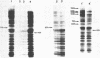Abstract
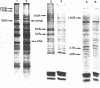
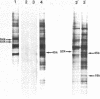
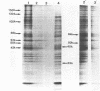
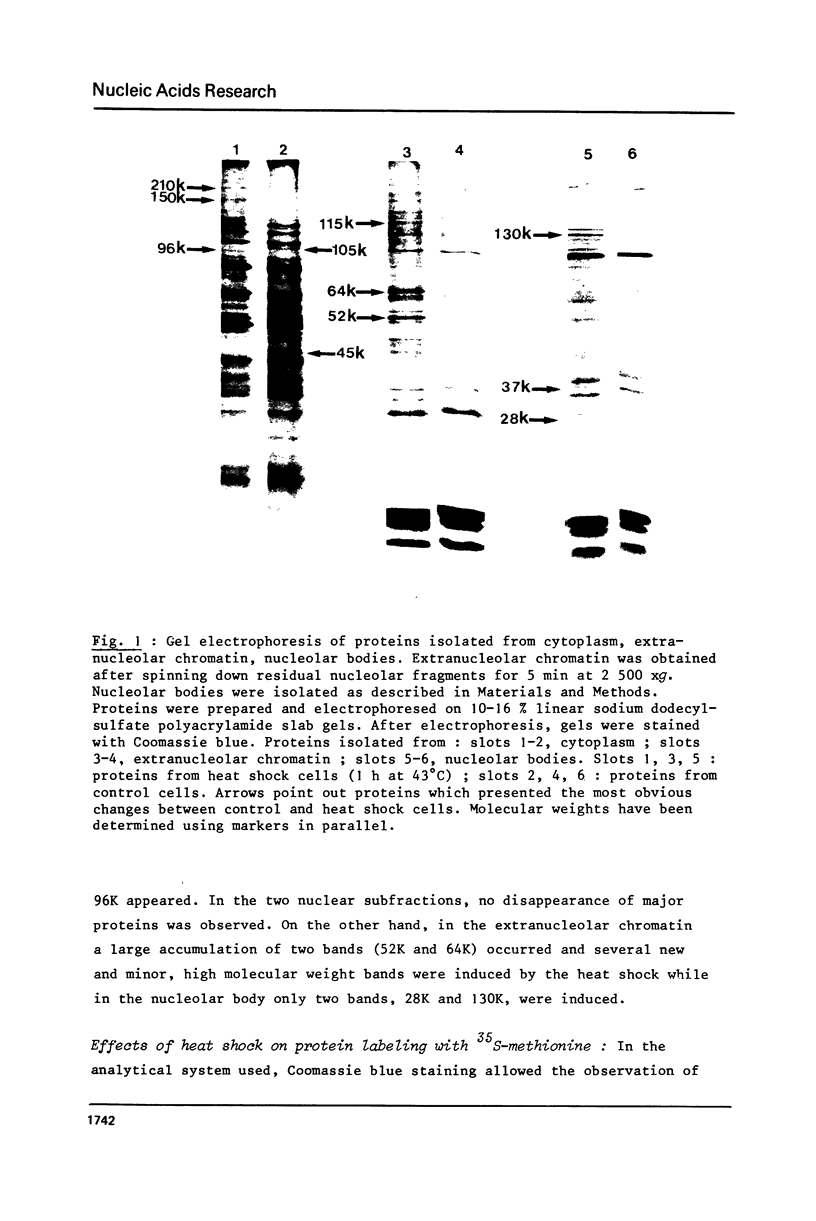
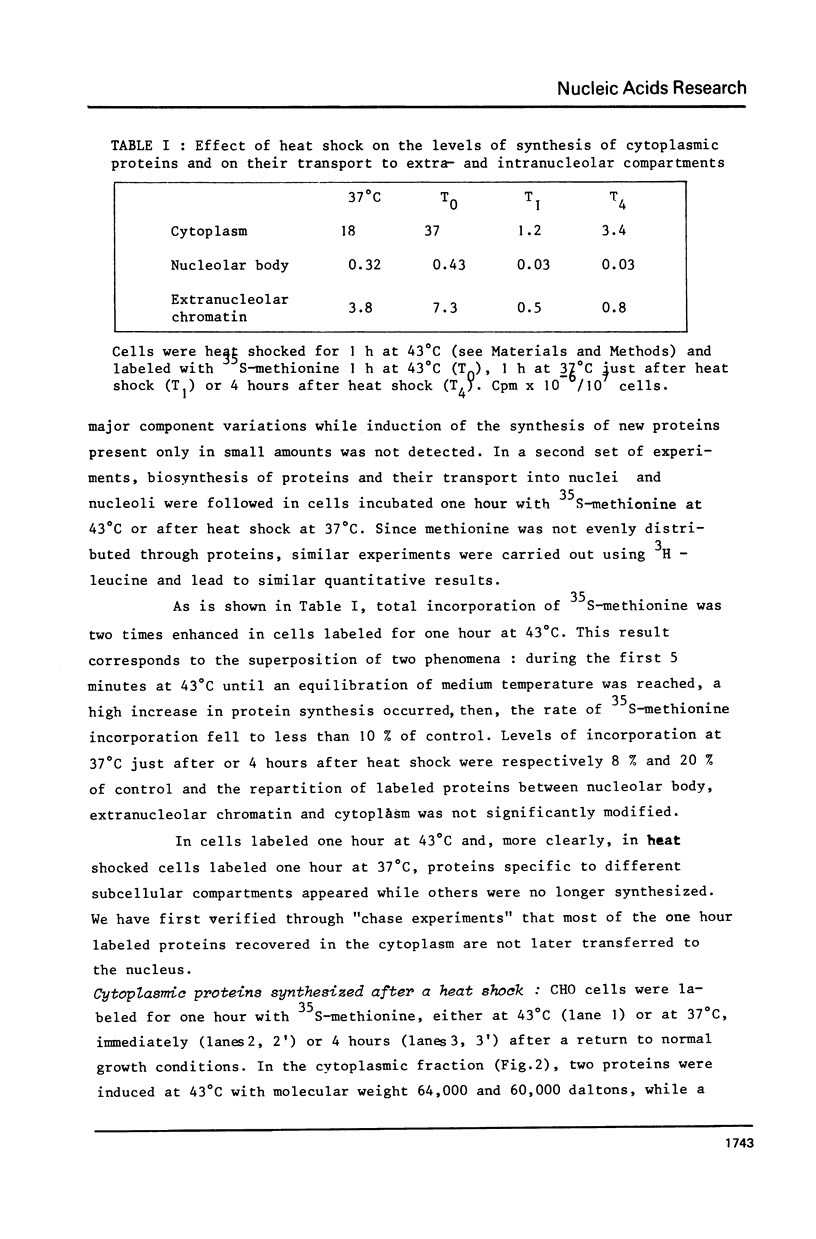
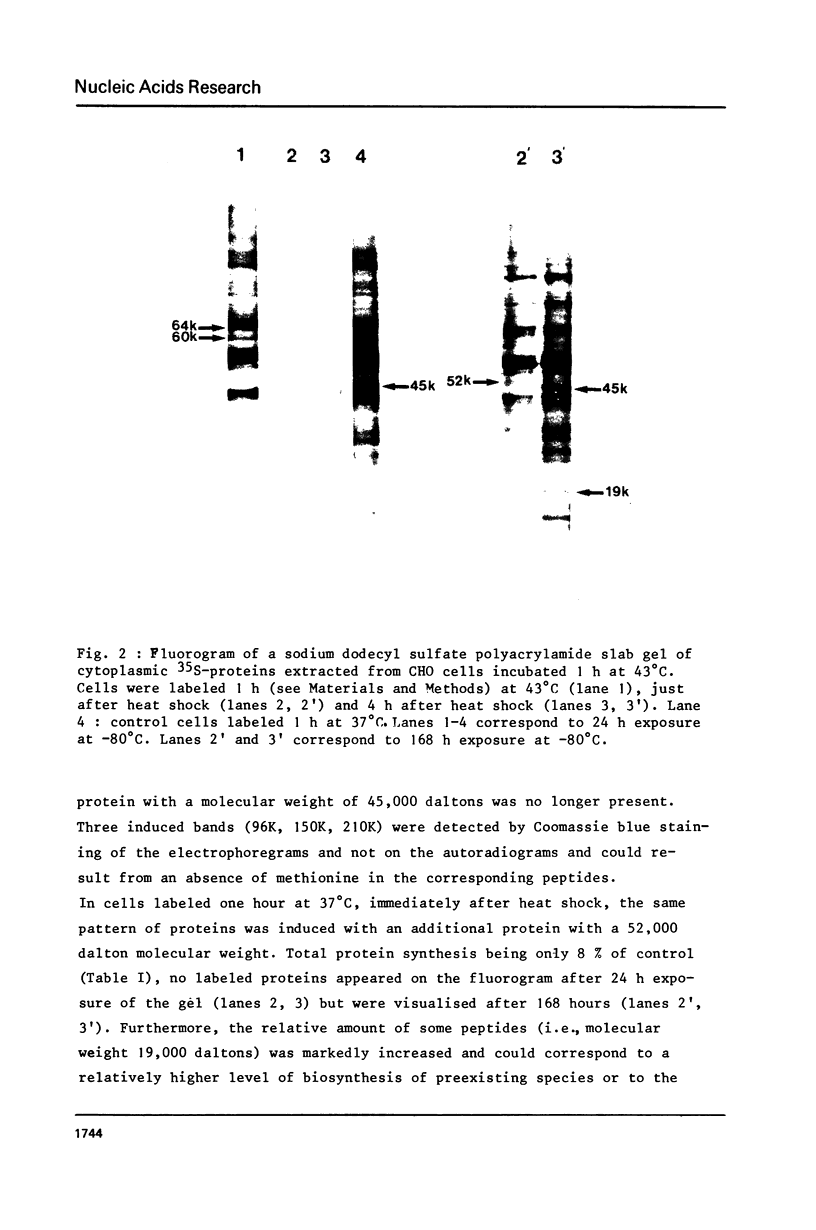
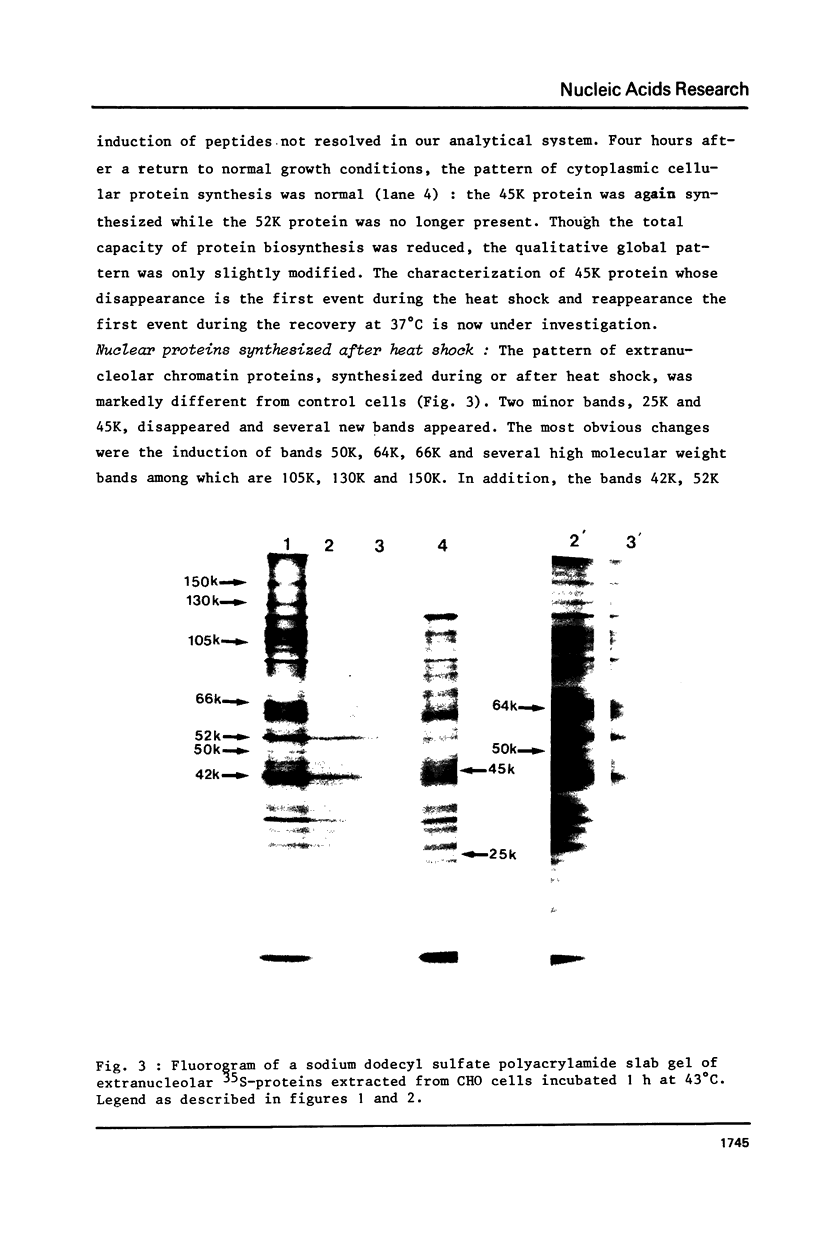
Incubation of Chinese Hamster Ovary (CHO) cells for one hour at 43 degrees C results in several obvious changes in protein distribution and protein synthesis. One major protein of the cytoplasm (molecular weight 45,000 daltions), also present as a minor component in the nucleus, rapidly disappeared while several proteins, especially high molecular weight peptides, were induced by heat shock. Localization of the proteins in the cytoplasm, extra-nucleolar chromatin and nucleolar bodies has been carried out. Different sets of induced proteins appear in each subcellular compartment. Four hours after restoration of the normal temperature, the normal pattern of protein synthesis was observed. The 45,000 dalton protein reappeared first. Relations between structural and functional alterations and changes in protein distribution are suggested.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amalric F., Simard R., Zalta J. P. Effet de la température supra-optimale sur les ribonucléoprotéines et le RNA nucléolaire. II. Etude biochimique. Exp Cell Res. 1969 Jun;55(3):370–377. doi: 10.1016/0014-4827(69)90571-0. [DOI] [PubMed] [Google Scholar]
- Bachellerie J. P., Nicoloso M., Zalta J. P. Nucleolar chromatin in Chinese hamster ovary cells. Topographical distribution of ribosomal DNA sequences and isolation of ribosomal transcription complexes. Eur J Biochem. 1977 Sep 15;79(1):23–32. doi: 10.1111/j.1432-1033.1977.tb11779.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Caboche M., Bachellerie J. P. RNA methylation and control of eukaryotic RNA biosynthesis. Effects of cycloleucine, a specific inhibitor of methylation, on ribosomal RNA maturation. Eur J Biochem. 1977 Mar 15;74(1):19–29. doi: 10.1111/j.1432-1033.1977.tb11362.x. [DOI] [PubMed] [Google Scholar]
- Craig E. A., McCarthy B. J., Wadsworth S. C. Sequence organization of two recombinant plasmids containing genes for the major heat shock-induced protein of D. melanogaster. Cell. 1979 Mar;16(3):575–588. doi: 10.1016/0092-8674(79)90031-x. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Lewis M., Helmsing P. J., Ashburner M. Parallel changes in puffing activity and patterns of protein synthesis in salivary glands of Drosophila. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3604–3608. doi: 10.1073/pnas.72.9.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis J. T., Prestidge L., Hogness D. S. A novel arrangement of tandemly repeated genes at a major heat shock site in D. melanogaster. Cell. 1978 Aug;14(4):901–919. doi: 10.1016/0092-8674(78)90345-8. [DOI] [PubMed] [Google Scholar]
- Simard R., Amalric F., Zalta J. P. Effet de la température supra-optimale sur les ribonucléoprotéines et le RNA nucléolaire. I. Etude ultrastructurale. Exp Cell Res. 1969 Jun;55(3):359–369. doi: 10.1016/0014-4827(69)90570-9. [DOI] [PubMed] [Google Scholar]
- Tissières A., Mitchell H. K., Tracy U. M. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974 Apr 15;84(3):389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- Van Zaane D., Dekker-Michielsen J. A., Bloemers H. P. Virus-specific precursor polypeptides in cells infected with Rauscher leukemia virus: synthesis, identification, and processing. Virology. 1976 Nov;75(1):113–129. doi: 10.1016/0042-6822(76)90011-8. [DOI] [PubMed] [Google Scholar]
- Wu C., Bingham P. M., Livak K. J., Holmgren R., Elgin S. C. The chromatin structure of specific genes: I. Evidence for higher order domains of defined DNA sequence. Cell. 1979 Apr;16(4):797–806. doi: 10.1016/0092-8674(79)90095-3. [DOI] [PubMed] [Google Scholar]
- Wu C., Wong Y. C., Elgin S. C. The chromatin structure of specific genes: II. Disruption of chromatin structure during gene activity. Cell. 1979 Apr;16(4):807–814. doi: 10.1016/0092-8674(79)90096-5. [DOI] [PubMed] [Google Scholar]
- Zaanie D., Gielkens A. L., Dekker-michielsen M. J., Bloemers H. P. Virus-specific precursor polypeptides in cells infected with Rauscher leukemia virus. Virology. 1975 Oct;67(2):544–552. doi: 10.1016/0042-6822(75)90454-7. [DOI] [PubMed] [Google Scholar]
- Zalta J., Zalta J. P. Basic principles of a method of nucleoli isolation. Methods Cell Biol. 1973;6:317–324. doi: 10.1016/s0091-679x(08)60055-2. [DOI] [PubMed] [Google Scholar]