Abstract
Radiation-induced heart disease (RIHD) is a serious side effect of radiotherapy for intrathoracic and chest wall tumors. The threshold dose for development of clinically significant RIHD is believed to be lower than previously assumed. Therefore, research into mechanisms of RIHD has gained substantial momentum. RIHD becomes clinically apparent ten to fifteen years after radiation exposure. Chronic manifestations of RIHD include accelerated atherosclerosis, cardiomyopathy, and valve abnormalities. Reducing exposure of the heart during radiotherapy is the only known method of preventing RIHD, and there are no approaches to reverse RIHD once it occurs. We use a combination of pharmacological and genetic animal models to determine biological mechanisms of RIHD. Major technological advances in small animal research have made this type of study more valuable. The long-term goal of this work is to identify targets for intervention in RIHD, thereby enhancing the efficacy and safety of thoracic radiotherapy.
It is truly a great honor to receive the Radiation Research Society’s 2011 Michael Fry Award, which recognizes the contributions of a junior investigator to the field of radiation research. The main focus of my research has always been radiation-induced heart disease (RIHD). This side effect of radiation therapy captured my attention both as a clinical problem and from a radiation biology standpoint. Here, I am very pleased to have the opportunity to present and describe this line of research, as I hope to convey to you my fascination with it.
Radiation-induced heart disease is a long-term side effect of radiotherapy of thoracic and chest wall tumors when all or part of the heart is exposed to radiation. For instance, RIHD can occur among survivors of Hodgkin’s disease (1,2) or breast cancer (3–5) because radiation therapy fields for those patients can encompass the heart. Manifestations of RIHD include accelerated atherosclerosis, pericardial and myocardial fibrosis, conduction abnormalities, and injury to cardiac valves (6, 7). Both incidence and severity of the disease increase with higher radiation dose, larger volume exposed, younger age at time of exposure, and greater time elapsed since treatment. From a clinical perspective, the only approach to reduce late complications in the heart is through efforts to reduce cardiac exposure during therapy. Indeed, radiotherapy has undergone many such improvements over the last decades. Nonetheless, recent studies indicate that despite safety advances in radiotherapy some patients with Hodgkin’s disease, lung, esophageal or proximal gastric cancers still receive either a high dose of radiation to a small part of the heart or a low dose to the whole heart (8–13). In addition, there is increasing use of concomitant therapies, with the consequences of many combinations yet to be determined. While certain cardio-toxic chemotherapeutic agents such as anthracyclines are known to exacerbate radiation injury in the heart, the effects of many other agents are still unknown.
Clinical studies into RIHD are complicated by the fact that the symptoms of RIHD are indistinguishable from those of other forms of heart disease. It is therefore difficult to unequivocally relate injury in the heart to prior radiation exposure, as opposed to radiation injury in certain other organ systems such as the lung or intestine. As a result, most of the time it is impossible to identify the individual patients for whom it is certain that radiation exposure caused their heart disease. Moreover, the incidence and severity of RIHD are influenced by many factors, most of which can be considered general cardiovascular risk factors such as hypertension, smoking, and obesity. To overcome these issues, some studies have compared outcomes between groups of left-sided and right-sided breast cancer patients. The anatomical location of the heart often results in left-sided breast cancer patients being exposed to higher doses to the heart than right-sided breast cancer patients, especially in tangential breast irradiation. Other risk factors are assumed to be evenly distributed between the two groups. Several studies have shown greater morbidity and mortality from cardiovascular disease after treatment for left-sided breast cancer patients compared to those patients treated for right-sided breast cancer, which illustrates the cardiotoxicity of ionizing radiation (5, 14–16).
Analyses of atomic bomb survivors show an increased incidence of cardiovascular disease in populations that have been exposed to low doses of ionizing radiation (17, 18). These outcomes significantly strengthened interest in determining the cardiovascular effects of low-dose ionizing radiation and rekindled debate over the magnitude of the threshold dose (a level below which no effect would be obtained) (19–22). Based on these studies and on studies in other epidemiological cohorts, some researchers have suggested that doses of 1 Gy or lower could result in increased incidence of heart disease (23–25). However, as previously mentioned, the existence of many confounding factors related to cardiovascular disease make it difficult to draw affirmative conclusions about the minimum dose that may cause clinically relevant injury in the heart and vasculature. Nevertheless, these outcomes have raised the issue of possible cardiovascular effects of exposure to ionizing radiation during space flights or other scenarios of low-dose exposure.
Pharmacological methods to prevent or reverse RIHD in humans are not yet available. Therefore, pre-clinical (in vitro or animal) studies have been used to unravel biological mechanisms of RIHD, with the ultimate goal of identifying potential targets for intervention (pharmacological or other) that could eventually be translated to human subjects. Studies using these models have shown that local heart irradiation causes long-term changes in cardiac function and adverse myocardial remodeling in dogs, rabbits, rats, and mice (26–30). Spontaneously hypertensive rats or animals on a high-fat diet have been used to study radiation-induced accelerated atherosclerosis in coronary arteries (31, 32). Some of the histopathological changes in pre-clinical models, such as myocardial degeneration and fibrosis, are described in human cases of RIHD after exposure to doses of ~30 Gy and above (1, 2, 33–35). Although clinical and pre-clinical data on the cardiovascular effects of lower radiation doses are growing (9, 18), there is still uncertainty with regard to biological mechanisms and species-specific threshold doses.
I was familiarized with the topic of RIHD during my graduate studies in the laboratory of Jan Wondergem, at the Leiden University Medical Centre in The Netherlands. Dr. Wondergem provided his graduate students much freedom in the design of their experiments, which gave us a positive sense. We determined that histopathological changes in our rat heart model of local heart irradiation could be identified more easily in longitudinal heart sections compared to one midventricular section (36). We observed that a dose-dependent increase in the number of mast cells in the heart, coinciding with histopathological signs of radiation injury (36). Mast cells are mostly known for their adverse role in allergic reactions, but they also play an important role in tissue homeostasis and remodeling. Hence, the potential role of mast cells in RIHD captured my attention.
Shortly before my graduation, Dr. Wondergem put me in contact with Dr. Martin Hauer-Jensen at the University of Arkansas for Medical Sciences (UAMS), and I was selected to do my postdoctoral studies in his laboratory. Much of what I know today about experimental design and scientific writing I learned during those postdoctoral years. In addition, I realized that I my interest in RIHD remained strong. I was very fortunate that the Department of Pharmaceutical Sciences in the College of Pharmacy at UAMS offered me a faculty position to further develop projects to identify biological mechanisms of and potential interventions for RIHD.
We studied the mechanistic role of mast cells in radiation injury in the heart with the use of a genetic mast cell-deficient rat model (37). After local heart irradiation, mast cell-deficient rats showed more severe changes in in vivo and ex vivo cardiac function, more pronounced myocardial deposition of collagen III, and less myocardial degeneration than their mast cell-competent littermates (38) (Fig. 1). From these somewhat surprising results we concluded that mast cells played a predominantly protective role in RIHD in the rat. Mast cells may have cardioprotective effects through several mechanisms, one of which involves the kallikrein-kinin pathway. Kinins are peptide hormones that may aggravate the effects of certain cardiac events such as myocardial infarction (39), but they also display several cardioprotective properties, partially through the induction of nitric oxide (40–42). Mast cell-derived proteases enhance the release of kinins from their precursors, which are high-molecular-weight and low-molecular-weight kininogen (43,44). We recently used a rat model deficient in the secretion of kininogens (45, 46) to start investigating the role of the kallikrein-kinin pathway in RIHD. Preliminary results indicated that early cardiac functional events and late changes in inflammatory infiltration after local heart irradiation are less pronounced in kininogen-deficient rats (unpublished data).
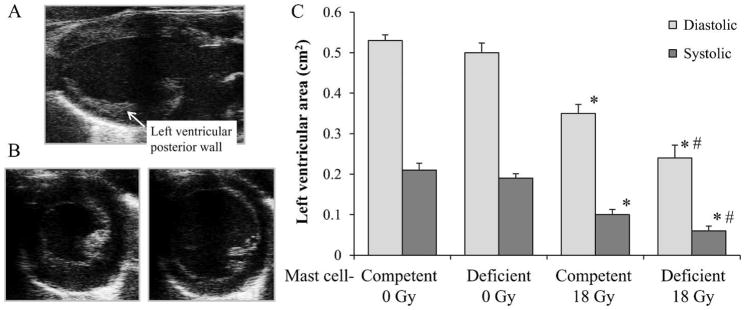
FIG. 1.
In vivo ultrasound at 6 months after local heart irradiation in mast cell-deficient and mast cell-competent rats. Panel A: Long axis view of a rat heart. Panel B: Short axis views of hearts in systole (left) and diastole (right) were used to measure left ventricular area. Panel C: Reductions in left ventricular diastolic area and left ventricular systolic area were more severe in mast cell-deficient rats. Data are shown as average ± SEM, n = 10, *P < 0.05 irradiated compare to sham irradiation, #P < 0.05 mast cell-deficient compare to mast cell-competent. This figure presents data from (38).
Mast cells may also display protective properties by releasing proteases that break down endothelin-1 (ET-1) (47). Interestingly, we found that left ventricular ET-1 gene expression was upregulated after local heart irradiation in mast cell-competent rats, but not in mast cell-deficient rats (48). These results sparked our interest in a potential pharmaceutical intervention in RIHD that would inhibit the effects of ET-1 in the heart. We tested the effects of bosentan, a dual inhibitor of the ET-1 receptors ETA and ETB, in our rat model of local heart irradiation. The effects of bosentan on late cardiac radiation injury in the rat were minimal, which may have been caused by the opposing roles that ETA and ETB are known to play in cardiovascular function and disease (48, 49).
Another pharmaceutical intervention that we are testing is the cyclic AMP phosphodiesterase inhibitor pentoxifylline. Studies by us and others suggest that pentoxifylline in combination with α-tocopherol can improve cardiac function and reduce adverse cardiac remodeling in the rat when administration starts before irradiation, also when administration starts several months after local heart irradiation (50,51) (Fig. 2). Some of our current studies focus on the effects of compounds that are related to pentoxifylline and α-tocopherol.
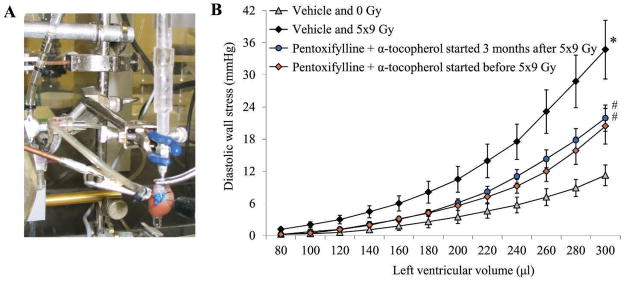
FIG. 2.
Effects of pentoxifylline and α-tocopherol on ex vivo cardiac function measurements at 6 months after local heart irradiation. Panel A: The Langendorff ex vivo perfused heart preparation is commonly used for investigation of cardiac physiology and disease. The picture reflects a rat heart in a Langendorff apparatus. A left ventricular balloon is connected to a pressure transducer to obtain real-time pressures generated inside the left ventricle. Panel B: Treatment with pentoxifylline and α-tocopherol reduced left ventricular diastolic wall stress at 6 months after local heart irradiation with 5 daily fractions of 9 Gy. Data are shown as average ± SEM (n =6–8). *P < 0.05 irradiated compared to sham irradiation, #P < 0.05 pentoxifylline + α-tocopherol compared to vehicle. This figure presents data from (50).
Small animal models of local heart irradiation have improved greatly. We are now able to perform real-time, image-guided localized irradiation that allows more precise targeting of the organ or tissue of interest (Fig. 3) (52). Ex vivo perfused heart preparations have long been used in biomedical research and are continuously updated to provide important insight into cardiac function and disease including RIHD (Fig. 1) (38, 53, 54). In addition, in the last decade tremendous advancements have been made in the development of noninvasive imaging technologies for use with small laboratory animals. High-resolution ultrasound, magnetic resonance imaging, and single photon emission computed tomography are only a few examples of tools that are now available to closely follow cardiac function in small laboratory animals (55, 56). Several of these technologies have been included in radiobiological studies of cardiovascular radiation injury (50, 57). Future developments will hopefully bring pre-clinical models closer to clinical applications. These are very exciting times to be involved in this type of research.
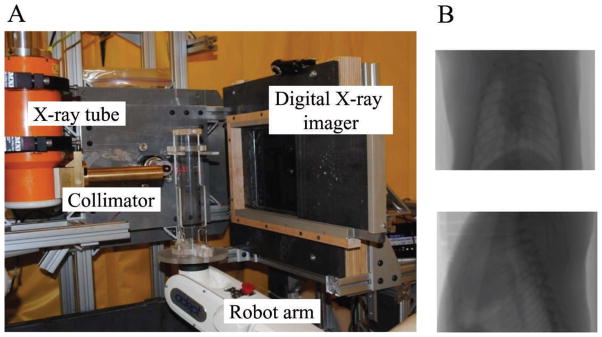
FIG. 3.
The Small Animal Conformal Radiation Therapy Device (SACRTD) (University of Arkansas for Medical Sciences, Department of Radiation Oncology) allows precise image-guided irradiation of the target of interest with minimal exposure of surrounding tissues in small laboratory animals. Panel A: Set-up for localized heart irradiation in the rat. Vertical positioning of the animal allows for X-ray imaging of the chest to delineate the heart. Panel B: Rat chest X rays taken in vertical position at different angles for heart delineation.
Once more I would like to end by expressing my deep appreciation for being honored with the 2011 Michael Fry Research Award. These are not the easiest times for junior investigators or those who are considering a career in the research. This award serves as a great motivation for young investigators to continue with their fascinating work in radiation research. One of the highlights since my nomination was an e-mail from Dr. Fry with his congratulations. The Radiation Research Society is one of a kind in this respect.
Acknowledgments
I would like to thank Martin Hauer-Jensen and the UAMS Office of Grants and Scientific Publications for reviewing this manuscript, and Viji Sridharan and Preeti Tripathi for performing much of the work in the laboratory. There is a wealth of articles related to the clinical and biological aspects of RIHD, and I apologize for not being able to cite all in this article. My work has been supported in part by the National Cancer Institute (CA148679), the American Cancer Society (RSG-10-125-01-CCE), and the Lance Armstrong Foundation (LAF06SY4).
References
- 1.Adams MJ, Lipsitz SR, Colan SD, Tarbell NJ, Treves ST, Diller L, et al. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–48. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Hancock SL, Vagelos RH, Lee BK, Schnittger I. Diastolic dysfunction after mediastinal irradiation. Am Heart J. 2005;150:977–82. doi: 10.1016/j.ahj.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists Collaborative Group. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Lancet. 2000;355:1757–70. [PubMed] [Google Scholar]
- 4.Hooning MJ, Aleman BM, van Rosmalen AJ, Kuenen MA, Klijn JG, van Leeuwen FE. Cause-specific mortality in long-term survivors of breast cancer: A 25-year follow-up study. Int J Radiat Oncol Biol Phys. 2006;64:1081–91. doi: 10.1016/j.ijrobp.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 5.McGale P, Darby SC, Hall P, Adolfsson J, Bengtsson NO, Bennet AM, et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol. 2011;100:167–75. doi: 10.1016/j.radonc.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Adams MJ, Hardenbergh PH, Constine LS, Lipshultz SE. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol. 2003;45:55–75. doi: 10.1016/s1040-8428(01)00227-x. [DOI] [PubMed] [Google Scholar]
- 7.Heidenreich PA, Kapoor JR. Radiation induced heart disease: systemic disorders in heart disease. Heart. 2009;95:252–8. doi: 10.1136/hrt.2008.149088. [DOI] [PubMed] [Google Scholar]
- 8.Kole TP, Aghayere O, Kwah J, Yorke ED, Goodman KA. Comparison of Heart and Coronary Artery Doses Associated with Intensity-Modulated Radiotherapy Versus Three-Dimensional Conformal Radiotherapy for Distal Esophageal Cancer. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2011.10.053. In press. [DOI] [PubMed] [Google Scholar]
- 9.Chera BS, Rodriguez C, Morris CG, Louis D, Yeung D, Li Z, et al. Dosimetric comparison of three different involved nodal irradiation techniques for stage II Hodgkin’s lymphoma patients: conventional radiotherapy, intensity-modulated radiotherapy, and three-dimensional proton radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75:1173–80. doi: 10.1016/j.ijrobp.2008.12.048. [DOI] [PubMed] [Google Scholar]
- 10.McGale P, Darby SC. Low doses of ionizing radiation and circulatory diseases: a systematic review of the published epidemiological evidence. Radiat Res. 2005;163:247–57. doi: 10.1667/rr3314. [DOI] [PubMed] [Google Scholar]
- 11.Tillman GF, Pawlicki T, Koong AC, Goodman KA. Preoperative versus postoperative radiotherapy for locally advanced gastro-esophageal junction and proximal gastric cancers: a comparison of normal tissue radiation doses. Dis Esophagus. 2008;21:437–44. doi: 10.1111/j.1442-2050.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 12.Weber DC, Peguret N, Dipasquale G, Cozzi L. Involved-node and involved-field volumetric modulated arc vs. fixed beam intensity-modulated radiotherapy for female patients with early-stage supra-diaphragmatic Hodgkin lymphoma: a comparative planning study. Int J Radiat Oncol Biol Phys. 2009;75:1578–86. doi: 10.1016/j.ijrobp.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Wu WC, Chan CL, Wong YW, Cuijpers JP. A study on the influence of breathing phases in intensity-modulated radiotherapy of lung tumours using four-dimensional CT. Br J Radiol. 2009;83:252–6. doi: 10.1259/bjr/33094251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–65. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 15.Haque R, Yood MU, Geiger AM, Kamineni A, Avila CC, Shi J, et al. Long-term safety of radiotherapy and breast cancer laterality in older survivors. Cancer Epidemiol Biomark Prev. 2011;20:2120–6. doi: 10.1158/1055-9965.EPI-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris EE, Correa C, Hwang WT, Liao J, Litt HI, Ferrari VA, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006;24:4100–6. doi: 10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 17.Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, et al. Studies of the mortality of atomic bomb survivors, report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res. 2011;177:229–43. doi: 10.1667/rr2629.1. [DOI] [PubMed] [Google Scholar]
- 18.Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950–1997. Radiat Res. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- 19.Adams MJ, Grant EJ, Kodama K, Shimizu Y, Kasagi F, Suyama A, et al. Radiation dose associated with renal failure mortality: a potential pathway to partially explain increased cardiovascular disease mortality observed after whole-body irradiation. Radiat Res. 2011;177:220–8. doi: 10.1667/rr2746.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Little MP, Tawn EJ, Tzoulaki I, Wakeford R, Hildebrandt G, Paris F, et al. A systematic review of epidemiological associations between low and moderate doses of ionizing radiation and late cardiovascular effects, and their possible mechanisms. Radiat Res. 2008;169:99–109. doi: 10.1667/RR1070.1. [DOI] [PubMed] [Google Scholar]
- 21.Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: Is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67:10–8. doi: 10.1016/j.ijrobp.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 22.Stewart AM, Kneale GW. A-bomb survivors: factors that may lead to a re-assessment of the radiation hazard. Int J Epidemiol. 2000;29:708–14. doi: 10.1093/ije/29.4.708. [DOI] [PubMed] [Google Scholar]
- 23.Azizova TV, Muirhead CR, Druzhinina MB, Grigoryeva ES, Vlasenko EV, Sumina MV, et al. Cardiovascular diseases in the cohort of workers first employed at Mayak PA in 1948–1958. Radiat Res. 2010;174:155–68. doi: 10.1667/RR1789.1. [DOI] [PubMed] [Google Scholar]
- 24.Hoel DG. Ionizing radiation and cardiovascular disease. Ann N Y Acad Sci. 2006;1076:309–17. doi: 10.1196/annals.1371.001. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu Y, Kodama K, Nishi N, Kasagi F, Suyama A, Soda M, et al. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950–2003. BMJ. 2010;340:b5349. doi: 10.1136/bmj.b5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fajardo LF, Stewart JR. Pathogenesis of radiation-induced myocardial fibrosis. Lab Invest. 1973;29:244–57. [PubMed] [Google Scholar]
- 27.Kruse JJ, Zurcher C, Strootman EG, Bart CI, Schlagwein N, Leer JW, et al. Structural changes in the auricles of the rat heart after local ionizing irradiation. Radiother Oncol. 2001;58:303–11. doi: 10.1016/s0167-8140(00)00327-3. [DOI] [PubMed] [Google Scholar]
- 28.Labudova O, Hardmeier R, Rink H, Lubec G. The transcription of the XRCC1 gene in the heart of radiation-resistant and radiation-sensitive mice after ionizing irradiation. Pediatr Res. 1997;41:435–9. doi: 10.1203/00006450-199703000-00022. [DOI] [PubMed] [Google Scholar]
- 29.Lauk S, Kiszel Z, Buschmann J, Trott KR. Radiation-induced heart disease in rats. Int J Radiat Oncol Biol Phys. 1985;11:801–8. doi: 10.1016/0360-3016(85)90314-1. [DOI] [PubMed] [Google Scholar]
- 30.McChesney SL, Gillette EL, Powers BE. Radiation-induced cardiomyopathy in the dog. Radiat Res. 1988;113:120–32. [PubMed] [Google Scholar]
- 31.Gold H. Atherosclerosis in the rat. Effect of x-ray and a high fat diet Proc Soc Exp Biol Med. 1962;111:593–5. doi: 10.3181/00379727-111-27865. [DOI] [PubMed] [Google Scholar]
- 32.Lauk S, Trott KR. Radiation induced heart disease in hypertensive rats. Int J Radiat Oncol Biol Phys. 1988;14:109–14. doi: 10.1016/0360-3016(88)90058-2. [DOI] [PubMed] [Google Scholar]
- 33.Brosius FC, Waller BF, Roberts WC. Radiation heart disease. Analysis of 16 young (aged 15 to 33 years) necropsy patients who received over 3,500 rads to the heart. Am J Med. 1981;70:519–30. doi: 10.1016/0002-9343(81)90574-x. [DOI] [PubMed] [Google Scholar]
- 34.Hamza A, Tunick PA, Kronzon I. Echocardiographic manifestations of complications of radiation therapy. Echocardiography. 2009;26:724–8. doi: 10.1111/j.1540-8175.2008.00878.x. [DOI] [PubMed] [Google Scholar]
- 35.Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol. 1996;27:766–73. doi: 10.1016/s0046-8177(96)90447-5. [DOI] [PubMed] [Google Scholar]
- 36.Boerma M, Zurcher C, Esveldt I, Schutte-Bart CI, Wondergem J. Histopathology of ventricles, coronary arteries and mast cell accumulation in transverse and longitudinal sections of the rat heart after irradiation. Oncol Rep. 2004;12:213–9. doi: 10.3892/or.12.2.213. [DOI] [PubMed] [Google Scholar]
- 37.Tsujimura T, Hirota S, Nomura S, Niwa Y, Yamazaki M, Tono T, et al. Characterization of Ws mutant allele of rats: a 12-base deletion in tyrosine kinase domain of c-kit gene. Blood. 1991;78:1942–6. [PubMed] [Google Scholar]
- 38.Boerma M, Wang J, Wondergem J, Joseph J, Qiu X, Kennedy RH, et al. Influence of mast cells on structural and functional manifestations of radiation-induced heart disease. Cancer Res. 2005;65:3100–7. doi: 10.1158/0008-5472.CAN-04-4333. [DOI] [PubMed] [Google Scholar]
- 39.Koike MK, de Carvalho FC, de Lourdes HM. Bradykinin B2 receptor antagonism attenuates inflammation, mast cell infiltration and fibrosis in remote myocardium after infarction in rats. Clin Exp Pharmacol Physiol. 2005;32:1131–6. doi: 10.1111/j.1440-1681.2005.04309.x. [DOI] [PubMed] [Google Scholar]
- 40.Sharma JN. Cardiovascular activities of the bradykinin system. Scientific World Journal. 2008;8:384–93. doi: 10.1100/tsw.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeh CH, Chen TP, Wang YC, Lin YM, Fang SW. Cardiomyocytic apoptosis limited by bradykinin via restoration of nitric oxide after cardioplegic arrest. J Surg Res. 2010;163:e1–e9. doi: 10.1016/j.jss.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Yin H, Chao L, Chao J. Nitric oxide mediates cardiac protection of tissue kallikrein by reducing inflammation and ventricular remodeling after myocardial ischemia/reperfusion. Life Sci. 2008;82:156–65. doi: 10.1016/j.lfs.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coffman LG, Brown JC, Johnson DA, Parthasarathy N, D’Agostino RB, Jr, Lively MO, et al. Cleavage of high-molecular-weight kininogen by elastase and tryptase is inhibited by ferritin. Am J Physiol Lung Cell Mol Physiol. 2008;294:L505–L515. doi: 10.1152/ajplung.00347.2007. [DOI] [PubMed] [Google Scholar]
- 44.Imamura T, Dubin A, Moore W, Tanaka R, Travis J. Induction of vascular permeability enhancement by human tryptase: dependence on activation of prekallikrein and direct release of bradykinin from kininogens. Lab Invest. 1996;74:861–70. [PubMed] [Google Scholar]
- 45.Damas J, Adam A. Congenital deficiency in plasma kallikrein and kininogens in the brown Norway rat. Experientia. 1980;36:586–7. doi: 10.1007/BF01965817. [DOI] [PubMed] [Google Scholar]
- 46.Kaschina E, Stoll M, Sommerfeld M, Steckelings UM, Kreutz R, Unger T. Genetic kininogen deficiency contributes to aortic aneurysm formation but not to atherosclerosis. Physiol Genomics. 2004;19:41–9. doi: 10.1152/physiolgenomics.00035.2004. [DOI] [PubMed] [Google Scholar]
- 47.Maurer M, Wedemeyer J, Metz M, Piliponsky AM, Weller K, Chatterjea D, et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432:512–6. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- 48.Boerma M, Wang J, Kulkarni A, Roberto KA, Qiu X, Kennedy RH, et al. Influence of endothelin-1 receptor inhibition on functional, structural and molecular changes in the rat heart after irradiation. Radiat Res. 2008;170:275–83. doi: 10.1667/RR1093.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clozel M, Gray GA, Breu V, Loffler BM, Osterwalder R. The endothelin ETB receptor mediates both vasodilation and vasoconstriction in vivo. Biochem Biophys Res Commun. 1992;186:867–73. doi: 10.1016/0006-291x(92)90826-7. [DOI] [PubMed] [Google Scholar]
- 50.Boerma M, Roberto KA, Hauer-Jensen M. Prevention and treatment of functional and structural radiation injury in the rat heart by pentoxifylline and alpha-tocopherol. Int J Radiat Oncol Biol Phys. 2008;72:170–7. doi: 10.1016/j.ijrobp.2008.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu H, Xiong M, Xia YF, Cui NJ, Lu RB, Deng L, et al. Studies on pentoxifylline and tocopherol combination for radiation-induced heart disease in rats. Int J Radiat Oncol Biol Phys. 2009;73:1552–9. doi: 10.1016/j.ijrobp.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Verhaegen F, Granton P, Tryggestad E. Small animal radiotherapy research platforms. Phys Med Biol. 2011;56:R55–R83. doi: 10.1088/0031-9155/56/12/R01. [DOI] [PubMed] [Google Scholar]
- 53.Bell RM, Mocanu MM, Yellon DM. Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. J Mol Cell Cardiol. 2011;50:940–50. doi: 10.1016/j.yjmcc.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 54.Wondergem J, van der Laarse A, Van Ravels FJ, van Wermeskerken AM, Verhoeve HR, de Graaf BW, et al. In vitro assessment of cardiac performance after irradiation using an isolated working rat heart preparation. Int J Radiat Biol. 1991;59:1053–68. doi: 10.1080/09553009114550931. [DOI] [PubMed] [Google Scholar]
- 55.Goergen CJ, Sosnovik DE. From molecules to myofibers: multiscale imaging of the myocardium. J Cardiovasc Transl Res. 2011;4:493–503. doi: 10.1007/s12265-011-9284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kagadis GC, Loudos G, Katsanos K, Langer SG, Nikiforidis GC. In vivo small animal imaging: current status and future prospects. Med Phys. 2010;37:6421–42. doi: 10.1118/1.3515456. [DOI] [PubMed] [Google Scholar]
- 57.Seemann I, Gabriels K, Visser NL, Hoving S, te Poele JA, Pol JF, et al. Irradiation induced modest changes in murine cardiac function despite progressive structural damage to the myocardium and microvasculature. Radiother Oncol. 2012;103:143–50. doi: 10.1016/j.radonc.2011.10.011. [DOI] [PubMed] [Google Scholar]