Abstract
Short DNA chains were purified from phage T7 infected E. coli cells and 5' ends were labeled with 32P. By an alkali-treatment, pNp's rich in pAp and pCp were liberated from the T7 short DNA chains. After digestion of the [5'-32P] short DNA with the 3' to 5' exonuclease of T4 DNA polymerase, [5'-32P] mono- to pentaribonucleotides tipped with a deoxyribonucleotide residue at their 3' ends were isolated. 5' terminal ribonucleotides were; exclusively AMP in the penta- and the tetraribonucleotides, mostly CMP in the triribonucleotide and mainly CMP and AMP in di- and monoribonucleotides. The 5' terminal dinucleotide of the penta- and the tetraribonucleotides was pApC. The nucleotide sequence of the tetraribonucleotide was mainly pApCpCpN and some pApCpApN, where N was mainly A and C. These results indicate that oligoribonucleotides shorter than trinucleotide may result from in vivo degradation of the tetra- and pentaribonucleotides. A possibility that the tetra- and pentaribonucleotides with a 5' triphosphate terminus are the intact primers for the discontinuous T7 DNA replication is discussed.
Full text
PDF




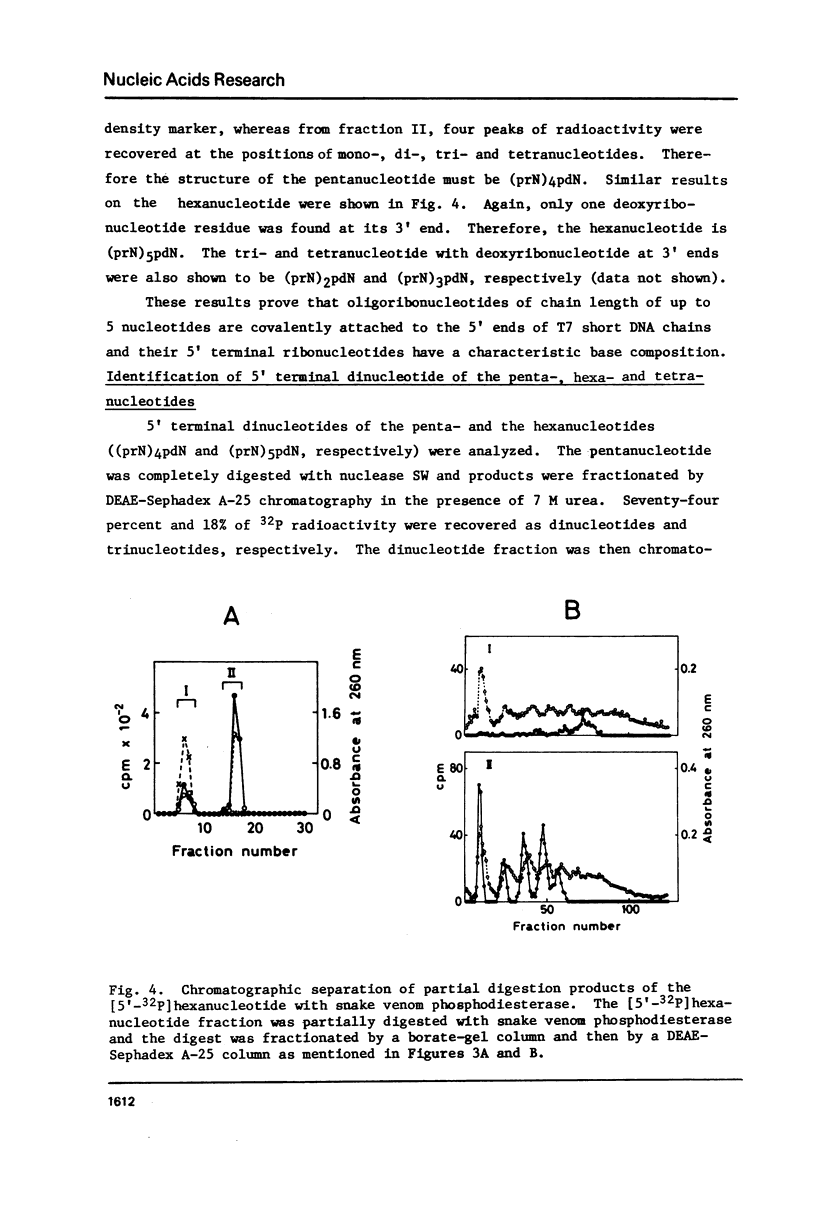
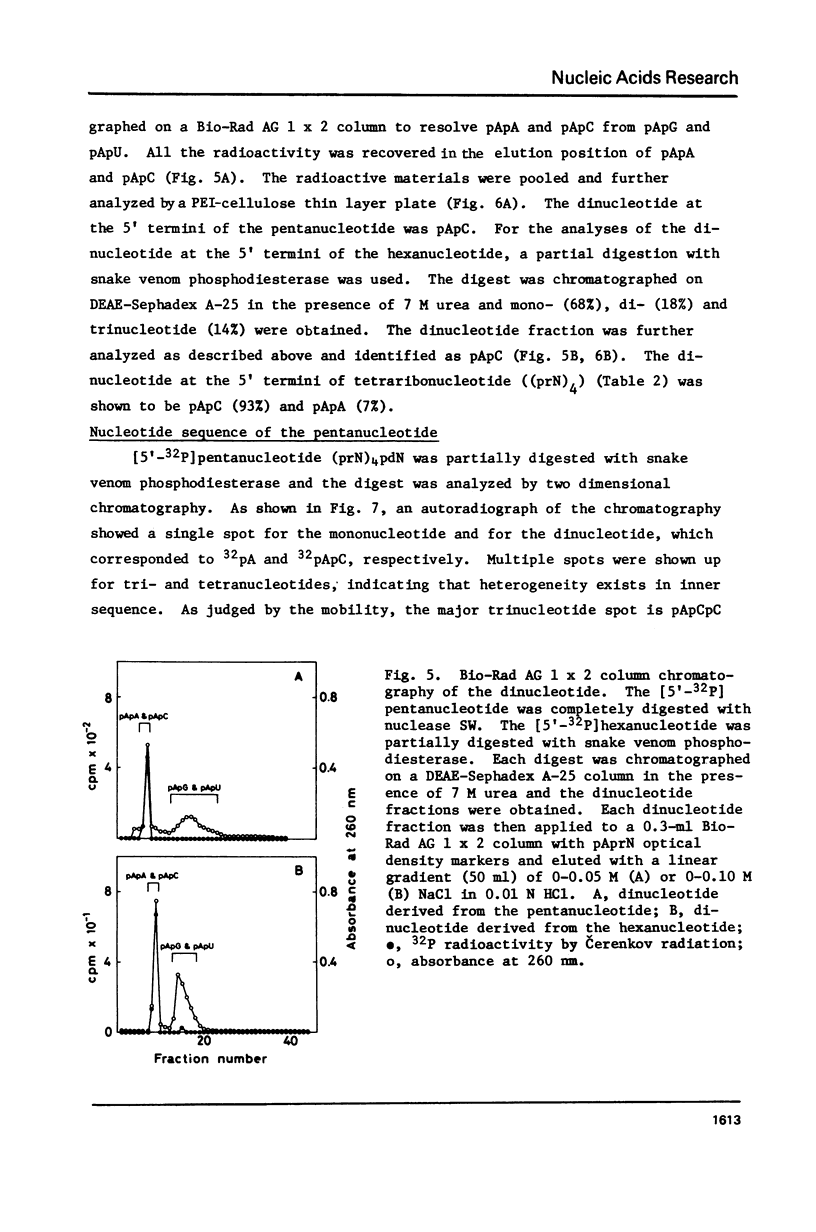
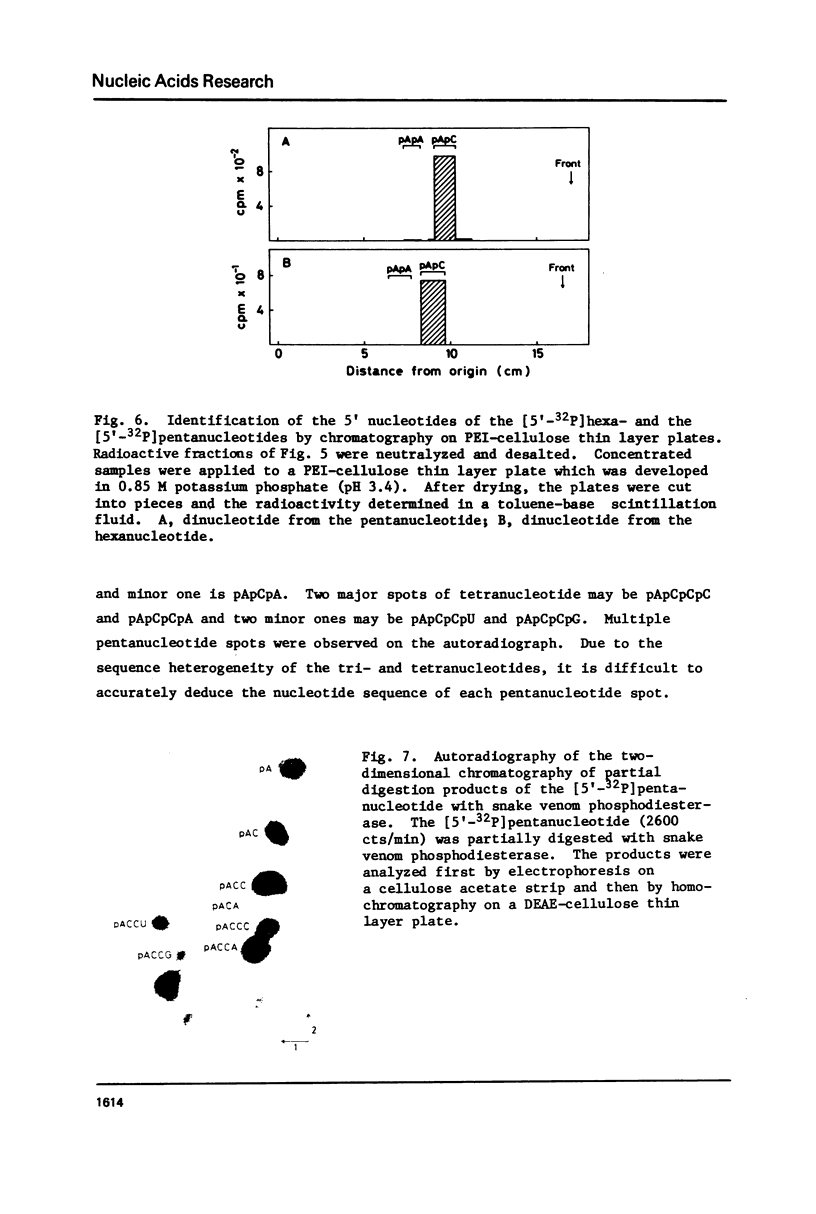





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cashel M., Lazzarini R. A., Kalbacher B. An improved method for thin-layer chromatography of nucleotide mixtures containing 32P-labelled orthophosphate. J Chromatogr. 1969 Mar 11;40(1):103–109. doi: 10.1016/s0021-9673(01)96624-5. [DOI] [PubMed] [Google Scholar]
- Clausen T. Measurement of 32P activity in a liquid scintillation counter without the use of scintillator. Anal Biochem. 1968 Jan;22(1):70–73. doi: 10.1016/0003-2697(68)90260-1. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillenbrand G., Morelli G., Lanka E., Scherzinger E. Bacteriophage T7 DNA primase: a multifunctional enzyme involved in DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):449–459. doi: 10.1101/sqb.1979.043.01.051. [DOI] [PubMed] [Google Scholar]
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainuma-Kuroda R., Okazaki R. Mechanism of DNA chain growth. XII. Asymmetry of replication of P2 phage DNA. J Mol Biol. 1975 May 15;94(2):213–228. doi: 10.1016/0022-2836(75)90079-0. [DOI] [PubMed] [Google Scholar]
- Liu C. C., Burke R. L., Hibner U., Barry J., Alberts B. Probing DNA replication mechanisms with the T4 bacteriophage in vitro system. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):469–487. doi: 10.1101/sqb.1979.043.01.053. [DOI] [PubMed] [Google Scholar]
- Masamune Y., Frenkel G. D., Richardson C. C. A mutant of bacteriophage T7 deficient in polynucleotide ligase. J Biol Chem. 1971 Nov 25;246(22):6874–6879. [PubMed] [Google Scholar]
- Mukai J. I. An endonuclease from silkworm---purification and mode of action. Biochem Biophys Res Commun. 1965 Dec 21;21(6):562–567. doi: 10.1016/0006-291x(65)90522-x. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Hirose S., Okazaki T., Okazaki R. Mechanism of DNA chain growth XVI. Analyses of RNA-linked DNA pieces in Escherichia coli with polynucleotide kinase. J Mol Biol. 1977 May 5;112(1):121–140. doi: 10.1016/s0022-2836(77)80160-5. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Okazaki T. RNA-linked nascent DNA pieces in phage T7-infected Escherichia coli. III. Detection of intact primer RNA. Nucleic Acids Res. 1979 Nov 24;7(6):1621–1633. doi: 10.1093/nar/7.6.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Ueda K., Hayaishi O. Purification of ADP-ribosylated nuclear proteins by covalent chromatography on dihydroxyboryl polyacrylamide beads and their characterization. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1111–1115. doi: 10.1073/pnas.75.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R., Hirose S., Okazaki T., Ogawa T., Kurosawa Y. Assay of RNA-linked nascent DNA pieces with polynucleotide kinase. Biochem Biophys Res Commun. 1975 Feb 17;62(4):1018–1024. doi: 10.1016/0006-291x(75)90424-6. [DOI] [PubMed] [Google Scholar]
- Okazaki T., Kurosawa Y., Ogawa T., Seki T., Shinozaki K., Hirose S., Fujiyama A., Kohara Y., Machida Y., Tamanoid F. Structure and metabolism of the RNA primer in the discontinuous replication of prokaryotic DNA. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):203–219. doi: 10.1101/sqb.1979.043.01.026. [DOI] [PubMed] [Google Scholar]
- Richardson C. C., Romano L. J., Kolodner R., LeClerc J. E., Tamanoi F., Engler M. J., Dean F. B., Richardson D. S. Replication of bacteriophage T7 DNA by purified proteins. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):427–440. doi: 10.1101/sqb.1979.043.01.049. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R. Transcriptional role in deoxyribonucleic acid replication. Nature of primer function of newly synthesized ribonucleic acid in vitro. J Biol Chem. 1973 Dec 25;248(24):8465–8473. [PubMed] [Google Scholar]
- Scherzinger E., Lanka E., Morelli G., Seiffert D., Yuki A. Bacteriophage-T7-induced DNA-priming protein. A novel enzyme involved in DNA replication. Eur J Biochem. 1977 Feb;72(3):543–558. doi: 10.1111/j.1432-1033.1977.tb11278.x. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Okazaki T. RNA-linked nascent DNA pieces in T7 phage-infected Escherichia coli cells. I. Role of gene 6 exonuclease in removal of the linked RNA. Mol Gen Genet. 1977 Sep 9;154(3):263–267. doi: 10.1007/BF00571281. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Okazaki T. T7 gene 6 exonuclease has an RNase H activity. Nucleic Acids Res. 1978 Nov;5(11):4245–4261. doi: 10.1093/nar/5.11.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinschneider A., Fraenkel-Conrat H. Studies of nucleotide sequences in tobacco mosaic virus ribonucleic acid. IV. Use of aniline in stepwise degradation. Biochemistry. 1966 Aug;5(8):2735–2743. doi: 10.1021/bi00872a034. [DOI] [PubMed] [Google Scholar]
- Sternglanz R., Wang H. F., Donegan J. J. Evidence that both growing DNA chains at a replication fork are synthesized discontinuously. Biochemistry. 1976 May 4;15(9):1838–1843. doi: 10.1021/bi00654a008. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]



