayek A. Nicotinamide is a potent inducer of endocrine differentiation in cultured human fetal pancreatic cells. J Clin Invest. 1993;92:1459–1466. doi: 10.1172/JCI116723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sjoholm A, Korsgren O, Andersson A. Polyamine requirements in nicotinamide-stimulated beta-cell differentiation in fetal porcine islet-like cell clusters. Endocrinology. 1994;135:1559–1565. doi: 10.1210/endo.135.4.7925118. [DOI] [PubMed] [Google Scholar]