Abstract
We have previously shown that the Coxsackievirus and adenovirus receptor (CAR) can interact with post-synaptic density 95 (PSD-95) and localize PSD-95 to cell-cell junctions. We have also shown that activity of the acid-sensing ion channel (ASIC3), a H+-gated cation channel that plays a role in mechanosensation and pain signaling, is negatively modulated by PSD-95 through a PDZ-based interaction. We asked whether CAR and ASIC3 simultaneously interact with PSD-95, and if so, whether co-expression of these proteins alters their cellular distribution and localization. Results indicate that CAR and ASIC3 co-immunoprecipitate only when co-expressed with PSD-95. CAR also brings both PSD-95 and ASIC3 to the junctions of heterologous cells. Moreover, CAR rescues PSD-95-mediated inhibition of ASIC3 currents. These data suggest that, in addition to activity as a viral receptor and adhesion molecule, CAR can play a role in trafficking proteins, including ion channels, in a PDZ-based scaffolding complex.
Keywords: Coxsackievirus and adenovirus receptor, ASIC3, PSD-95, protein trafficking
1. Introduction
The Coxsackievirus and adenovirus receptor (CAR) is both a cell adhesion protein and a viral receptor [1,2]. CAR is a developmentally essential member of the immunoglobulin superfamily and is present in numerous cell types, including many regions of the nervous system where it can play a role in endocytosis and cargo trafficking in neurons [3,4,5,6,7,8]. CAR contains a class 1 PSD-95/Drosophila discs-large protein/zonula occludens protein-1 (PDZ)-binding domain at its C-terminus and is known to interact with and affect the trafficking of several PDZ domain-containing scaffolding proteins, such as PSD-95, MAGI-1, PICK1, and MUPP-1 [2,9].
The PDZ domain-containing family is an important group of proteins involved in the transport and stabilization of channel complexes and adhesion molecules [10,11]. These are generally large scaffolding proteins containing one or more PDZ domains, along with several other protein domains. One key PDZ domain-containing protein involved in the localization and stability of many ion channels is post-synaptic density 95 (PSD-95). PSD-95 contains three PDZ domains, an SH3 domain, and a guanylate kinase domain, and each of these domains potentially interacts with several different partners. PSD-95 also interacts with itself increasing the potential number of simultaneous interactions. For example, PSD-95 is known to concentrate multiple different ion channels and other synaptic proteins at glutamatergic synapses [10]. However, it is unclear whether PSD-95 functions to traffic channels to the synapse, or whether it serves as a scaffold able to trap and retain channels there after arrival [12]. We have previously shown that CAR is able to direct PSD-95 localization to the junctions of heterologous cells [2], suggesting that CAR might also participate in the localization of other proteins, including cell surface signaling proteins, within a larger protein complex.
Acid-sensing ion channels (ASICs) are proton-gated cation channels with four alternatively spliced members (ASIC1a, 1b, 1b2, 2a, 2b, 3, and 4) [13,14]. ASIC channels function as homo- or heteromultimers, and they interact with multiple other modulatory proteins, including PDZ domain-containing proteins. ASIC channels are known to be involved in nociception and in fear response, and may be important for other pathogenic or psychiatric diseases. In particular, ASIC3 expression is primarily restricted to peripheral sensory neurons where it plays a role in sensing pain associated with modest drops in pH and may play a role in mechanosensation. PSD-95 is also implicated in pain pathways [15]. ASIC3 directly interacts with PSD-95 via a PDZ-PDZ binding domain interaction [16,17]. Interestingly, this interaction increases retention of ASIC3 within the reticular compartments of the cell where it strongly co-localizes with PSD-95. This interaction reduces ASIC3 cell surface levels and hence proton-gated current.
We asked whether CAR and ASIC3 could simultaneously interact with PSD-95, and if so, what the formation of this complex does to the localization and activity of the individual proteins. We show that CAR is able to bring both PSD-95 and ASIC3 to the junctions of heterologous cells resulting in restoration of ASIC3 current, as measured by whole-cell patch-clamp. These results suggest a novel function of CAR as a trafficking protein for cell surface signaling molecules.
2. Materials and methods
2.1. Materials
FLAG M2 antibody (Ab) was purchased from Sigma (F3165, St. Louis, MO). Guinea Pig ASIC3 Ab was purchased from Millipore (AB5927, Billerica, MA). Mouse anti-HA was purchased from Cell Signaling Technology (2367S, Danvers, MA). HRP conjugated donkey anti-guinea pig Ab was purchased from Jackson ImmunoResearch Laboratories, Inc (West Grove, PA). Alexa-488, -568 or -647 conjugated goat anti-mouse, -rabbit, or -guinea pig Abs, mouse and rabbit anti-GFP were from Molecular Probes (Eugene, OR). Mouse anti-CAR RmcB Ab (CRL-2379, ATCC, Manassas, VA) was produced by the University of Iowa Hybridoma Core. Rabbit anti-CAR 1605p was produced in rabbits immunized with a GST fusion to the intracellular CAR C-terminus (aa 261–365) as previously described [18]. COS-7 cells were from ATCC (Manassas, VA), and maintained under standard culture conditions (D-MEM with 10% FCS, penicillin and streptomycin).
2.2. Transfection
COS-7 cells were electroporated by standard methodologies [2]. Briefly, 10 million cells were mixed with 10 μg of each DNA for triple transfections, in 400 μl of cytomix (120 mM KCl, 0.15 mM CaCl2, 10 mM K2HPO4, 10 mM KH2PO4, 25 mM HEPES, 2 mM EGTA, 5 mM MgCl2, 2 mM ATP and glutathione) and incubated in an electroporation cuvette (Bio-Rad Laboratories, Hercules, CA) for 30 minutes on ice. For double or single transfections, 10 μg of the target plasmid DNA was balanced by either peGFP (Clontech) or parental pcDNA3.1. After electroporation, cells were seeded onto 10 cm dishes for immunoprecipitation (IP), collagen coated glass chamber slides for immunofluorescence, or glass coverslips for patch-clamp studies 2 days later.
2.3. Immunostaining
COS-7 cells grown on collagen coated chamber slides were washed once with PBS, fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and blocked with 2% BSA in SuperBlock (Pierce, Rockford, IL). Cells were incubated with primary Ab, washed extensively and incubated with Alexa-labeled secondary Ab. After washing, slides were coverslipped with Vectashield mounting media (Vector Laboratories, Burlingame, CA). Images were acquired with an FV1000 Laser Scanning Confocal Microscope (Olympus, CenterValley, PA) using a 60X oil immersion lens.
2.4. IP and Western Blot
COS-7 cells from two 100 mm plates were placed on ice, washed once with ice cold PBS, and lysed with lysis buffer [50 mM Tris-HCl, pH 7.5, 137 mM NaCl, 1% Triton X-100, 5 mM EDTA, 1 mM EGTA, protease inhibitors (10 μg/ml) leupeptin, aprotinin, pepstatin, and 1 mM phenylmethylsulfonyl fluoride] by rocking at 4°C. Cells were scraped, sonicated 5 times and spun in a microcentrifuge at full speed for 10 minutes. For co-IP, supernatant was incubated with the indicated Ab with rotation at 4°C overnight. Protein A or G conjugated sepharose (Amersham Biosciences, Uppsala Sweden) was added for 1–2 hours followed by a wash with lysis buffer, 10% lysis buffer in TBS [50 mM Tris-HCl, pH 7.5, 137 mM NaCl], and TBS. Beads were suspended in loading buffer [4% sodium dodecyl sulfate, 100 mM dithiothreitol, 20% Glycerol, 65 mM Tris, pH 6.8, 0.005% bromophenol blue] and proteins were separated by SDS-poly acrylamide gel electrophoresis. Gels were transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA), blocked with 5% BSA, washed, probed with primary Ab as indicated, followed by washing and incubation with HRP-conjugated donkey anti-mouse or -rabbit secondary Ab (Jackson ImmunoResearch Laboratories, West Grove, PA). Bands were detected with ECL reagents (Pierce, Rockford, IL) and imaged on the EpiChemi3 Darkroom (UVP Inc, Upland, CA).
2.5. Electrophysiology
Whole-cell patch-clamp recordings (at −70 mV) from COS-7 cells were performed at room temperature with an Axopatch 200B amplifier (Axon Instruments, Foster City, CA) and were acquired and analyzed with PULSE/PULSEFIT 8.70 (HEKA Electronics, Lambrecht, Germany) and IGOR Pro 6.01 (WaveMetrics, Lake Oswego, OR) software. Currents were filtered at 5 kHz and sampled at 2 kHz. Series resistance was compensated by at least 50%. Capacitive currents were compensated for and recorded for normalization of peak current amplitudes (reported as current densities). Micropipettes (2–4 MΩ) were filled with internal solution (mM): 100 KCl, 10 EGTA, 40 HEPES, and 5 MgCl2, pH 7.4 with KOH. External solutions contained (mM): 120 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, 10 MES; pH was adjusted with tetramethylammonium hydroxide, and osmolarity adjusted with tetramethylammonium chloride (TMA-Cl). Extracellular solutions were changed within 20 msec by using a computer-driven solenoid valve system [19]. Kinetics of desensitization were fit to with single exponential equations and time constants (τ) reported. Data are means ± SEM. Statistical significance was assessed using unpaired Student’s t-test.
3. Results
3.1. ASIC3, CAR, and PSD-95 interact in a trimolecular complex
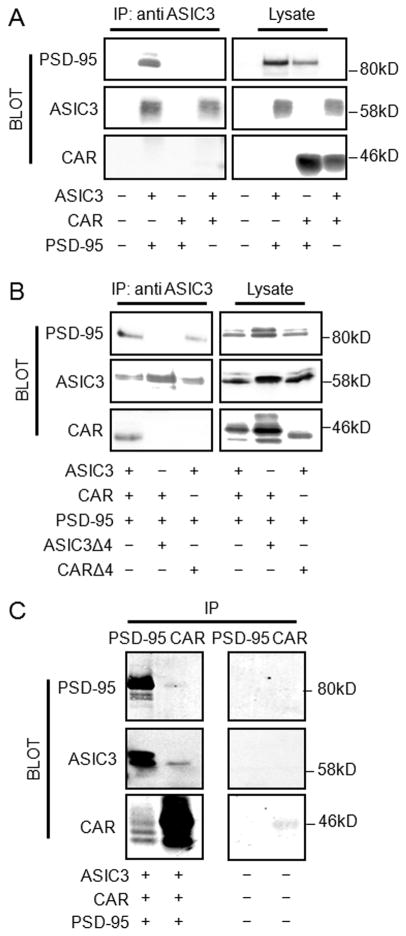
We have previously demonstrated interactions between ASIC3 and PSD-95, as well as CAR and PSD-95 [2,16]. Moreover, both interactions have been shown to be dependent on the functional PDZ-binding domains of ASIC3 and CAR. To investigate whether CAR interacts directly with ASIC3, COS-7 cells were co-transfected with paired combinations of plasmids encoding HA-tagged ASIC3, FLAG-tagged CAR, and GFP-tagged PSD-95 (Fig. 1A). As expected, coexpression of ASIC3 with PSD-95 allowed for co-immunoprecipitation of the two proteins. However, CAR, when coexpressed with ASIC3, did not co-immunoprecipitate with ASIC3 (Fig. 1A). In contrast, when all three proteins were coexpressed, ASIC3 co-immunoprecipitated both PSD-95 and CAR (Fig. 1B). Similarly, PSD-95 co-immunoprecipitated ASIC3 and CAR, and CAR co-immunoprecipitated ASIC3 and PSD-95 upon coexpression of all three proteins (Fig. 1C). We next investigated whether this trimolecular interaction was dependent upon PDZ-binding domain interactions. Deletion of the four terminal amino acids comprising the PDZ-binding domain of ASIC3 (ASIC3Δ4) abolished the capacity of ASIC3 to co-immunoprecipitate either PSD-95 or CAR (Fig. 1B). Moreover, deletion of the PDZ-binding domain of CAR (CARΔ4) abolished the capacity of ASIC3 to co-immunoprecipitate CAR; however it did not alter the ability of ASIC3 to co-immunoprecipitate PSD-95 (Fig. 1B). In summary, these data show that ASIC3, PSD-95, and CAR form a trimolecular complex that involves the binding of ASIC3 and CAR, via their PDZ-binding domains, to PSD-95.
Fig. 1.
CAR, ASIC3, and PSD-95 form a trimolecular complex. A) COS-7 cells were double transfected with GFP-tagged PSD-95, HA-tagged ASIC3, or FLAG-tagged CAR and subjected to IP with an antibody specific for ASIC3, followed by Western blot with tag-specific antibodies. Western blot of lysates demonstrated appropriate bands indicating successful transfection. Cells were triple transfected with GFP-PSD-95, HA-ASIC3, FLAG-CAR, HA-ASICΔ4 or FLAG-CARΔ4, and subjected to IP B) with an antibody specific for ASIC3 or C) antibodies against GFP or CAR (RmcB), followed by Western blot with GFP-, AISC3-, or CAR (1605p)-specific antibodies. Western blot of B) lysates or C) mock transfected cells subjected to immunoprecipitation. Transfections are indicated at the bottom of each blot. Blots are representative of 3 experimental replicates.
3.2. CAR alters the localization of the PSD-95-ASIC3 complex
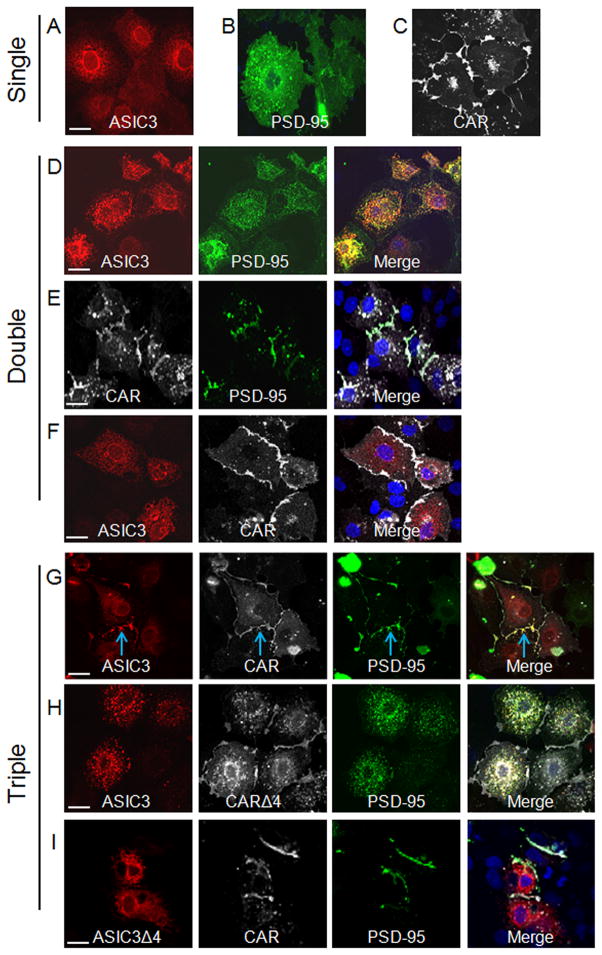
We next asked whether the cellular localization of these proteins was altered upon interaction. Previously we have shown that CAR and ASIC3 independently co-localize with PSD-95 and changed their distribution of expression [2,16]. Consistent with these previous findings, when each of the individual proteins was expressed alone, immunocytochemistry for ASIC3 demonstrated a strong reticular pattern (Fig. 2A), PSD-95 staining was largely diffuse throughout the cell (Fig. 2B), and CAR localized to the junctions between transfected cells (Fig. 2C). Coexpression of ASIC3 and PSD-95 together resulted in altered localization of both proteins so that they co-localized in small clusters throughout the cell (Fig. 2D). Coexpressing CAR and PSD-95 resulted in CAR-mediated recruitment of PSD-95 to the junctions of cells (Fig. 2E). Interestingly, coexpressed CAR and ASIC3 did not co-localize and the localization of the two proteins was unchanged; ASIC3 remained reticular, while CAR was primarily at the intercellular junctions (Fig. 2F). Surprisingly, coexpression of all three proteins together resulted in ASIC3, CAR, and PSD-95 co-localization at the junctions between cells (Fig. 2G, blue arrow). These data suggest that CAR plays a dominant role in the cellular localization of the complex, causing both PSD-95 and ASIC3 to either traffic to junctions, and/or be retained at junctions after arrival.
Fig. 2.
CAR localizes ASIC3 to cell junctions via PSD-95. Single transfected COS-7 cells were investigated for the localization of A) HA-ASIC3 (red), B) GFP-PSD-95 (green), C) FLAG-CAR (white) by GFP fluorescence or with tag-specific antibodies for ASIC3 (HA) or CAR (FLAG). Double transfected cells were investigated for co-localization of D) HA-ASIC3 and GFP-PSD-95, E) FLAG-CAR and GFP-PSD-95, or F) HA-ASIC3 and FLAG-CAR. Triple transfected cells were investigated for co-localization of G) HA-ASIC3, FLAG-CAR, and GFP-PSD-95, H) HA-ASIC3, FLAG-CARΔ4, and GFP-PSD-95, or I) HA-ASIC3Δ4, FLAG-CAR, and GFP-PSD-95. Merge images are shown in the right hand panel with nuclei counterstained with DAPI (blue). Representative X–Y images shown; 60X oil immersion confocal microscopy. White bar represents 20 μm.
3.3. Cellular co-localization of the CAR-ASIC3-PSD-95 complex requires PDZ-binding domains
We then tested if the junctional localization of the CAR-ASIC3-PSD-95 complex was dependent on the PDZ-binding domains of CAR and ASIC3. Coexpression of ASIC3, CARΔ4, and PSD-95 resulted in co-localization of ASIC3 and PSD-95 in small clusters throughout the cell, and neither co-localized with CAR at junctions (Fig. 2H). Moreover, coexpressing ASICΔ4, CAR, and PSD-95 showed that PDS-95 was now recruited to the junctions with CAR, while ASIC3 remained reticular (Fig. 2I). These data strongly suggest that these protein interactions and cellular localization patterns are dependent on the PDZ-binding domains of CAR and ASIC3 interacting with PSD-95.
3.4. CAR co-expression restores ASIC3 current in the presence of PSD-95
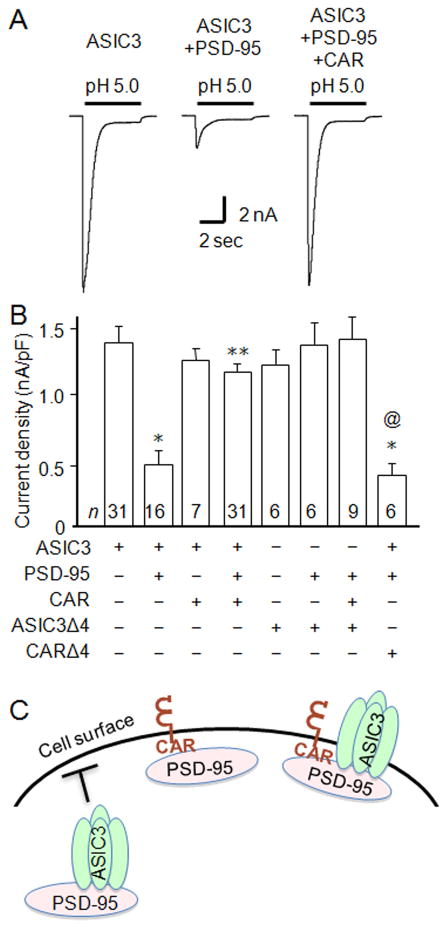
Even though heterologous ASIC3 staining appears largely reticular within COS-7 cells, some ASIC3 is at the cell surface since exposing cells to acidic pH evokes inward transient currents characteristic of ASIC3 currents, as measured by whole-cell patch-clamp (Fig. 3A, B). Acid application to COS-7 cells not transfected with ASIC3 generated no current (data not shown). It has also been shown that co-expression of ASIC3 with PSD-95 reduces ASIC3 expression at the cell surface and decreases current amplitude (Fig. 3A, B) [16]. Considering the dramatic change in ASIC3 localization upon co-expression with CAR and PSD-95, we asked what effect this would have upon ASIC3 current properties. Interestingly, pH 5.0-evoked current amplitudes were completely restored to the levels of ASIC3 alone (Fig. 3A, B). We studied pH 5-evoked currents because pH 5 maximally activates ASIC3. To confirm that these findings were dependent upon PDZ-PDZ-binding domain interactions between the three proteins, we investigated the effect of ASIC3Δ4 and CARΔ4. As previously shown, deleting the PDZ-binding domain of ASIC3 abolished PSD-95-mediated inhibition of ASIC3 current (Fig. 3B, compare bars 2 and 6). Moreover, mutating the PDZ-binding domain of CAR largely abolished its capacity to “reverse” the effect of PSD-95-mediated inhibition of ASIC3 (Fig. 3B, compare bars 4 and 8). We previously found that PDZ domain-containing proteins alter ASIC3 expression at the cell surface, without affecting gating properties [16]. Similarly, by fitting the falling phase (desensitization) of the acid-evoked currents to a single exponential, we found that the mean time constants of channel desensitization (τ) remained unchanged whether ASIC3 was expressed alone (τ = 501 ± 44 ms; n = 20), with PSD-95 (τ = 515 ± 44 ms; n = 8), or in a complex with both PSD-95 and CAR (τ = 495 ± 20 ms; n = 21), suggesting that the channel gating properties are not significantly affected by the complex formation. These data suggest that the functional interaction between ASIC3 and PSD-95 can be modulated by co-interaction with the cellular adhesion protein CAR.
Fig. 3.
CAR rescues ASIC3 current from PSD-95-mediated inhibition. A) Representative acid-evoked currents from COS-7 cells transfected with ASIC3 alone, ASIC3 and PSD-95, or ASIC3, PSD-95 and CAR. B) Mean current densities from cells transfected with the constructs indicated below the graph (n’s are indicated within each bar; *p < 0.01 vs. ASIC3 expressed alone; **p < 0.01 vs. ASIC3 and PSD-95 coexpressed; @p < 0.01 vs. coexpressed ASIC3, PSD-95, and CAR). C) Schematic representation of interactions between ASIC3, PSD-95, and CAR.
4. Discussion
Our results demonstrate that ASIC3 and CAR interact indirectly via the PDZ domain-containing protein PSD-95 to form a complex that localizes to cellular junctions. This interaction requires the PDZ-binding domains of ASIC3 and CAR, and suggests a simultaneous interaction with non-overlapping PDZ domains in PSD-95. When ASIC3 interacts with PSD-95 alone, it is functionally inhibited by being sequestered in the cell. However, when complexed with CAR, ASIC3 and PSD-95 localization is altered, and ASIC3 current is restored. We report for the first time that CAR can effect the cellular localization of an ion channel, and alter its function.
While our studies demonstrate functional interactions in heterologous cells, CAR, PSD-95, and ASIC3 are expressed in various cell types including astrocytes, dorsal root ganglia, and other sensory neurons [5,6,8,10,14,17,20]. In addition, CAR and ASIC3 expression overlap in other tissues that do not express PSD-95, such as epithelial cells of the lung and gastrointestinal tract. It is possible that indirect interactions in these other tissues may be mediated by similar interactions with other PDZ domain-containing proteins such as PICK1 [2,16]. Moreover, the related ASIC subunit, ASIC2, targets ASIC channels to synapses in the brain via its interaction with PSD-95 [21], and it is attractive to speculate that some of the functions of ASICs and PSD-95 in the brain are mediated through their interactions with CAR.
Increasing evidence suggests that CAR functions in the trafficking or cellular localization of different proteins in several cell types. For example, CAR is able to alter the localization of the cellular scaffolding protein MAGI-1, a protein known to interact with a diverse set of proteins, such as the renal tubule K+ channel Slo1 and glutamate transporter subtype 1 (GLT-1) [22,23]. Although CAR can interact with actin, microtubules, and the clathrin adaptors AP-1A and AP-1B, it is largely unknown how CAR functions in the transport of its interacting proteins [24,25,26]. Future work will aim to define these other potential mechanisms.
Studies in motor neurons have shown that CAR is localized at the synapse, where it facilitates endocytosis of adenovirus [6]. The complex of CAR and adenovirus can be followed through a series of endosomal maturation events ultimately ending up in the neuronal cell body. CAR is also critical for the structure and function of the intercalated discs in the heart. Conditional cardiac-specific knock-out of CAR in mice results in the loss of the gap junction protein, Connexin 45, at the AV-node of cardiac myocytes resulting in a block of atrioventricular conduction [27]. Connexin 45 contains a PDZ binding-domain similar to CAR and both proteins can co-IP together, thus, the authors speculated that these proteins may interact via a PDZ domain-containing protein, such as ZO-1. While a known function of PSD-95 is to cluster and alter the cellular localization of other proteins, it is interesting that CAR had a dominant role over PSD-95 in localization of the protein complex, and on ASIC3 function.
Several studies have shown that peripheral ASIC3 channels are sensors of acidic pain and integrators of molecular signals produced during inflammation [13]. PSD-95 has also been implicated in pain pathways. For example, reduced PSD-95 in the spinal cord attenuated nerve mechanical and thermal hyperalgesia during both the development and maintenance of chronic neuropathic pain [28]. In addition, pain signaling mechanisms may be organized within lipid rafts [29]. CAR, PSD-95, and ASIC3 have all been shown to reside in lipid rafts, a cellular environment that may enhance multimolecular complex formation or stability [17,30]. Taken together, it can be speculated that interaction with ASIC3 and PSD-95 may implicate CAR in pain sensitization.
In summary, apart from being a cell adhesion molecule and viral receptor, our findings indicate a new role for CAR as a trafficking molecule for cell surface signaling proteins. Our data suggest a model (Fig. 3D) whereby ASIC3 is retained within the cell when bound to PSD-95. However, when CAR is also bound to PSD-95, the complex now assumes the localization pattern of CAR, and the intracellular retention of the ASIC3-PSD-95 complex is relieved resulting in increased ASIC3 channel activity. These data present a novel function of CAR to modulate cell surface signaling, and have implications for neuronal development, plasticity, and pain, as well as understanding mechanisms behind changes in cellular signaling during viral infection.
Highlights.
CAR indirectly interacts with ASIC3 via a PDZ-based interaction with PSD-95
CAR mediates junctional localization of ASIC3 and PSD-95
CAR rescues PSD-95-mediated inhibition of ASIC3 currents
Acknowledgments
This work was supported by NIH/NIAID grant R15AI090625 to KJDAE, NIH/NHLBI grant HL076419 to CJB, and NIH grant R15NS070260 to EP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- CAR
Coxsackievirus and adenovirus receptor
- ASIC3
acid-sensing ion channel 3
- PSD-95
post-synaptic density 95
- PDZ
PSD-95/Drosophila discs-large protein/zonula occludens protein
- Ab
antibody
- IP
immunoprecipitation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 2.Excoffon KJ, Hruska-Hageman A, Klotz M, Traver GL, Zabner J. A role for the PDZ-binding domain of the coxsackie B virus and adenovirus receptor (CAR) in cell adhesion and growth. J Cell Sci. 2004;117:4401–4409. doi: 10.1242/jcs.01300. [DOI] [PubMed] [Google Scholar]
- 3.Honda T, Saitoh H, Masuko M, Katagiri-Abe T, Tominaga K, Kozakai I, Kobayashi K, Kumanishi T, Watanabe YG, Odani S, Kuwano R. The coxsackievirus-adenovirus receptor protein as a cell adhesion molecule in the developing mouse brain. Brain Res Mol Brain Res. 2000;77:19–28. doi: 10.1016/s0169-328x(00)00036-x. [DOI] [PubMed] [Google Scholar]
- 4.Soudais C, Laplace-Builhe C, Kissa K, Kremer EJ. Preferential transduction of neurons by canine adenovirus vectors and their efficient retrograde transport in vivo. FASEB J. 2001;15:2283–2285. doi: 10.1096/fj.01-0321fje. [DOI] [PubMed] [Google Scholar]
- 5.Persson A, Fan X, Widegren B, Englund E. Cell type- and region-dependent coxsackie adenovirus receptor expression in the central nervous system. J Neurooncol. 2006;78:1–6. doi: 10.1007/s11060-005-9055-3. [DOI] [PubMed] [Google Scholar]
- 6.Salinas S, Bilsland LG, Henaff D, Weston AE, Keriel A, Schiavo G, Kremer EJ. CAR-associated vesicular transport of an adenovirus in motor neuron axons. PLoS Pathog. 2009;5:e1000442. doi: 10.1371/journal.ppat.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patzke C, Max KE, Behlke J, Schreiber J, Schmidt H, Dorner AA, Kroger S, Henning M, Otto A, Heinemann U, Rathjen FG. The coxsackievirus-adenovirus receptor reveals complex homophilic and heterophilic interactions on neural cells. J Neurosci. 2010;30:2897–2910. doi: 10.1523/JNEUROSCI.5725-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sims K, Ahmed Z, Gonzalez AM, Read ML, Cooper-Charles L, Berry M, Logan A. Targeting adenoviral transgene expression to neurons. Mol Cell Neurosci. 2008;39:411–417. doi: 10.1016/j.mcn.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Coyne CB, Voelker T, Pichla SL, Bergelson JM. The coxsackievirus and adenovirus receptor interacts with the multi-PDZ domain protein-1 (MUPP-1) within the tight junction. J Biol Chem. 2004;279:48079–48084. doi: 10.1074/jbc.M409061200. [DOI] [PubMed] [Google Scholar]
- 10.Collins MO, Grant SG. Supramolecular signalling complexes in the nervous system. Subcell Biochem. 2007;43:185–207. doi: 10.1007/978-1-4020-5943-8_9. [DOI] [PubMed] [Google Scholar]
- 11.Han K, Kim E. Synaptic adhesion molecules and PSD-95. Prog Neurobiol. 2008;84:263–283. doi: 10.1016/j.pneurobio.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 13.Sluka KA, Winter OC, Wemmie JA. Acid-sensing ion channels: A new target for pain and CNS diseases. Curr Opin Drug Discov Devel. 2009;12:693–704. [PMC free article] [PubMed] [Google Scholar]
- 14.Qadri YJ, Rooj AK, Fuller CM. ENaCs and ASICs as therapeutic targets. Am J Physiol Cell Physiol. 2012;302:C943–965. doi: 10.1152/ajpcell.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Mello R, Marchand F, Pezet S, McMahon SB, Dickenson AH. Perturbing PSD-95 interactions with NR2B-subtype receptors attenuates spinal nociceptive plasticity and neuropathic pain. Mol Ther. 2011;19:1780–1792. doi: 10.1038/mt.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hruska-Hageman AM, Benson CJ, Leonard AS, Price MP, Welsh MJ. PSD-95 and Lin-7b interact with acid-sensing ion channel-3 and have opposite effects on H+-gated current. J Biol Chem. 2004;279:46962–46968. doi: 10.1074/jbc.M405874200. [DOI] [PubMed] [Google Scholar]
- 17.Eshcol JO, Harding AM, Hattori T, Costa V, Welsh MJ, Benson CJ. Acid-sensing ion channel 3 (ASIC3) cell surface expression is modulated by PSD-95 within lipid rafts. Am J Physiol Cell Physiol. 2008;295:C732–739. doi: 10.1152/ajpcell.00514.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Excoffon KJ, Gansemer N, Traver G, Zabner J. Functional effects of coxsackievirus and adenovirus receptor glycosylation on homophilic adhesion and adenoviral infection. J Virol. 2007;81:5573–5578. doi: 10.1128/JVI.02562-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: A possible mediator of myocardial ischemic sensation. Circ Res. 1999;84:921–928. doi: 10.1161/01.res.84.8.921. [DOI] [PubMed] [Google Scholar]
- 20.Huang C, Hu ZL, Wu WN, Yu DF, Xiong QJ, Song JR, Shu Q, Fu H, Wang F, Chen JG. Existence and distinction of acid-evoked currents in rat astrocytes. Glia. 2010;58:1415–1424. doi: 10.1002/glia.21017. [DOI] [PubMed] [Google Scholar]
- 21.Zha XM, Costa V, Harding AM, Reznikov L, Benson CJ, Welsh MJ. ASIC2 subunits target acid-sensing ion channels to the synapse via an association with PSD-95. J Neurosci. 2009;29:8438–8446. doi: 10.1523/JNEUROSCI.1284-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ridgway LD, Kim EY, Dryer SE. MAGI-1 interacts with Slo1 channel proteins and suppresses Slo1 expression on the cell surface. Am J Physiol Cell Physiol. 2009;297:C55–65. doi: 10.1152/ajpcell.00073.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou S, Pita-Almenar JD, Eskin A. Regulation of glutamate transporter GLT-1 by MAGI-1. J Neurochem. 2011;117:833–840. doi: 10.1111/j.1471-4159.2011.07250.x. [DOI] [PubMed] [Google Scholar]
- 24.Huang KC, Yasruel Z, Guerin C, Holland PC, Nalbantoglu J. Interaction of the Coxsackie and adenovirus receptor (CAR) with the cytoskeleton: binding to actin. FEBS Lett. 2007;581:2702–2708. doi: 10.1016/j.febslet.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Fok PT, Huang KC, Holland PC, Nalbantoglu J. The Coxsackie and adenovirus receptor binds microtubules and plays a role in cell migration. J Biol Chem. 2007;282:7512–7521. doi: 10.1074/jbc.M607230200. [DOI] [PubMed] [Google Scholar]
- 26.Carvajal-Gonzalez JM, Gravotta D, Mattera R, Diaz F, Perez Bay A, Roman AC, Schreiner RP, Thuenauer R, Bonifacino JS, Rodriguez-Boulan E. Basolateral sorting of the coxsackie and adenovirus receptor through interaction of a canonical YXXPhi motif with the clathrin adaptors AP-1A and AP-1B. Proc Natl Acad Sci U S A. 2012;109:3820–3825. doi: 10.1073/pnas.1117949109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim BK, Xiong D, Dorner A, Youn TJ, Yung A, Liu TI, Gu Y, Dalton ND, Wright AT, Evans SM, Chen J, Peterson KL, McCulloch AD, Yajima T, Knowlton KU. Coxsackievirus and adenovirus receptor (CAR) mediates atrioventricular-node function and connexin 45 localization in the murine heart. J Clin Invest. 2008;118:2758–2770. doi: 10.1172/JCI34777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao F, Tao YX, Mao P, Johns RA. Role of postsynaptic density protein-95 in the maintenance of peripheral nerve injury-induced neuropathic pain in rats. Neuroscience. 2003;117:731–739. doi: 10.1016/s0306-4522(02)00801-1. [DOI] [PubMed] [Google Scholar]
- 29.Dina OA, Hucho T, Yeh J, Malik-Hall M, Reichling DB, Levine JD. Primary afferent second messenger cascades interact with specific integrin subunits in producing inflammatory hyperalgesia. Pain. 2005;115:191–203. doi: 10.1016/j.pain.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 30.Ashbourne Excoffon KJ, Moninger T, Zabner J. The coxsackie B virus and adenovirus receptor resides in a distinct membrane microdomain. J Virol. 2003;77:2559–2567. doi: 10.1128/JVI.77.4.2559-2567.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]