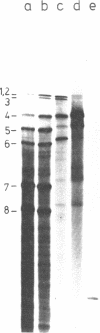
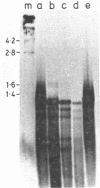
Abstract
A novel technique is described for the production of pure, full-length influenza virus ds DNA's corresponding to each segment of the influenza virus genome, and suitable for molecular cloning and restriction endonuclease mapping. The method involves the synthesis of DNA complementary to both virion (negative strand) and messenger (positive strand) RNA, gel purification and annealing. By avoiding the use of SI nuclease, which often removes the terminal regions of DNA duplexes, the method allows transcription of the total sequence information of influenza virion and messenger RNA's into a ds DNA form.
Full text
PDF







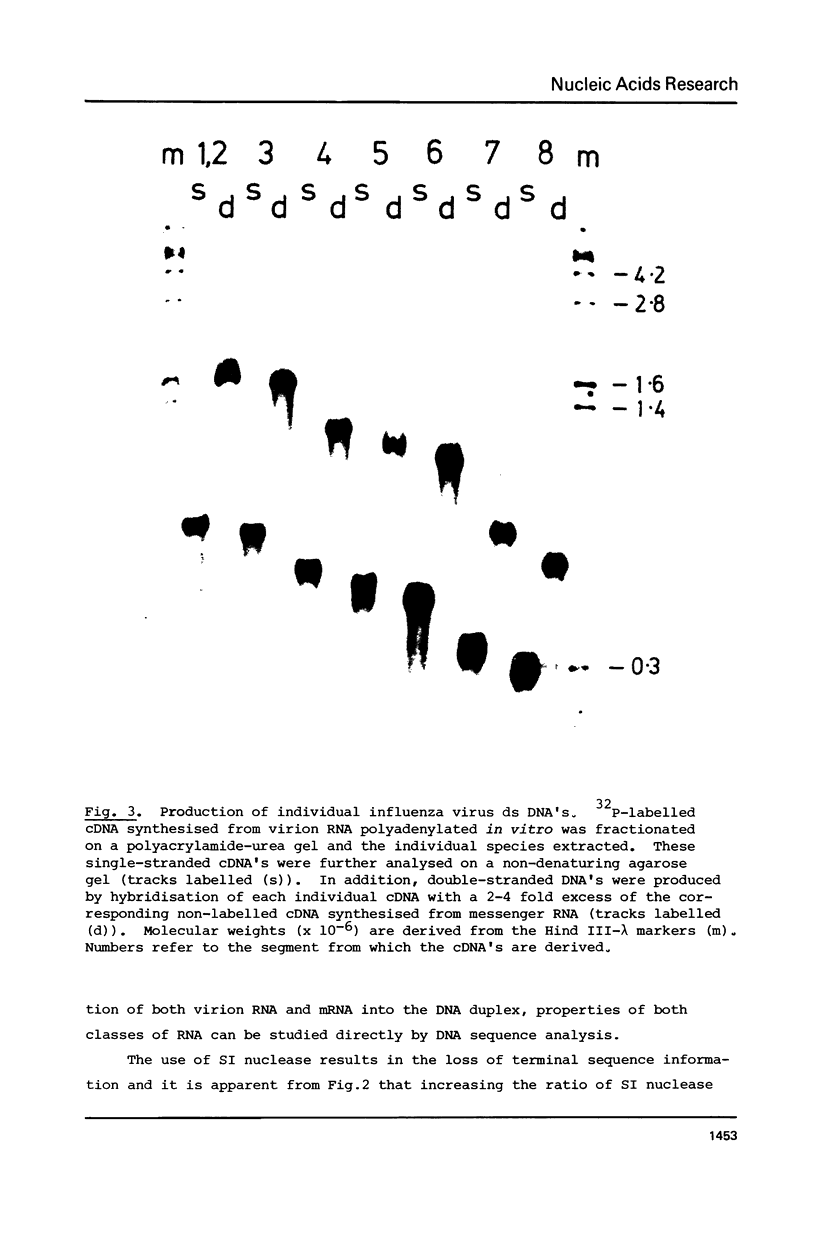



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett T., Wolstenholme A. J., Mahy B. W. Transcription and replication of influenza virus RNA. Virology. 1979 Oct 15;98(1):211–225. doi: 10.1016/0042-6822(79)90539-7. [DOI] [PubMed] [Google Scholar]
- Chang J. C., Temple G. F., Poon R., Neumann K. H., Kan Y. W. The nucleotide sequences of the untranslated 5' regions of human alpha- and beta-globin mRNAs. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5145–5149. doi: 10.1073/pnas.74.11.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maxam A. M., Maniatis T. Enzymatic in vitro synthesis of globin genes. Cell. 1976 Feb;7(2):279–288. doi: 10.1016/0092-8674(76)90027-1. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Maniatis T., Kafatos F. C., Jeffrey A., Vournakis J. N. Full length and discrete partial reverse transcripts of globin and chorion mRNAs. Cell. 1975 Apr;4(4):367–378. doi: 10.1016/0092-8674(75)90157-9. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Vournakis J. N., Donis-Keller H., Chaconas G., Dougall D. K., Kafatos F. C. End labeling of enzymatically decapped mRNA. Nucleic Acids Res. 1977 Dec;4(12):4165–4174. doi: 10.1093/nar/4.12.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emtage J. S., Catlin G. H., Carey N. H. Polyadenylation and reverse transcription of influenza viral RNA. Nucleic Acids Res. 1979 Apr;6(4):1221–1239. doi: 10.1093/nar/6.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkind P. R., Krug R. M. Purification of influenza viral complementary RNA: its genetic content and activity in wheat germ cell-free extracts. J Virol. 1975 Dec;16(6):1464–1475. doi: 10.1128/jvi.16.6.1464-1475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman E. Y., Rosbash M. The syntheiss of high yields of full-length reverse transcripts of globin mRNA. Nucleic Acids Res. 1977 Oct;4(10):3455–3471. doi: 10.1093/nar/4.10.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass S. E., McGeoch D., Barry R. D. Characterization of the mRNA of influenza virus. J Virol. 1975 Dec;16(6):1435–1443. doi: 10.1128/jvi.16.6.1435-1443.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A. J., Abraham G., Skehel J. J., Smith J. C., Fellner P. Influenza virus messenger RNAs are incomplete transcripts of the genome RNAs. Nucleic Acids Res. 1977 Dec;4(12):4197–4209. doi: 10.1093/nar/4.12.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A. J., Lomniczi B., Bellamy A. R., Skehel J. J. Transcription of the influenza virus genome. Virology. 1977 Dec;83(2):337–355. doi: 10.1016/0042-6822(77)90179-9. [DOI] [PubMed] [Google Scholar]
- Higuchi R., Paddock G. V., Wall R., Salser W. A general method for cloning eukaryotic structural gene sequences. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3146–3150. doi: 10.1073/pnas.73.9.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis S. C., Carroll A. R., Lamb R. A., Mahy B. W. Polypeptides specified by the influenza virus genome I. Evidence for eight distinct gene products specified by fowl plague virus. Virology. 1976 Oct 15;74(2):489–503. doi: 10.1016/0042-6822(76)90355-x. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., Mahy B. W. Polypeptides specified by the influenza virus genome. 3. Control of synthesis in infected cells. Virology. 1979 May;95(1):154–164. doi: 10.1016/0042-6822(79)90410-0. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., McGeoch D. J., Mahy B. W. Polypeptides specified by the influenza virus genoma. 2. Assignement of protein coding functions to individual genome segments by in vitro translation. Virology. 1977 May 15;78(2):522–536. doi: 10.1016/0042-6822(77)90128-3. [DOI] [PubMed] [Google Scholar]
- Lewandowski L. J., Content J., Leppla S. H. Characterization of the subunit structure of the ribonucleic acid genome of influenza virus. J Virol. 1971 Nov;8(5):701–707. doi: 10.1128/jvi.8.5.701-707.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D., Fellner P., Newton C. Influenza virus genome consists of eight distinct RNA species. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3045–3049. doi: 10.1073/pnas.73.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. R., Cartwright E. M., Brownlee G. G., Fedoroff N. V., Brown D. D. The nucleotide sequence of oocyte 5S DNA in Xenopus laevis. II. The GC-rich region. Cell. 1978 Apr;13(4):717–725. doi: 10.1016/0092-8674(78)90221-0. [DOI] [PubMed] [Google Scholar]
- Monahan J. J., McReynolds L. A., O'Malley B. W. The ovalbumin gene. In vitro enzymatic synthesis and characterization. J Biol Chem. 1976 Dec 10;251(23):7355–7362. [PubMed] [Google Scholar]
- Murray K., Murray N. E. Phage lambda receptor chromosomes for DNA fragments made with restriction endonuclease III of Haemophilus influenzae and restriction endonuclease I of Escherichia coli. J Mol Biol. 1975 Nov 5;98(3):551–564. doi: 10.1016/s0022-2836(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Plotch S. J., Bouloy M., Krug R. M. Transfer of 5'-terminal cap of globin mRNA to influenza viral complementary RNA during transcription in vitro. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1618–1622. doi: 10.1073/pnas.76.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougeon F., Mach B. Stepwise biosynthesis in vitro of globin genes from globin mRNA by DNA polymerase of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3418–3422. doi: 10.1073/pnas.73.10.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H., Feix G. Terminal riboadenylate transferase from Escherichia coli. Characterization and application. Eur J Biochem. 1976 Dec 11;71(2):577–583. doi: 10.1111/j.1432-1033.1976.tb11148.x. [DOI] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- Sippel A. E. Purification and characterization of adenosine triphosphate: ribonucleic acid adenyltransferase from Escherichia coli. Eur J Biochem. 1973 Aug 1;37(1):31–40. doi: 10.1111/j.1432-1033.1973.tb02953.x. [DOI] [PubMed] [Google Scholar]
- Sleigh M. J., Both G. W., Brownlee G. G. A new method for the size estimation of the RNA genome segments of influenza virus. Nucleic Acids Res. 1979 Apr;6(4):1309–1321. doi: 10.1093/nar/6.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. T., Forget B. G., Wilson L. B., Weissman S. M. Human globin messenger RNA: importance of cloning for structural analysis. Science. 1977 Apr 8;196(4286):200–202. doi: 10.1126/science.847468. [DOI] [PubMed] [Google Scholar]