Abstract
Black cohosh rhizome (Actaea racemosa) is used as a remedy for pain and gynecological ailments; modern preparations are commonly sold as ethanolic extracts available as dietary supplements. Black cohosh was nominated to the National Toxicology Program (NTP) for toxicity testing due to its widespread use and lack of safety data. Several commercially available black cohosh extracts (BCE) were characterized by the NTP, and one with chemical composition closest to formulations available to consumers was used for all studies. Female B6C3F1/N mice and Wistar Han rats were given 0, 15 (rats only), 62.5 (mice only), 125, 250, 500, or 1000 mg/kg/day BCE by gavage for 90 days starting at weaning. BCE induced dose-dependent hematological changes consistent with a non-regenerative macrocytic anemia and increased frequencies of peripheral micronucleated red blood cells (RBC) in both species. Effects were more severe in mice, which had decreased RBC counts in all treatment groups and increased micronucleated RBC at doses above 125 mg/kg. Dose-dependent thymus and liver toxicity was observed in rats but not mice. No biologically significant effects were observed in other organs. Puberty was delayed 2.9 days at the highest treatment dose in rats; a similar magnitude delay in mice occurred in the 125 and 250 mg/kg groups but not at the higher doses. An additional uterotrophic assay conducted in mice exposed for 3 days to 0.001, 0.01, 0.1, 1, 10, 100 and 500 mg/kg found no estrogenic or anti-estrogenic activity. These are the first studies to observe adverse effects of BCE in rodents.
Keywords: black cohosh, Actaea racemosa, non-regenerative macrocytic anemia, micronucleus, genetic toxicity
Introduction
Black cohosh (Actaea racemosa, previously Cimicifuga racemosa) is a perennial woodland plant native to North America. The rhizome has traditionally been used as a remedy for inflammatory pain, neuralgia, and many gynecological ailments, including pre-menstrual discomfort, dysmenorrhea, premature labor, “difficult” labor, pain after childbirth, and perimenopausal symptoms (American Herbal Pharmacopoeia 2002; Dugoua et al. 2006). Because of this usage pattern, there is potential for long-term exposure of women during childbearing years and perimenopause. Modern preparations are most commonly sold as dried ethanolic extract tablets or capsules, sometimes formulated in combination with other herbs and used as dietary supplements. The recommended intake of black cohosh extract (BCE) is 40 mg/day (~ 0.5 mg/kg/day) (American Herbal Pharmacopoeia 2002). Black cohosh was nominated to the National Toxicology Program (NTP) for general toxicity testing by both the National Cancer Institute and National Institute of Environmental Health Sciences due to its widespread use and lack of human or animal studies in the published literature demonstrating its safety. Both Institutes recommended that safety studies also focus on possible reproductive and developmental toxicities.
The current scientific literature suggests that black cohosh may be a liver toxicant; evaluation of its historic uses and chemical constituents suggests that it also has the potential for cardiac and reproductive toxicity. Several cases of human liver injury have been associated with black cohosh use, with pathologies ranging from transient autoimmune hepatitis (Pierard et al. 2009; Zimmermann et al. 2010) to centrilobular necrosis consistent with severe drug-induced liver injury (Guzman et al. 2009; Pierard et al. 2009). However at least two meta-analyses of black cohosh clinical data found no evidence of liver toxicity (Naser et al. 2011; Teschke and Schwarzenboeck 2009). Only one clinical report has associated heart disease (bradycardia) with black cohosh use (McKenzie and Rahman 2010). The use of black cohosh during labor and lactation is of concern because experimental data on reproductive and developmental effects is lacking (Dugoua et al. 2006).
While currently there is no adequate toxicity data for black cohosh, several studies have tried to elucidate its therapeutic mode of action. BCE appears to reduce luteinizing hormone secretion in ovariectomized rats but not in perimenopausal women (Chung et al. 2007; Jacobson et al. 2001; Jarry and Harnischfeger 1985; Nappi et al. 2005; Rachon et al. 2008; Reame et al. 2008; Seidlova-Wuttke et al. 2003); demonstrations of estrogenic/anti-estrogenic activity of black cohosh are inconclusive (Jarry and Harnischfeger 1985; Jarry et al. 2003). However, neurotransmitter activities that can modify activity of the hypothalamic-pituitary-gonadal (HPG) axis at the CNS level (reviewed by Rivier and Rivest 1991), including dopaminergic, serotonergic, and opioid activity, have been observed in vitro (Jarry et al. 2003; Powell et al. 2008; Rhyu et al. 2006).
Given the scientific literature to date, NTP designed studies focused on subchronic toxicity of BCE with special emphasis on possible toxic effects in the liver and reproductive systems. Here we report the results of subchronic toxicity studies in female weanling rats and mice, peripheral blood micronucleus tests for evaluation of chromosomal damage in rats and mice, and an uterotrophic assay in juvenile CD-1 mice, a sensitive and well characterize model of estrogenic activity (Newbold et al. 2001). The studies were conducted to characterize the general toxicity of BCE and address suspected estrogenic/anti-estrogenic activity. While typical NTP subchronic studies use adult animals, in these subchronic studies exposures were started at weaning in order to assess the effects of BCE on pubertal endpoints as well as general toxicology endpoints usually evaluated in NTP subchronic studies.
Materials and Methods
Chemical Characterization
Chemical procurement and characterization was conducted at Battelle Memorial Institute (Columbus, OH) under NTP contract # N01-ES-55551. Single component standards used in the chromatographic characterization were purchased from Chromadex (Irvine CA) and Sigma-Aldrich (St. Louis, Mo). The test article, black cohosh (CAS 84776-26-1) dried extract (BCE) lot number 3012782 (extracted using 1:1 ethanol/water) was obtained from PlusPharma, Inc. (Vista, CA) in six plastic bags. Three bags were randomly selected, combined in a larger high density polyethylene (HDPE) bag, homogenized by rolling for 5 minutes. Each of the 3 bags was sampled and subjected to Karl Fisher, Proton-induced x-ray emission (PIXE) spectroscopy, and infrared analysis. The remaining 3 bags were similarly homogenized and combined, and each of the larger bags was divided into multiple 4-L opaque HDPE bottles, which were sealed with lids and stored at approximately 25°C. All homogeneity samples gave consistent analytical results, indicating that potassium (2.5%), magnesium (0.3%), and calcium (0.3%) were the significant inorganic components. Infrared reference spectra for black cohosh were not available, but sample spectra showed absorption bands consistent with the presence of phenolic compounds, as well as plant components such as sugars, terpenes, and glycosides. Karl-Fisher analysis determined water content between 3.50 and 4.11%. Weight loss on drying indicated a loss of 7.9 %. High performance liquid chromatographic analysis with ultraviolet detection (HPLC/UV), HPLC with evaporative light scattering detection (HPLC/ELSD), and HPLC with mass spectrometry (HPLC/MS) were used to generate chromatographic profiles and identify components using retention time matching and spectral information. Seven compounds typical of BCE, caffeic acid, ferulic acid, isoferulic acid, cimiracemoside A, cimicifugin, actein, and 27-deoxyactein were identified. Standard addition experiments (HPLC/UV detection) also confirmed the presence of caffeic acid, ferulic acid and isoferulic acid, but did not confirm the presence of formononetin in the BCE samples. For both the rat and mouse 90-day studies, the stability of the BCE test article relative to a frozen reference sample, using isoferulic acid as a marker, was determined before study start, once during the study, and following terminal sacrifice. No degradation was observed.
Dose Formulations
Dose formulations for the 90-day studies were prepared by mixing the dry BCE powder in 0.5% methylcellulose (CAS 9004-67-5) purchased from Spectrum Chemical Mfg. Corp (Gardena, CA) for approximately 2 hours on a stir plate at concentrations of 0, 3 (rats only), 6.25 (B6C3F1/N mice only), 12.5, 25, 50, 100 and 200 mg/mL. Formulations were stored refrigerated (approximately 5°C), stirred at room temperature for a minimum of 2 hours before use, and used within 43 days (chemical stability was established for 43 days using isoferulic acid as a marker). Pre- and post-administration analyses by liquid chromatography with UV detection (using benzophenone as an internal standard and isoferulic acid as a marker for BCE concentration) confirmed that all dose formulations varied by < 4.0% from target concentrations. Dose formulations for the uterotrophic assay were prepared by mixing the dry BCE powder in corn oil.
Immature CD-1 Mouse Uterotrophic Assay
Uterotrophic studies were conducted at the National Institute of Environmental Health Sciences (NIEHS, Research Triangle Park, NC). Animal procedures complied with NIEHS/NIH animal care guidelines. Female CD-1 mice from the NIEHS breeding colony were weaned on postnatal day 17 (PND 17) as described previously (Newbold et al. 2001). Mice consumed ad libitum fresh reverse osmosis/deionized water and NIH-31 feed (Thigpen et al 1999). Estradiol (50 μg/kg/day, a concentration expected to produce 50% of maximum respose) and/or BCE (0.001, 0.01, 0.1, 1, 10, 100 and 500 mg/kg) were administered by subcutaneous injection once a day on PND 17, 18 and 19 using a 25 gauge needle (except for the 100 and 500 mg/kg doses a 23 gauge needle was required). Mice were killed by cervical dislocation on PND 20; body and uteri weight (wet weight) were recorded.
Animals and Animal Husbandry for 90-Day Study
Since BCE dietary supplements are used almost exclusively by women, subchronic (90-day) studies were conducted in female rats and mice only. The studies were conducted in compliance with the Food and Drug Administration’s Good Laboratory Practice Regulations (21 CFR 58) at Battelle Memorial Institute (Columbus, OH) under NTP contract #N01-ES-55536. Time-mated Wistar Han rats were received from Charles River Laboratories (Raleigh, NC) on gestation day (GD) 14. B6C3F1/N mouse dams with litters were received on postnatal day (PND) 8 from Taconic Farms (Germantown, NY). On arrival, rats and mice were quarantined for 13 days. The date of birth was designated PND 0. Litters were randomly standardized to a maximum of eight pups (three males and five females when possible) on PND 4, and litters containing fewer than 3 female pups were removed from the study. On PND 21, pups were weaned and dams were removed from the study. Male weanlings were also removed from the study except twenty male rats (10 per rack using 2 racks) and 3 male mice (3 per rack using 1 rack) were housed (up to three per cage for rats, one per cage for mice) in the same study room as the corresponding females to ensure the regular cyclicity of study animals. After weaning, females were randomly assigned to six treatment groups and housed five per cage. Irradiated NIH-07 (gestation and lactation phases) or NTP-2000 (13-week phase) wafer feed (Zeigler Brothers, Inc., Gardners, PA) and tap water were provided ad libitum. Animals were euthanized by carbon dioxide asphyxiation.
Animal Care for All Studies
Animal use was in accordance with the U.S. Public Health Service policy on humane care and use of laboratory animals and the Guide for the Care and Use of Laboratory Animals (National Research Council 1996). Animals were treated humanely and with regard for alleviation of pain and distress. All animals were housed in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care and all procedures were approved by the Institutional Animal Care and Use Committee of NIEHS or Battelle as appropriate.
90-Day Study Design
Animals were exposed to vehicle control (0.5% methylcellulose) or BCE by gavage starting on PND 21–22 for rats and PND 21 for mice. Groups of 10 female rats were dosed with vehicle or 15, 125, 250, 500, or 1000 mg/kg/day BCE in a volume of 5.0 mL/kg. Groups of 15 female mice were dosed with vehicle or 62.5, 125, 250, 500, or 1000 mg/kg/day BCE in a volume of 10.0 mL/kg. Dosing volume was adjusted based on each animal’s most recent body weight. Animal weights and clinical findings were recorded weekly. Body weights were also collected for each animal at vaginal patency and first estrus. Starting on the first day of exposure, animals were examined daily to determine vaginal patency and first estrus; vaginal smears were collected after patency for each rat through PND 43 and for each mouse through PND 42. In addition, vaginal smears for assessment of estrous cyclicity were obtained from mice in the control, 250, 500, and 1000 mg/kg groups for 16 consecutive days prior to termination. Estrous cyclicity was not assessed in rats.
Determination of Vaginal Patency, First Estrus, and Estrous Cyclicity
Each animal was held and the tail lifted to observe the urogenital area for vaginal opening. The day a complete opening was present was recorded as the day of patency. For rats, day of patency was considered the same as pubertal achievement because patency and estrous usually occur within 24 hours of each other in this species. Conversely, first day of estrus was considered the same as pubertal achievement for mice since estrus in mice may occur several days after patency. Daily vaginal lavage smears were collected between 8:00 and 10:00 AM. Slides were evaluated to determine estrous cycle stage as proestrus (P), estrus (E), metestrus (M), or diestrus (D), or they were described as having insufficient cells (IC). The first day of estrus was defined as the first occurrence of E alone or in transition between P and E (P/E). E was not captured between P and M for one rat in the 500 mg/kg/day group, and it was assumed that E was short and occurred late in the noted day of P. Vaginal smears were fixed on slides and processed through decreasing concentrations of ethanol, followed by staining with 0.5% toluidine blue. Smears for assessment of estrous cyclicity were blinded and evaluated by microscopic examination (Bennett and Vickery 1970; Bronson et al. 1968; Goldman et al. 2007) by the same person.
Necropsy and Histopathology
At necropsy, the following organs were weighed and examined for grossly visible lesions: liver, heart, lungs, right kidney, thymus, uterus with vagina (including cervix), pituitary (post-fixation), and right ovary; the following tissues were only examined for gross lesions: adrenal glands, brain, clitoral glands, esophagus, eyes, femur, gallbladder, Harderian glands, aorta, large intestine (cecum, colon, and rectum), small intestine (duodenum, jejunum, and ileum), left kidney, mainstem bronchi, lymph nodes (mandibular and mesenteric), mammary gland and adjacent skin, muscle (thigh), nasal cavity with turbinates, nerve (sciatic), oral cavity (larynx and pharynx), left ovary, pancreas, parathyroid glands, salivary glands, spinal cord, spleen, stomach (forestomach and glandular), thyroid gland, tongue, trachea, urinary bladder, and Zymbal’s gland. Microscopic evaluations were conducted on all organs from vehicle control and 1000 mg/kg dose groups and for all organs with gross lesions. In the rat study only, the liver, heart and thymus were examined microscopically in all dose groups. Organ tissues were fixed and preserved in 10% neutral buffered formalin (except eyes were initially fixed in Davidson’s solution), processed and stained with hematoxylin and eosin (H&E) for microscopic examination.
Hematology and Clinical Chemistry
At study termination, blood was collected from the retro-orbital plexus under carbon dioxide/oxygen anesthesia and placed into tubes containing ethylenediaminetetraacetic acid (EDTA) for hematology determination (mice and rats) and into serum separator tubes for clinical chemistry determination (rats only). Blood smears were made and stained with Wright’s. The following hematology parameters were analyzed using the Advia 120 hematology analyzer (Bayer Diagnostics Division, Tarrytown, NY): red blood cell (RBC) count, hemoglobin (Hgb) concentration, hematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), reticulocyte count, white blood cell count and differential, and platelet count. The following clinical chemistry parameters were analyzed using the cobas c501 Chemistry Analyzer (Roche, Indianapolis, IN): sorbitrol dehydrogenase, bile acids, alkaline phosphatase, alanine aminotransferase, total protein, albumin, glucose, blood urea nitrogen, creatinine, creatine kinase, triglycerides and cholesterol.
Micronucleus Assay
Micronucleus assays were conducted to assess for chromosomal damage. Micronuclei (MN) are biomarkers of chromosomal damage resulting from whole chromosome loss or chromosome breakage. Blood samples (60–120 μL) for determination of micronucleated reticulocyte and erythrocyte frequencies were collected at termination, within 24 hr of the final dosing, and were refrigerated immediately (2 – 8°C). Coded samples were shipped overnight in chilled packaging to the genotoxicity testing laboratory where they were fixed in ultracold methanol within 48 hr of collection, and stored at −80°C until analysis. Thawed blood samples were analyzed for frequency of micronucleated reticulocytes (MN-RET), micronucleated erythrocytes (MNE), and %RET using a flow cytometer (Witt et al. 2008); approximately 20,000 reticulocytes and 106 erythrocytes were evaluated in each of 5 animals per treatment group (Kissling et al. 2007). All samples were scored without knowledge of treatment group.
Statistical Methods
For body weights, organ weights, organ weight ratios, which typically are normally distributed, were analyzed using parametric methods. Specifically, homogeneity of variances was determined by Bartlett’s test. When variances were homogeneous, differences between control and treatment groups were compared using ANOVA with Dunnett’s test post-test. When variances were not homogeneous, differences between the control and treatment groups were compared using Cochran and Cox’s modified two-sample t-test. Clinical pathology and hematology data are typically non-normally distributed, so they were analyzed using nonparametric methods. Jonckheere’s test was used to assess the significance of dose-response trends. If a significant trend was detected at p < 0.01, Shirley’s test was used to compare each treatment group to the control group; if the trend was not significant, Dunn’s test was used to compare each treatment group to the control group. Uterotrophic data was analyzed by t-test with Welch’s correction for non-homogeneous data.
Attainment of puberty measures (age at vaginal patency or first estrous) were analyzed by ANCOVA with body weight at weaning as the covariate. If the regression slopes were not statistically different from zero, it was assumed that ANCOVA was not the appropriate statistical test and ANOVA with Dunnett’s was used instead, as described above.
Vaginal cytology data were analyzed using a Markov transition matrix approach (Girard and Sager 1987), in which treatment effects were investigated by testing for increased probabilities for deviations in cycling relative to the vehicle control group using the Chi-square Test. The number of females with regular cycles and the number of cycling females were analyzed using Fisher’s exact test (Conover 1971).
Micronucleus data was tested for equal variances using Levene’s test at α = 0.05. If variances were equal, linear trend was tested by linear regression and pairwise differences between control and each treatment group were tested by Williams’ test. If variances were unequal, linear trend was tested by Jonckheere’s test and pairwise differences by Dunn’s test.
Tests were considered significant at P < 0.05 except the micronucleus tests, which were considered significant at P < 0.025.
Results
CD-1 Juvenile Uterotrophic Assay
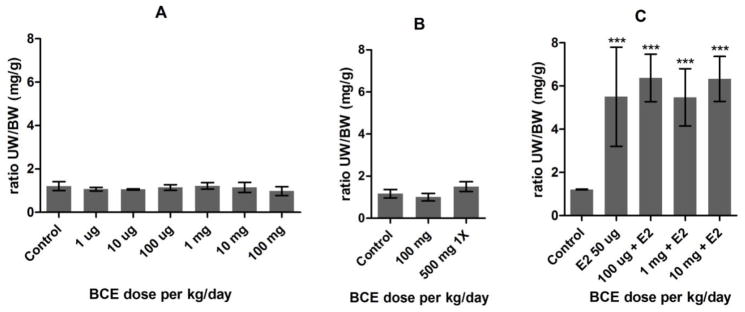
BCE treatment of juvenile CD-1 mice for 3 days by subcutaneous injection did not increase uterus size at doses up to 100 mg/kg (Figure 1A). A higher dose of 500 mg/kg was acutely toxic, causing lethargy and decreased motor activity, therefore animals were euthanized and data collected after only one exposure (Figure 1B). Co-treatment with 50 μg/kg/day 17β-estradiol and BCE did not modify the uterotrophic effect of 17β-estradiol (Figure 1C).
Figure 1. Relative uterus weight of juvenile CD-1 mice after 3-day subcutaneous treatment with black cohosh.
Each chart shows mean ± SEM of an individual experiment. N = 5 except for 500 mg n = 4. 1X = group dosed only once, all others dosed 3 times. (A) Experiment #1. (B) Experiment #2. (C) Experiment #3, E2 = co-treatment with 50 μg/kg/day estradiol.
B6C3F1/N Mice and Wistar Han Rat Survival, Body Weight and Clinical Observations
Survival of exposed groups of rats and mice was similar to that of the respective control groups. One 500 mg/kg rat was humanely terminated prior to the end of the study following an accidental injury. One 62.5 mg/kg mouse was removed due to being mis-sexed at weaning. There were no notable clinical observations in rats or mice. Mean weekly body weights were within 8% of control for rats and 7% of control for mice throughout the study (data not shown) and final body weights were not different from controls (Tables 5 and 6).
Table 5.
Wistar Han rat body and organ weights at necropsy after subchronic gavage treatment with black cohosh.
| Dose (mg/kg/day) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Organ | 0 | 15 | 125 | 250 | 500 | 1000 | |
|
| |||||||
| Body | (g) | 245.9 ± 3.4 | 240.2 ± 4.1 | 244.0 ± 4.4 | 238.1 ± 6.3 | 237.7 ± 5.8 | 241.9 ± 3.5 |
|
| |||||||
| Liver | (g) | 8.8 ± 0.2 | 8.4 ± 0.2 | 9.0 ± 0.3 | 8.8 ± 0.3 | 9.7 ± 0.3 | 10.1 ± 0.2** |
| (%) | 3.6 ± 0.07 | 3.5 ± 0.06 | 3.7 ± 0.06 | 3.7 ± 0.04 | 4.1 ± 0.08** | 4.2 ± 0.08** | |
|
| |||||||
| Heart | (g) | 0.80 ± 0.02 | 0.81 ± 0.02 | 0.81 ± 0.02 | 0.78 ± 0.03 | 0.78 ± 0.03 | 0.81 ± 0.02 |
| (%) | 0.33 ± 0.01 | 0.34 ± 0.01 | 0.33 ± 0.01 | 0.33 ± 0.01 | 0.33 ± 0.01 | 0.33 ± 0.01 | |
|
| |||||||
| Right kidney | (g) | 0.87 ± 0.02 | 0.84 ± 0.02 | 0.86 ± 0.02 | 0.85 ± 0.02 | 0.88 ± 0.04 | 0.94 ± 0.02 |
| (%) | 0.35 ± 0.01 | 0.35 ± 0.01 | 0.35 ± 0.01 | 0.36 ± 0.01 | 0.37 ± 0.01 | 0.39 ± 0.01* | |
|
| |||||||
| Thymus | (g) | 0.43 ± 0.02 | 0.44 ± 0.02 | 0.42 ± 0.02 | 0.37 ± 0.03 | 0.33 ± 0.03* | 0.33 ± 0.02** |
| (%) | 0.175 ± 0.005 | 0.183 ± 0.009 | 0.170 ± 0.005 | 0.154 ± 0.010 | 0.138 ± 0.009** | 0.1435 ± 0.007** | |
|
| |||||||
| Pituitary | (g) | 0.018 ± 0.001 | 0.016 ± 0.001 | 0.016 ± 0.001 | 0.015 ± 0.001* | 0.016 ± 0.001* | 0.013 ± 0.001** |
| (‰) | 0.074 ± 0.003 | 0.067 ± 0.003 | 0.065 ± 0.003 | 0.064 ± 0.003 | 0.066 ± 0.003 | 0.056 ± 0.003** | |
|
| |||||||
| Right ovary | (g) | 0.049 ± 0.003 | 0.050 ± 0.003 | 0.054 ± 0.004 | 0.053 ± 0.003 | 0.052 ± 0.005 | 0.057 ± 0.003 |
| (%) | 0.020 ± 0.001 | 0.021 ± 0.001 | 0.022 ± 0.002 | 0.022 ± 0.001 | 0.022 ± 0.002 | 0.024 ± 0.001 | |
|
| |||||||
| Uterus | (g) | 0.51 ± 0.05 | 0.48 ± 0.03 | 0.51 ± 0.07 | 0.60 ± 0.07 | 0.51 ± 0.04 | 0.47 ± 0.04 |
| (%) | 0.21 ± 0.02 | 0.20 ± 0.01 | 0.21 ± 0.03 | 0.25 ± 0.13 | 0.22 ± 0.02 | 0.19 ± 0.02 | |
|
| |||||||
| Vagina | (g) | 0.32 ± 0.02 | 0.31 ± 0.01 | 0.32 ± 0.02 | 0.24 ± 0.02 | 0.30 ± 0.01 | 0.32 ± 0.02 |
| +cervix | (%) | 0.128 ± 0.007 | 0.130 ± 0.007 | 0.131 ± 0.007 | 0.141 ± 0.010 | 0.125 ± 0.005 | 0.132 ± 0.008 |
All data shown as mean ± SEM, n = 10 except 500 mg/kg group n = 9. Relative organ weights expressed per 100 g of body weight (%) except pituitary weights are expressed per 1000 g of body weight (‰). Groups compared by ANOVA with Dunnett’s test.
p <0.05,
p < 0.01.
(g) = absolute organ weights in grams; (%) = organ weight in grams as percent of body weight in grams.
Table 6.
B6C3F1/N mouse body and organ weights at necropsy after subchronic gavage treatment with black cohosh.
| Dose (mg/kg/day) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Organ | 0 | 62.5 | 125 | 250 | 500 | 1000 | |
|
| |||||||
| Body | (g) | 22.4 ± 0.3 | 23.3 ± 0.3 | 23.5 ± 0.3 | 23.2 ± 0.2 | 23.6 ± 0.3 | 22.7 ± 0.4 |
|
| |||||||
| Liver | (g) | 1.08 ± 0.02 | 1.14 ± 0.03 | 1.16 ± 0.02 | 1.14 ± 0.02 | 1.24 ± 0.02** | 1.26 ± 0.03** |
| (%) | 4.8 ± 0.07 | 4.9 ± 0.08 | 4.9 ± 0.05 | 4.9 ± 0.07 | 5.3 ± 0.06** | 5.5 ± 0.08** | |
|
| |||||||
| Heart | (g) | 0.31 ± 0.005 | 0.14 ± 0.005 | 0.14 ± 0.006 | 0.13 ± 0.005 | 0.15 ± 0.004 | 0.14 ± 0.004 |
| (%) | 0.58 ± 0.02 | 0.61 ± 0.02 | 0.59 ± 0.02 | 0.56 ± 0.02 | 0.62 ± 0.02 | 0.60 ± 0.02 | |
|
| |||||||
| Right kidney | (g) | 0.142 ± 0.002 | 0.153 ± 0.003 | 0.157 ± 0.003** | 0.152 ± 0.002 | 0.156 ± 0.003** | 0.157 ± 0.004** |
| (%) | 0.63 ± 0.01 | 0.66 ± 0.01 | 0.67 ± 0.01** | 0.65 ± 0.01 | 0.66 ± 0.01* | 0.69 ± 0.01** | |
|
| |||||||
| Thymus | (g) | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.01 ± 0. 001 | 0.01 ± 0.001 | 0.01 ± 0.001 |
| (%) | 0.046 ± 0.003 | 0.046 ± 0.003 | 0.047 ± 0.002 | 0.045 ± 0.003 | 0.049 ± 0.003 | 0.047 ± 0.002 | |
|
| |||||||
| Pituitary | (g) | 0.002 ± 0.000 | 0.002 ± 0.000 | 0.003 ± 0.000* | 0.002 ± 0.000 | 0.002 ± 0.00 | 0.002 ± 0.000 |
| (%) | 0.0109 ± 0.000 | 0.010 ± 0.001 | 0.011 ± 0.000 | 0.010 ± 0.001 | 0.010 ± 0.001 | 0.010 ± 0.001 | |
|
| |||||||
| Right ovary | (g) | 0.047 ± 0.001 | 0.043 ± 0.002 | 0.045 ± 0.002 | 0.046 ± 0.001 | 0.045 ± 0.002 | 0.046 ± 0.001 |
| (%) | 0.208 ± 0.005 | 0.186 ± 0.006* | 0.189 ± 0.007 | 0.197 ± 0.005 | 0.192 ± 0.005 | 0.201 ± 0.004 | |
|
| |||||||
| Uterus | (g) | 0.161 ± 0.007 | 0.170 ± 0.010 | 0.203 ± 0.010* | 0.212 ± 0.015** | 0.197 ± 0.010 | 0.212 ± 0.014** |
| +vagina (%) | 0.71 ± 0.03 | 0.74 ± 0.05 | 0.87 ± 0.05 | 0.92 ± 0.07* | 0.84 ± 0.04 | 0.93 ± 0.05* | |
| +cervix | |||||||
All data shown as mean ± SEM, n = 15. Groups compared by ANOVA with Dunnett’s test.
p <0.05,
p < 0.01
B6C3F1/N Mice and Wistar Han Rat Vaginal Patency, First Estrus, and Estrous Cyclicity
Delayed vaginal patency was observed in 1000 mg/kg rats when compared to control animals, and 125 and 250 mg/kg mice showed statistically significant delays in attainment of vaginal patency and first estrus (Table 1). Estrous cyclicity did not differ between treated mice and controls.
Table 1.
Puberty acquisition and adult estrous cyclicity data for Wistar Han rats and B6C3F1/N mice after subchronic gavage treatment with black cohosh.
| Dose (mg/kg/day) | |||||||
|---|---|---|---|---|---|---|---|
| Rat | 0 | 15 | 125 | 250 | 500 | 1000 | |
| Weaning BW (g) | 61.1 ± 0.7 | 61.0 ± 0.8 | 60.1 ± 1.0 | 59.4 ± 1.1 | 61.1 ± 0.8 | 62.0 ± 1.5 | |
| Vaginal Patency PND | 34.9 ± 0.4 | 35.4 ± 0.5 | 35.7± 0.4 | 36.2 ± 0.7 | 35.6 ± 0.5 | 37.8 ± 0.7** | |
| Vaginal Patency BW (g) | 115.4 ± 2.2 | 114.4 ± 3.5 | 109.4 ± 2.0 | 114.6 ± 3.5 | 112.2 ± 2.3 | 119.4 ± 3.7 | |
| First Estrus PND | 36.6 ± 0.7 | 37.0 ± 0.9 | 36.5 ± 0.6 | 37.5 ± 0.8 | 37.4 ± 0.7 | 38.6 ± 0.7 | |
| First Estrus BW (g) | 124.2 ± 4.2 | 119.9 ± 3.8 | 111.8 ± 2.1 | 119.1 ± 4.1 | 119.0 ± 2.7 | 122.2 ± 3.8 | |
| Mice | 0 | 62.5 | 125 | 250 | 500 | 1000 | |
| Weaning BW (g) | 8.4 ± 0.4 | 8.5 ± 0.2 | 8.6 ± 0.3 | 8.6 ± 0.2 | 8.6 ± 0.2 | 8.3 ± 0.2 | |
| Vaginal Patency PND | 40.0 ± 0.7 | 41.0 ± 0.5 | 41.6 ± 0.6 # | 42.3 ± 0.4 * | 41.5 ± 0.6 | 41.7 ± 0.8 | |
| Vaginal Patency BW (g) | 16.1 ± 0.2 | 16.3 ± 0.2 | 16.6 ± 0.2 | 16.6 ± 0.2 | 16.9 ± 0.2 | 16.3 ± 0.2 | |
| First Estrus PND | 41.1 ± 0.7 | 41.8 ± 0.7 | 43.2 ± 0.9 # | 44.3 ± 0.5 ** | 42.9 ± 0.7 | 42.6 ± 0.8 | |
| First Estrus BW (g) | 16.3 ± 0.3 | 16.5 ± 0.3 | 16.8 ± 0.2 | 16.8 ± 0.2 | 16.9 ± 0.3 | 16.6 ± 0.3 | |
| %Time in each stage | Proestrus | 0.4 | Not measured | Not measured | 1.7 | 0.8 | 0.8 |
| Estrus | 42.9 | 46.7 | 47.1 | 46.3 | |||
| Metestrus | 37.1 | 32.1 | 32.1 | 30.0 | |||
| Diestrus | 18.8 | 19.2 | 18.3 | 22.1 | |||
| Not cleara | 0.8 | 0.4 | 1.7 | 0.8 | |||
| Cycle length (days) | 4.1 ± 0.1 | 4.2 ± 0.2 | 4.1 ± 0.1 | 4.1 ± 0.1 | |||
| Number of cycles | 2.9 ± 0.1 | 3.0 ± 0.1 | 3.1 ± 0.2 | 3.1 ± 0.2 | |||
| % animals cycling | 100 | 100 | 100 | 100 | |||
| % w/regular cycles | 87 | 87 | 93 | 93 | |||
Post natal day (PND) and weight data shown as mean ± SEM, n = 10 rats or 15 mice (except 62.5 mg/kg n = 14). Patency and first estrous data analyzed by ANOVA with Dunnett’s test; analysis of covariance was attempted using body weight at weaning or at puberty acquisition as covariate, however only mice control and two lowest doses had slopes significantly different from zero, for other mice groups and all rat groups puberty acquisition did not correlate with body weight at weaning or day of acquisition. Estrous cyclicity data was acquired from adult mice and analyzed as described in Methods.
p < 0.01 by ANOVA with Dunnett’s test;
p<0.05 by ANCOVA vs. BW at weaning.
Due to poor quality of slide or insufficient number of cells.
B6C3F1/N Mice and Wistar Han Rat Hematology and Clinical Chemistry
Hematology (mice and rats) and clinical chemistry (rats) data are shown in Tables 2 and 3. All treated groups of mice had a dose-dependent decrease in the RBC count that reached a 20% difference from the control group in the 1000 mg/kg dose group. Dose-dependent increases in MCV and MCH were observed in all treated groups of mice, up to 16% and 15% greater than the control group in the 1000 mg/kg group, respectively. Dose-dependent decreases in Hgb concentration and Hct were observed in the 125 mg/kg and greater mice, and 250 mg/kg and greater mice, respectively. There were no statistical differences in MCHC between treated and control mice. In addition, no changes were seen in the WBC count and differential of treated mice compared to concurrent controls (data not shown). Platelet counts showed treatment-related increases in the 125 mg/kg and higher mice; although the exact mechanism cannot be known, this observation may reflect altered peripheral distribution or increased production. Hyperplasia of the megakaryocytes, however, was not observed in the spleen or bone marrow.
Table 2.
Hematology parameters of Wistar Han rats and B6C3F1/N mice after subchronic gavage treatment with black cohosh.
| Dose (mg/kg/day) | ||||||
|---|---|---|---|---|---|---|
| Rats | 0 | 15 | 125 | 250 | 500 | 1000 |
| Red Blood Cells (106/uL) | 7.79 ± 0.06 | 7.97 ± 0.07 | 7.62 ± 0.13 | 7.41 ± 0.14 | 7.35 ± 0.15* | 7.30 ± 0.12** |
| Hemoglobin (g/dL) | 14.7 ± 0.2 | 15.1 ± 0.1 | 15.1 ± 0.2 | 15.0 ± 0.2 | 14.8 ± 0.2 | 14.2 ± 0.2 |
| Hematocrit (%) | 45.2 ± 0.3 | 46.6 ± 0.4 | 46.6 ± 0.6 | 45.7 ± 0.7 | 45.7 ± 0.7 | 44.7 ± 0.7 |
| MCV (fL/cell) | 58.0 ± 0.4 | 58.5 ± 0.4 | 61.2 ± 0.5** | 61.8 ± 0.4** | 62.2 ± 0.5** | 61.1 ± 0.3** |
| MCH (pg/cell) | 18.9 ± 0.2 | 18.9 ± 0.1 | 19.9 ± 0.3* | 20.2 ± 0.2** | 20.2 ± 0.3** | 19.5 ± 0.2* |
| MCHC (g/dL) | 32.6 ± 0.2 | 32.4 ± 0.2 | 32.5 ± 0.3 | 32.7 ± 0.2 | 32.4 ± 0.3 | 31.9 ± 0.3 |
| Reticulocytes (103/uL) | 183.67 ± 8.15 | 176.57 ± 8.47 | 148.36 ± 8.95 | 171.41 ± 10.77 | 136.81 ± 10.86** | 123.52 ± 7.10** |
| Platelets (103/uL) | 769 ± 26 | 779 ± 29 | 757 ± 37 | 812 ± 27 | 954 ± 34** | 1037 ± 38** |
| Mice | 0 | 62.5 | 125 | 250 | 500 | 1000 |
| Red Blood Cells (106/uL) | 10.49 ± 0.18 | 9.76 ± 0.09** | 9.39 ± 0.07** | 9.09 ± 0.07** | 8.64 ± 0.07** | 8.36 ± 0.05** |
| Hemoglobin (g/dL) | 15.5 ± 0.2 | 15.3 ± 0.2 | 15.0 ± 0.1* | 14.8 ± 0.1** | 14.4 ± 0.1** | 14.2 ± 0.1** |
| Hematocrit (%) | 47.4 ± 0.5 | 46.6 ± 0.5 | 45.9 ± 0.4 | 45.7 ± 0.4* | 44.2 ± 0.4** | 44.0 ± 0.3** |
| MCV (fL/cell) | 45.2 ± 0.4 | 47.7 ± 0.2** | 48.9 ± 0.2** | 50.2 ± 0.2** | 51.1 ± 0.2** | 52.6 ± 0.2** |
| MCH (pg/cell) | 14.8 ± 0.2 | 15.7 ± 0.1** | 15.9 ± 0.1** | 16.2 ± 0.1** | 16.6 ± 0.1** | 17.0 ± 0.1** |
| MCHC (g/dL) | 32.7 ± 0.1 | 32.8 ± 0.2 | 32.6 ± 0.1 | 32.3 ± 0.1 | 32.5 ± 0.1 | 32.4 ± 0.2 |
| Reticulocytes (103/uL) | 266.2 ± 10.5 | 300.6 ± 13.8 | 251.5 ± 14.3 | 233.6 ± 10.0 | 266.8 ± 11.2 | 249.7 ± 11.5 |
| Platelets (103/uL) | 672 ± 39 | 778 ± 44 | 774 ± 55* | 878 ± 34** | 824 ± 53** | 871 ± 31** |
All data shown as mean ± SEM, n = 10 rats (except 500 mg/kg group n = 9) and 15 mice (except 62.5 mg/kg group n = 11, 125 and 500 mg/kg n = 13). Groups compared by ANOVA with Dunnett’s test.
p <0.05,
p < 0.01. MCV = Mean Corpuscular Volume, MCH = Mean Corpuscular Hemoglobin, MCHC = Mean Corpuscular Hemoglobin Concentration
Table 3.
Total protein and albumin levels in Wistar Han rats after subchronic gavage treatment with black cohosh.
| Dose (mg/kg/day) | ||||||
|---|---|---|---|---|---|---|
| Parameter | 0 | 15 | 125 | 250 | 500 | 1000 |
| Total protein (g/dL) | 7.0 ± 0.1 | 6.9 ± 0.1 | 6.7 ± 0.1 | 6.7 ± 0.2 | 6.7 ± 0.1 | 6.2 ± 0.1** |
| Albumin (g/dL) | 5.1 ± 0.1 | 5.2 ± 0.1 | 5.0 ± 0.1 | 4.9 ± 0.1 | 4.9 ± 0.1 | 4.5 ± 0.1** |
All data shown as mean ± SEM, n = 10 except 500 mg/kg group n = 9. Groups compared by ANOVA with Dunnett’s test.
p < 0.01.
Similar hematologic changes were observed in rats, but were lower in magnitude. The RBC counts of the 500 mg/kg and 1000 mg/kg rats had treatment-related decreases reaching 6% of the control group. Minimal treatment-related increases (≤ 6.7%) of the MCV and MCH occurred in the 125 mg/kg and higher rats. Treatment-related decreases in reticulocytes were seen in rats in the 500 mg/kg and 1000 mg/kg dose groups. There were no statistical differences in Hgb concentration, Hct value and MCHC between treated and control rats. In addition, no changes were seen in the WBC count and differential of treated rats compared to concurrent controls (data not shown). Similar to the mice, platelet counts were increased in the 500 mg/kg and 1000 mg/kg rats.
There were no toxicologically relevant changes in biomarkers related to liver, kidney, muscle or lipid and carbohydrate metabolism (data not shown). A mild (11%) proportional decrease in the serum total protein and albumin in the 1000 mg/kg rats (Table 3) was suggestive of decreased feed intake or gastrointestinal loss. However, there was no corresponding significant difference between the body weights of control and 1000 mg/kg rats at study termination and no clinical observations of diarrhea. The lack of changes in renal and hepatic biomarkers is not consistent with kidney loss or decreased production by the liver as the cause for the decreased proteins.
Evaluation of the mouse peripheral blood smears revealed mild morphological changes in the 500 mg/kg and 1000 mg/kg treatment groups compared to concurrent controls. Specifically, there was a mild increase in anisocytosis with the presence of rare to occasional microcytes, poikilocytes (abnormally shaped erythrocytes not otherwise defined), schistocytes (red blood cell fragments), acanthocytes and basophilic stippling. The rat peripheral blood smears were not evaluated.
B6C3F1/N Mouse and Wistar Han Rat Micronucleus Tests
Treatment with BCE for 13 weeks via gavage resulted in highly significant dose-dependent increases in MN-RET and MNE in female mice (Table 4). In rats, significant increases were seen in MN-RET (Table 4). The %RET in peripheral blood of female mice and rats was not significantly affected by treatment with BCE.
Table 4.
Frequency of Micronucleated Red Blood Cells Per 1000 Cells in Peripheral Blood Following Administration of Black Cohosh by Dosed Feed for 13 weeksa.
| Dose mg/kg/d | Rats | Dose mg/kg/d | Mice | |
|---|---|---|---|---|
| Reticulocytes | Erythrocytes | Reticulocytes | ||
| 0 | 0.91 ± 0.11 | 0 | 1.03 ± 0.01 | 1.97 ± 0.25 |
| 15 | 0.90 ± 0.22 | 62.5 | 1.26 ± 0.01 | 1.90 ± 0.10 |
| 125 | 1.11 ± 0.18 | 125 | 1.41 ± 0.04 | 2.41 ± 0.07 |
| 250 | 1.45 ± 0.24 | 250 | 1.76 ± 0.11* | 2.79 ± 0.46 |
| 500 | 1.42 ± 0.26 | 500 | 2.53 ± 0.09** | 3.97 ± 0.37** |
| 1000 | 2.80 ± 0.75** | 1000 | 2.78 ± 0.10*** | 4.29 ± 0.17** |
| Trendb | p < 0.0001 | Trend | p < 0.0001 | p < 0.0001 |
All data shown as mean ± SEM; N = 5.
Jonckheere’s test was used to test for linear trend, if variances were unequal; if variances were equal, linear regression was used. Both tests are significant at P < 0.025.
p < 0.025,
p < 0.01,
p < 0.001 per Dunn’s test if unequal variances, or Williams’ test if variances were equal.
B6C3F1/N Mice and Wistar Han Rat Necropsy and Histopathology
Organ weights for the 13-week rat and mouse studies are summarized in Tables 5 and 6, respectively. There were no significant differences in terminal body weights of rats or mice between treated and control groups or differences in absolute or relative heart weights.
Absolute and relative thymus weights were lower than controls (23% and 22%, respectively) in 500 and 1000 mg/kg rats. Microscopic examination of the rat thymus consistently noted a combination of lymphocyte apoptosis, decreased cortical density and increase cortical macrophages in most treated rats. Apoptosis of the thymus was characterized by a combination of increased apoptotic bodies and tingible-body macrophages. Although categorized of minimal severity, the number of rats with lymphocytic apoptotic lesions was significantly different from control in the 500 and 1000 mg/kg treatment groups (Table 7). There were no significant microscopic or weight changes in the thymus of mice exposed to BCE.
Table 7.
Incidence of non-neoplastic lesions in Wistar Han rats after subchronic gavage treatment with black cohosh.
| Dose (mg/kg/day) | ||||||
|---|---|---|---|---|---|---|
| Organ | 0 | 15 | 125 | 250 | 500 | 1000 |
| Thymus, apoptosis | 1 | 0 | 1 | 0 | 8 *** (1.0) | 7 ** (1.0) |
| Liver, hemorrhage | 0 | 0 | 0 | 0 | 1 (3.0) | 0 |
| Liver, chronic inflammation | 1 (1.0) | 0 | 0 | 0 | 3 (1.0) | 4 (1.0) |
| Liver, necrosis | 0 | 0 | 0 | 0 | 0 | 2 (1.5) |
All data shown as number of animals with lesion out of a total n = 10 examined. Average severity is shown in parenthesis (1 = minimal, 2 = mild). Dose-response trends and pair-wise comparisons to control were assessed by continuity-corrected poly-3 test.
p < 0.05,
p < 0.01,
p < 0.001.
Average absolute liver weight was 15% and 16% higher than control in the 500 and 1000 mg/kg mice respectively, and 15% higher than control in the 1000 mg/kg rats; a 10% increase in average liver weight of rats in the 500 mg/kg group was not statistically significant. Two rats in the 1000 mg/kg group showed minimal to mild liver necrosis (Table 7). Microscopically, necrosis of the liver was characterized by two to three foci of coagulative necrosis surrounded and infiltrated by a mixture of inflammatory cells (predominantly macrophages, with a small number of lymphocytes and infrequent neutrophils). Severity scoring was based on the overall size of the foci; those smaller than 10 hepatocytes were graded minimal, those larger than 10 hepatocytes were graded mild. There were no significant microscopic changes in mouse liver.
Average kidney and pituitary weights were statistically different in some treatment groups compared to controls (Tables 5 and 6) however there were no histological correlates in those organs. Average absolute kidney weights were 11%, 10% and 11% higher in 125, 500 and 1000 mg/kg mice, respectively, compared to controls; however there were no related histopathological findings in the mouse kidney. In the rat the average relative kidney weight was 11% higher than control at the highest dose. Average absolute pituitary weights were decreased 17%, 11% and 28% in 250, 500 and 1000 mg/kg rats, respectively.
A 16% increase in ovarian weights from 1000 mg/kg rats compared to control was not statistically significant, and there were no significant differences in ovarian weights of mice (Table 5). The weight of the uterus or vagina/cervix in treated rats was not different from controls (Table 5). In the mice study the uterus, vagina and cervix were weighed together and there was a statistically significant trend of increasing average reproductive tract weight with increasing dose (ANOVA p = 0.0053 for absolute weights, p = 0.0074 for relative weights). Pairwise comparison found that the 125, 250 and 1000 mg/kg groups, but not the 500 mg/kg group, were statistically different from control (6%, 26%, 32% and 32% increase from control, respectively). Histopathological analysis determined that estrous cycle stage was synchronous between the ovaries, uterus and vagina of individual control and 1000 mg/kg rats and mice (other groups were not assessed). There were no significant microscopic changes in rat or mouse ovaries or reproductive tract.
Discussion
Ethanolic extracts of black cohosh (BCE) are currently widely available as herbal supplements and have been used historically to treat several ailments, including pain, menstrual discomfort, and perimenopausal symptoms. The NTP conducted subchronic and uterotrophic studies to determine possible adverse effects of BCE in rodents. The subchronic studies showed hematological changes consistent with mild anemia and chromosomal damage in mice; similar effects of lesser severity were observed also in rats. These studies provide the first evidence of BCE effects on the erythron and suggest possible bone marrow toxicity. Since BCE is suspected to have estrogenic activity, weanlings were used for the subchronic studies in order to observe pubertal development. However no effects expected from an estrogen were observed in the subchronic studies.
The test article was selected after characterizing samples of alcohol extracts from 3 different suppliers and comparing results to those of alcohol extracts of two lots of Remifemin Menopause tablets, a product containing black cohosh extract which is available to the general public in the United States. Chromatographic profiles of two of the three samples analyzed were similar to Remifemin Menopause tablets purchased at the time of the study (data not shown).
The characterization of the test article for this study was approached in a generally qualitative rather than quantitative manner. Extraction of plant materials with alcohol carries over a mixture of non-species specific compounds, such as sugars and terpenes, along with desired compounds in the end product. Many of these are unidentifiable as specific compounds due to the size of the compound classes and lack of single component standards but appear as major components in chromatographic analyses of the extract. The characteristic components of BCE have differing chemical properties such that more than one analytical method is required for their detection and quantitation across methods is not possible. For instance, components that were detected by ELSD were not detected by UV because they did not have chromophores that absorbed at 317 nm. Others had strong absorbances at this wavelength. Seven components characteristic of black cohosh were identified using retention time and spectral matching with single component standards. In addition, the HPLC/UV chromatographic profile matched well with literature fingerprints for black cohosh (Jiang, et al, 2011).
Oral administration of BCE caused a dose-dependent non-regenerative normochromic macrocytic anemia in B6C3F1/N mice, consisting of decreases in the RBC count, Hgb concentration and Hct with an increase in the MCV. Similar changes were observed in the Wistar Han rats, but were mild in comparison. The RBC count, Hgb concentration and Hct are quantitative estimates of the circulating erythroid mass. Changes in these parameters generally parallel each other; however, the magnitude of change between these parameters may differ when, for example, there are abnormal erythrocytes (Stockham 2008). As such, the observed decreases in the Hct do not accurately represent the decreases in the RBC concentration due to the presence of macrocytic erythrocytes. While the degree of anemia observed was not considered life–threatening, the finding is significant in that it indicates the bone marrow as a target site of BCE induced toxicity, raising the possibility of more detrimental effects on longer exposure duration.
The development of a non-regenerative macrocytic anemia indicates ineffective erythropoiesis and is consistent with a megaloblastic disorder. These disorders are a result of impaired DNA synthesis, which leads to increased rates of erythroid precursor cell death (apoptosis) and the observed ineffective erythropoiesis (Koury and Ponka 2004). In addition to the complete blood count changes, the mice peripheral blood smears also revealed changes consistent with disruption of erythropoiesis (basophilic stippling, erythrocyte morphology changes). A definitive diagnostic feature of megaloblastic disorders or anemias is the finding of what is called megaloblastic precursor cells in the bone marrow. Although examination of the bone marrow was not performed, the hematological findings in this study are highly compatible with this diagnosis and alternative explanations (e.g., myelodyplastic syndrome) for the findings are improbable.
Another finding of the current studies that may share a common etiology with the non-regenerative macrocytic anemia is the dose-dependent incidence of micronucleated peripheral red blood cells. Following 13 weeks of exposure to BCE via gavage, highly significant dose-dependent increases in the frequencies of micronucleated reticulocytes and erythrocytes were observed in peripheral blood of female B6C3F1/N mice. Evaluation of the reticulocyte population in mice allows assessment of chromosomal damage induced within the preceding 48 hr, while evaluation of the mature erythrocyte population permits assessment of chromosomal damage that has occurred during the preceding 4 weeks, as the mouse spleen, in contrast to the rat spleen, does not effectively remove damaged erythrocytes from circulation. Consistent with the observations in the mice, a significant increase in MN-RET was observed in peripheral blood of female Wistar Han rats exposed to BCE for 13 weeks. However, unlike the mouse spleen, the rat spleen efficiently and rapidly removes micronucleus-bearing red blood cells from circulation, depleting the circulating population of micronucleated cells very rapidly. Therefore, in rats, although data from the mature erythrocyte population was collected to permit calculation of the percentage of RET in circulation, only the CD-71 positive (transferrin receptor active) subpopulation of very young reticulocytes can be accurately assessed for micronucleus frequency as this cell population is too young to be appreciably altered by splenic selection (MacGregor et al. 2006).
There are several well-known causes of macrocytic anemia with megaloblastic marrow (megaloblastic anemia), all of which involve impairment of DNA synthesis and could also increase the incidence of micronucleated cells in the peripheral blood. Erythropoiesis, in particular, is susceptible to disruption from agents that directly or indirectly interfere with DNA synthesis or induce chromosomal breakage, as the progenitor cell population is rapidly dividing. Metabolic derangements of the two vitamins, folate and cobalmin (vitamin B12) can cause a megaloblastic anemia, as they are both essential for several reactions involved in DNA synthesis. In addition to outright folate or cobalamin deficiency, malabsorption syndromes, changes in bacterial gut flora and altered enterobiliary secretions (intrinsic factor) can all alter folate and cobalamin metabolism (Bills et al. 1992; Evans 2009; Wickramasinghe 2006). In addition, compounds may directly interfere with their uptake (e.g., colchicine, phenylhydantoin) or inhibit enzymes involved in their normal metabolism (e.g., methotrexate) (Evans 2009; Wickramasinghe 2006). Compounds that directly affect DNA synthesis can also cause a megaloblastic anemia and increased micronucleus frequencies in RBCs. These include purine analogues (e.g. azathioprine, 6-mercaptopurine) and pyrimidine analogues (e.g. 5 – fluorouracil). In mice, administration of azidothymidine (AZT) or 1,3 –butadiene have been shown to cause a nonregenerative macrocytic/megaloblastic anemia by directly affecting DNA metabolism (Irons et al. 1986a, b; NTP 1999); both these compounds also increased the frequencies of micronucleated erythrocytes (Tice et al. 1987; Witt et al. 2004). While it is suspected that BCE affects folate or cobalamin metabolism, further studies are needed to elucidate the mechanism by which it causes a macrocytic anemia.
Large, comprehensive cytogenetics studies of human populations have shown that increased frequencies of chromosomal aberrations or micronuclei in peripheral lymphocytes are associated with an increased risk for cancer in these populations, as the elevated incidences of cytogenetic abnormalities indicate exposure to genotoxicants (Bonassi et al. 2007). Other studies have shown that chemicals that induce micronuclei in red blood cells of laboratory rodents can also do so in humans (Bishop et al. 2004; Witt et al. 2007; Witt et al. 2004), and positive results in rodent micronucleus tests are highly correlated with rodent carcinogenicity (Witt et al. 2000). Thus, the demonstrated association between cytogenetic endpoints of DNA damage in humans and cancer risk, together with the observations of dose-dependent increases in micronucleated red blood cells, indicative of chromosomal aberration induction, in two different rodent species in the current study, raises a concern for potentially adverse health consequences related to exposure to black cohosh. Evaluation of MN-RET or micronucleated lymphocyte frequencies in a cohort of human females who regularly use black cohosh products might be useful as part of an effort to better characterize the potential for genotoxicity after consumption of recommended doses of BCE. Given the widespread use of this herbal product, additional efforts to further define potential risks from exposure seems warranted.
Outside the hematopoietic system, toxicity in the thymus of rats and the liver of rats and mice were observed; however these effects were less apparent than the hematological effects. Thymus weights were decreased in rats in a dose-dependent manner and microscopic lesions were observed in rats treated with the top two doses of BCE. To address possible immune toxicity of BCE, the NTP conducted immunotoxicity studies in female mice that will be reported separately. Liver weights were increased at the two top BCE doses in mice but only at the top dose in rat, and 2/10 rats had minimal to mild liver necrosis. However, there were no changes in clinical chemistry parameters that would indicate liver toxicity.
In the current studies BCE treatment did not produce the well-characterized estrogenic effect of increasing uterus weight in juvenile CD-1 mice (Newbold et al. 2001), nor display other estrogenic effects in B6C3F1/N or Wistar Han rats treated from weaning to adulthood. This was expected since BCE used in these studies did not contain detectable levels of formononetin, an estrogenic isoflavone reported in some black cohosh samples (Jarry et al. 1985). Furthermore, the highest dose used seemed to delay puberty in rats by 3 days while a similar delay in B6C3F1/N mouse puberty attainment was not dose related; the observed delays in rodent pubertal attainment are not expected from an estrogenic compound but may be produced by chemicals that block estrogen or prevent its biosynthesis (Marty et al. 1999). However, the potential of BCE to behave as an antiestrogen was examined by cotreating CD-1 mice with a dose of estradiol that produces 50% response in the uterotrophic assay. In this experiment, BCE was unable to block the effect of estradiol. Other endocrine effects observed in the subchronic assays were discordant and/or of marginal biological significance, and included decreased pituitary weights in Wistar Han rats and increased reproductive tract weights in B6C3F1/N mice. Estrogen treatment in rats leads to lactotroph hyperplasia, decreased numbers of somatotropes and enlarged pituitary (Lloyd 1983), while pure antiestrogens like fluvestrant (ICI 182,780) inhibit lactotroph proliferation, and selective estrogen receptor modulators (SERMs) may be less potent than fluvestrant or have no effect (Kansra et al. 2005). Decreased pituitary weights may indicate decreased ability of the gland to release hormones needed for normal pubertal attainment. However, while the pubertal delay and decreased pituitary weights point towards an antiestrogenic or SERM-type effect, the magnitude of the pituitary weight change in the current study is of questionable biological significance given the lack of a clear dose-dependent response and the absence of microscopic changes in the same organs. Furthermore, without more definitive data, other potential activities of BCE at the hypothalamus or CNS level (i.e. dopaminergic, opioid) cannot be discounted as causing delayed puberty in rats. In addition, the increases in reproductive organ weights of B6C3F1/N mice could also suggest a SERM-type effect (i.e. agonism at the reproductive tract at the same time as antagonism at the pituitary or hypothalamus). However the increases in reproductive tract weight are difficult to interpret since they lack a clear dose-dependent pattern and occurred in intact animals which were necropsied on the same calendar date regardless of the estrous cycle stage for each individual mouse.
While the current studies provide the first comprehensive assessment of the subchronic toxicity of BCE in rodents, direct extrapolation of the results to humans is difficult since BCE preparations are variable natural mixtures of chemicals (many still unidentified) and it is not known which compounds in the mixture are active. In the current studies the lowest external dose that had an effect (62.5 mg/kg/day) was 125 times the currently recommended amount for daily human consumption (~ 0.5 mg/kg/day for a 70 kg human). Pharmacokinetic studies of one or several marker compounds could permit a comparison of internal doses of BCE between rodents and humans.
In conclusion, BCE treatment for 90 days starting at weaning caused a dose-dependent increase in peripheral micronucleated RBCs indicative of chromosomal damage and hematological changes consistent with a non-regenerative macrocytic anemia in both female rats and female mice. Both the chromosomal damage and hematological effects were more severe in mice than rats. Less apparent toxicity was also observed in the thymus of rats and the liver of rats and mice. Delayed puberty was observed at the highest treatment dose in rats but a similar delay in mice was not dose-dependent. Neither analysis of onset of puberty or uterotrophic assay demonstrated estrogenic or antiestrogenic activity; however other hormonal or CNS/hypothalamus activities were not measured and cannot be discounted as a cause of delayed puberty. Biologically significant effects were not observed in the cardiovascular system or in other tissues. Further studies are underway to determine the reproductive toxicity, immune toxicity and carcinogenicity of BCE.
Highlights.
Mice and rats were dosed with black cohosh extract for 90 days starting at weaning.
Hematological changes were consistent with a non-regenerative macrocytic anemia.
Peripheral micronucleated red blood cells frequencies increased.
Puberty was delayed 2.9 days in rats.
No estrogenic/anti-estrogenic activity was seen in the uterotrophic assay.
Acknowledgments
This article may be the work product of an employee or group of employees of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), however, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions or conclusions of NIEHS, NIH or the United States government.
The authors want to thank Drs. Chad Blystone, Grace Kissling, Barry McIntyre, and Nigel Walker for their critical review of this article. The subchronic assays were conducted at the facilities of Battelle, Columbus OH. Estrous cycle determinations were conducted by RTI International, Research Triangle Park, NC. The juvenile uterotrophic assay was conducted by Dr. Wendy Jefferson and Elizabeth Padilla-Banks at the laboratory of Dr. Retha Newbold, National Institute of Environmental Health Sciences.
Funding Information
This work was supported by the Division of the National Toxicology Program at the National Institute of Environmental Health Sciences under Contract Numbers N01-ES-55536, N01-ES-55551, N01-ES-35514, and Research Project Number 1 Z01 ESO45004-11 BB.
Abbreviations
- BCE
black cohosh extract
- PND
postnatal day
- MN
micronucleus
- RET
reticulocytes
- MN-RET
micronucleated reticulocytes
- MNE
micronucleated erythrocytes
- SEM
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Herbal Pharmacopoeia. Black Cohosh Rhizome, Actaea racemosa L. syn. Cimicifuga racemosa (L.) Nutt., Standards of Analysis, Quality Control, and Therapeutics. In: Upton R, editor. American Herbal Pharmacopoeia and Therapeutic Compendium. American Herbal Pharmacopoeia; Santa Cruz, California: 2002. [Google Scholar]
- Bain BJ, Clark DM, Lampert IA, Wilkins BS. Bone Marrow Pathology. Blackwell Sciences; Oxford, UK: 2001. [Google Scholar]
- Bennett J, Vickery B. Rats and Mice. In: Hafez E, editor. Reproduction and Breeding Techniques for Laboratory Animals. Lea and Febiger; Philadelphia: 1970. pp. 299–315. [Google Scholar]
- Bills ND, Koury MJ, Clifford AJ, Dessypris EN. Ineffective hematopoiesis in folate-deficient mice. Blood. 1992;79:2273–2280. [PubMed] [Google Scholar]
- Bishop JB, Witt KL, Tice RR, Wolfe GW. Genetic damage detected in CD-1 mouse pups exposed perinatally to 3′-azido-3′-deoxythymidine and dideoxyinosine via maternal dosing, nursing, and direct gavage. Environ Mol Mutagen. 2004;43:3–9. doi: 10.1002/em.10210. [DOI] [PubMed] [Google Scholar]
- Bonassi S, Znaor A, Ceppi M, Lando C, Chang WP, Holland N, Kirsch-Volders M, Zeiger E, Ban S, Barale R, Bigatti MP, Bolognesi C, Cebulska-Wasilewska A, Fabianova E, Fucic A, Hagmar L, Joksic G, Martelli A, Migliore L, Mirkova E, Scarfi MR, Zijno A, Norppa H, Fenech M. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis. 2007;28:625–631. doi: 10.1093/carcin/bgl177. [DOI] [PubMed] [Google Scholar]
- Bronson F, Dagg C, Snell G. Reproduction. In: Green E, editor. Biology of the Laboratory Mouse. Dover; New York: 1968. pp. 187–204. [Google Scholar]
- Chung DJ, Kim HY, Park KH, Jeong KA, Lee SK, Lee YI, Hur SE, Cho MS, Lee BS, Bai SW, Kim CM, Cho SH, Hwang JY, Park JH. Black cohosh and St. John’s wort (GYNO-Plus) for climacteric symptoms. Yonsei Med J. 2007;48:289–294. doi: 10.3349/ymj.2007.48.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover W. Practical Nonparametric Statistics. John Wiley & Sons; New York: 1971. [Google Scholar]
- Dugoua JJ, Seely D, Perri D, Koren G, Mills E. Safety and efficacy of black cohosh (Cimicifuga racemosa) during pregnancy and lactation. Can J Clin Pharmacol. 2006;13:e257–261. [PubMed] [Google Scholar]
- Evans GO. Animal Clinical Chemistry: A Practical Guide for Toxicologists and Biomedical Researchers. Taylor and Francis Group; Boca Raton, FL: 2009. [Google Scholar]
- Food and Drug Administration. 21 CFR 58, Federal Register. 1987. Good laboratory practices regulations; p. 12. [Google Scholar]
- Girard DM, Sager DB. The use of Markov chains to detect subtle variation in reproductive cycling. Biometrics. 1987;43:225–234. [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Guzman G, Kallwitz ER, Wojewoda C, Chennuri R, Berkes J, Layden TJ, Cotler SJ. Liver Injury with Features Mimicking Autoimmune Hepatitis following the Use of Black Cohosh. Case Report Med. 2009;2009:918156. doi: 10.1155/2009/918156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons RD, Smith CN, Stillman WS, Shah RS, Steinhagen WH, Leiderman LJ. Macrocytic-megaloblastic anemia in male B6C3F1 mice following chronic exposure to 1,3-butadiene. Toxicol Appl Pharmacol. 1986a;83:95–100. doi: 10.1016/0041-008x(86)90326-1. [DOI] [PubMed] [Google Scholar]
- Irons RD, Smith CN, Stillman WS, Shah RS, Steinhagen WH, Leiderman LJ. Macrocytic-megaloblastic anemia in male NIH Swiss mice following repeated exposure to 1,3-butadiene. Toxicol Appl Pharmacol. 1986b;85:450–455. doi: 10.1016/0041-008x(86)90352-2. [DOI] [PubMed] [Google Scholar]
- Jacobson JS, Troxel AB, Evans J, Klaus L, Vahdat L, Kinne D, Lo KM, Moore A, Rosenman PJ, Kaufman EL, Neugut AI, Grann VR. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol. 2001;19:2739–2745. doi: 10.1200/JCO.2001.19.10.2739. [DOI] [PubMed] [Google Scholar]
- Jarry H, Harnischfeger G. Studies on the endocrine effects of the contents of Cimicifuga racemosa. Planta Med. 1985;51:46–49. doi: 10.1055/s-2007-969390. [DOI] [PubMed] [Google Scholar]
- Jarry H, Metten M, Spengler B, Christoffel V, Wuttke W. In vitro effects of the Cimicifuga racemosa extract BNO 1055. Maturitas. 2003;44(Suppl 1):S31–38. doi: 10.1016/s0378-5122(02)00346-8. [DOI] [PubMed] [Google Scholar]
- Jiang B, Ma C, Motley T, Kronenberg F, Kennelly EJ. Phytochemical fingerprinting to thwart black cohosh adulteration: a 15 Actaea species analysis. Phytochem Anal. 2011;22:339–51. doi: 10.1002/pca.1285. [DOI] [PubMed] [Google Scholar]
- Kansra S, Yamagata S, Sneade L, Foster L, Ben-Jonathan N. Differential effects of estrogen receptor antagonists on pituitary lactotroph proliferation and prolactin release. Mol Cell Endocrinol. 2005;239:27–36. doi: 10.1016/j.mce.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Kissling GE, Dertinger SD, Hayashi M, MacGregor JT. Sensitivity of the erythrocyte micronucleus assay: dependence on number of cells scored and inter-animal variability. Mutat Res. 2007;634:235–240. doi: 10.1016/j.mrgentox.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koury MJ, Ponka P. New insights into erythropoiesis: the roles of folate, vitamin B12, and iron. Annu Rev Nutr. 2004;24:105–131. doi: 10.1146/annurev.nutr.24.012003.132306. [DOI] [PubMed] [Google Scholar]
- Lloyd RV. Estrogen-induced hyperplasia and neoplasia in the rat anterior pituitary gland. An immunohistochemical study. Am J Pathol. 1983;113:198–206. [PMC free article] [PubMed] [Google Scholar]
- MacGregor JT, Bishop ME, McNamee JP, Hayashi M, Asano N, Wakata A, Nakajima M, Saito J, Aidoo A, Moore MM, Dertinger SD. Flow cytometric analysis of micronuclei in peripheral blood reticulocytes: II. An efficient method of monitoring chromosomal damage in the rat. Toxicol Sci. 2006;94:92–107. doi: 10.1093/toxsci/kfl076. [DOI] [PubMed] [Google Scholar]
- Marty MS, Crissman JW, Carney EW. Evaluation of the EDSTAC female pubertal assay in CD rats using 17beta-estradiol, steroid biosynthesis inhibitors, and a thyroid inhibitor. Toxicol Sci. 1999;52:269–277. doi: 10.1093/toxsci/52.2.269. [DOI] [PubMed] [Google Scholar]
- McKenzie SC, Rahman A. Bradycardia in a patient taking black cohosh. Med J Aust. 2010;193:479–481. doi: 10.5694/j.1326-5377.2010.tb04006.x. [DOI] [PubMed] [Google Scholar]
- Nappi RE, Malavasi B, Brundu B, Facchinetti F. Efficacy of Cimicifuga racemosa on climacteric complaints: a randomized study versus low-dose transdermal estradiol. Gynecol Endocrinol. 2005;20:30–35. doi: 10.1080/09513590400020922. [DOI] [PubMed] [Google Scholar]
- Naser B, Schnitker J, Minkin MJ, de Arriba SG, Nolte KU, Osmers R. Suspected black cohosh hepatotoxicity: no evidence by meta-analysis of randomized controlled clinical trials for isopropanolic black cohosh extract. Menopause. 2011 doi: 10.1097/gme.0b013e3181fcb2a6. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. National Academies Press; Washington DC: 1996. [Google Scholar]
- National Toxicology Program (NTP) NTP Toxicology and Carcinogenesis Studies of AZT (CAS No. 30516-87-1) and AZT/alpha-Interferon A/D B6C3F1 Mice (Gavage Studies) Natl Toxicol Program Tech Rep Ser. 1999;469:1–361. [PubMed] [Google Scholar]
- Newbold RR, Jefferson WN, Padilla-Banks E, Walker VR, Pena DS. Cell response endpoints enhance sensitivity of the immature mouse uterotropic assay. Reprod Toxicol. 2001;15:245–252. doi: 10.1016/s0890-6238(01)00130-7. [DOI] [PubMed] [Google Scholar]
- Pierard S, Coche JC, Lanthier P, Dekoninck X, Lanthier N, Rahier J, Geubel AP. Severe hepatitis associated with the use of black cohosh: a report of two cases and an advice for caution. Eur J Gastroenterol Hepatol. 2009;21:941–945. doi: 10.1097/MEG.0b013e3283155451. [DOI] [PubMed] [Google Scholar]
- Powell SL, Godecke T, Nikolic D, Chen SN, Ahn S, Dietz B, Farnsworth NR, van Breemen RB, Lankin DC, Pauli GF, Bolton JL. In vitro serotonergic activity of black cohosh and identification of N(omega)-methylserotonin as a potential active constituent. J Agric Food Chem. 2008;56:11718–11726. doi: 10.1021/jf803298z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachon D, Vortherms T, Seidlova-Wuttke D, Wuttke W. Effects of black cohosh extract on body weight gain, intra-abdominal fat accumulation, plasma lipids and glucose tolerance in ovariectomized Sprague-Dawley rats. Maturitas. 2008;60:209–215. doi: 10.1016/j.maturitas.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Reame NE, Lukacs JL, Padmanabhan V, Eyvazzadeh AD, Smith YR, Zubieta JK. Black cohosh has central opioid activity in postmenopausal women: evidence from naloxone blockade and positron emission tomography neuroimaging. Menopause. 2008;15:832–840. doi: 10.1097/gme.0b013e318169332a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhyu MR, Lu J, Webster DE, Fabricant DS, Farnsworth NR, Wang ZJ. Black cohosh (Actaea racemosa, Cimicifuga racemosa) behaves as a mixed competitive ligand and partial agonist at the human mu opiate receptor. J Agric Food Chem. 2006;54:9852–9857. doi: 10.1021/jf062808u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Rivest S. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biol Reprod. 1991;45:523–532. doi: 10.1095/biolreprod45.4.523. [DOI] [PubMed] [Google Scholar]
- Seidlova-Wuttke D, Hesse O, Jarry H, Christoffel V, Spengler B, Becker T, Wuttke W. Evidence for selective estrogen receptor modulator activity in a black cohosh (Cimicifuga racemosa) extract: comparison with estradiol-17beta. Eur J Endocrinol. 2003;149:351–362. doi: 10.1530/eje.0.1490351. [DOI] [PubMed] [Google Scholar]
- Stockham SL. Fundamentals of Veterinary Clinical Pathology. Blackwell Publishing; Ames, IA: 2008. [Google Scholar]
- Teschke R, Schwarzenboeck A. Suspected hepatotoxicity by Cimicifugae racemosae rhizoma (black cohosh, root): critical analysis and structured causality assessment. Phytomedicine. 2009;16:72–84. doi: 10.1016/j.phymed.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Tice RR, Boucher R, Luke CA, Shelby MD. Comparative cytogenetic analysis of bone marrow damage induced in male B6C3F1 mice by multiple exposures to gaseous 1,3-butadiene. Environ Mutagen. 1987;9:235–250. doi: 10.1002/em.2860090303. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe SN. Diagnosis of megaloblastic anaemias. Blood Rev. 2006;20:299–318. doi: 10.1016/j.blre.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Witt KL, Cunningham CK, Patterson KB, Kissling GE, Dertinger SD, Livingston E, Bishop JB. Elevated frequencies of micronucleated erythrocytes in infants exposed to zidovudine in utero and postpartum to prevent mother-to-child transmission of HIV. Environ Mol Mutagen. 2007;48:322–329. doi: 10.1002/em.20266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt KL, Knapton A, Wehr CM, Hook GJ, Mirsalis J, Shelby MD, MacGregor JT. Micronucleated erythrocyte frequency in peripheral blood of B6C3F(1) mice from short-term, prechronic, and chronic studies of the NTP carcinogenesis bioassay program. Environ Mol Mutagen. 2000;36:163–194. [PubMed] [Google Scholar]
- Witt KL, Livanos E, Kissling GE, Torous DK, Caspary W, Tice RR, Recio L. Comparison of flow cytometry- and microscopy-based methods for measuring micronucleated reticulocyte frequencies in rodents treated with nongenotoxic and genotoxic chemicals. Mutat Res. 2008;649:101–113. doi: 10.1016/j.mrgentox.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt KL, Tice RR, Wolfe GW, Bishop JB. Genetic damage detected in CD-1 mouse pups exposed perinatally to 3′-azido-3′-deoxythymidine or dideoxyinosine via maternal dosing, nursing, and direct gavage: II. Effects of the individual agents compared to combination treatment. Environ Mol Mutagen. 2004;44:321–328. doi: 10.1002/em.20048. [DOI] [PubMed] [Google Scholar]
- Zimmermann R, Witte A, Voll RE, Strobel J, Frieser M. Coagulation activation and fluid retention associated with the use of black cohosh: a case study. Climacteric. 2010;13:187–191. doi: 10.3109/13697130902939921. [DOI] [PubMed] [Google Scholar]