Abstract
Diabetic neuropathy is associated with functional and morphological changes of the neuromuscular junction (NMJ) associated with muscle weakness. This study examines the effect of type 1 diabetes on NMJ function. Swiss Webster mice were made diabetic with three interdaily ip injections of streptozotocin (STZ). Mice were severely hyperglycemic within 7 days after the STZ treatment began. Whereas performance of mice on a rotating rod remained normal, the twitch tension response of the isolated extensor digitorum longus to nerve stimulation was reduced significantly at 4 wk after the onset of STZ-induced hyperglycemia. This mechanical alteration was associated with increased amplitude and prolonged duration of miniature end-plate currents (mEPCs). Prolongation of mEPCs was not due to expression of the embryonic acetylcholine receptor but to reduced muscle expression of acetylcholine esterase (AChE). Greater sensitivity of mEPC decay time to the selective butyrylcholinesterase (BChE) inhibitor PEC suggests that muscle attempts to compensate for reduced AChE levels by increasing expression of BChE. These alterations of AChE are attributed to STZ-induced hyperglycemia since similar mEPC prolongation and reduced AChE expression were found for db/db mice. The reduction of muscle end-plate AChE activity early during the onset of STZ-induced hyperglycemia may contribute to endplate pathology and subsequent muscle weakness during diabetes.
Keywords: butyrylcholinesterase, streptozotocin, type 1 diabetes
hyperglycemia associated with diabetes mellitus produces long-term damage and failure of various tissues (10). In particular, diabetes-induced neural damage is a predominant form of neuropathy in the Western world. Changes of neuromuscular transmission would contribute to the progressive weakness of extensor and flexor muscles during diabetes (4). Therefore, the goal of this study was to further explore the effects of diabetes on the neuromuscular junction (NMJ).
A limited number of studies have examined the impact of experimental diabetic neuropathy on pre- and postsynaptic elements of the NMJ. For example, type 1 diabetic rodents have decreased numbers of acetylcholine (ACh)-containing vesicles as well as degeneration of mitochondria within motor nerve endings. These changes are associated with reduced chemical transmission across the NMJ (17, 18, 33). At the postsynaptic level, the pattern of ACh receptor (AChR) distribution is altered, and discrete AChR islands form on the muscle end-plate surface (43). These experimental studies are clinically significant since muscle end-plate remodeling also occurs during human diabetes (49, 56, 57).
In this study, we examined muscles from mice at various times after the onset of streptozotocin (STZ)-induced hyperglycemia. This broad-spectrum antibiotic is toxic to pancreatic β-cells and produces an experimental model of type 1 diabetes (28). Recently, we reported (67) electrophysiological alterations of the NMJ of mice with STZ-induced type 1 diabetes. In particular, miniature end-plate current (mEPC) amplitude increased significantly at early times after the onset of STZ-induced hyperglycemia. Further analyses demonstrated a significant prolongation of end-plate current decay time. The goal of the present study was to understand the molecular basis and functional relevance of these diabetes-induced changes.
Our data demonstrate that the increase in mEPC amplitude and decay time for the fast-twitch extensor digitorum longus (EDL) muscle of STZ-treated mice is due primarily to a decline of endplate acetylcholinesterase (AChE) expression and activity. These data suggest that plasticity of the NMJ allows remodeling of this synapse in an attempt to adapt to hyperglycemia/hypoinsulinemia-induced suppression of ACh release from motor nerve endings. Although loss of AChE activity could cause muscle weakness (30), the initial alteration of this enzyme during diabetes does not impair motor performance in the whole animal. However, there is a gradual loss of muscle twitch tension along with a reduction in the number of functional motor units (66). Therefore, we conclude that diabetes-induced loss of end-plate AChE activity initiates a sequence of pathophysiological changes in the muscle end-plate that could lead to muscle weakness.
MATERIALS AND METHODS
Mice.
All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experimental protocols were approved by the University of Medicine and Dentistry of New Jersey Institutional Animal Care and Use Committee. Adult male Swiss Webster (SW) mice were purchased from Taconic Farms and housed in our institutional animal care facility. Mice weighing 25–30 g received three interdaily intraperitoneal (ip) injections of STZ (Sigma-Aldrich, St. Louis, MO) according to the following schedule: day 1, 100 mg/kg; day 3, 60 mg/kg; day 5, 60 mg/kg. Control mice received citrate buffer (ip), following the same schedule. Weekly blood glucose levels were monitored with a commercial glucometer (One Touch Ultra). Male db/db mice, a model of type 2 diabetes that harbors a spontaneous mutation in the leptin receptor, as well as control C57BL/6 mice, were purchased from The Jackson Laboratory (Bar Harbor, ME). They were euthanized at 5–6 wk of age. All rodents were housed in a pathogen-free environment with continuous access to food and water on a 12:12-h light-dark schedule.
Rotorod analysis.
Mice were trained to walk on a horizontal rod rotating at 4 rpm (Rotamex; Columbus Instruments, Columbus, OH). This initial phase of training lasted 3 days and involved three trials of 5 min/trial. On day 4, the rotation rate was progressively increased by 4 rpm every 40 s until a maximum velocity of 40 rpm was reached or the mouse fell from the rod. Mice continued training for an additional 3 days under these conditions. The rotation velocity at the moment mice fell from the rotating rod was recorded in control and diabetic mice at 1, 2, and 4 wk after the onset of hyperglycemia. To estimate end rotorod velocity, mice were subjected to three trials/wk three times/day, and the average terminal velocity was calculated.
In vivo electrodiagnostic studies.
Mice were anesthetized with an ip injection of anesthetic containing ketamine (80 mg/kg)-xylazine (10 mg/kg). After the abdomen and distal hindlimbs were shaved, mice were taped prone to a polystyrene foam board. Skin temperature was maintained at 32°C with a heating pad. The stimulating electrodes were 0.7-mm needles insulated with Teflon (Dantec sensory needle; Dantec, Skovlunde, Denmark). The cathode was placed close to the sciatic nerve at the proximal thigh, and the anode was placed subcutaneously 1 cm proximal to the anode. Motor responses were recorded from a ring electrode (Hush micro digital rings; Alpine Biomed, Fountain Valley, CA) that was placed circumferentially around the hindlimb at 1–1.5 cm from the stimulating electrode. The reference ring electrode was placed circumferentially around the hindlimb, 2 cm distal to the recording electrode; electrical activity was recorded in both flexor and extensor compartments of both hindlimbs. Nerve stimuli were monophasic pulses delivered from a Medtronic Keypoint (Medtronic, Minneapolis, MN) through a constant current stimulator with fine intensity control. Recordings were acquired with Medtronic Keypoint electromyography amplifiers (500 Hz/5 kHz) and stored for subsequent analysis. For all studies, the position of the stimulating electrode was optimized so that the threshold for evoking a motor response was <0.7 mA. Stimulus intensity was increased to produce maximal compound muscle action potential (CMAP) amplitude. Integration of the maximal CMAP record indicated the maximum CMAP area. Recording of amplitude (peak-peak) and distal incremental motor unit number estimate (MUNE) was then performed at a standard amplifier gain, using a modification of the technique described previously by McComas et al. (48) and used by Shefner (65) in mice. Briefly, at a stimulation rate of 1 s, the stimulus intensity was slowly increased from subthreshold levels until a small all-or-none response was evoked. The response was digitally recorded after its stability was established by three to four identical repeats. The intensity was slowly augmented until the response increased in a quantal fashion. The increased response was again monitored for stability before a tracing was acquired for analysis. This process was repeated for a total of 10 increments. Individual motor unit area was determined by subtracting the CMAP area of each response from that of the prior response. The average of the 10 individual values yielded an estimate of average single motor unit action potential area. The area of the maximum CMAP was divided by the preceding value to yield the MUNE.
In vitro evaluation of muscle performance.
The fast-twitch EDL nerve muscle preparation was dissected and mounted in a glass chamber (Rodnoti Glass Technology, Monrovia, CA) filled with oxygenated (95% O2-5% CO2) normal Ringer solution (pH 7.4, room temperature) containing (in mM) 135 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 1 Na2HPO4, 15 NaHCO3, and 5.5 glucose.
The EDL nerve was drawn into a suction electrode for indirect activation of muscle twitches. One tendon of the muscle was tied to a Grass Force transducer connected to a Digidata 1440A (Axon Instruments; Molecular Devices, Sunnyvale, CA). This permitted acquisition and analysis of muscle mechanical responses to nerve stimulation with PCLAMP software (Axon Instruments). Isolated preparations were adjusted to optimal length for force generation and equilibrated for 15 min prior to supramaximal nerve stimulation (1 Hz).
Physiological recording at NMJs.
The EDL muscle was removed from isoflurane-anesthetized mice and pinned to a Sylgard-lined chamber containing HEPES-Ringer (HR) solution (135 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 5.5 mM dextrose, and 5 mM HEPES; pH 7.3–7.4). Preparations were bathed in HR solution containing 0.75 μM μ-conotoxin GIIIB to inhibit muscle action potentials and mechanical responses to nerve stimulation. mEPCs and end-plate currents (EPCs; 1 Hz) were recorded at a holding potential of −75 mV with the two-electrode voltage clamp technique (Axoclamp 2B; Axon Instruments, Foster City, CA). PCLAMP software (version 9.2; Axon Instruments) was used for acquisition and analyses of EPCs. Voltage clamp recording minimized concerns introduced by diabetes-induced changes of muscle fiber size, membrane capacitance, or input resistance. EPCs were also recorded in the presence of 1 μM αA OIVA[K15N][N16K] from cone snail venom (71) or Waglerin-1 from snake venom (46) to pharmacologically dissect the contribution of embryonic and adult AChRs to the currents (72). Some muscles were exposed to either 5 μM phenserine tartrate (PT) or 5 μM phenethylcymserine tartrate (PEC), which are selective inhibitors of AChE and butyrylcholinesterase (BChE), respectively. PT and PEC were kindly donated by Dr. Nigel H. Greig (National Institute on Aging/NIH, Baltimore, MD).
The sensitivity of the motor end plate to iontophoretically applied ACh was evaluated in the Triangularis sterni (TS) muscle, in which end plates are readily observed (31, 45). Square pulses through a pipette containing 3 M ACh and having a resistance of 200 MOhms delivered ACh onto muscle fibers; end-plate regions were identified by the presence of miniature end-plate potentials. To prevent ACh leakage and undesirable desensitization of the AChR, a constant breaking current of 10–30 nA was applied to the pipette tip. ACh sensitivity was calculated as the ratio of membrane potential response to nCoul of charge passed through the ACh pipette (46).
Quantitative reverse transcription PCR.
RNA was isolated from the EDL muscle using Qiagen RNeasy Fibrous Tissue Mini Kit reagents (Qiagen, Valencia, CA). One microgram of RNA was converted to cDNA with the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen, Grand Island, NY) according to the manufacturer's instructions. Primers to amplify AChE (Mm 00477275_m1), AChR γ-subunit (Mm00437417_m1), and the housekeeping gene GAPDH (Mm99999915_g1) were purchased from Applied Biosystems. AChE probes were considered to detect all mRNA isoforms since they bind to sequences encoding the esterase domain of the enzyme. Real-time PCR was performed with the Taq PCR Master Mix Kit (Qiagen). Each sample was run in duplicate using the Light Cycler II (Roche) programmed with a denaturation step at 95°C, followed by 35 cycles of 15 s at 95°C and a final 60-s extension at 60°C. Relative expression was calculated by the “ΔΔCT method” (42).
Muscle end-plate imaging.
Motor end plates of EDL muscles were equilibrated for 30 min with 5 μM Alexa-647 αA OIVA[K15N][N16K], which binds selectively to embryonic AChRs (71). Because of the technical difficulty of dissecting EDL muscles from mouse pups, diaphragm muscle from 4-day-old mice was stained as positive control for Alexa-647 αA OIVA[K15N][N16K]. After a 20-min wash in toxin-free physiological saline, preparations were visualized using an upright fluorescence microscope (Olympus BX61WI). The presence of end plates was confirmed by a subsequent labeling of all AChRs with 1 μM FITC α-bungarotoxin for 30 min. Microscopic images were acquired and analyzed with Metamorph Software (Molecular Devices, Downingtown, PA).
EDL muscles were stained for AChE with the method of Koelle and Horn (35), as modified by Gautron (22). Acetylthiocholine acid was the substrate for the staining reaction. AChE labeling was performed at room temperature. After a 30-min reaction period, preparations were washed with physiological saline. Images of randomly selected end plates were collected immediately using an upright microscope (Olympus BX61WI).
AChE activity.
EDL muscles were dissected, weighed, and homogenized in 9 volumes of 100 mM sodium phosphate buffer (pH 7.4). AChE activity was measured by the Ellman reaction at 24°C in a Shimadzu UV2550 Spectrophotometer (Kyoto, Japan), as described by Sultatos and Kaushik (70). The reaction volume in the cuvette was 1 ml and contained 0.44 mM acetylthiocholine and 0.1 mM 5,5′-dithio-bis(2-nitrobenzoic acid). The reaction was initiated by the addition of 50 μl of homogenate, and the increase in optical density at 412 nm (which was an indicator of thiocholine production) was followed for 10 min. The slopes of these rate curves were determined by linear regression analyses using Sigmaplot 8 (Systat Software, Chicago, IL).
Statistics.
Biochemical and electrophysiological data were analyzed using GraphPad Prism Software. Data are presented as means ± SE. One-way ANOVA with Tukey post hoc test compared experimental and control mean values; P < 0.05 indicated significant difference.
RESULTS
Glucose levels.
One week after STZ treatment, SW diabetic mice exhibited severe hyperglycemia; that is, plasma glucose was 130 ± 22.2 mg/dl for control mice and >450 mg/dl (maximum reading of the commercial glucometer) for mice having STZ-induced diabetes. Weekly tests indicated that hyperglycemia was maintained throughout the time course of the study. Additionally, the average blood glucose level in 5- to 6-wk-old db/db mice (217.3 ± 11.9 mg/dl) was significantly (P < 0.01) greater than that of wild-type C57BL/6 mice (157.5 ± 4.7 mg/dl).
Body weight did not differ significantly for the control (38.25 ± 1.61 g) or the 4-wk diabetic (37.25 ± 0.92 g) groups of SW mice. Similarly, EDL muscle weight for hyperglycemic (11.9 ± 0.4 mg) and control (10.9 ± 0.8 mg) mice was the same at this early stage of diabetes. Thus, muscle atrophy was not detected at this early time of the STZ-induced diabetes.
STZ-induced diabetes did not alter rotarod performance at a time when twitch tension and MUNE were reduced.
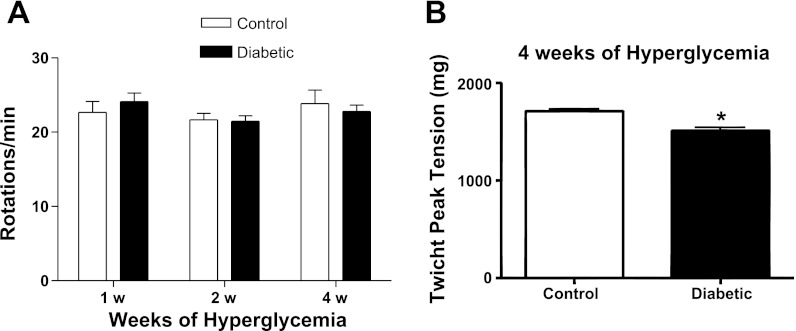
Figure 1A summarizes the performance of SW mice on a rotating rod. The velocity at which mice fell from the rod when the speed was increased by 4 rpm every 40 s was equivalent to control for mice at 2 and 4 wk after the onset of STZ-induced hyperglycemia. However, maximal neurally evoked twitch tension of the EDL muscle declined significantly to 1,514 ± 23.16 g from the control value of 1,711 ± 24.5 g at the 4-wk time point (Fig. 1B) when MUNE also had declined to 187.2 ± 10.6 from the control value of 298.9 ± 8.55 (67).
Fig. 1.
Rotorod performance was unaltered after 4 wk of hyperglycemia, although twitch tension of the extensor digitorum longus (EDL) muscle decreased significantly. A: rotorod performance was evaluated with an incremental velocity protocol. The end velocity at which mice remained on the rotorod did not significantly change at 1, 2, or 4 wk after the onset of hyperglycemia. B: twitch tension of the EDL muscle was reduced at 4 wk after the onset of hyperglycemia. *P < 0.05. Bars represent the mean + SE obtained from 10 mice.
STZ-induced diabetes increases amplitude and decay time of end-plate currents.
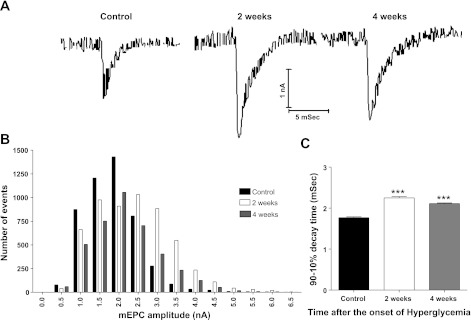
The representative mEPCs of Fig. 2A suggest that amplitude and decay time of mEPCs increase at 2 and 4 wk after the onset of STZ-induced hyperglycemia. Statistical analysis (Fig. 2B) confirmed this suggestion; that is, the amplitude distribution of the mEPCs shifted to the right to give a mean mEPC amplitude of 2.4 ± 0.01 and 2.1 ± 0.01 nA at 2 and 4 wk, respectively, after the onset of hyperglycemia; these values were significantly (P < 0.01) greater than the control mEPC amplitude of 1.9 ± 0.01 nA. Similarly, the mean 90 to 10% decay time for mEPCs increased to 2.2 ± 0.03 and 2.1 ± 0.02 ms at 2 and 4 wk, respectively, after the onset of hyperglycemia; these values (Fig. 2C) were significantly (P < 0.01) greater than the control value of 1.7 ± 0.02 ms.
Fig. 2.
Miniature end-plate current (mEPC) 90–10% decay time and amplitude increased for EDL muscles removed from diabetic mice at 2 and 4 wk after the onset of hyperglycemia. A: representative mEPCs recorded at end plates of control as well as mice having 2 or 4 wk of streptozotocin (STZ)-induced hyperglycemia. These records illustrate the increased decay time and amplitude of mEPCs during diabetes. B: representative histograms of mEPC amplitude distribution showing a shift to larger amplitudes at 2- (open bars) and 4-wk (gray bars) diabetic neuromuscular junctions (NMJs) compared with controls (black bars). C: mEPC 90–10% decay time is significantly (***P < 0.01) longer than control at 2 and 4 wk after the onset of STZ-induced hyperglycemia. Each bar represents the mean + SE obtained from 4 to 5 EDL fibers for each of 4 mice (40–200 mEPCs/fiber).
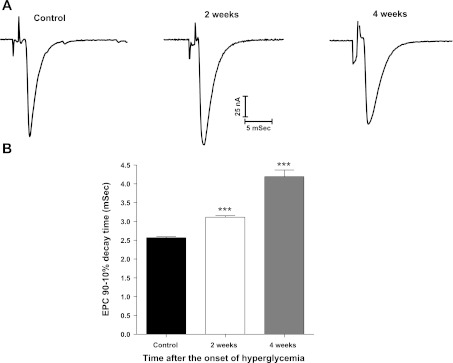
Decay time of neurally evoked EPCs (Fig. 3A) also increased in response to diabetes; that is, 90 to 10% decay time of EPCs increased significantly (P < 0.001) from the control value of 2.6 ± 0.02 ms to 3.1 ± 0.04 and 4.2 ± 0.18 ms at 2 and 4 wk, respectively, after onset of STZ-induced hyperglycemia (Fig. 3B). EPCs were not studied further because stimulus-evoked transmitter release declines progressively during diabetes (67).
Fig. 3.
End-plate current (EPC) 90–10% decay time is prolonged for EDL muscles removed from diabetic mice. A: representative EPCs recorded for control as well as mice having 2 or 4 wk of STZ-induced hyperglycemia. B: EPC 90–10% decay time is significantly (***P < 0.01) greater than control at 2 and 4 wk after the onset of STZ-induced hyperglycemia. Bars represent the mean + SE obtained from 4 to 5 EDL fibers of 4 mice (10–11 EPCs/fiber).
Alterations in time course and amplitude of mEPCs at the onset of the diabetic state are postsynaptic in origin.
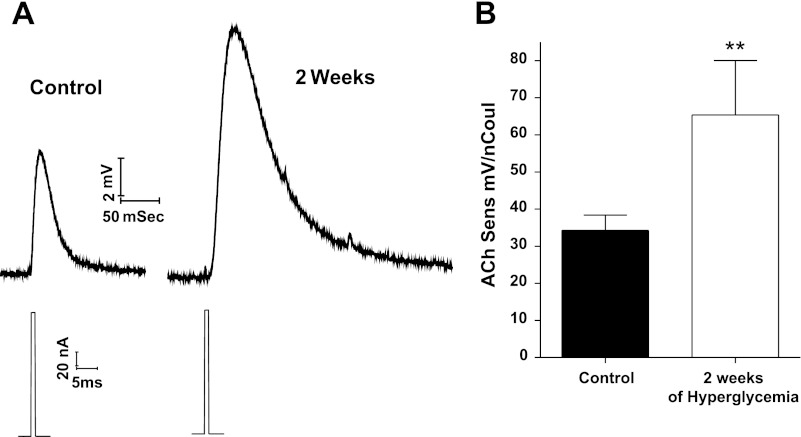
To exclude diabetes-induced presynaptic alterations to the increase in mEPC amplitude and decay time, we tested the response of motor end plates to iontophoretically applied ACh. The representative end-plate responses of Fig. 4A show that both peak amplitude and decay time of the end-plate response to exogenous ACh increased at 2 wk after the induction of hyperglycemia; mean ACh sensitivity increased from the control value of 34.3 ± 4.1 to 65.4 ± 14.6 mV/nCoul (P < 0.05; Fig. 4B). We were unable to detect membrane potential changes when ACh was applied iontophoretically to the non-end plate membrane.
Fig. 4.
End-plate sensitivity to iontophoretically applied acetylcholine (ACh) increased for muscle end plates of mice at 2 wk after the onset of STZ-induced hyperglycemia. A: representative recordings of iontophoretically evoked ACh potentials recorded at end plates of the Triangularis sterni muscle of control mice and mice with 2 wk of STZ-induced hyperglycemia. For each recording, the top trace is the end-plate potential response and the bottom trace the current injected through the iontophoretic pipette containing 3 M ACh. These records illustrate the increased amplitude and prolonged decay time of the ACh-induced potential. B: average ACh sensitivity increased at 2 wk after onset of STZ-induced hyperglycemia. ACh sensitivity was calculated as the ratio of membrane potential response to nCoul of charge passed through the ACh pipette. Bars represent ACh sensitivity (mean + SE) obtained from 14 to 19 recordings from different end plates in 3 muscles from the control and the hyperglycemic groups. **P < 0.05.
STZ-induced diabetes reduces expression and activity of AChE at the NMJ of 4-wk diabetic animals.
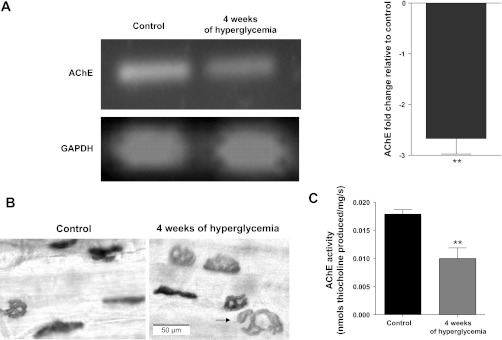
Semiquantitative PCR indicated a 2.8-fold downregulation of transcripts encoding for AChE in the EDL muscles of 4-wk diabetic mice (Fig. 5A). Likewise, histochemical staining suggested that end-plate AChE reaction product was reduced in both size and intensity for the EDL muscle of 4-wk diabetic mice (Fig. 5B). In agreement with this qualitative histochemical observation, biochemical evaluation demonstrated that EDL cholinesterase activity decreased significantly from a control value of 0.01785 ± 0.0008 to 0.009995 ± 0.001 nmol·mg−1·s−1 thiocholine produced at 4 wk after the onset of hyperglycemia (Fig. 5C).
Fig. 5.
Diabetes reduced acetylcholinesterase (AChE) expression and activity of the EDL muscle. A: expression of mRNA coding for AChE declines within 4 wk of STZ-induced hyperglycemia. Left: representative agarose gel showing the products for RT-PCR analysis of AChE and GAPDH. Right: histogram expressing the fold change of AChE mRNA expression relative to control and normalized to the housekeeping gene GAPDH. Relative expression was calculated by the “ΔΔCT method” (42) from real-time PCR data. The bar presents the mean of 6 different experiments. The error bar represents standard deviation. B: histochemical detection of AChE with the Koelle reaction for the end plate region of the EDL muscle of control and 4-wk diabetic mice. Incubation time was 30 min for all muscles. The reduced staining intensity and size for EDL of diabetic animals suggest decreased AChE activity. The black arrow within the image of the 4-wk diabetic end plate indicates discontinuity of AChE. C: biochemical determination of AChE activity in EDL muscle homogenates. The y-axis is AChE activity normalized to muscle weight (nmol·mg−1·s−1 thiocholine produced). Bars represent the mean + SE obtained for EDL muscles from 6 control and 9 diabetic mice. **P < 0.05.
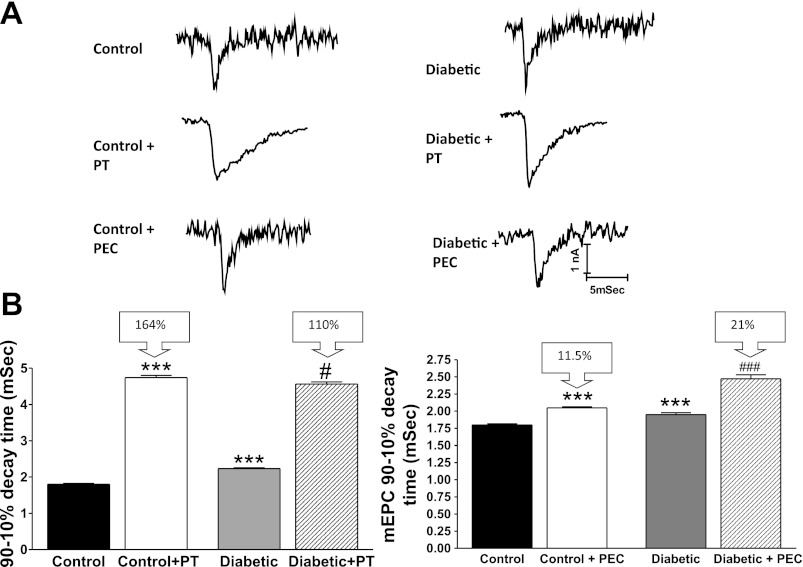
Consistent with partial AChE deficiency, mEPC decay time for end plates of diabetic mice became less sensitive to PT, a potent and selective inhibitor of AChE (27); that is, PT prolonged mEPC decay time by 4.75 ± 0.06 and 4.56 ± 0.06 ms for control and 4-wk diabetic EDL preparations, respectively (Fig. 6). Normalization of the decay time to the value prior to application of PT indicates that PT prolonged mEPC decay time by only 110% for the diabetic vs. 164% for control end plates. This observation suggests that AChE termination of ACh action at the end plate is reduced significantly during diabetes.
Fig. 6.
mEPC decay time for EDL muscles of 4-wk diabetic mice is less sensitive to phenserine tartrate (PT), a selective inhibitor of AChE, and more sensitive to phenethylcymserine tartrate (PEC), a selective inhibitor of butyrylcholinesterase. A: representative mEPCs recorded from EDL muscles of control and 4-wk diabetic mice before and after in vitro application of 5 μM PEC or 5 μM PT. B: histograms of mEPC decay time (mean ± SE) for EDL muscles of control and diabetic mice before and at 30 min after exposure to 5 μM PEC or 5 μM PT. PT significantly prolonged the 90–10% decay time for mEPCs of EDL muscle preparations from both control and diabetic mice. However, PT prolonged decay time by 164 and 110% for control and diabetic preparations, respectively. Additionally, prolongation of mEPC 90–10% decay time was more apparent for muscles of diabetic mice when PEC was used. Bars represent the mean + SE obtained from 4 to 5 end plates before and after PT or PEC for each of 4 muscles from different mice. ***P < 0.01 compared with control; #P < 0.05 for the difference in decay time between control + PT and diabetic + PT. ###P < 0.01 for the difference in decay time between control + PEC and diabetic + PEC.
STZ-induced diabetes increases end-plate BChE activity.
Since AChE expression and activity decline, we hypothesized that diabetic muscle may compensate by increasing the activity of BChE. To test this hypothesis, we examined the effect of a selective BChE inhibitor (PEC) on mEPC decay time. Representative mEPCs (Fig. 6A) suggest that PEC prolongs decay time for EDL muscles excised from both control and diabetic mice. Statistical analysis confirmed this suggestion (Fig. 6B); that is, PEC increased mEPC decay time for the normal EDL muscle from 1.8 ± 0.01 to 2.04 ± 0.01 ms. For the 4-wk diabetic EDL, PEC increased mEPC decay time from 2.0 ± 0.03 to 2.5 ± 0.05 ms. However, mEPC decay time was prolonged by 11.5 and 21% for the control and diabetic EDL preparations, respectively (Fig. 6B). These data suggest that diabetic muscle compensates for the decline of end-plate AChE by increasing BChE activity.
The prolonged decay time of mEPCs during diabetes is not due to functional expression of the embryonic AChR.
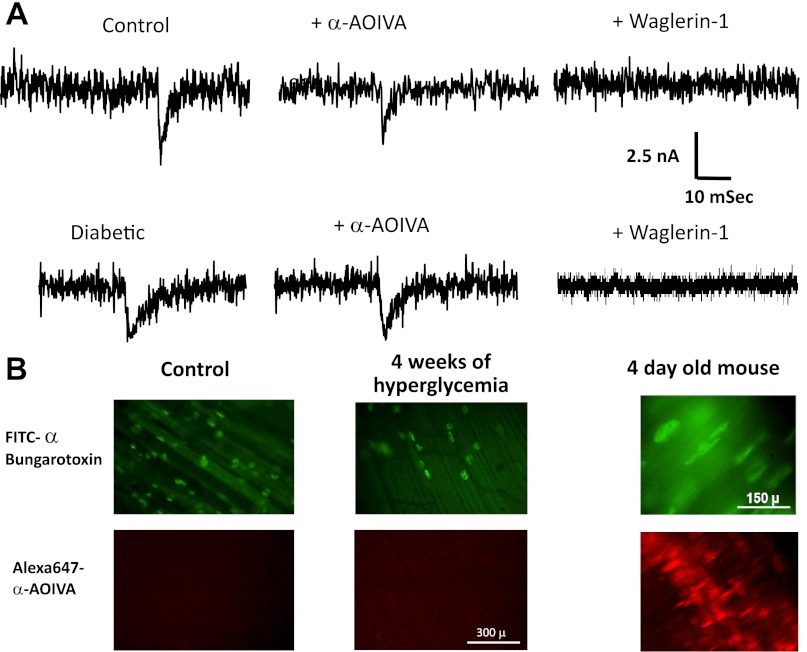
If skeletal muscle produced the γ-subunit-containing embryonic form of the AChR during diabetes, then this would contribute to prolonged mEPCs (59, 73). We tested for this possibility in two ways. First, we utilized toxins that selectively interact with the adult (Waglerin-1) or embryonic (αA OIVA) AChR (46, 71, 72). Figure 7A shows that 1 μM αA OIVA did not alter the amplitude or time course of mEPCs recorded in the EDL of either control or diabetic mice. In particular, diabetic mEPC amplitude and decay time before (2.1 ± 0.01 nA and 2.0 ± 0.03 ms) and after (2.1 ± 0.02 nA and 2.1 ± 0.03 ms) exposure to αA OIVA were not significantly different. In contrast, mEPCs were abolished for both control and diabetic EDL preparations bathed in 1 μM Waglerin-1. In agreement with these physiological observations, fluorescently labeled αA OIVA detected embryonic AChRs at NMJs of 4-day-old mice but not at end plates of diabetic mice (Fig. 7B); FITC α-bungarotoxin staining confirmed the presence of AChRs in these EDL preparations. Finally, we did not detect a significant difference in the level of transcripts coding for the AChR γ-subunit in control and 4-wk diabetic EDL muscles.
Fig. 7.
End plates in the EDL muscle of diabetic mice do not express immature ACh receptors (AChRs). A: representative records of mEPCs recorded from EDL muscles removed from control and 4-wk diabetic mice before and after exposure to Waglerin-1 or αA OIVA. Amplitude and decay times of mEPCs were not sensitive to 1 μM of the neonatal AChR antagonist αA OIVA. In contrast, 1 μM Waglerin-1 completely inhibited mEPCs in EDL muscles of both control and diabetic mice. B: end plates of EDL muscles of control and diabetic mice were not labeled with Alexa 647 αA OIVA applied with a protocol that strongly labeled immature AChRs at end plates of the diaphragm muscle of a 4-day-old mouse. The presence of end plates was evident in all of the preparations bathed in FITC α-bungarotoxin.
Prolonged mEPC decay time and AChE expression reduction result from hyperglycemia/hypoinsulinemia.
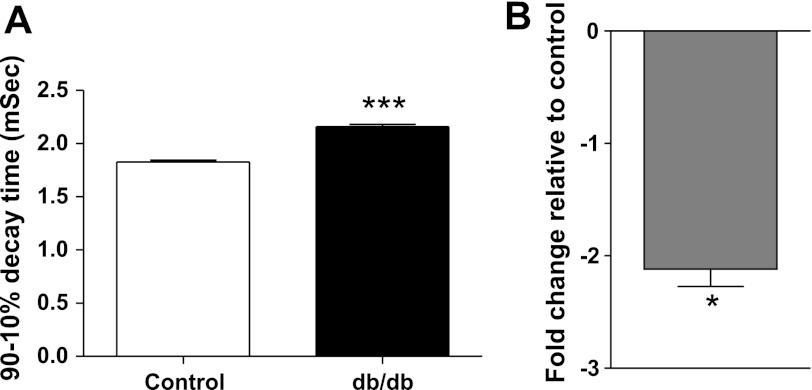
mEPC decay time and AChE expression were also evaluated in db/db mice that exhibit a congenital form of type 2 diabetes characterized by hyperglycemia and insulin resistance. This model of diabetes serves to control for any effects of STZ alone. As summarized in Fig. 8A, mEPC decay time in the EDL muscle of db/db mice (2.2 ± 0.02 ms) was significantly (P < 0.01) longer than mEPC decay time for EDL muscles of age-matched C57BL/6 background mice (1.8 ± 0.02 ms). The prolonged mEPC decay time for the db/db mice was associated with a 2.2-fold reduction of AChE expression (Fig. 8B).
Fig. 8.
EDL muscles of db/db mice have prolonged mEPCs and reduced AChE expression. A: mEPCs in the EDL of db/db mice have a significantly prolonged 90–10% decay time relative to that of control C57 mice. Each bar represents the mean + SE obtained from 4 to 5 fibers for 4 EDL muscles excised from control and db/db mice. ***P < 0.01. B: expression of mRNA coding for AChE also declines in EDL muscle of db/db mice. The histogram represents the fold change of AChE mRNA expression relative to control and normalized to the housekeeping gene GAPDH. Relative expression was calculated by the ΔΔCT method from real-time PCR data. The bar presents the mean of 5 different experiments. The error bar represents standard deviation. *P < 0.05.
DISCUSSION
Diabetic patients suffer from progressive peripheral neuropathy (58). Treatment of this debilitating comorbidity necessitates understanding of the cellular and molecular basis of diabetic neuropathy. Experimental models of diabetes facilitate such understanding. Although congenital animal models of diabetes are important, poor understanding of the impact of hyperglycemia/hypoinsulinemia on development complicates interpretation of the findings. In contrast, the STZ-induced model of diabetes offers several advantages. In particular, STZ treatment of normal adult mice with a standard protocol induces a reproducible model of type 1 diabetes. It is significant that we observed similar changes of muscle AChE expression and mEPC decay times during the hyperglycemia of STZ-treated SW as well as db/db mice.
Previous studies show that the NMJ of mice with STZ-induced diabetes undergoes dramatic changes of function and morphology (17, 19, 33, 43, 63, 67). The present study was stimulated by the need to understand the molecular basis for a postsynaptic alteration of neuromuscular transmission that could contribute to long-term weakness of skeletal muscles during diabetes. Specifically, the amplitude and decay time of mEPCs were significantly greater than control beginning at 2 wk after the onset of STZ-induced hyperglycemia. Since the amplitude and decay time of the end-plate response to ACh applied iontophoretically to the muscle were also increased at this time, the mEPC changes were attributed to diabetes-induced modifications of the postsynaptic apparatus.
Two postsynaptic alterations could account for the mEPC decay time and amplitude changes. First, diabetes might induce expression of embryonic AChRs that have a longer open time that accounts for the prolonged decay of mEPCs at developing and regenerating NMJs (6, 20, 51, 59, 73). In normally active mature muscle, expression of the adult AChR isoform containing the ε-subunit is dominant, and there are very low levels of γ-subunit transcripts (9, 75). Reexpression of the fetal AChR isoform (74) and prolongation of end-plate current decay occur after experimental denervation of adult skeletal muscle (6). In addition, expression of the γ-subunit has been reported for humans and animal models with neurogenic disorders (21, 36). However, muscle of diabetic mice with altered mEPCs did not express the γ-subunit. Furthermore, our iontophoretic technique failed to detect functional extrajunctional AChRs, and the diabetes-altered mEPCs remained resistant to αA OIVA, which selectively blocks the embryonic AChR (71). These observations indicate that expression of the embryonic AChR does not account for prolonged mEPCs during STZ-induced diabetes.
A second mechanism that would account for the mEPC changes is the reduction of the end-plate enzyme that inactivates ACh. In support of this hypothesis, we observed a significant decrease in AChE mRNA expression for EDL muscles in association with increased mEPC amplitude and decay time at 2 and 4 wk after STZ-induced hyperglycemia. At the same time, histochemical staining suggested a decline of end-plate AChE level, and biochemical assay of EDL homogenates showed a quantitatively significant reduction of AChE activity. To further test for changes of AChE as a determinant of mEPC properties, we compared the effects of PE, a selective inhibitor of AChE, on control and 4-wk diabetic mice. This comparison indicated that mEPCs of muscles from diabetic mice are significantly less prolonged by PE. Thus, AChE activity makes a smaller contribution to mEPC decay time during diabetes. Overall, these data substantiate the earlier suggestion (34) that AChE activity declines for skeletal muscle of rats with STZ-induced diabetes.
The decline of AChE activity may be a compensatory response of muscle to preserve functional neuromuscular transmission; that is, a primary motor neuron pathology that reduces stimulus-evoked ACh release during diabetes (12, 19, 33, 67) may initiate downregulation of muscle AChE expression. Thus, reduced AChE expression may be an early stage in diabetes-induced molecular disorganization of the muscle end plate (43). Conversely, the reduction of ACh release in response to nerve terminal excitation may be a compensatory response to transmitter accumulation at NMJs as a result of reduced AChE activity.
Related to the observed diabetes-induced reduction of AChE, it is important to note that AChE at the NMJ is regulated by the nerve-induced pattern of muscle activation (13, 25, 66). Specifically, AChE activity and mRNA levels of rat fast muscle are reduced after denervation, paralysis, or alteration of activity patterns (13, 32, 66). Although we did not specifically evaluate the EDL activity, an earlier study demonstrated that hindlimb compound muscle action potential amplitude values and motor strength were relatively spared in 4-wk diabetic mice (67). Furthermore, we failed to detect expression or function of embryonic AChRs in muscles with diabetes-induced reduction of AChE. Because extrajunctional embryonic AChRs are induced following muscle disuse, we hypothesize that diabetes-induced muscle inactivity is not responsible for the decline of AChE. In any case, our results indicate the need for cautious use of AChE inhibitors in diabetic patients, for example, in comorbidity with Alzheimer's disease, myasthenia gravis, or neuropathic pain treatment.
The collagen-tailed AChE is the predominant form of the enzyme at the NMJ (37, 38, 44). This form of AChE consists of three ColQ units that attach tetramers of AChE to the basal lamina. Among the molecules responsible for this interaction are perlecan, α-dystroglycan, and MuSK. In fact, a decrease in NMJ perlecan expression may reduce AChE in a mouse model of Schwartz-Jample syndrome (68). The integrity of the preceding molecules in diabetic neuropathy has not yet been investigated.
Previous studies show that diabetes changes AChE activity of a variety of tissues, including red blood cells (62, 69) and nervous tissue. For example, AChE activity increases for brain extracts of diabetic rodents (40, 62, 64, 76). The increased AChE activity has been proposed to decrease functional levels of ACh in critical brain structures and contribute to neurological dysfunction of diabetic patients and animals (8, 39). Conversely, other studies report a decrease in brain AChE activity during experimental diabetes (7, 14, 23, 55). The reduced metabolism of ACh would increase cholinergic transmission and contribute to behavioral disturbances. Impaired AChE activity has also been reported for diabetic retina (55, 61) and heart (14).
In addition to AChE, mammalian NMJs contain BChE (41, 50). Normally, hydrolysis of ACh by BChE is inefficient, and the enzyme may have only a minor impact on neuromuscular transmission. Individuals having mutations that totally inactivate BChE have no significant phenotype other than increased susceptibility to succinylcholine (54). However, BChE may significantly control transmission across some cholinergic synapses. For example, selective inhibition of BChE produced a 43% prolongation in the half-relaxation time of tracheal smooth muscle contractions (2). In Alzheimer's disease, there is a progressive increase in BChE activity as AChE declines (5). This accounts for the finding that selective BChE inhibitors (e.g., PEC) improve cognitive function in animal models of Alzheimer's disease (27). Furthermore, whereas BChE inhibitors are harmless to wild-type mice, they are fatal to AChE-null mice (16), where expression and activity of BChE is essential to survival (3). Further evidence supporting the functional significance of BChE is the prolongation of miniature end-plate potential decay for muscles of AChE-null mice exposed to the BChE inhibitor iso-OMPA (47). However, other investigators have not confirmed this effect of BChE inhibitors on muscle of AChE-null mice (24).
In the present study, we used the selective inhibitor PEC to pharmacologically test for changes in functional BChE activity. Whereas PEC prolonged the decay time of mEPCs for both control and diabetic end plates, the diabetic EDL was more sensitive. This difference implies increased BChE regulation of ACh concentration at NMJs of diabetic mice. Our finding is significant since other investigators have reported increased activity of BChE during diabetes. For example, BChE increased in serum and cardiac muscle of rats with aloxan-induced diabetes (15). Similarly, serum BChE activity is elevated in type 1 and type 2 diabetic patients (1). In contrast, BChE activity is decreased in retina and hippocampus of rats with STZ-induced diabetes (60). Further studies are needed to assess the tissue-specific expression of AChE and BChE during diabetes.
Besides reducing AChE activity, diabetes-induced morphological alterations might also contribute to mEPC alterations as well as decreased neurally evoked twitch tension by changing ACh diffusion along the synaptic cleft. However, we have not detected a significant change in motor end plate size in EDL muscles at 4 wk after the onset of STZ-induced hyperglycemia (67).
Together, our data suggest that impaired ACh metabolism could lead to diabetes-induced weakness of skeletal muscle, as demonstrated by the statistically significant decrease in neurally evoked twitch tension for the STZ diabetic mice. In fact, muscle weakness is a feature of two direct AChE deficit pathologies, i.e., human congenital end-plate AChE deficiency attributed to ColQ mutations (53) as well as irreversible organophosphate poisoning (29). In both cases, the safety factor for neuromuscular transmission is compromised. Specifically, the mEPC amplitude is a direct measurement of synaptic strength and is a critical component of the safety factor of neuromuscular transmission. Muscle weakness, delayed mEPC decay time, and decreased amplitude of the EPC could be the result of Ca2+ overload within the end plate cytosol (11, 26). Since endplate Ca2+ overload has been demonstrated in congenital slow-channel myasthenic syndromes due to different mutations of the AChR, the same pathophysiological mechanism may occur during AChE deficiency, where AChR activity and Ca2+ influx are presumably increased. Ca2+ overload would lead to further end plate degeneration as diabetes progresses. The apparent normal gross motor performance for the STZ-treated mice is likely due to compensatory mechanisms of motor neurons, other functional muscle groups, and/or upper motor centers. It is of interest that similar decreases in EDL in vitro twitch tension with preserved gross in vivo motor performance has been reported for mice lacking mitsugumin 29, a synaptophysin-related protein (52).
Our study of db/db mice implies that the reduction of end-plate AChE function is a feature of type 2 as well as type 1 diabetes. However, it remains to be determined whether reduced cholinesterase expression is due to hypoinsulinemia or hyperglycemia. Muscles from type 2 diabetic animals can be considered somewhat hypoinsulinemic since the insulin action in muscle is impaired due to insulin resistance. Nevertheless, the finding that AChE function declines prior to muscular atrophy or apparent motor dysfunction (67) implies that a therapeutic window exists for prevention of these components of diabetic neuropathy.
GRANTS
This work was supported by an Alfred P. Sloan Foundation Integrative Neuroscience Training Grant, the National Institutes of Health (Grant R01-NS-045979), the Kirby Foundation, and the Marie and Jerry Toohey Neuroscience Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.C.G., B.T., L.G.S., N.S., V.H.R., and J.J.M. did the conception and design of the research; C.C.G., J.G.P., K.H., B.T., L.G.S., and N.S. performed the experiments; C.C.G., J.G.P., K.H., and L.G.S. analyzed the data; C.C.G., L.G.S., N.S., and J.J.M. interpreted the results of the experiments; C.C.G. and J.G.P. prepared the figures; C.C.G. drafted the manuscript; C.C.G., N.S., V.H.R., and J.J.M. edited and revised the manuscript; C.C.G., J.G.P., K.H., B.T., L.G.S., N.S., V.H.R., and J.J.M. approved the final version of the manuscript.
ACKNOWLEDGMENTS
Present address of C. G. Garcia: Cátedra de Fisiopatología, Instituto de Medicina Experimental, Universidad Central de Venezuela, Caracas, Venezuela.
REFERENCES
- 1. Abbott CA, Mackness MI, Kumar S, Olukoga AO, Gordon C, Arrol S, Bhatnagar D, Boulton AJ, Durrington PN. Relationship between serum butyrylcholinesterase activity, hypertriglyceridaemia and insulin sensitivity in diabetes mellitus. Clin Sci (Lond) 85: 77–81, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Adler M, Filbert MG. Role of butyrylcholinesterase in canine tracheal smooth muscle function. FEBS Lett 267: 107–110, 1990 [DOI] [PubMed] [Google Scholar]
- 3. Adler M, Manley HA, Purcell AL, Deshpande SS, Hamilton TA, Kan RK, Oyler G, Lockridge O, Duysen EG, Sheridan RE. Reduced acetylcholine receptor density, morphological remodeling, and butyrylcholinesterase activity can sustain muscle function in acetylcholinesterase knockout mice. Muscle Nerve 30: 317–327, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Andersen H. Motor function in diabetic neuropathy. Acta Neurol Scand 100: 211–220, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Arendt T, Bruckner MK, Lange M, Bigl V. Changes in acetylcholinesterase and butyrylcholinesterase in Alzheimer's disease resemble embryonic development—a study of molecular forms. Neurochem Int 21: 381–396, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Argentieri TM, Aiken SP, Laxminarayan S, McArdle JJ. Characteristics of synaptic transmission in reinnervating rat skeletal muscle. Pflugers Arch 421: 256–261, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Ashokkumar N, Pari L, Ramkumar KM. N-Benzoyl-d-phenylalanine attenuates brain acetylcholinesterase in neonatal streptozotocin-diabetic rats. Basic Clin Pharmacol Toxicol 99: 246–250, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol 7: 184–190, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Buonanno A, Mudd J, Merlie JP. Isolation and characterization of the beta and epsilon subunit genes of mouse muscle acetylcholine receptor. J Biol Chem 264: 7611–7616, 1989 [PubMed] [Google Scholar]
- 10. Calcutt NA, Cooper ME, Kern TS, Schmidt AM. Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat Rev Drug Discov 8: 417–429, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chevessier F, Peter C, Mersdorf U, Girard E, Krejci E, McArdle JJ, Witzemann V. A new mouse model for the slow-channel congenital myasthenic syndrome induced by the AChR epsilonL221F mutation. Neurobiol Dis 45: 851–861, 2012 [DOI] [PubMed] [Google Scholar]
- 12. Constantini S, Schiller Y, Cohen AM, Rahamimoff R. Pathophysiology of the neuromuscular junction in diabetic rats. Isr J Med Sci 23: 101–106, 1987 [PubMed] [Google Scholar]
- 13. Cresnar B, Crne-Finderle N, Breskvar K, Sketelj J. Neural regulation of muscle acetylcholinesterase is exerted on the level of its mRNA. J Neurosci Res 38: 294–299, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Dash NK, Azam M, Gupta G, Baquer NZ. Effect of hyperglycemia on acetylcholinesterase and catecholamine levels in rat brain and heart. Biochem Int 23: 261–269, 1991 [PubMed] [Google Scholar]
- 15. Dave KR, Katyare SS. Effect of alloxan-induced diabetes on serum and cardiac butyrylcholinesterases in the rat. J Endocrinol 175: 241–250, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Duysen EG, Li B, Xie W, Schopfer LM, Anderson RS, Broomfield CA, Lockridge O. Evidence for nonacetylcholinesterase targets of organophosphorus nerve agent: supersensitivity of acetylcholinesterase knockout mouse to VX lethality. J Pharmacol Exp Ther 299: 528–535, 2001 [PubMed] [Google Scholar]
- 17. Fahim MA, el-Sabban F, Davidson N. Muscle contractility decrement and correlated morphology during the pathogenesis of streptozotocin-diabetic mice. Anat Rec 251: 240–244, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Fahim MA, Hasan MY, Alshuaib WB. Cadmium modulates diabetes-induced alterations in murine neuromuscular junction. Endocr Res 26: 205–217, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Fahim MA, Hasan MY, Alshuaib WB. Early morphological remodeling of neuromuscular junction in a murine model of diabetes. J Appl Physiol 89: 2235–2240, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Fischbach GD, Schuetze SM. A post-natal decrease in acetylcholine channel open time at rat end-plates. J Physiol 303: 125–137, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gattenlohner S, Schneider C, Thamer C, Klein R, Roggendorf W, Gohlke F, Niethammer C, Czub S, Vincent A, Muller-Hermelink HK, Marx A. Expression of foetal type acetylcholine receptor is restricted to type 1 muscle fibres in human neuromuscular disorders. Brain 125: 1309–1319, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Gautron J. Ultrastructural localization of acetylcholinesterase. A direct method for light and electron microscopy. Histochemistry 76: 469–478, 1982 [DOI] [PubMed] [Google Scholar]
- 23. Ghareeb DA, Hussen HM. Vanadium improves brain acetylcholinesterase activity on early stage alloxan-diabetic rats. Neurosci Lett 436: 44–47, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Girard E, Bernard V, Minic J, Chatonnet A, Krejci E, Molgo J. Butyrylcholinesterase and the control of synaptic responses in acetylcholinesterase knockout mice. Life Sci 80: 2380–2385, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Glisovic S, Trinkaus M, Pregelj P, Sketelj J. The asymmetric molecular forms of AChE and the expression of collagen Q in mature and immature fast and slow rat muscles. Chem Biol Interact 187: 90–95, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Gomez CM, Maselli RA, Groshong J, Zayas R, Wollmann RL, Cens T, Charnet P. Active calcium accumulation underlies severe weakness in a panel of mice with slow-channel syndrome. J Neurosci 22: 6447–6457, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Greig NH, Utsuki T, Ingram DK, Wang Y, Pepeu G, Scali C, Yu QS, Mamczarz J, Holloway HW, Giordano T, Chen D, Furukawa K, Sambamurti K, Brossi A, Lahiri DK. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc Natl Acad Sci USA 102: 17213–17218, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Homs J, Ariza L, Pagès G, Verdú E, Casals L, Udina E, Chillón M, Bosch A, Navarro X. Comparative study of peripheral neuropathy and nerve regeneration in NOD and ICR diabetic mice. J Peripher Nerv Syst 16: 213–227, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Jayawardane P, Senanayake N, Dawson A. Electrophysiological correlates of intermediate syndrome following acute organophosphate poisoning. Clin Toxicol (Phila) 47: 193–205, 2009 [DOI] [PubMed] [Google Scholar]
- 30. John M, Oommen A, Zachariah A. Muscle injury in organophosphorous poisoning and its role in the development of intermediate syndrome. Neurotoxicology 24: 43–53, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Kerschensteiner M, Reuter MS, Lichtman JW, Misgeld T. Ex vivo imaging of motor axon dynamics in murine triangularis sterni explants. Nat Protoc 3: 1645–1653, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kiauta T, Brzin M, Dettbarn WD. Synthesis of neuromuscular cholinesterases in innervated and denervated rat diaphragm. Exp Neurol 56: 281–288, 1977 [DOI] [PubMed] [Google Scholar]
- 33. Kimura I, Okazaki M, Kimura M. Streptozocin-diabetes modifies acetylcholine release from mouse phrenic nerve terminal and presynaptic sensitivity to succinylcholine. Jpn J Pharmacol 62: 35–41, 1993 [DOI] [PubMed] [Google Scholar]
- 34. Kiss G, Somogyi J, Csermely P, Szelenyi J, Ver A. Streptozotocin-induced diabetes alters the oligomerization pattern of acetylcholinesterase in rat skeletal muscle. Diabetologia 44: 220–223, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Koelle GB, Horn RS. Acetyl disulfide, (CH3COS)2, a major active component in the thiolacetic acid histochemical method for acetylcholinesterase. J Histochem Cytochem 16: 743–753, 1968 [DOI] [PubMed] [Google Scholar]
- 36. Kong L, Wang X, Choe DW, Polley M, Burnett BG, Bosch-Marcé M, Griffin JW, Rich MM, Sumner CJ. Impaired synaptic vesicle release and immaturity of neuromuscular junctions in spinal muscular atrophy mice. J Neurosci 29: 842–851, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Krejci E, Legay C, Thomine S, Sketelj J, Massoulie J. Differences in expression of acetylcholinesterase and collagen Q control the distribution and oligomerization of the collagen-tailed forms in fast and slow muscles. J Neurosci 19: 10672–10679, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krejci E, Thomine S, Boschetti N, Legay C, Sketelj J, Massoulie J. The mammalian gene of acetylcholinesterase-associated collagen. J Biol Chem 272: 22840–22847, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Kuhad A, Chopra K. Curcumin attenuates diabetic encephalopathy in rats: behavioral and biochemical evidences. Eur J Pharmacol 576: 34–42, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Lakhman SS, Kaur G. Effect of alloxan-induced diabetes on acetylcholinesterase activity from discrete areas of rat brain. Neurochem Int 24: 159–163, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Li B, Stribley JA, Ticu A, Xie W, Schopfer LM, Hammond P, Brimijoin S, Hinrichs SH, Lockridge O. Abundant tissue butyrylcholinesterase and its possible function in the acetylcholinesterase knockout mouse. J Neurochem 75: 1320–1331, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[−Delta Delta C(T)] Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Marques MJ, Santo Neto H. Acetylcholine receptors and nerve terminal distribution at the neuromuscular junction of non-obese diabetic mice. Anat Rec 267: 112–119, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Massoulié J, Anselmet A, Bon S, Krejci E, Legay C, Morel N, Simon S. Acetylcholinesterase: C-terminal domains, molecular forms and functional localization. J Physiol Paris 92: 183–190, 1998 [DOI] [PubMed] [Google Scholar]
- 45. McArdle JJ, Angaut-Petit D, Mallart A, Bournaud R, Faille L, Brigant JL. Advantages of the triangularis sterni muscle of the mouse for investigations of synaptic phenomena. J Neurosci Methods 4: 109–115, 1981 [DOI] [PubMed] [Google Scholar]
- 46. McArdle JJ, Lentz TL, Witzemann V, Schwarz H, Weinstein SA, Schmidt JJ. Waglerin-1 selectively blocks the epsilon form of the muscle nicotinic acetylcholine receptor. J Pharmacol Exp Ther 289: 543–550, 1999 [PubMed] [Google Scholar]
- 47. McArdle JJ, Sellin LC, Coakley KM, Potian JG, Quinones-Lopez MC, Rosenfeld CA, Sultatos LG, Hognason K. Mefloquine inhibits cholinesterases at the mouse neuromuscular junction. Neuropharmacology 49: 1132–1139, 2005 [DOI] [PubMed] [Google Scholar]
- 48. McComas AJ, Fawcett PR, Campbell MJ, Sica RE. Electrophysiological estimation of the number of motor units within a human muscle. J Neurol Neurosurg Psychiatry 34: 121–131, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miglietta O. Neuromuscular junction defect in diabetes. Diabetes 22: 719–723, 1973 [DOI] [PubMed] [Google Scholar]
- 50. Minic J, Chatonnet A, Krejci E, Molgo J. Butyrylcholinesterase and acetylcholinesterase activity and quantal transmitter release at normal and acetylcholinesterase knockout mouse neuromuscular junctions. Br J Pharmacol 138: 177–187, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mishina M, Takai T, Imoto K, Noda M, Takahashi T, Numa S, Methfessel C, Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature 321: 406–411, 1986 [DOI] [PubMed] [Google Scholar]
- 52. Nishi M, Komazaki S, Kurebayashi N, Ogawa Y, Noda T, Iino M, Takeshima H. Abnormal features in skeletal muscle from mice lacking mitsugumin29. J Cell Biol 147: 1473–1480, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ohno K, Brengman J, Tsujino A, Engel AG. Human endplate acetylcholinesterase deficiency caused by mutations in the collagen-like tail subunit (ColQ) of the asymmetric enzyme. Proc Natl Acad Sci USA 95: 9654–9659, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Primo-Parmo SL, Bartels CF, Wiersema B, van der Spek AF, Innis JW, La Du BN. Characterization of 12 silent alleles of the human butyrylcholinesterase (BCHE) gene. Am J Hum Genet 58: 52–64, 1996 [PMC free article] [PubMed] [Google Scholar]
- 55. Ramkumar KM, Latha M, Ashokkumar N, Pari L, Ananthan R. Modulation of impaired cholinesterase activity in experimental diabetes: effect of Gymnema montanum leaf extract. J Basic Clin Physiol Pharmacol 16: 17–35, 2005 [DOI] [PubMed] [Google Scholar]
- 56. Reske-Nielsen E, Gregersen G, Harmsen A, Lundbaek K. Morphological abnormalities of the terminal neuromuscular apparatus in recent juvenile diabetes. Diabetologia 6: 104–109, 1970 [DOI] [PubMed] [Google Scholar]
- 57. Reske-Nielsen E, Lundbaek K, Gregersen G, Harmsen A. Pathological changes in the central and peripheral nervous system of young long-term diabetics. The terminal neuro-muscular apparatus. Diabetologia 6: 98–103, 1970 [DOI] [PubMed] [Google Scholar]
- 58. Said G. Diabetic neuropathy—a review. Nat Clin Pract Neurol 3: 331–340, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Sakmann B, Brenner HR. Change in synaptic channel gating during neuromuscular development. Nature 276: 401–402, 1978 [DOI] [PubMed] [Google Scholar]
- 60. Sánchez-Chávez G, Salceda R. Acetyl- and butyrylcholinesterase in normal and diabetic rat retina. Neurochem Res 26: 153–159, 2001 [DOI] [PubMed] [Google Scholar]
- 61. Sánchez-Chávez G, Salceda R. Acetyl- and butyrylcholinesterase molecular forms in normal and streptozotocin-diabetic rat retinal pigment epithelium. Neurochem Int 39: 209–215, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Sánchez-Chávez G, Salceda R. Effect of streptozotocin-induced diabetes on activities of cholinesterases in the rat retina. IUBMB Life 49: 283–287, 2000 [DOI] [PubMed] [Google Scholar]
- 63. Schiller Y, Rahamimoff R. Neuromuscular transmission in diabetes: response to high-frequency activation. J Neurosci 9: 3709–3719, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schmatz R, Mazzanti CM, Spanevello R, Stefanello N, Gutierres J, Corrêa M, da Rosa MM, Rubin MA, Chitolina Schetinger MR, Morsch VM. Resveratrol prevents memory deficits and the increase in acetylcholinesterase activity in streptozotocin-induced diabetic rats. Eur J Pharmacol 610: 42–48, 2009 [DOI] [PubMed] [Google Scholar]
- 65. Shefner JM. Motor unit number estimation in human neurological diseases and animal models. Clin Neurophysiol 112: 955–964, 2001 [DOI] [PubMed] [Google Scholar]
- 66. Sketelj J, Crne-Finderle N, Strukelj B, Trontelj JV, Pette D. Acetylcholinesterase mRNA level and synaptic activity in rat muscles depend on nerve-induced pattern of muscle activation. J Neurosci 18: 1944–1952, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Souayah N, Potian JG, Garcia CC, Krivitskaya N, Boone C, Routh VH, McArdle JJ. Motor unit number estimate as a predictor of motor dysfunction in an animal model of type 1 diabetes. Am J Physiol Endocrinol Metab 297: E602–E608, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stum M, Girard E, Bangratz M, Bernard V, Herbin M, Vignaud A, Ferry A, Davoine CS, Echaniz-Laguna A, Rene F, Marcel C, Molgo J, Fontaine B, Krejci E, Nicole S. Evidence of a dosage effect and a physiological endplate acetylcholinesterase deficiency in the first mouse models mimicking Schwartz-Jampel syndrome neuromyotonia. Hum Mol Genet 17: 3166–3179, 2008 [DOI] [PubMed] [Google Scholar]
- 69. Suhail M, Rizvi SI. Regulation of red cell acetylcholinesterase activity in diabetes mellitus. Indian J Exp Biol 28: 234–236, 1990 [PubMed] [Google Scholar]
- 70. Sultatos LG, Kaushik R. Altered binding of thioflavin t to the peripheral anionic site of acetylcholinesterase after phosphorylation of the active site by chlorpyrifos oxon or dichlorvos. Toxicol Appl Pharmacol 230: 390–396, 2008 [DOI] [PubMed] [Google Scholar]
- 71. Teichert RW, Garcia CC, Potian JG, Schmidt JJ, Witzemann V, Olivera BM, McArdle JJ. Peptide-toxin tools for probing the expression and function of fetal and adult subtypes of the nicotinic acetylcholine receptor. Ann NY Acad Sci 1132: 61–70, 2008 [DOI] [PubMed] [Google Scholar]
- 72. Teichert RW, Rivier J, Torres J, Dykert J, Miller C, Olivera BM. A uniquely selective inhibitor of the mammalian fetal neuromuscular nicotinic acetylcholine receptor. J Neurosci 25: 732–736, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang X, Engisch KL, Teichert RW, Olivera BM, Pinter MJ, Rich MM. Prolongation of evoked and spontaneous synaptic currents at the neuromuscular junction after activity blockade is caused by the upregulation of fetal acetylcholine receptors. J Neurosci 26: 8983–8987, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Witzemann V, Barg B, Criado M, Stein E, Sakmann B. Developmental regulation of five subunit specific mRNAs encoding acetylcholine receptor subtypes in rat muscle. FEBS Lett 242: 419–424, 1989 [DOI] [PubMed] [Google Scholar]
- 75. Witzemann V, Barg B, Nishikawa Y, Sakmann B, Numa S. Differential regulation of muscle acetylcholine receptor gamma- and epsilon-subunit mRNAs. FEBS Lett 223: 104–112, 1987 [DOI] [PubMed] [Google Scholar]
- 76. Zarros A, Liapi C, Galanopoulou P, Marinou K, Mellios Z, Skandali N, Al-Humadi H, Anifantaki F, Gkrouzman E, Tsakiris S. Effects of adult-onset streptozotocin-induced diabetes on the rat brain antioxidant status and the activities of acetylcholinesterase, [Na(+),K (+)]- and Mg(2+)-ATPase: modulation by l-cysteine. Metab Brain Dis 24: 337–348, 2009 [DOI] [PubMed] [Google Scholar]