Abstract
AIM: To determine the functional significance of TC21 in esophageal squamous cell carcinoma (ESCC).
METHODS: TC21 siRNA transfection was carried out using Hyperfectamine to knock down TC21, and transcripts were analyzed by reverse transcription-polymerase chain reaction and protein by Western blotting. We demonstrated the effect of TC21 downregulation of cell signaling in esophageal cancer cells by assessing the phosphorylation status of its downstream targets, phosphoinositide 3-kinase (PI3K), phosphatase and tensin homolog (PTEN), protein kinase B (pAkt), nuclear factor-κB (NF-κB) and cyclinD1 using specific antibodies. Cell survival analysis after cisplatin treatment was carried out by cell viability assay and cell cycle analysis using flow cytometry.
RESULTS: TC21 knockdown in human ESCC cell line TE13 cells, showed only a marginal increase (14.2%) in cell death compared with control cells. The expressions of the signaling proteins PI3K and pAkt, transcription factor NF-κB, and cell cycle protein cyclin D1 were markedly decreased in response to TC21 downregulation, whereas the level of pPTEN, an antagonist of PI3K, was increased. In addition, we evaluated the potential of TC21 as a putative target for sensitizing ESCC cells to the chemotherapeutic agent cisplatin. Increased cell death (38.4%) was observed in cells treated with cisplatin after TC21 knockdown compared with cells which were treated with cisplatin alone (20% cell death).
CONCLUSION: Results suggest that TC21 mediates its effects via the PI3K-Akt pathway, NF-κB and cyclin D1, and enhances chemoresistance in esophageal cancer cells.
Keywords: TC21, Esophageal squamous cell carcinoma, siRNA, Cisplatin, Chemosensitivity
INTRODUCTION
Esophageal cancer is one of the most aggressive malignancies of the gastrointestinal tract, ranking as the sixth most common cancer among males and ninth most common cancer among females globally. Esophageal squamous cell carcinoma (ESCC) is the predominant histological subtype of esophageal cancer in India, characterized by a high mortality rate and strong association with dietary habits and lifestyle[1,2]. It is the second most common cancer among males and fourth most common cancer among females in India[3]. Despite advances in multimodality therapy, because of its insidious symptomatology, late stage of diagnosis and poor efficacy of treatment, the prognosis of patients with ESCC still remains poor, with an average 5-year survival of < 10% globally[4,5] and 12% in India[6,7].
TC21/R-Ras2 is the only member of the R-Ras subfamily for which overexpression or mutants were detected in human tumor cells, including cells derived from uterine sarcoma[8], ovarian cancer[9] and mammary tumors[10]. Increased expression of TC21 was found in breast cancer cells[11-13]. Our laboratory identified TC21 overexpression in human oral squamous cell carcinomas using differential display and verified its increased expressions independently in oral cancer[14,15]; as well as in esophageal carcinomas[16]. These clinical studies suggested that deregulated TC21 activity might contribute to human oncogenesis, however like R-Ras, the functions of TC21 are not completely understood.
The R-Ras family of Ras-related proteins, including R-Ras, TC21 (R-Ras2), and M-Ras (R-Ras3), share 55% amino acid identity with H-Ras, including identical core effector regions[17]. Besides H-, K-, and N-Ras, TC21 is the only Ras superfamily member known to transform epithelial and fibroblast cell lines[18], and induce tumor formation in vivo[9]. Despite their similarities, R-Ras and TC21/R-Ras2 exhibited differential transforming properties in a variety of cell lines. In NIH 3T3 fibroblasts, TC21/R-Ras2 induced foci formation and tumor growth more efficiently than R-Ras[18]. TC21/R-Ras2 also potently transformed Rat-1 fibroblasts and various epithelial cell lines, including MCF-10A, RIE-1, and EpH4[19,20]. In comparison, R-Ras was unable to transform Rat-1 fibroblasts, but promoted tumor growth in cervical epithelial cells[21,22]. Phosphoinositide 3-kinase (PI3K) is the predominant effector of R-Ras and TC21/R-Ras2 transforming activity; however, these oncogenes also activate Raf1, Ral-GDS, extracellular signal-regulated kinase (ERK) 1/2, c-Jun NH2-terminal kinase, and p38 mitogen-activated protein kinase (MAPK) in a cell type-specific manner[23-25]. Previous reports suggested that TC21 induces its effects in multiple ways in different cell types, for example TC21 has been shown to activate p38 MAPK in Cos7 cells[18], and p38 MAPK activation is important for TC21-induced ureteric bud cell proliferation[26], therefore, TC21-mediated signaling is tissue specific[20-22,26,27]. We have earlier identified TC21 to be overexpressed in ESCC[16], but its role in ESCC is not well understood. This study was designed to determine the functional significance of TC21 protein in esophageal cancer. Further, we aimed to analyze the effect of TC21 downregulation on the sensitivity of ESCC cells to chemotherapeutic agents using cisplatin as a prototype.
MATERIALS AND METHODS
Reagents
Cisplatin, propidium iodide and protease inhibitor cocktail were procured from Sigma-Aldrich (St. Louis, MO, United States); protein assay reagent was obtained from Bio-Rad Laboratories (Hercules, CA, United States); lipofectamine, TC21 small interfering RNA (siRNA) and scrambled sequence siRNA from Qiagen, RPMI 1640 (Invitrogen); primary antibodies directed against protein kinase B [pAkt, (Ser 473)], pAkt (Thr 308), protein Glycogen synthase kinase 3β [pGSK3β (Ser9)], total Akt, phosphatase and tensin homolog [pPTEN, (Ser 380)], and protein Phosphoinositide-dependent kinase-1 [pPDK1 (Ser 241)] were procured from Cell Signaling Technology (Beverly, MA, United States); cyclin D1 and GSK3β were obtained from Santa Cruz Biotechnology Inc., (California, United States), β-actin (AC-15), GAPDH (Abcam Inc. Cambridge, MA, United States). Secondary antibodies, alkaline phosphatase conjugated goat anti-mouse IgG, goat anti rabbit IgG or rabbit anti-goat IgG, were from Cell Signaling Technology (Beverly, MA, United States). Enhanced chemiluminescence (ECL) Western blotting detection reagents were obtained from Santa Cruz Biotechnology Inc., CA, United States.
Cells and cell culture
A human ESCC line, TE13, were grown in RPMI-1640 supplemented with 10% heat inactivated fetal bovine serum, 100 units/mL penicillin and 100 μg/mL streptomycin. Cells were incubated at 37 °C in 5% humidified CO2 enriched atmosphere and routinely sub-cultured every 2 d by trypsinization.
Cisplatin treatment and cell viability assay
Cells were seeded and grown to 60%-70% confluence in triplicates prior to addition of cisplatin. The cells were treated with varying doses of cisplatin (0-10 μg/mL) for 24 h. Thereafter, the cells were harvested and LD50 was determined by measuring the cell viability using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent according to the manufacturer's instructions. The conversion of MTT to formazan by mitochondrial dehydrogenases was measured at wavelength 570 nm in a microtiter plate reader. The percentage cell death was calculated individually for each dose as follows: (Acontrol-Atreated/Acontrol) × 100, as described earlier[28]. For further experiments, the cisplatin concentration required to inhibit cell growth by 50% (LD50) was determined by interpolation from dose-response curves.
RNA interference
TE13 cells were seeded and grown to 70% confluence in growth medium without antibiotics. Transfection was carried out using Hyperfectamine according to the manufacturer’s instructions, using TC21 siRNA or scrambled sequence siRNA at final concentrations of 20 nmol/L and 10 nmol/L, respectively. After 24 h, 48 h and 72 h cells were harvested for reverse transcription-polymerase chain reaction (RT-PCR) and Western blotting analysis for detection of TC21. For cisplatin treatment the cells were transfected with TC21 siRNA for 48 h, followed by cisplatin treatment for 24 h and compared to untreated cells as controls, transfected with a scrambled sequence siRNA (negative control), or with the transfection reagent alone (mock). At least three independent experiments were performed with reproducible results. MAPK siRNA was used as a positive control and was detected by Western blotting for ERK.
RNA isolation
Total RNA was isolated from TE13 cells (untreated and TC21 siRNA transfected cells) using Qiagen kit following the manufacturer’s instructions or the standard protocol. DNase I treatment of RNA was carried out using the Message Clean Kit (Gen Hunter Corp., Brookline, MASS, United States). RNA was quantified using formaldehyde agarose gel and by measuring spectrophometrically the absorbance at 260 nm and 280 nm (ND-1000 UV-Vis Spectrophotometer from NanoDrop Technologies).
RT-PCR analysis
Briefly, cDNA was prepared using 1 μg of total RNA and Moloney murine leukemia virus reverse transcriptase (Gibco BRL, Life Technologies Inc., Gaithersburg, MD, United States) with oligo dT as the primer. Primers used for amplification of TC21-specific sequences were forward 5’-CCTTAGACCAAGAAGCTGGC-3’ and reverse 5’-CAGGCATTTGGTATTTTGGC-3’. The PCR cycling parameters were initial denaturation at 94 °C for 5 min; 30 cycles of 94 °C for 1 min, 54 °C for 1 min and 72 °C for 2 min and final extension at 72 °C for 10 min. PCR for β-actin was reverse transcribed for all the samples to check for the quality and quantity of the initial RNA used. The PCR-amplified products were electrophoresed on 1.2% agarose gels and bands were visualized by ethidium bromide staining.
Protein extraction and Western blotting
After the above described TC21 siRNA treatment with or without cisplatin, TE13 cells were harvested and protein extracts were made by lysing cells in buffer, containing 50 mmol Tris-HCl, pH 7.5, 150 mmol NaCl, 1% Triton X-100, 10% glycerol, 1 mmol DTT, 10 mmol NaF, supplemented with 1 mmol activated Na3VO4 and 1 × protease inhibitor cocktail. Protein concentration was measured using Bradford reagent (Sigma) and bovine serum albumin as standard. Proteins were separated by Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes by electroblotting. Membranes were blocked with phosphate-buffered saline containing 1% bovine serum albumin, followed by incubation with primary and secondary antibodies. Detection of antibody-protein complexes was done using an ECL Western blotting kit according to the manufacturer’s instructions.
Flow cytometry
TE13 cells were transfected with TC21 siRNA, cisplatin alone, or a combination of siRNA and cisplatin for specific time intervals as described above, cells were harvested and resuspended in phosphate-buffered saline. For cell cycle analysis, cells were fixed in 70% ethanol overnight at -20 °C and stained with propidium iodide (20 μg/mL) and RNaseA (10 μg/mL). All flow cytometric analyses were done using a FACS Caliber flow cytometer (San Jose, CA, United States). The acquired data were analyzed using BD FACS Diva software. For each measurement 10 000 cells were analyzed.
Statistical analysis
The Western blotting data were subjected to statistical analyses using SPSS 10.0 software (Chicago). Densitometry analysis of Western blotting was carried out using ChemiImager 4400 software. The integrated density value was compared with the loading control. The protein expression of 4 different groups (mock, scrambled sequence, TC21siRNA 48 h treatment and TC21siRNA 72 h treatment) were compared using one-way analysis of variance (ANOVA). ANOVA was applied for statistical analysis with the post hoc (Bonferroni) multiple range test. P < 0.05 was considered to be significant.
RESULTS
TC21/R-Ras2 gene silencing using siRNA
Evaluation of TC21 mRNA and protein levels 48 h and 72 h post-transfection of TC21 siRNA revealed an 80% reduction in mRNA levels and a 95% reduction in TC21 protein levels as compared with the respective controls transfected with scrambled siRNA (negative control) or with transfection reagent alone (mock) as shown in Figure 1A, B and Figure 2A, B. MAPK siRNA was used as a positive control and its detection was carried out by Western blotting for ERK (Figure 2C, D).
Figure 1.
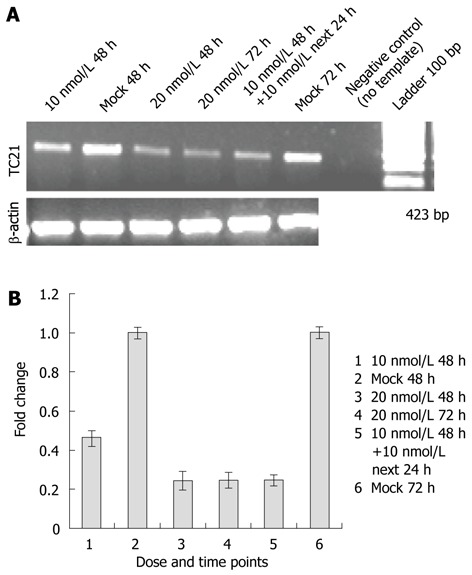
Small interfering RNA-targeting TC21/R-Ras2 was transfected in TE13 cells. A: Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis: Small interfering RNA (siRNA)-targeting TC21/R-Ras2 was transfected in TE13 cells in a dose-dependent manner. After 48 h and 72 h, cells were lysed, RNA was isolated and the mRNA level was determined semiquantitatively by RT-PCR using TC21-specific primers; B: Bar diagram showing fold change in level of TC21 transcripts after TC21 knockdown.
Figure 2.
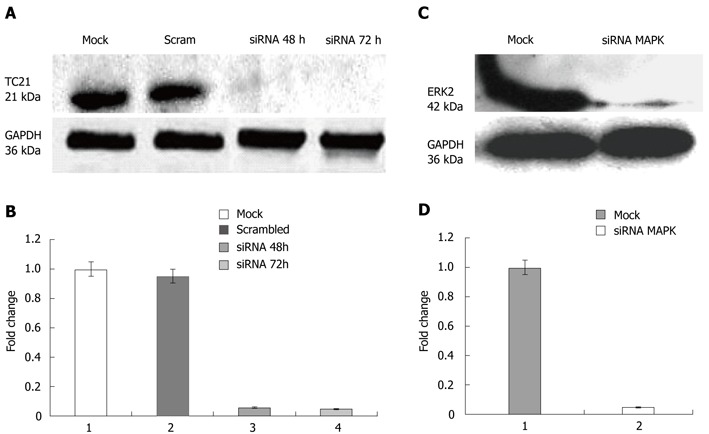
TC21 protein and extracellular signal-regulated kinase 2 level. A: Western blotting was carried out using specific antibody; the panel shows inhibition of TC21 protein compared with cells without transfection or transfection with a negative control siRNA (scrambled sequence); B: Bar diagram showing more than 95% decrease in TC21 protein level after TC21 knockdown compared with untreated mock and nonspecific scrambled sequence; C: Western blotting analyses show inhibition of ERK2 protein by targeted siRNA as positive control; D: Bar diagram showing more than 98% decrease in ERK2 level after MAPK knockdown compared with untreated mock and nonspecific scrambled siRNA sequence. ERK: Extracellular signal-regulated kinase; MAPK: Mitogen-activated protein kinase; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
TC21 activates the Akt pathway in ESCC
Since TC21 has been shown to activate PI3K, we investigated the role of the PI3K pathway in TC21-mediated esophageal tumorigenesis. siRNA-mediated TC21 downregulation resulted in a significant decrease in the expression of phosphorylated Akt/PKB with P < 0.001 (Ser 473, Thr 308 showed equal reduction) and phosphorylated glycogen synthase kinase-3β, pGSK3β (Ser 9), without any change in the levels of total Akt (Figure 3A, B). A significant increase in expression of PTEN was observed in TC21 siRNA-treated TE13 cells compared with controls (P < 0.001). Since PTEN is a PI3K antagonist and inhibits downstream signaling through Akt, its upregulation in siRNA-treated cells suggests the involvement of PI3K in TC21-mediated esophageal tumorigenesis. Moreover, knockdown of TC21 resulted in a significant decrease in PDK1 expression which may be responsible for the decrease in the expression of pAkt/PKB, resulting in reduced levels of pGSK3β (Figure 3A, B).
Figure 3.
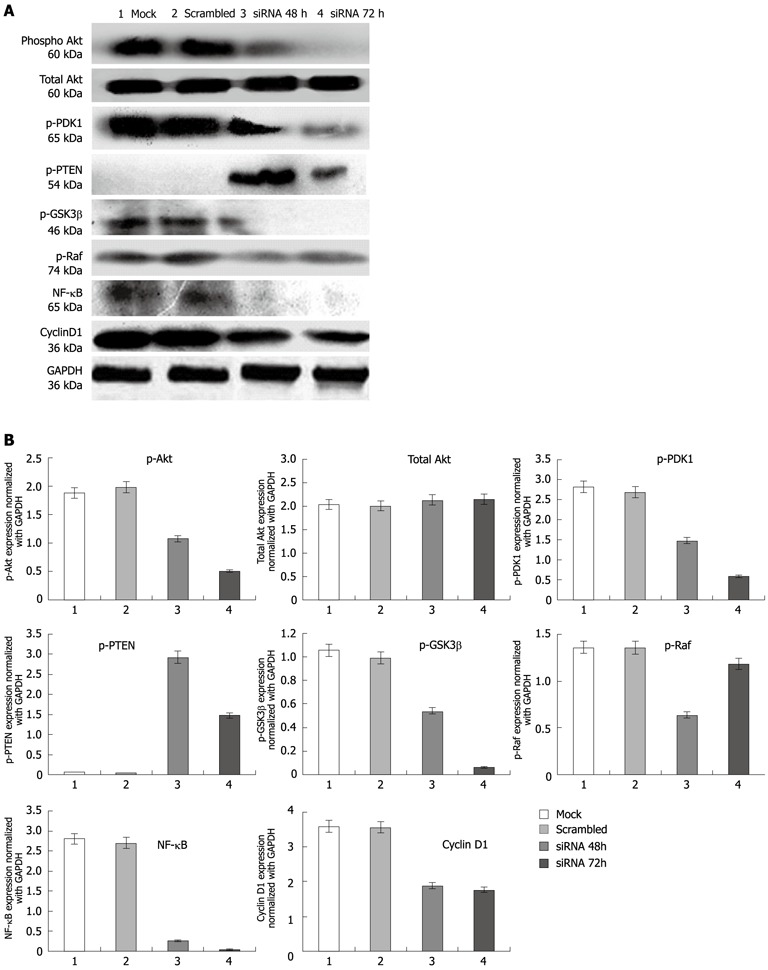
Expression of proteins in esophageal squamous cancer cells TE13 and compared with control. A: Expression analysis of protein kinase B (pAkt), total Akt, protein Glycogen synthase kinase 3β (pGSK3β), pRaf, protein Phosphoinositide-dependent kinase-1 (pPDK1), phosphatase and tensin homolog (pPTEN), nuclear factor-κB (NF-κB) and cyclin D1 proteins compared in esophageal squamous cancer cells TE13. Lane 1: Mock; Lane 2: Scrambled (non-target siRNA); Lane 3: TC21 siRNA transfected for 48 h; Lane 4: TC21 siRNA transfected for 72 h; B: Bar diagram showing relative levels of proteins in comparison with control protein GAPDH. GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
TC21 activates the anti-apoptotic factor nuclear factor-κB and cyclin D1
Western blotting analysis of whole cell lysates from TC21-knockdown TE13 cells probed with antibodies specific for the p65 subunit of nuclear factor-κB (NF-κB) and cyclin D1 showed significant decrease in NF-κB and cyclin D1 proteins compared with untransfected controls (Figure 3A, B). Our results suggest that NF-κB targeting the growth promoting protein cyclin D1, may be the downstream targets of TC21 signaling through the PI3-K/Akt pathway, thereby increasing survival of esophageal cancer cells.
TC21 knockdown does not affect P-Raf protein
There was no significant change in phosphorylated Raf protein expression observed in TC21-knockdown esophageal cancer cells for 72 h of transfection in comparison with control cells, but there was a decreased P-Raf protein level observed in TC21 siRNA treated for 48 h (Figure 3A, B).
Knockdown of TC21 results in decreased G1/S population
TC21-knockdown TE13 cells resulted in a marginal increase (14.2%) in the subG0 population (cell death) compared with the mock control cells (7.9%), while the G1/S population decreased from 44% to 35% in the siRNA-treated cells (Figure 5A-2, B and Table 1).
Figure 5.
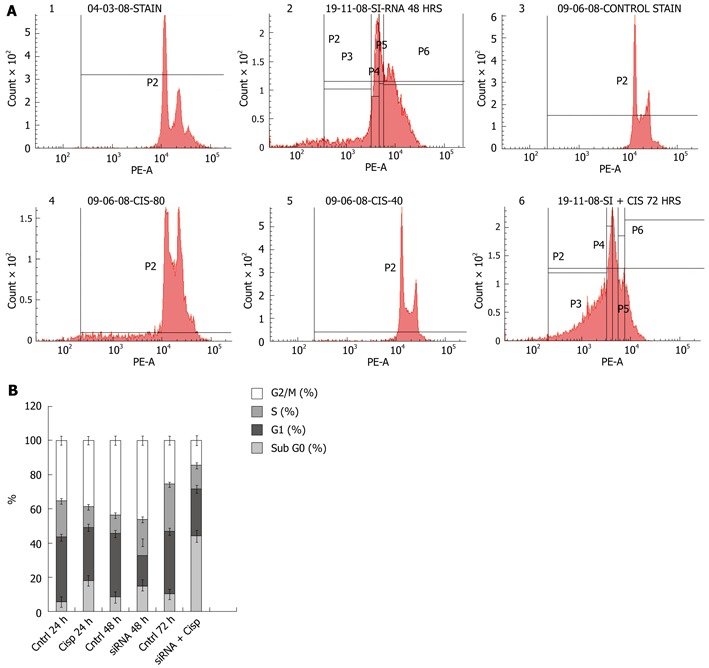
Cell cycle analyses. A: TC21-knockdown cells (20 nmol siRNA for 48 h) were treated with cisplatin (8 μg/mL) for 24 h and cell cycle analysis was performed by labeling cells with DNA binding dye propidium iodide. The panel shows 1: DNA histograms of mock-transfected TE13 cells for 48 h; 2: TE13 cells transfected with TC21 siRNA for 48 h showing increased cell death; 3: TE13 cells without transfection for 24 h; 4: TE13 cells were treated with cisplatin alone for 24 h; 5: TE13 cells without transfection for 72 h; 6: TE13 cells were transfected with TC21siRNA for 48 h for optimal knockdown followed by cisplatin at 8 μg/mL for 24 h; B: Stacked bar graph depicts the cell cycle distribution. Data presented here as mean of percentage events from 3 independent experiments.
Table 1.
Cell cycle analyses of siRNA-treated TE13 cells using flow cytometry
| Experimental parameters | SubG0 (%) | G1 (%) | S (%) | G2/M (%) |
| Untreated TE131 cells for 24 h | 5.7 | 37 | 21 | 34.8 |
| Cisplatin treatment (8 μg/mL) for 24 h | 17.8 | 30.6 | 11.8 | 38.2 |
| Untreated TE13 cells for 48 h | 7.9 | 34.7 | 10 | 41 |
| TC21 siRNA2 (20 nmol) transfection for 48 h | 14.2 | 22.8 | 12.2 | 42.3 |
| Untreated TE13 cells for 72 h | 10.3 | 36.7 | 27.7 | 25.8 |
| TC21 siRNA transfection (20 nmol) for 48 h followed by cisplatin treatment for 24 h (total time of treatment is 72 h) | 38.4 | 23.8 | 11.9 | 12.8 |
Esophageal squamous cell carcinoma cells;
Small interfering RNA.
Effect of cisplatin treatment on TE13 cells
The TE13 cells were treated with varying doses of cisplatin (0-10 μg/mL) for 24 h and LD50 was calculated by measuring the cell viability using MTT (Figure 4). LD50 was found to be 9 μg/mL. TE13 were treated with 8 μg/mL cisplatin for 24 h and flow cytometric analysis was performed to determine the cell cycle distribution. Cisplatin-treated TE13 cells showed a marginal increase in cell death (17.8%) compared with untreated cells (5.7%). There was a decrease in the S phase (11.8%) compared with 21% in the untreated control cells (Figure 5A-4, B and Table 1).
Figure 4.

Cell viability assay. TE13 cells were treated with cisplatin in a dose-dependent manner for 24 h. Cell viability was determined using the MTT assay, LD50 was found to be 9 μg/mL. Further treatment with cisplatin was carried out at less than the LD50 dose.
Knockdown of TC21 sensitizes TE13 cells to cisplatin treatment
The combined effect of TC21 siRNA and cisplatin treatment on TE13 cells was determined. In TC21 siRNA-transfected TE13 cells, 38.4% cell death was observed after exposure to 8 μg/mL cisplatin compared with 10.3% cell death in the untreated controls; 23.8% cells were found in the G1 phase compared with 36.7% in the controls. Further, a decrease in S phase fraction (11.9%) was observed compared with the untreated control cells (27.7%) (Figure 5A-6, B and Table 1).
DISCUSSION
This study was focused on the effect of TC21 downregulation on cell signaling in ESCC and its effect on the cisplatin response by downregulation of TC21 targets, both individually and in combination. In our study, TC21 protein expression correlated with cisplatin sensitivity. Earlier studies have shown that overexpression of TC21 resulted in cisplatin resistance to apoptosis[29]. Therefore, we investigated whether TC21 downregulation would sensitize TE13 cells to cisplatin. Our results demonstrated that TC21 downregulation resulted in a significant reduction in the expression of the Akt pathway components, supporting that Akt pathway proteins serve as important downstream targets of TC21. Akt, an oncogenic protein implicated in human cancer development, is a key component of the PI3-K signaling pathway[30,31]. Knockdown of TC21 decreased the expression of pAkt/PKB (antibodies specific for Ser 473 and Thr 308 showed similar effects), while the total Akt/PKB levels remained unaffected.
Furthermore, TC21 knockdown decreased the NF-κB levels. The TC21 oncogenic signals are mediated via the PI3K/Akt, NF-κB pathway, whereas the role of TC21 in activation of the extracellular signal-regulated kinase/MAPK pathway is less clear[20].
Significant down-regulation of pGSK3β (Ser 9) in TC21-knockdown cells suggests a role of GSK3β downstream from Akt in TC21-mediated esophageal cancer. TC21 knockdown also suppressed the phosphorylation of the upstream kinase PDK1 (P-Serine 241). The phosphorylation of the p85 subunit of another upstream kinase, PI3K was also suppressed upon TC21 knockdown (data not shown). Suppression of Akt by TC21 siRNA led to the suppression of phosphorylation of GSK3β, the substrate for Akt. The serine/threonine kinase Akt, a key target of PIP3, is activated by TC21[29], resulting in increased cell proliferation, transformation and survival through numerous effectors, including Bad, GSK-3β and mTOR[32]. Notably, a significant increase in pPTEN expression was observed in TC21 siRNA-treated esophageal cancer cells compared with controls. Since pPTEN is a PI3K antagonist and inhibits downstream signaling through Akt, its upregulation in siRNA-treated cells suggests the involvement of PI3K in TC21-mediated esophageal tumorigenesis, suggesting that the PI3K/Akt is a downstream target of TC21.
TC21-knockdown esophageal cancer cells did not affect the level of P-Raf, suggesting that the TC21 pathway is independent of Raf. Our results support the previous report that TC21 is regulated similar to Ras except that it does not interact with full-length Raf 1, B raf, and A raf, suggesting that TC21 uses a Raf-independent pathway to induce oncogenic transformation[33]. It also interacts with Ral GDS in vitro, which may be responsible for the Raf-independent pathway[34].
Cyclin D1 is a major regulator of the progression of cells into the proliferative stage of the cell cycle[35]. Interestingly, TC21 knockdown resulted in reduced expression of cyclin D1, suggesting TC21 may increase cell survival of esophageal cancer by targeting cyclin D1. Thus cyclin D1 may be a target of TC21 signaling through the PI3K/Akt/NF-κB/cyclin D1 pathway. We observed that TC21 gene knockdown by RNAi alone induces an increase in the subG0 population of TE13 cells and a decrease in cyclin D1. Cell cycle progression from G0/G1 to the S phase requires cyclin/cyclin-dependent kinase (CDK) complexes and hyperphosphorylated retinoblastoma protein (Rb). In the early G1 phase, the cyclin D1/CDK 4 complex phosphorylates Rb, triggering a cascade of events, including the dissociation of E2F from hyperphosphorylated Rb, the activation of E2F transcription, and progression to the S phase[36]. Keeping in view the above discussed facts it is possible that a TC21 knockdown-induced decrease in cyclin D1 expression may block transition from G1 to the S phase.
Overexpression of TC21 activated its downstream targets resulting in translocation of NF-κB to the nucleus and stimulated the transcription of anti-apoptotic genes including cyclin D1. Low cellular levels of cyclin D1 have been reported to potentially contribute to increased cisplatin sensitivity in human breast cancer cells CAL-148[37]. Our results suggest that TC21 may enhance cell survival against cisplatin-induced cell death through activation of the PI3-K/Akt/NF-κB/cyclin D1 signaling pathway in esophageal cancer cells. Knockdown of TC21 sensitized TE-13 cells to cisplatin treatment and resulted in an increase in the subG0 population (cell death), a decrease in G1/S-phase and an increase in G2/M-phase populations. Further studies are needed to reveal new downstream targets of NF-κB responsible for TC21-mediated cell survival.
In summary, cyclin D1 is a downstream target for TC21 and TC21 may be a candidate marker for prediction of cisplatin treatment outcome in esophageal cancer patients. Our study draws attention to the relevance of TC21 in the context of cisplatin pharmacogenetics of esophageal cancer.
COMMENTS
Background
Esophageal cancer is one of the most aggressive malignancies of the gastrointestinal tract, ranking as the sixth most common cancer among males and ninth most common cancer among females globally. Esophageal squamous cell carcinoma (ESCC) is the predominant histological subtype of esophageal cancer in India, characterized by high mortality rate and strong association with dietary habits and lifestyle.
Research frontiers
TC21/R-Ras2 is the only member of the R-Ras subfamily for which overexpression or mutants were detected in human tumor cells, including cells derived from uterine sarcoma, ovarian cancer and mammary tumors. Increased expression of TC21 was found in breast cancer cells.
Innovations and breakthroughs
The effect of TC21 downregulation on cell signaling in esophageal cancer cells was determined by assessing the phosphorylation status of its downstream targets, phosphoinositide 3-kinase, phosphatase and tensin homolog, protein kinase B, nuclear factor-κB (NF-κB), and cyclin D1 using specific antibodies. Cell survival analysis after cisplatin treatment was carried out by a cell viability assay and cell cycle analysis using flow cytometry.
Applications
Cyclin D1 is a downstream target for TC21 and TC21 may be a candidate marker for prediction of cisplatin treatment outcome in esophageal cancer patients. The study draws attention to the relevance of TC21 in the context of cisplatin pharmacogenetics of esophageal cancer.
Peer review
This is a good descriptive study in which authors determine the functional significance of TC21 in ESCC. The results are interesting and suggest that that TC21 mediates its effects via PI3K-Akt pathway, NF-κB and cyclin D1 and enhances chemoresistance in esophageal cancer cells.
Footnotes
Supported by Department of Science and Technology, Government of India
Peer reviewer: Itaru Endo, Professor, Gastroenterological Surgery, Graduate School of Medicine, Yokohama City University, 3-9 Fukuura, Kanazawa-ku, Yokohama 2360004, Japan
S-Editor Gou SX L-Editor Cant MR E-Editor Zhang DN
References
- 1.Malkan G, Mohandas KM. Epidemiology of digestive cancers in India. I. General principles and esophageal cancer. Indian J Gastroenterol. 1997;16:98–102. [PubMed] [Google Scholar]
- 2.Phukan RK, Chetia CK, Ali MS, Mahanta J. Role of dietary habits in the development of esophageal cancer in Assam, the north-eastern region of India. Nutr Cancer. 2001;39:204–209. doi: 10.1207/S15327914nc392_7. [DOI] [PubMed] [Google Scholar]
- 3.Gajalakshmi V, Swaminathan R, Shanta V. An Independent Survey to Assess Completeness of Registration: Population Based Cancer Registry, Chennai, India. Asian Pac J Cancer Prev. 2001;2:179–183. [PubMed] [Google Scholar]
- 4.Montesano R, Hall J. Environmental causes of human cancers. Eur J Cancer. 2001;37 Suppl 8:S67–S87. doi: 10.1016/s0959-8049(01)00266-0. [DOI] [PubMed] [Google Scholar]
- 5.Parkin DM, Moss SM. Lung cancer screening: improved survival but no reduction in deaths--the role of “overdiagnosis”. Cancer. 2000;89:2369–2376. doi: 10.1002/1097-0142(20001201)89:11+<2369::aid-cncr10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 6.Gupta D, Boffetta P, Gaborieau V, Jindal SK. Risk factors of lung cancer in Chandigarh, India. Indian J Med Res. 2001;113:142–150. [PubMed] [Google Scholar]
- 7.Yeole BB, Kumar AV. Population-based survival from cancers having a poor prognosis in Mumbai (Bombay), India. Asian Pac J Cancer Prev. 2004;5:175–182. [PubMed] [Google Scholar]
- 8.Huang J, Roby KF, Pace JL, Russell SW, Hunt JS. Cellular localization and hormonal regulation of inducible nitric oxide synthase in cycling mouse uterus. J Leukoc Biol. 1995;57:27–35. doi: 10.1002/jlb.57.1.27. [DOI] [PubMed] [Google Scholar]
- 9.Chan LC, Cheung A, Cheng D. Small cell carcinoma of the ovary associated with ins(10; 5)(p13; q31q13) Cancer Genet Cytogenet. 1994;77:89–90. doi: 10.1016/0165-4608(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 10.Barker KT, Crompton MR. Ras-related TC21 is activated by mutation in a breast cancer cell line, but infrequently in breast carcinomas in vivo. Br J Cancer. 1998;78:296–300. doi: 10.1038/bjc.1998.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark GJ, Kinch MS, Gilmer TM, Burridge K, Der CJ. Overexpression of the Ras-related TC21/R-Ras2 protein may contribute to the development of human breast cancers. Oncogene. 1996;12:169–176. [PubMed] [Google Scholar]
- 12.Movilla N, Crespo P, Bustelo XR. Signal transduction elements of TC21, an oncogenic member of the R-Ras subfamily of GTP-binding proteins. Oncogene. 1999;18:5860–5869. doi: 10.1038/sj.onc.1202968. [DOI] [PubMed] [Google Scholar]
- 13.Rokavec M, Schroth W, Amaral SM, Fritz P, Antoniadou L, Glavac D, Simon W, Schwab M, Eichelbaum M, Brauch H. A polymorphism in the TC21 promoter associates with an unfavorable tamoxifen treatment outcome in breast cancer. Cancer Res. 2008;68:9799–9808. doi: 10.1158/0008-5472.CAN-08-0247. [DOI] [PubMed] [Google Scholar]
- 14.Arora S, Matta A, Shukla NK, Deo SV, Ralhan R. Identification of differentially expressed genes in oral squamous cell carcinoma. Mol Carcinog. 2005;42:97–108. doi: 10.1002/mc.20048. [DOI] [PubMed] [Google Scholar]
- 15.Macha MA, Matta A, Sriram U, Thakkar A, Shukla NK, Datta Gupta S, Ralhan R. Clinical significance of TC21 overexpression in oral cancer. J Oral Pathol Med. 2010;39:477–485. doi: 10.1111/j.1600-0714.2009.00854.x. [DOI] [PubMed] [Google Scholar]
- 16.Sharma R, Sud N, Chattopadhyay TK, Ralhan R. TC21/R-Ras2 upregulation in esophageal tumorigenesis: potential diagnostic implications. Oncology. 2005;69:10–18. doi: 10.1159/000087283. [DOI] [PubMed] [Google Scholar]
- 17.Ehrhardt A, Ehrhardt GR, Guo X, Schrader JW. Ras and relatives--job sharing and networking keep an old family together. Exp Hematol. 2002;30:1089–1106. doi: 10.1016/s0301-472x(02)00904-9. [DOI] [PubMed] [Google Scholar]
- 18.Graham SM, Oldham SM, Martin CB, Drugan JK, Zohn IE, Campbell S, Der CJ. TC21 and Ras share indistinguishable transforming and differentiating activities. Oncogene. 1999;18:2107–2116. doi: 10.1038/sj.onc.1202517. [DOI] [PubMed] [Google Scholar]
- 19.Graham SM, Cox AD, Drivas G, Rush MG, D’Eustachio P, Der CJ. Aberrant function of the Ras-related protein TC21/R-Ras2 triggers malignant transformation. Mol Cell Biol. 1994;14:4108–4115. doi: 10.1128/mcb.14.6.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erdogan M, Pozzi A, Bhowmick N, Moses HL, Zent R. Signaling pathways regulating TC21-induced tumorigenesis. J Biol Chem. 2007;282:27713–27720. doi: 10.1074/jbc.M703037200. [DOI] [PubMed] [Google Scholar]
- 21.Marte BM, Rodriguez-Viciana P, Wennström S, Warne PH, Downward J. R-Ras can activate the phosphoinositide 3-kinase but not the MAP kinase arm of the Ras effector pathways. Curr Biol. 1997;7:63–70. doi: 10.1016/s0960-9822(06)00028-5. [DOI] [PubMed] [Google Scholar]
- 22.Rincon JC, Haase HR, Bartold PM. Effect of Emdogain on human periodontal fibroblasts in an in vitro wound-healing model. J Periodontal Res. 2003;38:290–295. doi: 10.1034/j.1600-0765.2003.00610.x. [DOI] [PubMed] [Google Scholar]
- 23.Rosário M, Paterson HF, Marshall CJ. Activation of the Raf/MAP kinase cascade by the Ras-related protein TC21 is required for the TC21-mediated transformation of NIH 3T3 cells. EMBO J. 1999;18:1270–1279. doi: 10.1093/emboj/18.5.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy GA, Graham SM, Morita S, Reks SE, Rogers-Graham K, Vojtek A, Kelley GG, Der CJ. Involvement of phosphatidylinositol 3-kinase, but not RalGDS, in TC21/R-Ras2-mediated transformation. J Biol Chem. 2002;277:9966–9975. doi: 10.1074/jbc.M109059200. [DOI] [PubMed] [Google Scholar]
- 25.Cox ME, Deeble PD, Lakhani S, Parsons SJ. Acquisition of neuroendocrine characteristics by prostate tumor cells is reversible: implications for prostate cancer progression. Cancer Res. 1999;59:3821–3830. [PubMed] [Google Scholar]
- 26.Pozzi A, Coffa S, Bulus N, Zhu W, Chen D, Chen X, Mernaugh G, Su Y, Cai S, Singh A, et al. H-Ras, R-Ras, and TC21 differentially regulate ureteric bud cell branching morphogenesis. Mol Biol Cell. 2006;17:2046–2056. doi: 10.1091/mbc.E05-08-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erdogan M, Karadeniz M, Ozbek M, Ozgen AG, Berdeli A. Interleukin-10 gene polymorphism in patients with papillary thyroid cancer in Turkish population. J Endocrinol Invest. 2008;31:750–754. doi: 10.1007/BF03349252. [DOI] [PubMed] [Google Scholar]
- 28.Macha MA, Matta A, Chauhan SS, Siu KW, Ralhan R. Guggulsterone targets smokeless tobacco induced PI3K/Akt pathway in head and neck cancer cells. PLoS One. 2011;6:e14728. doi: 10.1371/journal.pone.0014728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rong R, He Q, Liu Y, Sheikh MS, Huang Y. TC21 mediates transformation and cell survival via activation of phosphatidylinositol 3-kinase/Akt and NF-kappaB signaling pathway. Oncogene. 2002;21:1062–1070. doi: 10.1038/sj.onc.1205154. [DOI] [PubMed] [Google Scholar]
- 30.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 31.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wasylyk C, Wasylyk B, Heidecker G, Huleihel M, Rapp UR. Expression of raf oncogenes activates the PEA1 transcription factor motif. Mol Cell Biol. 1989;9:2247–2250. doi: 10.1128/mcb.9.5.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westwick JK, Cox AD, Der CJ, Cobb MH, Hibi M, Karin M, Brenner DA. Oncogenic Ras activates c-Jun via a separate pathway from the activation of extracellular signal-regulated kinases. Proc Natl Acad Sci USA. 1994;91:6030–6034. doi: 10.1073/pnas.91.13.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 36.Vermeulen K, Berneman ZN, Van Bockstaele DR. Cell cycle and apoptosis. Cell Prolif. 2003;36:165–175. doi: 10.1046/j.1365-2184.2003.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yde CW, Issinger OG. Enhancing cisplatin sensitivity in MCF-7 human breast cancer cells by down-regulation of Bcl-2 and cyclin D1. Int J Oncol. 2006;29:1397–1404. [PubMed] [Google Scholar]


