Summary
Upon activation, quiescent naive T cells undergo a growth phase followed by massive clonal expansion and differentiation that are essential for appropriate immune defense and regulation. Accumulation of cell biomass during the initial growth and rapid proliferation during the expansion phase is associated with dramatically increased bioenergetic and biosynthetic demands. This not only requires a metabolic rewiring during the transition between resting and activation, but also ‘addicts’ active T cells to certain metabolic pathways in ways that naive and memory T cells are not. We consider such addiction in terms of the biological effects of deprivation of metabolic substrates or inhibition of specific pathways in T cells. In this review, we illustrate the relevant metabolic pathways revealed by recent metabolic flux analysis and discuss the consequences of metabolic intervention on specific metabolic pathways in T lymphocytes.
Keywords: metabolism, T lymphocytes, T-cell activation
Introduction
As this volume attests, there is a renewed intellectual excitement that surrounds the metabolic events that empower immune responses. Indeed, here we will devote several pages to the metabolic changes that accompany and support T-cell activation. Before doing so, however, it may be worthwhile to ask why we should care that is, why any aspect of T-cell metabolism should distinguish it from that of any other cell type in the body.
There are at least three ways to address this question. First, we may maintain that T-cell metabolism holds a great deal in common with that of other cells (and little that is distinct), and thus manipulating the cell (e.g. by activation) provides a convenient, powerful, general model for exploring how signaling events impact on metabolic processes and vice versa. Alternatively, we may assert that T cells are ‘special’, having the property (which they share with B cells but few, if any, others) that following signals to engage proliferation, they do not do so directly but instead engage a long lag period during which they grow (increase in mass). This is then followed by a period of multiple rounds of cell division in which the cell cycle can be extraordinarily short (4–6 h). These properties are illustrated in the simple experiment shown in Fig. 1. Therefore, the metabolic processes associated with these growth and proliferation phases may have unique (or at least unusual) features. Finally, as a third type of answer, we might speculate that whether or not lymphocytes or other cells of the immune system have distinct metabolisms, these nevertheless have the potential to act as targets for therapeutic manipulation to promote or inhibit immune responses.
Fig. 1. Growth and proliferation of T cells following activation.
T-cell proliferation (A) and cell size (B) at the indicated time points following activation with anti-CD3 plus anti-CD28 were determined by CFSE dilution and FSC, respectively.
We do not know the answers to these questions, nor do we know which of these viewpoints hold the most value, although we suspect that they are all correct to some extent. Therefore, we simply overview what is known about metabolic events associated with T-cell activation and functions in different contexts and what happens when they are perturbed.
Several studies in the second half of the last century examined the changes in T-cell metabolism upon activation with polyclonal mitogens, and these have been reviewed extensively (1, 2). More recent studies have confirmed these findings in the context of defined T-cell activation events, and we mostly focus on these studies here, recognizing the important contributions of these earlier efforts.
Metabolic reprogramming upon T-cell activation
Quiescent naive and memory T cells rely on metabolism largely for housekeeping functions, such as the transportation and turnover of biomaterials, maintenance of the cytoskeleton, and regulation of ion gradients across the plasma membrane and intracellular organelle membranes. Mitochondria-dependent catabolic pathways such as glucose oxidation through the tricarboxylic acid (TCA) cycle and fatty acid beta-oxidation provide most of the metabolic support for these basic cellular functions in naive and memory cells (3, 4).
Upon antigen exposure, immune signaling from the T-cell receptor (TCR), costimulatory molecules, and cytokine receptors drives T cells into rapid growth and proliferation, requiring a duplication of its biomass for every cell division, and meanwhile instructs T cells to differentiate into their various functional populations. The active T cell must acquire metabolic support for converting available metabolic resources into biomass, producing sufficient adenosine triphosphate (ATP) as a source of free energy (for thermodynamically unfavorable cellular processes), and finally, generating metabolic products as signals for triggering intra- and inter-cellular signaling events (5, 6).
This change in metabolic activity and biomass generation can readily be observed in activated T cells prior to the first cell division at approximately 24 h post activation (4, 7–9). In the experiments illustrated in Figs 2 and 3, T cells were activated by ligation of CD3 and CD28 and evaluated for metabolic flux and the production of biomolecules. Not surprisingly, during the period of cell growth prior to the first cell division, there is dramatically elevated synthesis of proteins and lipids. Despite the energetic demands of such synthesis, ATP levels are elevated, indicative of expanded metabolic activity. Upon activation, T cells dramatically increase their uptake and catabolism of glucose and glutamine, and their consumption of oxygen, while quickly decreasing uptake and catabolism of free fatty acids (FFA) by β-oxidation.
Fig. 2. Increased biosynthesis and energy production upon T-cell activation.
Protein and lipid biosynthesis were determined by the incorporation of [14C]-acetate into the chloroform soluble lipid fraction and [3H]-amino acid mixture into the 10%TCA insoluble protein fraction, respectively (A). The cellular ATP content was determined using a luciferase-based bioluminescence assay (B). Methods are described elsewhere (4). The value in resting T cells was set to 1 in all cases.
Fig. 3. Metabolic reprogramming in activated T cells.
The indicated metabolic activities in resting T cells and activated T cells (collected at 24 h after activation) were determined by previously described metabolic flux assays (4).
These changes require a complex rewiring of multiple major catabolic pathways upon T-cell activation. In unicellular organisms, the control of such metabolic processes rests with the effects of substrate and product concentrations on the enzymes of the pathway. We have come to view these processes, then, as the proteins of ‘housekeeping’ genes, and their expression (even in animal cells) are routinely used as experimental controls. In multicellular organisms, however, gene expression of metabolic pathways is actively regulated, and this is readily apparent in T cells. Upon activation, most of the enzymes of the pathways of glycolysis and glutaminolysis are elevated, as are key enzymes in the pentose phosphate pathway (PPP). Similarly, many key enzymes for biosynthesis of macromolecules are coordinately expressed. In contrast, the expression of enzymes for FFA oxidation is repressed (4). The control of these transcriptional events is discussed in more detail in the next section.
One component of the metabolic reprogramming of activated T cells involves a dramatic increase in the rate of uptake and consumption of glucose and a change in the fate of glucose carbons (4, 7, 8). Glucose is first catabolized through a multi-step set of reactions resulting in an intermediate metabolite, pyruvate (glycolysis). Upon activation, some of the glucose catabolic flux diverts to the PPP at the point of glucose-6-phosphate. This leads to the production of NADPH for redox balance and 5-carbon ribose for nucleotide biosynthesis. Through various metabolic interconnections, the carbons of glucose also feed into different synthetic pathways to generate the precursors of hexamine, amino acids and lipids (see also below). Thus, the glucose catabolic pathway in active T cells is not optimized to produce ATP.
In naive and memory T cells, the majority of pyruvate enters the mitochondria, where it is converted to acetyl-CoA through oxidative decarboxylation and consequentially fluxes into the tricarboxylic acid (TCA) cycle to generate ATP. However, in active T cells, a major portion of pyruvate detours away from the TCA (Fig. 3) toward the production of lactate through the action of lactate dehydrogenase (LDH). Therefore, the production of lactate via glycolysis is significantly upregulated following T-cell activation. Importantly, this change is not restricted to low oxygen (anaerobic) environments and is actively regulated by signal transduction pathways when oxygen is plentiful (aerobic glycolysis) (4, 7, 8).
Another major carbohydrate catabolic pathway that is significantly elevated following T-cell activation is glutaminolysis, the glutamine catabolic pathway (4, 9, 10). Glutamine is not only an important nitrogen and carbon donor for the synthesis of many other amino acids but also a major carbon resource for α-ketoglutarate (α-KG), an anapleurotic substrate of the TCA cycle (11). In this process, glutamine is converted to glutamate and subsequently to α-KG. α-KG can then be metabolized through the TCA cycle to generate citrate and pyruvate. Recent glutamine carbon tracing studies further demonstrated the incorporation of glutamine carbons into essentially all of the intermediate metabolites in the TCA cycle (12). Thus, glutamine can fuel mitochondrial ATP production in active T cells when glucose is largely diverted to lactate by aerobic glycolysis.
At this point, however, we do not know which pathways in activated T cells are most responsible for the generation of energy (ATP and NADH/NADPH), in part because the pathways are intimately interconnected. The metabolic pathways in active T cells are optimized to convert the carbons from glucose and glutamine into macromolecular synthetic pathways rather than maximize ATP yield. Since ATP is an allosteric inhibitor of phosphofructokinase (PFK), the rate limiting enzyme in glycolysis, the appropriate balance between ATP production and ATP consumption is the key to maintaining a high glycolytic flux in activated T cells. Branching from glycolysis, the PPP is considered as the major catabolic pathway that generates ribose (for de novo nucleoside/nucleotide synthesis) and NADPH. While the contribution of de novo synthesis of nucleotides to the nucleotide pool in proliferating T cells remains unclear, cytosolic NADPH is essential for cell proliferation. It provides reducing equivalents for FFA and cholesterol biosynthesis, as well as for modulating oxidative stress through regulating gluthione production. T-cell activation-induced metabolic reprogramming is reminiscent of metabolic changes associated with oncogenic transformation (13, 14) and likely represents general metabolic signatures associated with cell growth and proliferation.
Glutaminolysis and glycolysis in active T cells provide carbon and nitrogen for other proliferation-associated biosynthetic pathways, such as the hexosamine and nucleotide biosynthetic pathways (2). Consistent with this, both the levels of intermediate metabolites and the transcription of key metabolic enzymes involved in these biosynthetic pathways are highly upregulated following T-cell activation (4). The hexosamine pathway is tightly coupled with protein glycosylation, which is involved in almost every aspect of T-cell biology (15). While the secretion of cytokines and the expression of their receptors are influenced by their glycosylation, recent evidence also suggests that intracellular proteins such as nuclear factor-κB (NF-κB) and nuclear factor of activated T cells (NFAT) are glycosylated to regulate T-cell activation (16).
In active T cells, a portion of carbon flux from glucose and glutamine catabolic pathways is diverted to support lipid biosynthesis. Our recent work revealed a striking accumulation of lipid metabolites that are involved in fatty acid synthesis following T-cell activation (4). This is associated with the induction of key metabolic enzymes required for lipid biosynthesis such as fatty acid synthase (FASN) (our unpublished result). Conversely, lipid catabolic metabolism by fatty acid β-oxidation (FAO) is rapidly suppressed following T-cell activation (4). Intriguingly, the inhibition of FAO occurs within two hours after T-cell activation, which precedes the changes of other metabolic pathways and cell growth. This indicates that the rapid inhibition of FAO is unlikely to be a metabolic adaptation due to the upregulation of fatty acid synthesis and glucose catabolism. However, the molecular mechanism behind this regulation remains to be identified.
As one of the major types of lipid, cholesterol is an essential structural component of T-cell membrane and is particularly enriched in certain cell membrane microdomains, such as lipid rafts. The formation and aggregation of lipid rafts following T-cell activation plays an essential role in early T-cell receptor-mediated signaling (17). One recent study revealed that the genes that are associated with de novo cholesterol biosynthesis and transport are downregulated following T-cell activation (18). This suggests that the suppression of cholesterol biosynthesis is associated with T-cell activation, implicating a direct link between cholesterol metabolism and T-cell immune function.
Polyamines (putrescine, spermidine, and spermine) are another group of metabolites, the biosynthesis of which is coupled with glutaminolysis in active T cells. Through an unbiased metabolomics approach, we recently found that the intracellular levels of polyamines and ornithine (Orn), the precursor of polyamines, are significantly elevated following T-cell activation (4). In liver, ornithine is generated largely from arginine via the action of arginase as part of the urea cycle (19). However, none of the metabolic genes in urea cycle including arginase are upregulated following T-cell activation, nor is the proliferation of arginase I (Arg I)-deficient T lymphocytes compromised (4). These data suggest that other metabolic resources can provide carbons for the biosynthesis of ornithine in active T lymphocytes. One of the putative polyamine biosynthetic pathways involves the conversion of glutamine to glutamate, followed by the conversion of glutamate to (S)-1-pyrroline-5-carboxylate (P-5-C), and finally to ornithine (20). Importantly, the expression of metabolic enzymes involved in the above steps is elevated following T-cell activation (4). This idea was further confirmed by metabolic tracer analysis. Using 13C-isotope-labeled glutamine as a tracer, metabolic flux analysis revealed the incorporation of 13C into all five carbons of ornithine, which accounted for up to 30% of total ornithine in active T cells but only 1% in resting T cells. The remaining unlabeled ornithine in active T cells may come from proline, which can be converted to ornithine through two consecutive reactions. These two reactions are catalyzed by proline dehydrogenase (PRODH) and ornithine aminotransferase (OAT), both of which are rapidly induced following T-cell activation (4).
The intracellular level of polyamines influences many cellular processes including cell growth and proliferation. Polyamines are highly charged polycations, and the majority of intracellular polyamines are associated with polyanionic molecules (DNA, RNA, and proteins). Thus, it has been postulated that polyamines exert their cellular function mainly through ionic interactions. However, one early study found that spermidine could be incorporated predominantly into one cellular protein in active T cells (21). This is through a unique posttranslational modification, hypusination, which involves in the transformation of 4-amino butyl moiety of spermidine to the ε-amino group of a lysine residue. The eukaryotic translation initiation factor 5A (eIF5A) was later identified as the only substrate of hypusination to date (22, 23). Intriguingly, recent evidence suggests that eIF5A is a translation elongation factor rather than an initiation factor and the hypusination of eIF5A plays a critical role in regulating its cellular function (24–26). As both the hypusination and the expression of eIF5A is rapidly engaged following T-cell activation, eIF5A is likely to mediate some of the effects of polyamines on -cell activation (21, 27).
Transcriptional control of metabolic reprogramming in activated T cells
Upon T-cell activation, the enzymes of the above catabolic and biosynthetic pathways are regulated at the transcriptional level, as outlined above. Interrogation of the promoters of these genes revealed a number of candidate transcription factors that might be involved in this reprogramming of metabolic function, and this was tested by acute ablation of the two most promising candidates, hypoxia-induced factor 1α (HIF-1α) and myelocytomatosis oncogene homolog (c-Myc) in resting T cells prior to activation (4). Remarkably, acute ablation of HIF-1α had little effect on the expression of metabolic enzymes, the metabolic changes, or the proliferation of T cells in the early expansion phase (4). In contrast, a substantial portion of elevated glycolysis, glutaminolysis, and their associated biosynthetic pathways were compromised in cells lacking c-Myc (4)(Fig. 4). This included the noncanonical pathway of polyamine synthesis from glutamine, outlined above (4).
Fig. 4. The role of c-Myc in activation-induced T-cell metabolic reprogramming.
The indicated metabolic activities in WT (black bar) and Myc-KO (white bar) active T cells were determined by tracer-based (red) metabolic assays. See (4) for methods. The value in WT samples was set to 1 in all cases.
Some pathways, however, were only minimally affected (or unaffected) by c-Myc ablation. These include the repression of FFA oxidation, the elevated oxygen consumption, and most of the PPP activity (4)(Fig. 4). It is possible that other recently revealed metabolic regulators such as estrogen-related receptor α (ERRα) and mTor complex 1 (TORC1) control these pathways following T-cell activation (28, 29).
Although HIF-1α appears to be dispensable in the metabolic events following activation of naive T cells, it is important in subsequent functional differentiation. Upon differentiation, T-helper 1 (Th1), Th2, and Th17 cells display dramatically elevated glycolysis well above that of freshly activated cells, while T-regulatory cells (Tregs) show decreased glycolysis (30, 31). In Th17 cells, the elevated glycolysis is dependent on the function of HIF-1α, and this transcription factor is required for their differentiation (30). This activation of HIF-1α under aerobic conditions is dependent on the function of TORC1. While this enhanced glycolysis is necessary for Th17 differentiation and function (30), HIF-1α appears to also directly regulate Th17 differentiation, at least in part through direct antagonism of the Treg master transcription factor forkhead box protein 3 (Foxp3) (32).
Either hypoxia or a forced expression of HIF1α (a major sensor of hypoxia) is sufficient to drive a noncanonical branched TCA cycle, converting glutamine-derived α-KG directly to isocitrate and finally citrate (33–35). This reduced glutamine catabolic pathway has also been found in adipose cells and in transformed cancer cells (36, 37), and is likely to be an evolutionally conserved metabolic branch in lower organisms, such as the human malaria parasite Plasmodium falciparum (38). In this pathway, a portion of mitochondrial citrate is exported to the cytoplasm and converted into acetyl-coA, the precursor of de novo lipid synthesis. Recently, this pathway was also revealed in T cells and removal of glutamine from T-cell culture medium significantly impaired lipids biosynthesis following T-cell activation (12, 34). While glucose is generally considered as a major carbon resource for lipid biosynthesis, hypoxia or aberrant upregulation of HIF1α results in a switch from glucose to glutamine as the carbon resource of lipogenic acetyl-coA (34, 35). It is likely that this is also the case for activated Th17 cells, although this has not been formally evaluated.
Metabolic addiction in T-cell responses
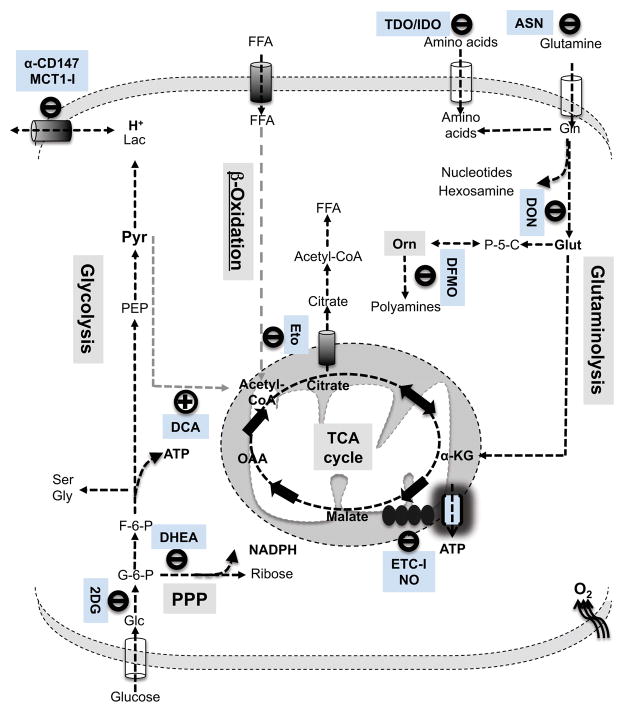
T-cell metabolic reprogramming is not only required for fulfilling increased bioenergetic and biosynthetic demands following activation but also is actively integrated into the signaling cascades that dictate T-cell fate. Emerging evidence suggests that such metabolic rewiring eventually renders active T cells highly dependent on certain metabolic pathways in a way that resting T cells are not. An understanding of metabolic ‘addiction’ of T cells may help to reveal the impact on immune responses under conditions of metabolic disease, nutritional imbalances, and cancer. In addition, it holds the promise for novel manipulation of immune responses by targeting metabolic pathways or signaling molecules that control metabolic pathways. Here we consider such addiction in terms of the effects of deprivation of metabolic substrates or inhibition of specific pathways. Fig. 5 shows several relevant pathways in T-cell metabolism and some of the inhibitors that affect them, discussed in more detail below.
Fig. 5. Metabolic targets during T-cell activation.
Relevant pharmacological compounds are shown that target the indicated metabolic steps in the metabolic pathways that are either elevated (illustrated in black) or inhibited (illustrated in gray) following T-cell activation.
Glucose and lactate
As we have seen, some of the most striking metabolic changes following T-cell activation occur in the glucose catabolic pathway. Both aerobic glycolysis and the PPP are remarkably elevated during the initial T-cell growth phase following activation. Subsequently, aerobic glycolysis is sustained at a high rate during cell expansion and differentiation. These findings raise the possibility that T-cell activation and differentiation is highly dependent on glucose catabolism. Supporting this idea, recent studies have demonstrated a requirement for glucose-dependent metabolism for various aspects of T-cell function in vitro. Constraining glucose availability by using glucose free media or high doses of the glycolysis inhibitor 2-deoxy-D-glucose (2-DG, 4–10mM) profoundly inhibits activation-induced T-cell proliferation (4, 39, 40) (Fig. 6). Similarly, a potent PPP inhibitor, dihydroepiandrosterone (DHEA), significantly suppresses activation-induced T cell proliferation (4) (Fig. 6). Intriguingly, CD8+ T cells that are activated under such conditions display compromised IFNγ but not IL2 production, implicating a requirement for glucose catabolism for this aspect of CD8+ T-cell effector function (40, 41). However, other studies suggest that the cytotoxic activity of mature CD8+ cytotoxic T lymphocytes (CTLs) is largely unperturbed in the absence of glucose (42).
Fig. 6. Metabolic ‘addiction’ in activated T cells.
T-cell proliferation of nutrient starved cells (A) or inhibitor-treated cells (B) was determined by CFSE dilution 48hr after activation. Proliferation is indicated as the percentage of cells with CFSE diluted more than one peak.
We have recently found that low dose 2-DG (1mM) treatment only moderately inhibits activation-induced glycolysis without blocking T-cell proliferation but significantly impaired the in vitro differentiation of Th1, Th2, and Th17 cells under polarizing conditions (310). In the case of impaired Th17 generation, there is a concurrent enhancement of inducible Treg (iTreg) differentiation, indicating that glycolysis directs a reciprocal modulation of these two T-cell lineages. As noted above, Th17 differentiation depends on the function of HIF-1α, but low dose 2-DG did not affect the stabilization of HIF-1α or the expression of its target genes (30).
Such in vitro findings further implicate the modulation of the glucose catabolic pathway as a potential therapeutic strategy for autoimmune and infection diseases. To test this idea, we have examined the effect of inhibiting glucose metabolism in experimental autoimmune encephalitis (EAE), an inflammatory demyelinating disease of the central nervous system, the development of which is largely dependent on the induction of autoreactive Th1 and Th17 cells. In a Th17- polarized transfer model of EAE, pretreatment with 2-DG significantly reduced the ability of Th17 to cause EAE. This was associated with diminished leukocyte infiltration and spinal cord inflammation (30).
In another mouse model, however, 2-DG pretreatment promoted immune responses in the resistance to Listeria monocytogenes infection (43). This appeared to be due to a selective modulation of macrophage function, since the bacterial resistance correlated with an enhanced macrophage activation following exposure to 2-DG. Together, these studies suggest that the modulation of glucose catabolism can elicit either positive or negative effects on immunity depending on the nature of immune response.
As the end product of glycolysis, lactate is rapidly exported outside of the cell after its generation and is therefore generally considered metabolic ‘waste’. However, numerous studies had shown that lactate could serve as a prominent substrate that fuels oxidative metabolism in muscle cells, neurons, and some tumor cells (44–46). Similarly, in an early study, it was found that uptake of lactate was induced in T cells following activation with lectin (47). If lactate is actually utilized as an alternative energy source by T cells (which has not been examined), it is possible that this may occur in an inflamed or otherwise glucose-deficient microenvrioment. The concentration of lactate in vertebrate plasma ranges from 1 to 30mM under physiological and pathological conditions (48). Early studies showed that high but physiologically relevant lactate concentrations (20 to 30mM) stimulate IL-2 production in murine CD4+ T cells following in vitro mitogen stimulation (49, 50). However, the same treatment inhibits mitogen-induced DNA synthesis in murine CD8+ T cells. A recent study also showed that sodium lactate, the sodium salt of lactic acid, can either enhance or impair human CD4+ T-cell proliferation at low (< 1mM) or moderately high concentrations (> 5mM), respectively (51). Paradoxically, another report suggested a dose-dependent effect on human CTLs from lactic acid but not sodium lactate (52). Antigen-stimulated human CTL proliferation, cytokine production, and cytotoxic activity were impaired by lactic acid, at levels ranging from 5 to 20mM. While lactic acid treatment inhibits the proliferation of Th1 and Th17 cells, the same treatment promotes the production of IL17A from Th17 cells, which is likely mediated through both IL23-dependent and independent mechanisms (53, 54).
These results raise the idea that the acidification of the T-cell microenvironment may partially mediate the effects of lactate acid on T-cell function (52, 55, 56). If so, this may be relevant to the intra-tumoral hypoxic environment, which is highly acidic. Finally, other mechanisms have also been postulated to explain the in vitro effects of lactate on T-cell function, including feedback inhibition on T-cell glycolysis, and oxidative stress due to the decrease of intracellular glutathione level and the increase of lactate oxidation (49, 50). However, the immunoregulatory effects of lactate on T cells seem to be dependent on the cellular context and the dose of lactate applied in these studies.
Many if not all the effects of lactate on T-cell immune function can be recapitulated through modulating lactate transport across the plasma membrane. While the monocarboxylate transport (MCT) family is largely responsible for lactate transport in most cells (57), the predominant MCTs in T cells are MCT-1, MCT-2, and MCT-4 (58, 59). Recent studies showed that the expression and function of MCT-1 and MCT-4 require the ancillary cell surface glycoprotein CD147 (also known as Basigin and EMMPRIN) (60, 61), and both MCTs and CD147 are rapidly elevated upon T-cell activation (4, 30, 62–64). Consistent with these results, the pharmacologic inhibition of MCT-1 impairs human CTL function and mouse T-cell proliferation in vitro (52, 64). In mouse or rat models of graft versus host response and allograft survival, the in vivo administration of the same inhibitor significantly suppressed disease (64–66).
CD147 represents another tempting target for modulating T-cell lactate transport. Recent studies showed that anti-CD147 antibody impairs T-cell activation in vitro and relieves asthmatic lung inflammation and acute lung injury associated inflammation in mouse models in vivo (67–69). Mechanistically, the immune suppressive effect of targeting MCT1/4 and CD147 may be achieved through regulating lactate influx or efflux. However, the anti-CD147 antibody may also exert its immune modulation through interfering with the downstream signaling of cyclophilin A (CypA), which was postulated as one of the potential ligands of CD147 (70, 71). Therefore, it is also conceivable that both lactate itself and MCT inhibitor elicit their immunosuppressive effect via modulating CypA and CD147. Further studies are warranted to determine the lactate flux and the potential crosstalk between lactate metabolism and CypA signaling in active T cells.
The intracellular concentration of lactate is also determined by the metabolic fate of its precursor, pyruvate, which can be preferentially converted to either lactate or acetyl-CoA depending on the activity of pyruvate dehydrogenase (PDH). In active T cells and tumor cells, PDH kinase (PDK) phosphorylates and inhibits PDH, resulting in a high metabolic flux from pyruvate to lactate. Thus, the inhibition of PDK would favor the conversion of pyruvate to acetyl-CoA with a concurrent reduction of intracellular lactate production from pyruvate. A well-characterized PDK inhibitor, dichloroacetate (DCA), has been applied in the treatment of lactic acidosis in patients and more recently been tested in targeting the tumor metabolic phenotype (72, 73). One recent study showed that DCA treatment impairs activation-induced T-cell proliferation and lactate production and promotes the generation of Foxp3+ T cells in vitro (51). Intriguingly, CD147 was recently identified as a marker of activated Tregs in a subset of human Treg cells (74). In agreement with these findings, the administration of DCA in vivo significantly reduced airway inflammation and T-cell hyperreactivity in a mouse model of asthma (51). While the underlying mechanisms remain to be determined, the above studies implicate a potent immunosuppressive property of targeting lactate transport or metabolism.
Glutamine and other amino acids
As the building blocks of proteins, the uptake and biosynthesis of amino acids are dramatically increased following T cell activation. Half of the amino acids in proteins are considered essential, as they cannot be synthesized de novo by the organism. This definition may not be applicable to active T cells, in which the capability of de novo synthesis of some amino acids may not meet the increased demand. Thus, some of the’non-essential’ amino acids are conditionally essential in active T cells. One example is glutamine, the catabolism of which is dramatically induced following T-cell activation, as discussed above.
In active T cells, the glutamine catabolic pathway provides precursors for multiple biosynthetic pathways as well as a substrate for mitochondria. In the absence of glutamine, cell growth, proliferation, and cytokine production are all significantly compromised following T-cell activation in vitro (4, 9, 75, 76). While glutamine is one of the most abundant amino acids in plasma, its concentration can become limiting following severe burns, major trauma, or surgery. Generally, a decreased plasma glutamine concentration is associated with impaired immune response in these patients, which can be partially improved following glutamine supplementation (77–81).
As an important chemotherapeutic regimen for acute lymphoblastic leukemia (ALL), asparginase (ASN) elicits its anti-leukemic effect through its dual enzymatic activity, i.e. hydrolyzing asparagine and glutamine, the latter activity of which varies depending on the source of the enzyme (82, 83). This results in the depletion of plasma asparagine and glutamine. Notably, treatment with ASN also causes immunosuppression, which is likely due to the inhibition of both T and B-cell-mediated immune responses (84–87). However, the immunosuppressive properties of ASN are largely due to its glutamine depletion activity in vitro and in vivo (88–92). Consistent with these studies, the acute treatment of a glutamine antagonist, 6-Diazo-5-oxo-L-norleucine (DON), suppresses antigen-specific T-cell proliferation in vitro and in mice (4) (Figure 6). Of note, DON may function as an inhibitor of various glutamine-utilizing enzymes because of its structural similarity to glutamine.
In vitro, glutamine is absolutely required for T-cell growth and proliferation following activation (4) (Fig. 6). Strikingly, we have observed that the expression of c-Myc is not only required for the uptake and catabolism of glutamine (4) but that glutamine is required for sustained expression of c-Myc (Fig. 7). This finding suggests a feedback mechanism, in which c-Myc induces the expression of the glutamine transporter, CD98 (Slc3a2/Slc7a5), and, in turn, glutamine subsequently enforces c-Myc expression (4). This may be through the function of TORC1, as the failure to express CD98 may result in amino acid deficiency with resulting inhibition of TORC1 activity (4, 93). This idea remains to be tested.
Fig. 7. Glutamine is required for sustained expression of c-Myc.
Myc expression was determined by Western blot in cells collected at the indicated time following T-cell activation. T cells were activated with anti-CD3 plus anti-CD28 in either complete or glutamine-free medium as described (4).
Following glutamine catabolic flux, a portion of glutamine carbon is diverted toward the biosynthesis of polyamines through a non-canonical pathway engaged in active T cells (4). The inhibition of ornithine decarboxylase (ODC), a rate-limiting enzyme in the biosynthesis of polyamines, by α-difluoromethylornithine (DFMO) (94), attenuates mitogen-induced T-cell proliferation and suppresses CTL differentiation without impairing IL-2 production (4, 95–98). These inhibitory effects of DFMO can be reversed by exogenous polyamines, suggesting that the biosynthesis of polyamines is required for normal lymphocyte functions. In agreement with these in vitro results, DFMO also partially inhibited the proliferation of antigen-specific active T cells in vivo (4).
In addition to glutamine, extracellular restriction of tryptophan and arginine has been suggested to regulate T-cell immune responses (99–101). This has been invoked to explain the immunosuppressive role of tumor cells, dendritic cells (DCs), and macrophages that express the amino acid catabolic enzymes, indoleamine 2,3-dioxygenase (IDO), tryptophan 2,3-dioxygenase (TDO), and arginase I (Arg I) (99, 102, 103). Consequentially, the local depletion of tryptophan and arginine diverts T-cell responses toward tolerance and modulates Th17 and Treg development (104–108). One putative downstream molecular mechanism triggered by amino acid starvation is the activation of GCN2. Supporting this idea, the pharmacological stimulation of GCN2 inhibits Th17 cell differentiation and Th17-associated EAE in vivo (105). However, the mechanism by which IDO/TDO-expressing cells suppress immune responses also appears to be related to the production of tryptophan-derived metabolites (109–112).
Mitochondrial substrates
In addition to the elevation of aerobic glycolysis, the activation of T cells in vitro also results in an increase of mitochondrial respiration (4), which is likely associated with dynamic mitochondrial fusion and biogenesis (113). This phenomena has been recapitulated in alloantigen-driven proliferating T cells and chronically activated T cells (114, 115). As a potentinhibitor of the electron transport chain (ETC) and F1F0-ATPase, nitric oxide (NO) suppresses T-cell respiration, reduces cellular ATP content, and subsequently inhibits cell proliferation and cytokine production following T-cell activation (116–119). In vivo, NO-mediated inhibition of T-cell responses has been postulated as one of the T-cell suppression mechanisms elicited by macrophages and myeloid-derived suppressor cells (MDSCs) in cancer, inflammation, and infection (120–122). Similarly, pharmacologic inhibition of the mitochondrial F1F0 adenosine triphosphate synthase (F1F0-ATPase) selectively induces apoptosis of alloreactive donor T cells but not resting T cells in graft-versus-host disease (GVHD) models. Consequentially, such treatment significantly reduces GVHD clinical scores and promotes mouse survival (114).
A reciprocal increase of intracellular fatty acid oxidation intermediates, acylcarnitines, and decrease of extracellular fatty acids has been recently revealed in alloreactive T cells (114). This result suggests that fatty acids may serve as a mitochondrial substrate in some T cells. Supporting this idea, fatty acids have been suggested as a preferred fuel for Treg and T-memory cells (3, 28, 31). Targeting carnitine palmitoyltransferase 1 (CPT-1), the rate-limiting enzyme in FFA β-oxidation, via CPT1a shRNA or the pharmacologic inhibitor Etomoxir (Eto), reduces oxygen consumption in CD8+ T memory cells in vitro. Conversely, overexpression of CPT1a enhanced oxygen consumption in vitro and promoted CD8+ T-memory cell development in vivo (3). Similarly, etoxomir significantly inhibited Treg differentiation in vitro (28, 31). Paradoxically, in a mouse model of EAE, etomoxir treatment after disease induction remarkably suppressed disease progression (123). Given that EAE is dominated by Th1 and Th17 cells, this finding is at odds with the fact that both Th1 and Th17 cells display a high glycolytic activity with a concurrent low fatty acid oxidation activity (30, 31). Therefore, further studies are warranted to solve this discrepancy.
Conclusion
To win the battle over rapidly replicating invading pathogens, T lymphocytes have evolved mechanisms to sense nutrient cues and to tightly couple their metabolic programs with cell mass accumulation, cell division. and immune functions. Inevitably, this addicts active T cells to specific nutrient supplies and their downstream metabolic pathways. However, this also leads to the hope that novel therapeutic regimes for the control of inflammation and autoimmune diseases will emerge from our ongoing understanding of metabolic’addictio’ of active T cells.
Acknowledgments
We thank the members of the Green laboratory for valuable discussions. This work was supported by the George J. Mitchell fellowship from St Jude Children Hospital (R.W.), NIH grants AI40646 and GM52735 (D.R.G.) and the American Lebanese and Syrian Associated Charities. The authors declare they have no competing financial interests.
References
- 1.Brand K, Williams JF, Weidemann MJ. Glucose and glutamine metabolism in rat thymocytes. Biochem J. 1984;221:471–475. doi: 10.1042/bj2210471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newsholme EA, Crabtree B, Ardawi MS. The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells. Biosci Rep. 1985;5:393–400. doi: 10.1007/BF01116556. [DOI] [PubMed] [Google Scholar]
- 3.van der Windt GJ, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang R, et al. The Transcription Factor Myc Controls Metabolic Reprogramming upon T Lymphocyte Activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michalek RD, Rathmell JC. The metabolic life and times of a T-cell. Immunol Rev. 2010;236:190–202. doi: 10.1111/j.1600-065X.2010.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang R, Green DR. The immune diet: meeting the metabolic demands of lymphocyte activation. F1000 Biology Reports. 2012:4. doi: 10.3410/B4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frauwirth KA, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs SR, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr EL, et al. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol. 2011;185:1037–1044. doi: 10.4049/jimmunol.0903586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newsholme EA, Crabtree B, Ardawi MS. Glutamine metabolism in lymphocytes: its biochemical, physiological and clinical importance. Q J Exp Physiol. 1985;70:473–489. doi: 10.1113/expphysiol.1985.sp002935. [DOI] [PubMed] [Google Scholar]
- 11.Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. The Journal of biological chemistry. 2002;277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 12.Le A, et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell metabolism. 2012;15:110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dang CV. Links between metabolism and cancer. Genes & development. 2012;26:877–890. doi: 10.1101/gad.189365.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniels MA, Hogquist KA, Jameson SC. Sweet ’n’ sour: the impact of differential glycosylation on T cell responses. Nature immunology. 2002;3:903–910. doi: 10.1038/ni1002-903. [DOI] [PubMed] [Google Scholar]
- 16.Golks A, Tran TT, Goetschy JF, Guerini D. Requirement for O-linked N-acetylglucosaminyltransferase in lymphocytes activation. The EMBO journal. 2007;26:4368–4379. doi: 10.1038/sj.emboj.7601845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nature reviews Molecular cell biology. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 18.Bensinger SJ, et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris SM., Jr Recent advances in arginine metabolism. Curr Opin Clin Nutr Metab Care. 2004;7:45–51. doi: 10.1097/00075197-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Wu G, Haynes TE, Li H, Meininger CJ. Glutamine metabolism in endothelial cells: ornithine synthesis from glutamine via pyrroline-5-carboxylate synthase. Comp Biochem Physiol A Mol Integr Physiol. 2000;126:115–123. doi: 10.1016/s1095-6433(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 21.Park MH, Cooper HL, Folk JE. Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Prioc Natl Acad Sci USA. 1981;78:2869–2873. doi: 10.1073/pnas.78.5.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper HL, Park MH, Folk JE, Safer B, Braverman R. Identification of the hypusine-containing protein hy+ as translation initiation factor eIF-4D. Proc Natl Acad Sci USA. 1983;80:1854–1857. doi: 10.1073/pnas.80.7.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park MH, Nishimura K, Zanelli CF, Valentini SR. Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids. 2010;38:491–500. doi: 10.1007/s00726-009-0408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chattopadhyay MK, Park MH, Tabor H. Hypusine modification for growth is the major function of spermidine in Saccharomyces cerevisiae polyamine auxotrophs grown in limiting spermidine. Proc Natl Acad Sci USA. 2008;105:6554–6559. doi: 10.1073/pnas.0710970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saini P, Eyler DE, Green R, Dever TE. Hypusine-containing protein eIF5A promotes translation elongation. Nature. 2009;459:118–121. doi: 10.1038/nature08034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel PH, Costa-Mattioli M, Schulze KL, Bellen HJ. The Drosophila deoxyhypusine hydroxylase homologue nero and its target eIF5A are required for cell growth and the regulation of autophagy. J Cell Biol. 2009;185:1181–1194. doi: 10.1083/jcb.200904161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bevec D, et al. Induced gene expression of the hypusine-containing protein eukaryotic initiation factor 5A in activated human T lymphocytes. Proc Natl Acad Sci USA. 1994;91:10829–10833. doi: 10.1073/pnas.91.23.10829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michalek RD, et al. Estrogen-related receptor-alpha is a metabolic regulator of effector T-cell activation and differentiation. Proc Natl Acad Sci USA. 2011;108:18348–18353. doi: 10.1073/pnas.1108856108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang K, Neale G, Green DR, He W, Chi H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol. 2011;12:888–897. doi: 10.1038/ni.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi LZ, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michalek RD, et al. Cutting edge: Distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dang EV, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wise DR, et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci USA. 2011;108:19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metallo CM, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mullen AR, et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012;481:385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Des Rosiers C, Fernandez CA, David F, Brunengraber H. Reversibility of the mitochondrial isocitrate dehydrogenase reaction in the perfused rat liver. Evidence from isotopomer analysis of citric acid cycle intermediates. J Biol Chem. 1994;269:27179–27182. [PubMed] [Google Scholar]
- 37.Yoo H, Antoniewicz MR, Stephanopoulos G, Kelleher JK. Quantifying reductive carboxylation flux of glutamine to lipid in a brown adipocyte cell line. J Biol Chem. 2008;283:20621–20627. doi: 10.1074/jbc.M706494200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olszewski KL, et al. Branched tricarboxylic acid metabolism in Plasmodium falciparum. Nature. 2010;466:774–778. doi: 10.1038/nature09301. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Miller ES, Klinger JC, Akin C, Koebel DA, Sonnenfeld G. Inhibition of murine splenic T lymphocyte proliferation by 2-deoxy-D-glucose-induced metabolic stress. J Neuroimmunol. 1994;52:165–173. doi: 10.1016/0165-5728(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 40.Cham CM, Gajewski TF. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J Immunol. 2005;174:4670–4677. doi: 10.4049/jimmunol.174.8.4670. [DOI] [PubMed] [Google Scholar]
- 41.Cham CM, Driessens G, O’Keefe JP, Gajewski TF. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur J Immunol. 2008;38:2438–2450. doi: 10.1002/eji.200838289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacDonald HR. Energy metabolism and T-cell-mediated cytolysis. II. Selective inhibition of cytolysis by 2-deoxy-D-glucose. J Exp Med. 1977;146:710–719. doi: 10.1084/jem.146.3.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller ES, Bates RA, Koebel DA, Fuchs BB, Sonnenfeld G. 2-deoxy-D-glucose-induced metabolic stress enhances resistance to Listeria monocytogenes infection in mice. Physiol Behav. 1998;65:535–543. doi: 10.1016/s0031-9384(98)00199-1. [DOI] [PubMed] [Google Scholar]
- 44.Gladden LB. Lactate metabolism: a new paradigm for the third millennium. J Physiol. 2004;558:5–30. doi: 10.1113/jphysiol.2003.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Philp A, Macdonald AL, Watt PW. Lactate--a signal coordinating cell and systemic function. J Exp Biol. 2005;208:4561–4575. doi: 10.1242/jeb.01961. [DOI] [PubMed] [Google Scholar]
- 46.Dhup S, Dadhich RK, Porporato PE, Sonveaux P. Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Curr Pharm Design. 2012;18:1319–1330. doi: 10.2174/138161212799504902. [DOI] [PubMed] [Google Scholar]
- 47.Sommer F, Bischof S, Rollinghoff M, Lohoff M. Demonstration of organic anion transport in T lymphocytes. L-lactate and fluo-3 are target molecules. J Immunol. 1994;153:3523–3532. [PubMed] [Google Scholar]
- 48.Merezhinskaya N, Fishbein WN. Monocarboxylate transporters: past, present, and future. Histol Histopathol. 2009;24:243–264. doi: 10.14670/HH-24.243. [DOI] [PubMed] [Google Scholar]
- 49.Roth S, Droge W. Regulation of interleukin 2 production, interleukin 2 mRNA expression and intracellular glutathione levels in ex vivo derived T lymphocytes by lactate. Eur J Immunol. 1991;21:1933–1937. doi: 10.1002/eji.1830210823. [DOI] [PubMed] [Google Scholar]
- 50.Roth S, Gmunder H, Droge W. Regulation of intracellular glutathione levels and lymphocyte functions by lactate. Cell Immunol. 1991;136:95–104. doi: 10.1016/0008-8749(91)90384-n. [DOI] [PubMed] [Google Scholar]
- 51.Ostroukhova M, et al. The role of low-level lactate production in airway inflammation in asthma. Am J Physiol. 2012;302:L300–307. doi: 10.1152/ajplung.00221.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fischer K, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 53.Shime H, et al. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J Immunol. 2008;180:7175–7183. doi: 10.4049/jimmunol.180.11.7175. [DOI] [PubMed] [Google Scholar]
- 54.Yabu M, et al. IL-23-dependent and -independent enhancement pathways of IL-17A production by lactic acid. Int Immunol. 2011;23:29–41. doi: 10.1093/intimm/dxq455. [DOI] [PubMed] [Google Scholar]
- 55.Feder-Mengus C, et al. Multiple mechanisms underlie defective recognition of melanoma cells cultured in three-dimensional architectures by antigen-specific cytotoxic T lymphocytes. Br J Cancer. 2007;96:1072–1082. doi: 10.1038/sj.bjc.6603664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mendler AN, Hu B, Prinz PU, Kreutz M, Gottfried E, Noessner E. Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. Int J Cancer. 2011 doi: 10.1002/ijc.26410. [DOI] [PubMed] [Google Scholar]
- 57.Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447:619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 58.Merezhinskaya N, Ogunwuyi SA, Mullick FG, Fishbein WN. Presence and localization of three lactic acid transporters (MCT1, -2, and -4) in separated human granulocytes, lymphocytes, and monocytes. J Histochem Cytochem. 2004;52:1483–1493. doi: 10.1369/jhc.4A6306.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999;343(Pt 2):281–299. [PMC free article] [PubMed] [Google Scholar]
- 60.Le Floch R, et al. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc Natl Acad Sci USA. 2011;108:16663–16668. doi: 10.1073/pnas.1106123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, Halestrap AP. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000;19:3896–3904. doi: 10.1093/emboj/19.15.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kasinrerk W, Fiebiger E, Stefanova I, Baumruker T, Knapp W, Stockinger H. Human leukocyte activation antigen M6, a member of the Ig superfamily, is the species homologue of rat OX-47, mouse basigin, and chicken HT7 molecule. J Immunol. 1992;149:847–854. [PubMed] [Google Scholar]
- 63.Koch C, et al. T cell activation-associated epitopes of CD147 in regulation of the T cell response, and their definition by antibody affinity and antigen density. Int Immunol. 1999;11:777–786. doi: 10.1093/intimm/11.5.777. [DOI] [PubMed] [Google Scholar]
- 64.Murray CM, et al. Monocarboxylate transporter MCT1 is a target for immunosuppression. Nat Chem Biol. 2005;1:371–376. doi: 10.1038/nchembio744. [DOI] [PubMed] [Google Scholar]
- 65.Bueno V, et al. The specific monocarboxylate transporter (MCT1) inhibitor, AR-C117977, a novel immunosuppressant, prolongs allograft survival in the mouse. Transplantation. 2007;84:1204–1207. doi: 10.1097/01.tp.0000287543.91765.41. [DOI] [PubMed] [Google Scholar]
- 66.Ekberg H, et al. The specific monocarboxylate transporter-1 (MCT-1) inhibitor, AR-C117977, induces donor-specific suppression, reducing acute and chronic allograft rejection in the rat. Transplantation. 2007;84:1191–1199. doi: 10.1097/01.tp.0000287541.53389.be. [DOI] [PubMed] [Google Scholar]
- 67.Staffler G, et al. Selective inhibition of T cell activation via CD147 through novel modulation of lipid rafts. J Immunol. 2003;171:1707–1714. doi: 10.4049/jimmunol.171.4.1707. [DOI] [PubMed] [Google Scholar]
- 68.Arora K, et al. Extracellular cyclophilins contribute to the regulation of inflammatory responses. J Immunol. 2005;175:517–522. doi: 10.4049/jimmunol.175.1.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gwinn WM, et al. Novel approach to inhibit asthma-mediated lung inflammation using anti-CD147 intervention. J Immunol. 2006;177:4870–4879. doi: 10.4049/jimmunol.177.7.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yurchenko V, et al. Active site residues of cyclophilin A are crucial for its signaling activity via CD147. The Journal of biological chemistry. 2002;277:22959–22965. doi: 10.1074/jbc.M201593200. [DOI] [PubMed] [Google Scholar]
- 71.Yurchenko V, Constant S, Eisenmesser E, Bukrinsky M. Cyclophilin-CD147 interactions: a new target for anti-inflammatory therapeutics. Clin Exp Immunol. 2010;160:305–317. doi: 10.1111/j.1365-2249.2010.04115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bonnet S, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 73.Stacpoole PW. The pharmacology of dichloroacetate. Metabolism. 1989;38:1124–1144. doi: 10.1016/0026-0495(89)90051-6. [DOI] [PubMed] [Google Scholar]
- 74.Solstad T, et al. CD147 (Basigin/Emmprin) identifies FoxP3+CD45RO+CTLA4+-activated human regulatory T cells. Blood. 2011;118:5141–5151. doi: 10.1182/blood-2011-02-339242. [DOI] [PubMed] [Google Scholar]
- 75.Newsholme EA, Calder PC. The proposed role of glutamine in some cells of the immune system and speculative consequences for the whole animal. Nutrition. 1997;13:728–730. doi: 10.1016/s0899-9007(97)83034-1. [DOI] [PubMed] [Google Scholar]
- 76.Horig H, et al. Exogenous glutamine requirement is confined to late events of T cell activation. J Cell Biochem. 1993;53:343–351. doi: 10.1002/jcb.240530412. [DOI] [PubMed] [Google Scholar]
- 77.Parry-Billings M, Evans J, Calder PC, Newsholme EA. Does glutamine contribute to immunosuppression after major burns? Lancet. 1990;336:523–525. doi: 10.1016/0140-6736(90)92083-t. [DOI] [PubMed] [Google Scholar]
- 78.Wischmeyer PE. Clinical applications of L-glutamine: past, present, and future. Nutr Clin Pract. 2003;18:377–385. doi: 10.1177/0115426503018005377. [DOI] [PubMed] [Google Scholar]
- 79.Peng X, Yan H, You Z, Wang P, Wang S. Glutamine granule-supplemented enteral nutrition maintains immunological function in severely burned patients. Burns. 2006;32:589–593. doi: 10.1016/j.burns.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 80.O’Riordain MG, et al. Glutamine-supplemented total parenteral nutrition enhances T-lymphocyte response in surgical patients undergoing colorectal resection. Ann Surg. 1994;220:212–221. doi: 10.1097/00000658-199408000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Newsholme EA. The possible role of glutamine in some cells of the immune system and the possible consequence for the whole animal. Experientia. 1996;52:455–459. doi: 10.1007/BF01919315. [DOI] [PubMed] [Google Scholar]
- 82.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 83.Broome JD. Studies on the mechanism of tumor inhibition by L-asparaginase. Effects of the enzyme on asparagine levels in the blood, normal tissues, and 6C3HED lymphomas of mice: differences in asparagine formation and utilization in asparaginase-sensitive and -resistant lymphoma cells. J Exp Med. 1968;127:1055–1072. doi: 10.1084/jem.127.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chakrabarty AK, Friedman H. L-asparaginase-induced immunosuppression: effects on antibody-forming cells and serum titers. Science. 1970;167:869–870. doi: 10.1126/science.167.3919.869. [DOI] [PubMed] [Google Scholar]
- 85.Gieldanowski J. Studies on the immunosuppressive and anti-inflammatory action of L-asparaginase. Arch Immunol Ther Exp. 1976;24:243–247. [PubMed] [Google Scholar]
- 86.Berenbaum MC, Cope WA, Jeffery W. Differential asparaginase sensitivity of T-cell and B-cell responses. Clin Exp Immunol. 1973;15:565–572. [PMC free article] [PubMed] [Google Scholar]
- 87.Kitoh T, Asai S, Akiyama Y, Kubota M, Mikawa H. The inhibition of lymphocyte blastogenesis by asparaginase: critical role of glutamine in both T and B lymphocyte transformation. Acta paediatrica Japonica. 1992;34:579–583. doi: 10.1111/j.1442-200x.1992.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 88.Kafkewitz D, Bendich A. Enzyme-induced asparagine and glutamine depletion and immune system function. The American journal of clinical nutrition. 1983;37:1025–1030. doi: 10.1093/ajcn/37.6.1025. [DOI] [PubMed] [Google Scholar]
- 89.Schwartz RS. Immunosuppression by L-asparaginase. Nature. 1969;224:275–276. doi: 10.1038/224275a0. [DOI] [PubMed] [Google Scholar]
- 90.Benezra D, Pitaro R, Birkenfeld V, Hochman A. Reversal of the immunosuppressive effect of L-asparaginase by L-glutamine. Nat New Biol. 1972;236:80–82. doi: 10.1038/newbio236080a0. [DOI] [PubMed] [Google Scholar]
- 91.Durden DL, Distasio JA. Comparison of the immunosuppressive effects of asparaginases from Escherichia coli and Vibrio succinogenes. Cancer Res. 1980;40:1125–1129. [PubMed] [Google Scholar]
- 92.Reinert RB, et al. Role of glutamine depletion in directing tissue-specific nutrient stress responses to L-asparaginase. The Journal of biological chemistry. 2006;281:31222–31233. doi: 10.1074/jbc.M604511200. [DOI] [PubMed] [Google Scholar]
- 93.Nicklin P, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jung MJ, Metcalf BW, Lippert B, Casara P. Mechanism of the stereospectific irreversible inhibition of bacterial glutamic acid decarboxylase by (R)-(−)-4-aminohex-5-ynoic acid, an analogue of 4-aminobutyric acid. Biochemistry. 1978;17:2628–2632. doi: 10.1021/bi00606a026. [DOI] [PubMed] [Google Scholar]
- 95.Mamont PS, Duchesne MC, Grove J, Bey P. Anti-proliferative properties of DL-alpha-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Comm. 1978;81:58–66. doi: 10.1016/0006-291x(78)91630-3. [DOI] [PubMed] [Google Scholar]
- 96.Holtta E, Janne J, Hovi T. Suppression of the formation of polyamines and macromolecules by DL-alpha-difluoromethylornithine and methylglyoxal bis(guanylhydrazone) in phytohaemagglutinin-activated human lymphocytes. Biochem J. 1979;178:109–117. doi: 10.1042/bj1780109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fillingame RH, Jorstad CM, Morris DR. Increased cellular levels of spermidine or spermine are required for optimal DNA synthesis in lymphocytes activated by concanavalin A. Proc Natl Acad Sci USA. 1975;72:4042–4045. doi: 10.1073/pnas.72.10.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bowlin TL, McKown BJ, Babcock GF, Sunkara PS. Intracellular polyamine biosynthesis is required for interleukin 2 responsiveness during lymphocyte mitogenesis. Cell Immunol. 1987;106:420–427. doi: 10.1016/0008-8749(87)90184-5. [DOI] [PubMed] [Google Scholar]
- 99.Huang L, Baban B, Johnson BA, 3rd, Mellor AL. Dendritic cells, indoleamine 2,3 dioxygenase and acquired immune privilege. Int Rev Immunol. 2010;29:133–155. doi: 10.3109/08830180903349669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bunpo P, Cundiff JK, Reinert RB, Wek RC, Aldrich CJ, Anthony TG. The eIF2 kinase GCN2 is essential for the murine immune system to adapt to amino acid deprivation by asparaginase. J Nutr. 2010;140:2020–2027. doi: 10.3945/jn.110.129197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nicholson LB, Raveney BJ, Munder M. Monocyte dependent regulation of autoimmune inflammation. Curr Mol Med. 2009;9:23–29. doi: 10.2174/156652409787314499. [DOI] [PubMed] [Google Scholar]
- 102.Edinger AL, Thompson CB. Antigen-presenting cells control T cell proliferation by regulating amino acid availability. Proc Natl Acad Sci USA. 2002;99:1107–1109. doi: 10.1073/pnas.042707999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nature reviews Immunology. 2008;8:74–80. doi: 10.1038/nri2233. [DOI] [PubMed] [Google Scholar]
- 104.Yan Y, et al. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J Immunol. 2010;185:5953–5961. doi: 10.4049/jimmunol.1001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sundrud MS, et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science. 2009;324:1334–1338. doi: 10.1126/science.1172638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sharma MD, et al. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113:6102–6111. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Munn DH, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 108.Mellor AL, et al. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol. 2001;2:64–68. doi: 10.1038/83183. [DOI] [PubMed] [Google Scholar]
- 109.Opitz CA, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 110.Terness P, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fallarino F, et al. Functional expression of indoleamine 2,3-dioxygenase by murine CD8 alpha(+) dendritic cells. Int Immunol. 2002;14:65–68. doi: 10.1093/intimm/14.1.65. [DOI] [PubMed] [Google Scholar]
- 113.Garedew A, Andreassi C, Moncada S. Mitochondrial dynamics, biogenesis, and function are coordinated with the cell cycle by APC/C CDH1. Cell Metab. 2012;15:466–479. doi: 10.1016/j.cmet.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 114.Gatza E, et al. Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease. Sci Transl Med. 2011;3:67ra68. doi: 10.1126/scitranslmed.3001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wahl DR, Petersen B, Warner R, Richardson BC, Glick GD, Opipari AW. Characterization of the metabolic phenotype of chronically activated lymphocytes. Lupus. 2010;19:1492–1501. doi: 10.1177/0961203310373109. [DOI] [PubMed] [Google Scholar]
- 116.Fiorucci S, Mencarelli A, Distrutti E, Baldoni M, del Soldato P, Morelli A. Nitric oxide regulates immune cell bioenergetic: a mechanism to understand immunomodulatory functions of nitric oxide-releasing anti-inflammatory drugs. J Immunol. 2004;173:874–882. doi: 10.4049/jimmunol.173.2.874. [DOI] [PubMed] [Google Scholar]
- 117.Bredt DS. Endogenous nitric oxide synthesis: biological functions and pathophysiology. Free Radical Res. 1999;31:577–596. doi: 10.1080/10715769900301161. [DOI] [PubMed] [Google Scholar]
- 118.Beltran B, Quintero M, Garcia-Zaragoza E, O’Connor E, Esplugues JV, Moncada S. Inhibition of mitochondrial respiration by endogenous nitric oxide: a critical step in Fas signaling. Proc Natl Acad Sci. 2002;99:8892–8897. doi: 10.1073/pnas.092259799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Beltran B, Mathur A, Duchen MR, Erusalimsky JD, Moncada S. The effect of nitric oxide on cell respiration: A key to understanding its role in cell survival or death. Proc Natl Acad Sci. 2000;97:14602–14607. doi: 10.1073/pnas.97.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mazzoni A, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 121.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bingisser RM, Tilbrook PA, Holt PG, Kees UR. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol. 1998;160:5729–5734. [PubMed] [Google Scholar]
- 123.Shriver LP, Manchester M. Inhibition of fatty acid metabolism ameliorates disease activity in an animal model of multiple sclerosis. Sci Rep. 2011;1:79. doi: 10.1038/srep00079. [DOI] [PMC free article] [PubMed] [Google Scholar]