Abstract
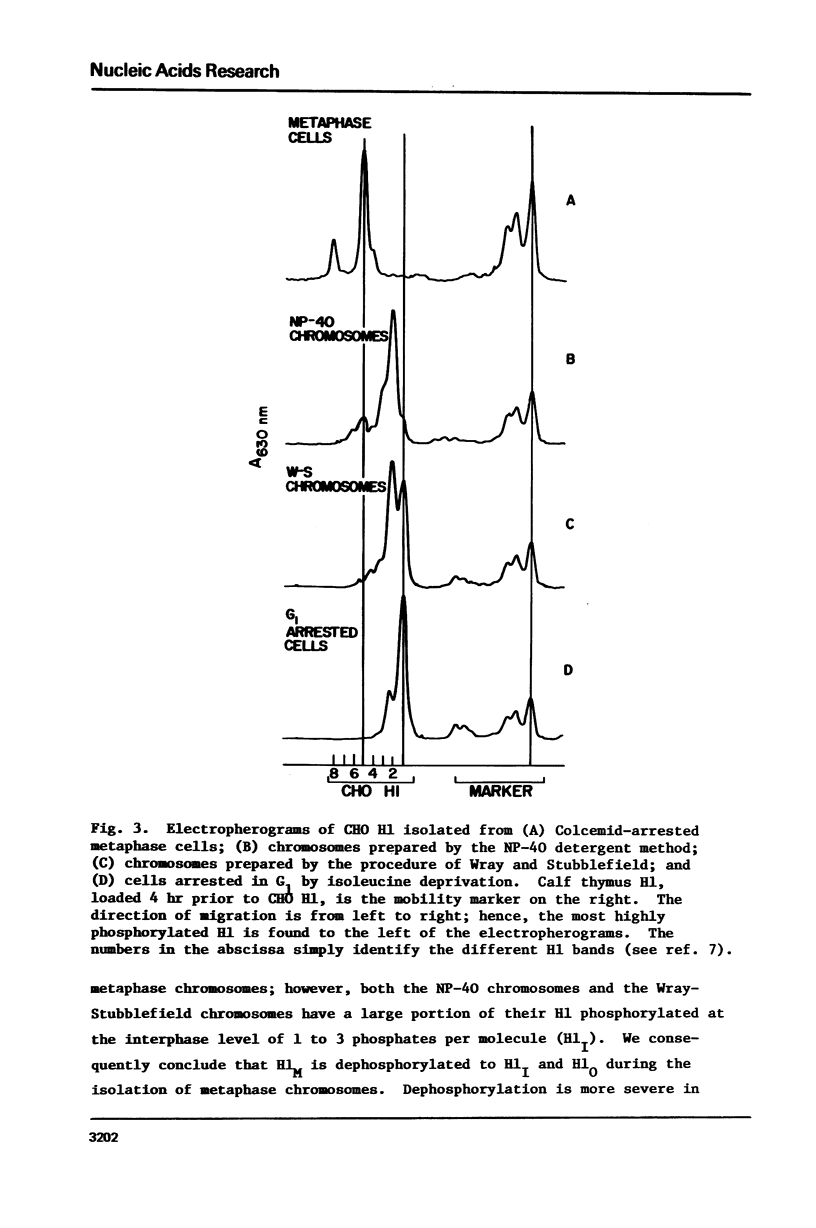
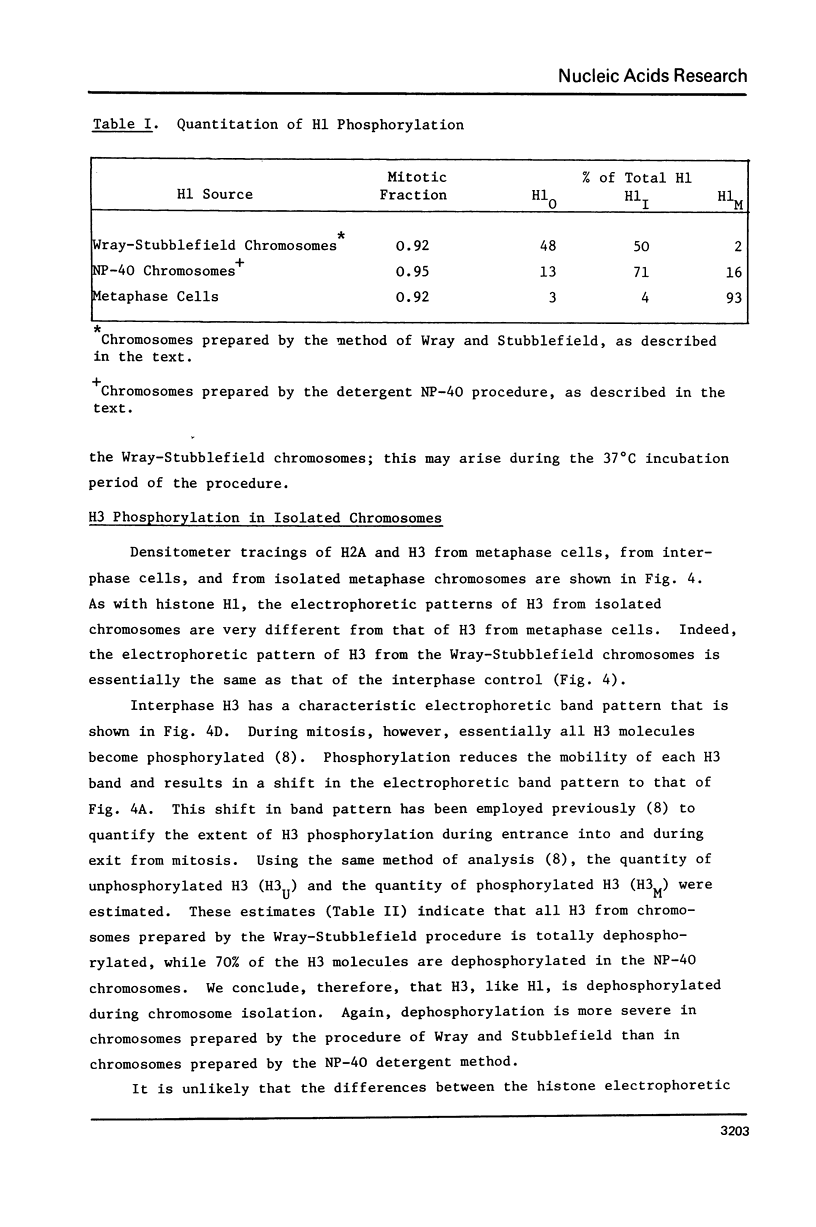
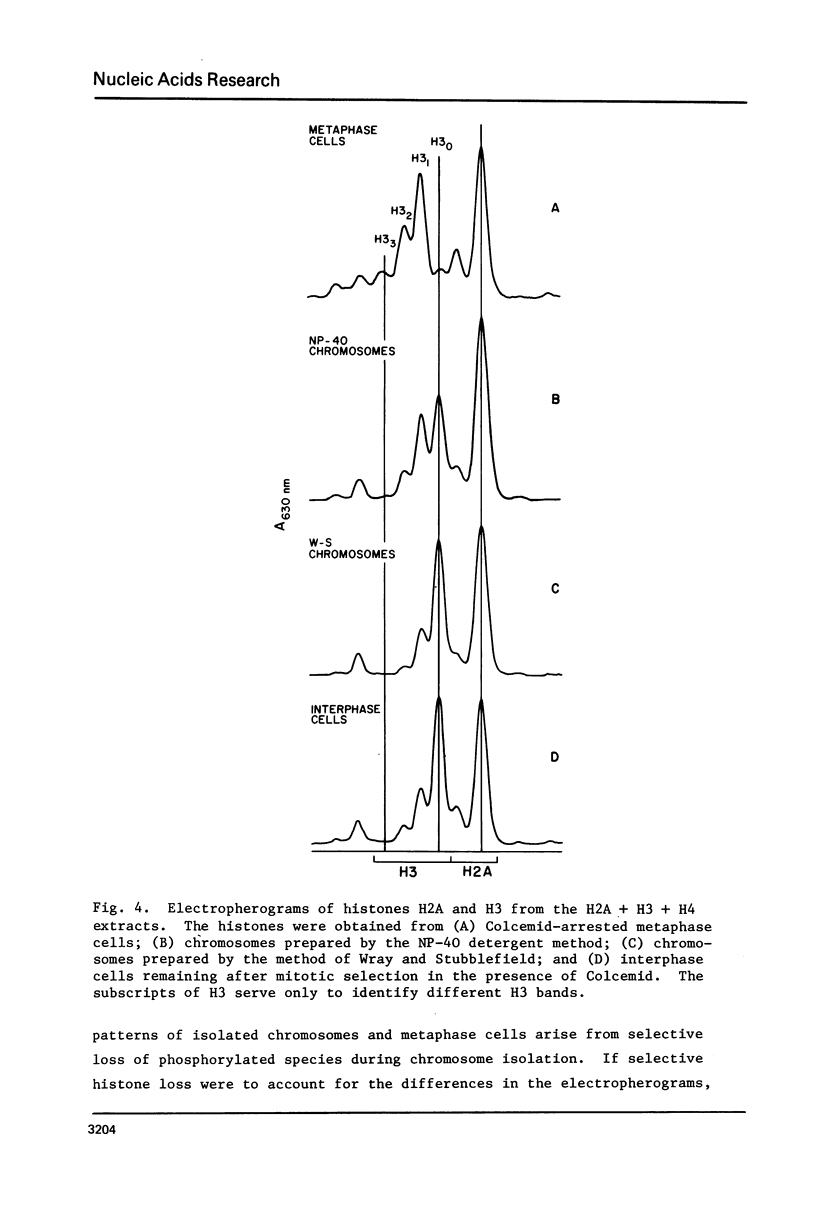
Histones have been extracted from isolated metaphase chromosomes prepared by the method of Wray and Sutbblefield [Exp. Cell Res 59, 469-478 (1970)] and by a Nonidet P-40 detergent procedure based on the method of Wigler and Axel [Nucleic Acids Res. 3, 1463-1471 (1976)]. Analysis of the densitometer profiles of long polyacrylamide gels shows that the mitotic phosphorylations of histone H1 (H1M) and histone H3 are extensively depleted during chromosome isolation. These data indicate that CHO metaphase chromosomes prepared by standard methodologies do not represent in vivo chromosomes with respect to their histone phosphorylations; therefore, current chemical and structural studies of isolated metaphase chromosomes may require further clarification.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bak A. L., Zeuthen J., Crick F. H. Higher-order structure of human mitotic chromosomes. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1595–1599. doi: 10.1073/pnas.74.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock C. J., Christie S., Hatch F. T. Accessibility of DNA in condensed chromatin to nuclease digestion. Nature. 1976 Aug 5;262(5568):516–519. doi: 10.1038/262516a0. [DOI] [PubMed] [Google Scholar]
- Compton J. L., Hancock R., Oudet P., Chambon P. Biochemical and electron-microscopic evidence that the subunit structure of Chinese-hamster-ovary interphase chromatin is conserved in mitotic chromosomes. Eur J Biochem. 1976 Nov 15;70(2):555–568. doi: 10.1111/j.1432-1033.1976.tb11047.x. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Klug A. Solenoidal model for superstructure in chromatin. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1897–1901. doi: 10.1073/pnas.73.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley L. R., D'Anna J. A., Barham S. S., Deaven L. L., Tobey R. A. Histone phosphorylation and chromatin structure during mitosis in Chinese hamster cells. Eur J Biochem. 1978 Mar;84(1):1–15. doi: 10.1111/j.1432-1033.1978.tb12135.x. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., Hardin J. M. The metabolism of histone fractions. I. Synthesis of histone fractions during the life cycle of mammalian cells. Arch Biochem Biophys. 1968 Nov;128(2):285–292. doi: 10.1016/0003-9861(68)90034-9. [DOI] [PubMed] [Google Scholar]
- Hohmann P., Tobey R. A., Gurley L. R. Phosphorylation of distinct regions of f1 histone. Relationship to the cell cycle. J Biol Chem. 1976 Jun 25;251(12):3685–3692. [PubMed] [Google Scholar]
- Hozier J. C., Kaus R. Subunit structure of chromosomes in mitotic nuclei of physarum polycephalum. Chromosoma. 1976 Aug 4;57(1):95–102. doi: 10.1007/BF00292952. [DOI] [PubMed] [Google Scholar]
- Johns E. W. Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J. 1964 Jul;92(1):55–59. doi: 10.1042/bj0920055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. The heterogeneity of histones. I. A quantitative analysis of calf histones in very long polyacrylamide gels. Biochemistry. 1969 Oct;8(10):3972–3979. doi: 10.1021/bi00838a013. [DOI] [PubMed] [Google Scholar]
- Renz M., Nehls P., Hozier J. Involvement of histone H1 in the organization of the chromosome fiber. Proc Natl Acad Sci U S A. 1977 May;74(5):1879–1883. doi: 10.1073/pnas.74.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubblefield E., Cram S., Deaven L. Flow microfluorometric analysis of isolated Chinese hamster chromosomes. Exp Cell Res. 1975 Sep;94(2):464–468. doi: 10.1016/0014-4827(75)90519-4. [DOI] [PubMed] [Google Scholar]
- Stubblefield E., Wray W. Architecture of the Chinese hamster metaphase chromosome. Chromosoma. 1971;32(3):262–294. doi: 10.1007/BF00284839. [DOI] [PubMed] [Google Scholar]
- Thoma F., Koller T. Influence of histone H1 on chromatin structure. Cell. 1977 Sep;12(1):101–107. doi: 10.1016/0092-8674(77)90188-x. [DOI] [PubMed] [Google Scholar]
- Tobey R. A., Anderson E. C., Petersen D. F. Properties of mitotic cells prepared by mechanically shaking monolayer cultures of Chinese hamster cells. J Cell Physiol. 1967 Aug;70(1):63–68. doi: 10.1002/jcp.1040700109. [DOI] [PubMed] [Google Scholar]
- Tobey R. A., Ley K. D. Isoleucine-mediated regulation of genome repliction in various mammalian cell lines. Cancer Res. 1971 Jan;31(1):46–51. [PubMed] [Google Scholar]
- Tobey R. A., Petersen D. F., Anderson E. C., Puck T. T. Life cycle analysis of mammalian cells. 3. The inhibition of division in Chinese hamster cells by puromycin and actinomycin. Biophys J. 1966 Sep;6(5):567–581. doi: 10.1016/s0006-3495(66)86678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Braun R. Repeated structure of chromatin in metaphase nuclei of Physarum. FEBS Lett. 1976 Apr 15;64(1):190–192. doi: 10.1016/0014-5793(76)80280-3. [DOI] [PubMed] [Google Scholar]
- Wigler M. H., Axel R. Nucleosomes in metaphase chromosomes. Nucleic Acids Res. 1976 Jun;3(6):1463–1471. doi: 10.1093/nar/3.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke K., Ruddle F. H. Transfer of the human gene for hypoxanthine-guanine phosphoribosyltransferase via isolated human metaphase chromosomes into mouse L-cells. Proc Natl Acad Sci U S A. 1975 May;72(5):1792–1796. doi: 10.1073/pnas.72.5.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A., Benyajati C. Higher order coiling of DNA in chromatin. Cell. 1977 Sep;12(1):83–100. doi: 10.1016/0092-8674(77)90187-8. [DOI] [PubMed] [Google Scholar]
- Wray W., Stubblefield E. A new method for the rapid isolation of chromosomes, mitotic apparatus, or nuclei from mammalian fibroblasts at near neutral pH. Exp Cell Res. 1970 Mar;59(3):469–478. doi: 10.1016/0014-4827(70)90656-7. [DOI] [PubMed] [Google Scholar]




