Abstract
The incidence of cardiac hypertrophy, an established risk factor for heart failure, is generally lower in women compared with men, but this advantage is lost after menopause. Although it is widely believed that estrogens are cardioprotective, there are contradictory reports, including increased cardiac events in postmenopausal women receiving estrogens and enhanced cardiac protection from ischemic injury in female mice without estrogens. We exposed aromatase knockout (ArKO) mice, which produce no estrogens, to both pathologic and physiologic stimuli. This model allows an investigation into the effects of a complete, chronic lack of estrogens in male and female hearts. At baseline, female ArKO mice had normal-sized hearts but decreased cardiac function and paradoxically increased phosphorylation of many progrowth kinases. When challenged with the pathological stimulus, isoproterenol, ArKO females developed 2-fold more hypertrophy than wild-type females. In contrast, exercise-induced physiological hypertrophy was unaffected by the absence of estrogens in either sex, although running performance was blunted in ArKO females. Thus, loss of estrogen signaling in females, but not males, impairs cardiac function and sensitizes the heart to pathological insults through up-regulation of multiple hypertrophic pathways. These findings provide insight into the apparent loss of cardioprotection after menopause and suggest that caution is warranted in the long-term use of aromatase inhibitors in the setting of breast cancer prevention.
The heart responds to many stimuli by increasing in size via cardiac myocyte hypertrophy. There are two types of hypertrophy: pathologic and physiologic. Pathologic hypertrophy is characterized by an initial adaptive phase that can progress to dilation and cardiac dysfunction. Common causes of pathologic hypertrophy are hypertension, myocardial infarction, and mutations in sarcomeric proteins (1). On the other hand, physiologic hypertrophy resulting from chronic exercise remains adaptive and does not involve eventual dilation or cardiac dysfunction. Importantly, the signaling pathways that regulate pathologic and physiologic hypertrophy are distinct (2).
The actions of estrogens on the heart have been mainly studied in rodent models in the context of many pathologic stimuli, including myocardial infarction, aortic constriction, genetic lesions, and chemical treatments (reviewed in Ref. 3). However, animal studies regarding estrogens' role as pro- or antihypertrophic in response to pathologic stimuli have been conflicting. For example, some studies using ovariectomized females have suggested antihypertrophic effects of estrogens in pathologic cardiac hypertrophy (4, 5), whereas others have suggested a prohypertrophic role for estrogens (6, 7). Studies using female estrogen receptor (ER)α and ERβ knockout (KO) mice implicate ERβ as antihypertrophic, whereas ERα deletion has little effect on pathologic hypertrophy (8–10). There are many proposed mechanisms for modulation of cardiac hypertrophy by estrogens. These include activation of AKT (protein kinase B) (11), its activity as an antioxidant (12), modulation of cellular pH (13), regulation of calcium signaling (14), and inhibition of calcineriun degradation (4). Because pathways are considered individually in these studies, it is difficult to integrate them into a unified bigger picture. The question of whether estrogens modulate hypertrophy through the above mechanisms in response to all pathologic stimuli, or whether estrogens modulate different hypertrophic pathways depending on the type of pathologic stimulus, remains to be answered.
Each model of estrogen deprivation described above has significant limitations, because they rely on either ovariectomy or global ER deletions, neither of which completely abrogates estrogen action. Ovariectomy reduces circulating 17β-estradiol by only 50–80% and does not block local conversion of estrogen from testosterone, which may play an important role in the heart (15), and greatly reduces circulating progesterone, a hormone that can influence cardiac function (16). Circulating estrogen levels in ERαKO females are 10 times higher than normal (17), and although the ERβKO mice have normal circulating estrogen, they have elevated blood pressure and systemic hypoxia (8, 18). In addition, ERs have been shown to have ligand-independent actions (19); the phenotypes could therefore be due to blockade of the ligand-independent actions of the receptor rather than signaling initiated by interaction between the receptors and estrogen.
To address the effect of a chronic and complete lack of estrogens on the heart, we evaluated the pathologic and physiologic hypertrophic responses of the hearts of aromatase KO (ArKO) mice. ArKO mice lack an enzyme essential for production of estrogens, thus they completely lack circulating and locally produced estrogens (20). In addition to increased adiposity, impaired sexual behavior, increased testosterone, and compulsive behavior (21, 22), the hearts from female ArKO mice are protected from short-term ischemic injury (23). To our knowledge, we are the first to study cardiac hypertrophy and hypertrophic signaling in ArKO mice.
Materials and Methods
Experimental animals
Male and female C57Bl/6J mice that were heterozygous for a mutant ArKO gene were bred to generate the experimental groups: ArKO and wild type (WT) (20). 17β-Estradiol was administered sc to a subset of 2-month-old ArKO females via a 0.25-mg sustained release (90 d) pellet. Similar doses have been shown to result in circulating estradiol in the physiologic range (24). Because phytoestrogen-containing diets can partially compensate for the lack of estrogens in ArKO females (25), mice were fed ad libitum a phytoestrogen-free diet for at least one generation (Research Diets AIN76-A; Research Diets, Inc., New Brunswick, NJ). Mice were euthanized at 4 months of age, and morphometric parameters were recorded (Supplemental Tables 1–3, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Colorado at Boulder.
Isoproterenol (ISO) treatment
ISO was chronically administered to 4-month-old mice using a surgically implanted sc miniosmotic pump (model 2001; Alzet, Cupertino, CA) that released ISO in 0.9% NaCl at a rate of 30 mg/kg of body weight per day. Control pumps delivered 0.9% NaCl solution alone. Seven days after implantation of pumps, mice were euthanized and morphometric parameters were recorded.
Voluntary cage-wheel exercise
Four-month-old mice were given access to a cage wheel for seven or 21 d as described previously (26). Seven or 21 d after the initiation of exercise, hearts were harvested, and morphometric parameters were recorded and compared between control and exercised animals.
Echocardiography
Echocardiography was performed on animals at 4 months of age as previously described (27).
Quantitative PCR
Total RNA was isolated from mouse hearts using TRIzol-based RNA isolation (Roche Molecular Biochemicals, Indianapolis, IN). Real-time PCR was used to determine the expression levels of GATA-binding protein 4 (GATA-4), atrial natriuretic factor (ANF), NK2 homeobox 5 (Nkx2.5), modulatory calcineurin-interacting protein (MCIP)1.4, sarcoplasmic reticulum Ca2+-ATPase (SERCA), phospholambon, ERα, and G protein-coupled ER (GPER)1. All genes were normalized to 18s rRNA levels except for ERα and GPER1, which were normalized to glyceraldehyde 3-phosphate dehydrogenase. The primer sequences used are listed in Supplemental Table 4.
Phospho-kinase array
Human phospho-kinase antibody arrays (R&D Systems, Minneapolis, MN) were used to determine changes in phosphorylation of 46 different kinases according to the manufacturer's protocol. Cardiac lysates from three animals were pooled, and the arrays were incubated with equivalent amounts of protein.
Western blot analysis
Western immunoblotting was performed as previously described (28). The antibodies used were the following: phospho (p) AKT (193H12; Cell Signaling, Beverly, MA), pERK (9101; Cell Signaling), glycogen synthase kinase (GSK)3β (sc-7291; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), AKT (9272; Cell Signaling), ERK1/2 (9102; Cell Signaling), and pGSK3β (sc-11757; Santa Cruz Biotechnology, Inc.).
Ca2+/calmodulin-dependent protein kinase II (CaMK II) activity assay
CaMK II activity was determined using the SignaTECTCaMK II assay kit (Promega, Madison, WI) according to the manufacturer's protocol.
Statistical analyses
All data are reported as mean ± sem. The number of animals for each group is indicated in tables and figures. Significance was determined by using a two-way ANOVA for experiments that involved two or more independent variables, which was followed by a Tukey's post hoc test to examine interactions between specific groups. For experiments with only two experimental groups, a Student's t test was used. P < 0.05 was considered significant.
Results
Baseline morphologic and functional characteristics
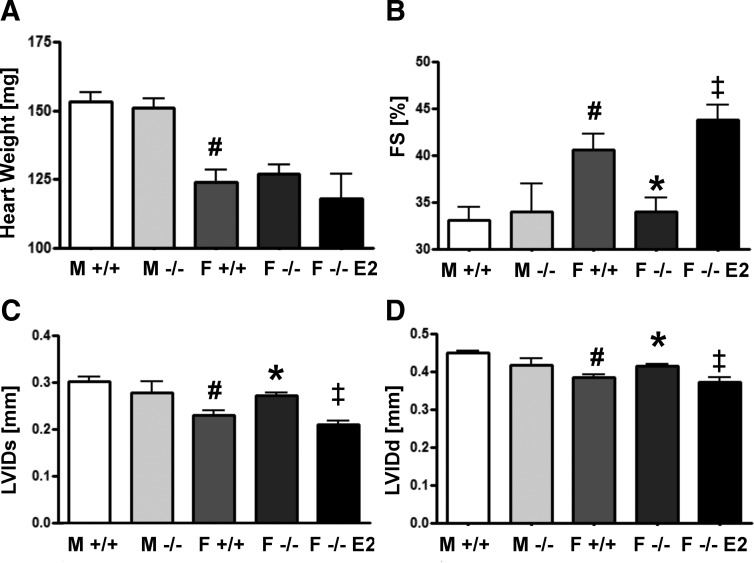
Male mice had increased cardiac mass (18%) compared with females, regardless of genotype. Neither deletion of aromatase in females nor treatment of ArKO females with 17β-estradiol affected cardiac mass (Fig. 1A). However, cardiac function, as measured by in vivo echocardiography, was affected by the presence of estrogens in females. Fractional shortening was reduced in ArKO females compared with WT females (34.0 vs. 40.6%). ArKO females also displayed significantly increased left ventricular internal dimension at systole (LVIDs) (0.27 vs. 0.23 mm) and at diastole (LVIDd) (0.41 vs. 0.38 mm) (Fig. 1, B–D). Treating ArKO females with 17β-estradiol for 2 months restored echocardiographic parameters to WT levels (Fig. 1, B–D), showing that 17β-estradiol supports cardiac function in females. Compared with WT males, WT females exhibited higher fractional shortening (40.6 vs. 33.1%), reduced LVIDs (0.23 vs. 0.30 mm), and reduced LVIDd (0.39 vs. 0.45 mm) (Fig. 1, B–D). In males, cardiac mass and function were unaffected by aromatase deletion (Fig. 1, A–D).
Fig. 1.
Baseline functional and morphometric parameters in 4-month-old mice. A, Heart weight of male and female mice of the indicated groups. In vivo cardiac functional parameters measured by echocardiography. B, Fractional shortening (FS%). C, LVIDs. D, LVIDd. M, Male; F, female. #, P < 0.05 relative to M +/+; *, P < 0.05 relative to F +/+; ‡, P < .05 relative to F −/−; n = 6–9 mice per group. Data are reported as mean ± sem. E2, 17β-estradiol.
Baseline differences in hypertrophic signaling pathways
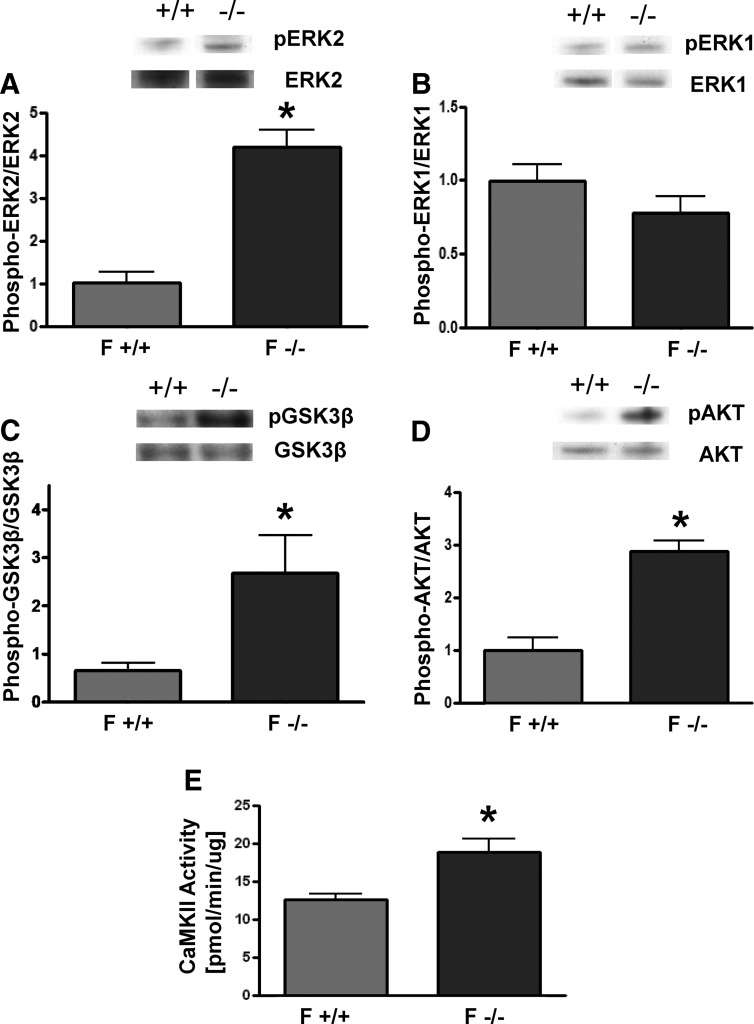
To determine the role of estrogens in modulating intracellular signaling, the phosphorylation states of 46 intracellular signaling kinases in the hearts of these mice were measured. Despite their equivalent weights, the hearts of ArKO females displayed a vastly different profile of activated kinases implicated in cardiovascular physiology when compared with their WT counterparts. In ArKO females compared with WT females, 21 kinases had elevated (>20% increased) phosphorylation, whereas only six had reduced (>20% decreased) phosphorylation (Table 1 and Supplemental Fig. 1). The up-regulation of pAKT and pGSK3β in ArKO females was confirmed by Western blotting (Fig. 2, C and D). Because the antibody array measured ERK1 and ERK2 together, we considered phosphorylation of these isoforms separately. The ratio of pERK2/ERK2 was 4-fold higher in ArKO females (Fig. 2A), whereas the ratio of pERK1/ERK1was unchanged (Fig. 2B) compared with WT females. CaMK II activity, which has been shown to be prohypertrophic (29), was also elevated in ArKO females (Fig. 2E). In contrast to females, male ArKO mice did not display increased pAKT/AKT or pERK2/ERK2 when compared with WT males (Supplemental Fig. 2).
Table 1.
Phosphorylation of selected kinases in ArKO and WT female mice
| Kinase: Phosphorylation site | Fold change ArKO/WT |
|---|---|
| Lower in ArKO | |
| P53: S392 | 0.52 |
| TOR: S2448 | 0.69 |
| c-Jun: S63 | 0.71 |
| HSP27: S78/S82 | 0.74 |
| p70 S6 kinase: T229 | 0.74 |
| PLCγ-1: Y783 | 0.79 |
| Unchanged in ArKO | |
| STAT4: Y693 | 0.81 |
| ERK1/2: T202/Y204, T185/Y187 | 0.83 |
| Src: Y419 | 0.89 |
| RSK1/2: S221 | 0.92 |
| Paxillin: Y118 | 0.93 |
| MSK1/2: S376/S360 | 0.97 |
| Pyk2: Y402 | 0.97 |
| MEK1/2: S218/S222 | 1.00 |
| P70 S6 kinase: T421/S424 | 1.02 |
| Hck: Y411 | 1.06 |
| STAT1: Y701 | 1.09 |
| STAT2: Y689 | 1.11 |
| eNOS: S1177 | 1.12 |
| Lyn: Y397 | 1.13 |
| P53: S15 | 1.15 |
| Yes: Y426 | 1.18 |
| Higher in ArKO | |
| Akt: Thr308 | 1.21 |
| P53: S46 | 1.22 |
| RSK1/2/3: S380 | 1.22 |
| P27: T157 | 1.23 |
| STAT5a/b: Y699 | 1.24 |
| STAT5b: Y699 | 1.28 |
| Chk-2: T68 | 1.36 |
| AMPKα2: T172 | 1.38 |
| STAT6: Y641 | 1.39 |
| CREB: S133 | 1.47 |
| β-Catenin | 1.52 |
| STAT5a: Y699 | 1.65 |
| P27: T198 | 1.74 |
| P38α: T180/Y182 | 1.77 |
| Fgr: Y412 | 2.79 |
| GSK-3α/&β: S21/S9 | 2.94 |
| Akt: Ser473 | 3.23 |
| FAK: Y397 | 3.73 |
| JNK pan: T183/Y185, T221/Y223 | 3.85 |
| Ampka1: T174 | 5.10 |
| STAT3: Y705 | 5.73 |
| Fyn: Y420 | 8.77 |
CREB, cAMP response element-binding protein; AMPKα2, AMP-activated protein kinase catalytic subunit 2.
Fig. 2.
Activity of selected kinases in female ArKO mouse hearts. A–D, above, Representative Western blottings in female WT and ArKO mice. Below, Quantification of pERK2/ERK2 (A), pERK1/ERK1 (B), pGSK3β/GSK3β (C), and pAKT/AKT (D). E, Quantification of CAMKII activity in cardiac lysates of female WT and ArKO mice. F, Female. *, P < 0.05 relative to F +/+, n = 4–5 animals per group. Data are reported as mean ± sem.
Response to ISO
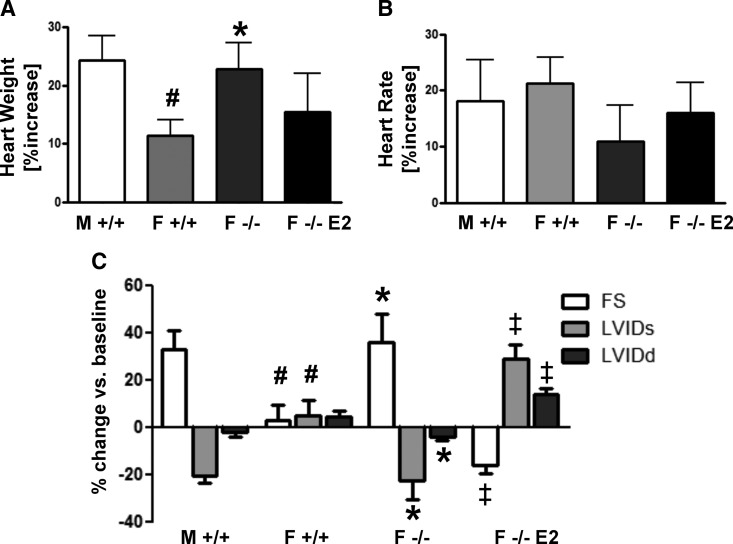
To test the hypothesis that estrogens protect female hearts from pathologic cardiac hypertrophy, WT and ArKO male and female mice were implanted with minipumps delivering a dose of ISO known to cause pathologic cardiac hypertrophy (30 mg/kg·d for 7 d) (29). Significant cardiac hypertrophy was induced in all groups studied when compared with vehicle controls. WT females developed less hypertrophy than WT males (11 vs. 24%) (Fig. 3A). This sex difference was abrogated in ArKO female hearts, which exhibited 2-fold more hypertrophy than WT females (23 vs. 11%) (Fig. 3A). 17β-Estradiol supplementation of ArKO females reduced the hypertrophic response to ISO (from 23 to 15%) (Fig. 3A). Importantly, no difference in cardiac growth in response to ISO was observed between male WT and male ArKO mice (Supplemental Table 1).
Fig. 3.
Morphometric and functional changes of WT and ArKO mice to 7 d of ISO treatment. Mice of the indicated sexes and genotypes treated with ISO for 7 d using implantable osmotic minipumps at a dose of 30 mg/kg·d. A, Percentage increase in absolute heart weight compared with vehicle controls. B, Percentage increase in heart rate compared with vehicle controls. C, The percent change after 7 d of ISO treatment compared with baseline of the following echocardiographic parameters. FS, Fractional shortening; M, male; F, female; E2, estrogen. #, P < .05 relative to M +/+; *, P < .05 relative to F +/+; ‡, P < .05 relative to F −/−; n = 5–10 animals per group. Data are reported as mean ± sem.
Pathologic hypertrophy is mediated by activation of the calcineurin/nuclear factor of activated T-cells, cytoplasmic 3 (NFATc3) pathway (30), and 17β-estradiol has been shown to negatively regulate the calcineriun/NFATc3 pathway in a pressure overload model (4). Therefore, we evaluated the expression levels of MCIP1.4, which is a direct transcriptional target of NFATc3, as a measure of pathway activation. MCIP1.4 was significantly lower at baseline in ArKO females compared with WT females and was induced 3-fold with ISO treatment in ArKO females. It was not significantly induced in WT females or ArKO females treated with estrogen (Supplemental Fig. 3, A and B). MCIP1.4 was also significantly elevated in males of both genotypes with ISO treatment (data not shown).
Cardiac function was monitored before and after ISO treatment by echocardiography. In general, the magnitude of ISO-induced changes in cardiac function paralleled the magnitude of the hypertrophic response. In male WT mice, fractional shortening increased by 33% and LVIDs decreased by 20% (Fig. 3C). This is in contrast to female WT mice, which did not have any significant change in fractional shortening or LVIDs (Fig. 3C), despite the increase in heart mass induced by ISO. As with heart mass, the functional response to ISO of the hearts of ArKO females was similar to WT males, with a 36% increase in fractional shortening and a 22% decrease in LVIDs (Fig. 3C). 17β-Estradiol treatment of ArKO females significantly changed their response to ISO, with an unexpected decrease in fractional shortening of 16% and an increase in LVIDs of 29%. The functional differences observed were not due to differences in heart rate differences under anesthesia between the groups studied (Fig. 3B).
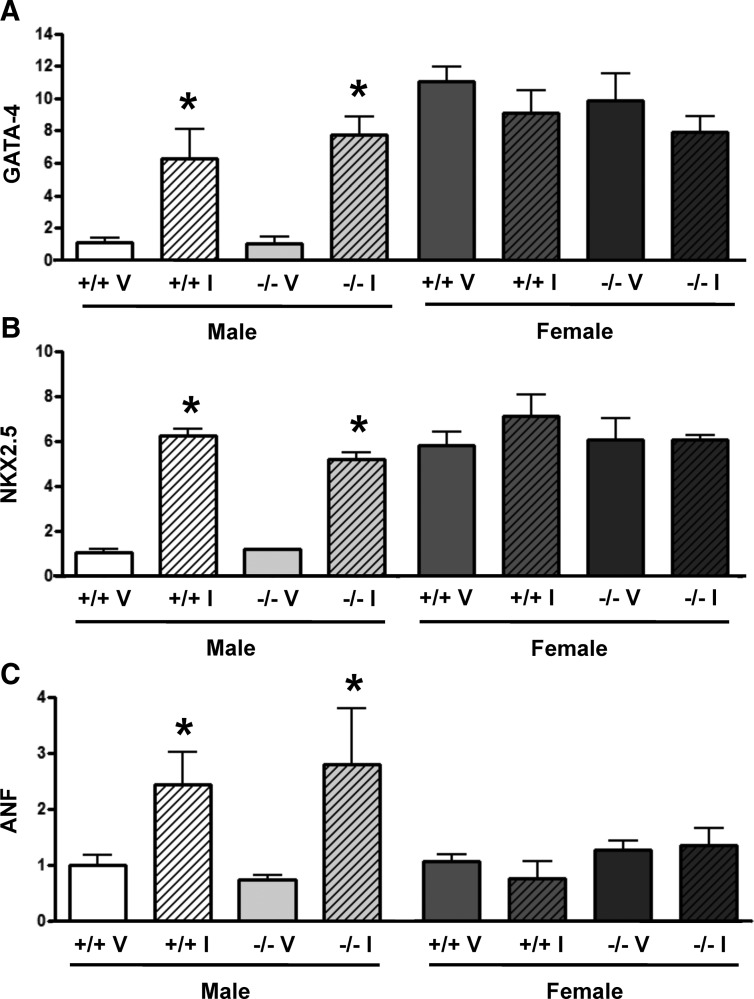
ISO infusion of male WT and ArKO mice induced GATA-4 (WT, 6.2-fold; ArKO, 7.4-fold), Nkx2.5 (WT, 5.1-fold; ArKO, 5.1-fold.), and ANF (WT, 2.4-fold; ArKO, 3.8-fold) (Fig. 4, A–C). In female WT and ArKO mice, ISO infusion did not induce expression of Gata-4, NKX2.5, or ANF (Fig. 4, A–C). However, the baseline levels of GATA-4 and NKX2.5 in the WT and ArKO females were approximately equivalent to males treated with ISO (Fig. 4, A and B).
Fig. 4.
Transcriptional response of WT and ArKO mice to 7 d of ISO (I) treatment. The RNA levels of GATA-4 (A), NKX2.5 (B), and ANF (C) were measured using quantitative RT-PCR and normalized to 18s. Data are reported as fold change of the indicated genes compared with male WT vehicle (V)-treated mice. *, P < 0.05 vs. vehicle control, n = 3–4 animals per group. Data are reported as mean ± sem.
Exercise response
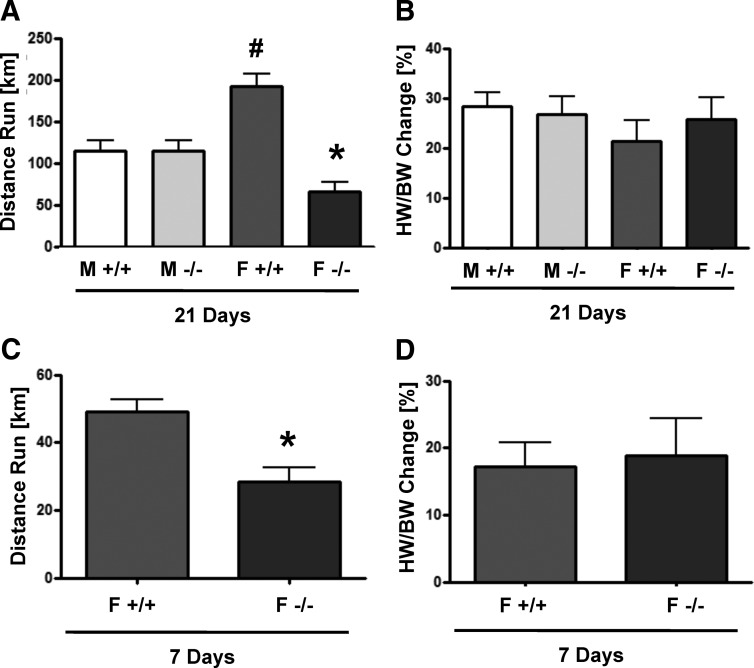
Because physiologic hypertrophy is mediated by different pathways compared with pathologic hypertrophy (2), we also tested the hypothesis that estrogens are antihypertrophic in the context of physiologic hypertrophy. WT and ArKO mice of both sexes were exercised for 21 d using a cage wheel. Voluntary wheel running induced significant cardiac hypertrophy in both sexes and genotypes. As has been previously reported (28), female WT mice ran farther than male WT mice (192 vs. 114 km) (Fig. 5A). ArKO females ran significantly less than WT females (66 vs. 192 km); a result that is in agreement with previous studies showing that estrogenic signaling in the brain increases wheel-running distance (31, 32). Although it seems logical to normalize to the amount run and conclude that ArKO mice had a greater hypertrophic response to exercise, we do not believe this is scientifically accurate. In both the current study and past studies in our lab (28), we do not see any correlation between the distance an individual animal run and the amount of its hypertrophy, thus we do not believe that this is a valid way to represent the data. Despite the difference in running distance, there was no significant difference in the hypertrophic response as measured by the percentage increase in heart weight normalized to body weight compared with sedentary controls (Fig. 5B).
Fig. 5.
Voluntary wheel-running performance and hypertrophic response of WT and ArKO mice. Mice of the indicated sexes and genotypes were exercised using a voluntary wheel. Total distance run after 21 d (A) and 7 d (C) of exercise. The percent increase in heart weight/body weight (HW/BW) compared with sedentary controls after 21 d (B) and 7 d (D) of exercise. M, Male; F, female. #, P < 0.05 relative to M +/+; *, P < 0.05 relative to F +/+, n = 6–11 animals per group. Data are reported as mean ± sem.
Due to concerns that 21 d of wheel running may represent a hypertrophic plateau and that significant differences in hypertrophy may have been masked at this late time point, we also compared the hypertrophic response of WT and ArKO females at 7 d of voluntary wheel running. Similar to the results obtained from 21 d of exercise, ArKO females ran less than WT females but developed equivalent hypertrophy (Fig. 5, C and D). These results support the conclusion that although estrogens clearly influence running distance, they do not seem to influence cardiac growth in response to a physiologic stimulus.
Discussion
The complete lack of estrogens modulates cardiac function and signaling in female mice
The effects of estrogens on baseline cardiac function have been investigated using various models. Global deletion of ERβ does not significantly affect baseline cardiac function in males or females (8, 9, 33, 34). To our knowledge, baseline cardiac function of global ERα nulls has not been published. However, multiple reports show that long-term reduction of estrogens by ovariectomy reduces cardiac contractility (35, 36). Our results provide further evidence that at baseline, loss of estrogens leads to reduced cardiac function in females that could account for increased incidence of cardiac disease in postmenopausal women. Further research is needed to demonstrate the roles of different ERs in this modulation of cardiac function.
Our study reveals a much broader down-regulation of prohypertrophic signaling pathways in female animals by estrogens than has been previously reported using ovariectomy or ERKOs. These include the Janus kinase/signal transducer and activator of transcription (STAT), MAPK, AKT, and focal adhesion kinase (FAK) pathways. Specific prohypertrophic kinases that are more phosphorylated in ArKO females include STAT3, AKT, cAMP response element-binding protein, GSK3α/β, P38α, c-Jun N-terminal kinase, FAK, and AMP-activated protein kinase catalytic subunit α1/2. Previous studies have investigated the effects of reducing circulating estrogens on hypertrophic cardiac signaling using various models of incomplete estrogen depletion (11–14). However, in contrast to the current study, these studies did not investigate the complicated interactions of the many kinase signaling pathways in the heart, which makes comparisons with our results difficult. Baseline differences were not seen in pERK1/ERK1 or pERK2/ERK2 in ERα or ERβ null females (8). Chronic depletion of estrogens in female mice by ovariectomy leads to increased levels of pp38/p38 but no change in levels of pAKT/AKT (both Ser473 and Thr308) (11). ERαKO mice display decreased pAKT/AKT at both Ser473 and Thr308 at baseline (37). Although this would seem to contradict our results, it should be noted that 17β-estradiol levels in the ERαKO are 10-fold higher than in WT females (17), thus the decreased levels of pAKT could be caused by a ERα-independent activity of increased circulating 17β-estradiol, such as increased signaling through other ERs, instead of reduced estrogen signaling through ERα. The effects of estrogen on hypertrophic signaling in myocytes in culture have also been studied (reviewed in Refs. 1, 38). These studies suggest that estrogen activates the phosphatidylinositol 3 kinase pathway and increases endothelial nitric oxide expression among many others. However, we do not believe that such short-term experiments using cultured myocytes, many of which use supraphysiologic levels of estrogen, are relevant to long-term in vivo studies.
It does not seem that estrogens directly regulate heart mass at baseline. ArKO females do not demonstrate increased cardiac mass (Fig. 1) (23), despite having many up-regulated hypertrophic pathways. Baseline differences in cardiac mass are also not observed in female ERαKO (8) or ERβKO mice (9). Understanding the mechanism for the resistance to these prohypertrophic pathways requires further research but is likely due to a compensatory down-regulation of other pathways that regulate cardiac mass, which may include reduced calcineriun/NFAT signaling at baseline in ArKO females, as shown by decreased MCIP1.4 expression (Supplemental Fig. 3A). To address possible compensatory up-regulation of ER that could possibly contribute to our phenotype, expression of ER or GPER1 in the hearts of WT and ArKO females was measured. No changes in the transcript levels of ER or GPER1 in ArKO females were observed (Supplemental Fig. 4); ER transcript levels were below the detection limit in this assay.
A possible confounding factor in the current study is the increased serum testosterone in ArKO mice (22). However, based on the available literature, we do not believe that increased serum testosterone affects the phenotype of ArKO mice. Androgen receptor KO mice do not display any difference in cardiac function or pAKT levels (39). Additionally, pAKT is only transiently activated in H9c2 cells treated with testosterone (39). In a separate study in neonatal rat ventricular myocytes, testosterone also only transiently increases pAKT and pERK (40). Both castration and flutamide treatment augment the increase of pAKT after ischemia reperfusion, suggesting that testosterone inhibits pAKT (41). In men, endogenous testosterone levels are inversely correlated with the incidence of cardiac hypertrophy, arguing against a direct prohypertrophic role of testosterone (42).
Effect of estrogens on hypertrophic signaling pathways and the hypertrophic response to ISO
Not surprisingly, given the broad up-regulation of prohypertrophic signaling pathways in ArKO females, these mice developed 2-fold more hypertrophy in response to ISO. Many different signaling pathways are involved in the induction of hypertrophy by ISO, including autocrine/paracrine signaling through IGF-I, angiotensin II, TGFβ, and intracellular signaling involving increased calcineurin, MAPK kinase 1, and rat sarcoma protein (reviewed in Ref. 43). Although the exact mechanism by which the up-regulated hypertrophic signaling molecules at baseline in ArKO females interact with the further activation of hypertrophic signaling molecules induced by ISO is not known, it is likely a cumulative effect of the many different pathways involved in the hypertrophic response, rather than just one pathway.
Our results indicate that estrogens act through multiple pathways to protect the female heart from pathologic cardiac growth and argue against the interpretation of estrogens as acting only through one pathway to influence cardiac growth, as is implied by many studies. Experiments are in progress to identify the mechanism for the broad modulation of hypertrophic signaling by estrogens.
Functional response to ISO
ISO, a β1- and β2-adrenoceptor agonist, is commonly used to experimentally induce pathologic cardiac hypertrophy characterized by increased wall thickness and decreased chamber size. The increase in fractional shortening induced by ISO is thought to be due to a prolonged action potential caused by increased cytosolic Ca2+ (43). ArKO females show some evidence of Ca2+ handling abnormalities in response to ISO that could lead to increased cytosolic Ca2+, specifically increased phospholambon expression without a concurrent increase in SERCA2A expression (Supplemental Fig. 3C). This is in agreement with abundant literature describing 17β-estradiol regulation of SERCA2A expression and activity (44, 45). We hypothesize that this may be responsible for the increased fractional shortening in response to ISO observed in ArKO females. Although supplementing ArKO females with 17β-estradiol decreased their hypertrophic response to ISO, it also led to reduced cardiac function and cardiac dilation. It is possible that the reduced cardiac function and increased dilation is related to the arrhythmia-inducing effect of exogenous 17β-estradiol (46).
Pathologic response is different between the sexes
WT males developed greater hypertrophy than WT females in response to ISO. In agreement with these results, other models of pathologic hypertrophy have shown greater hypertrophy in males than females, including Calstabin2 (FKB12.6) KO (14), pressure overload (8, 9), and β2-adrenergic receptor overexpression (6). However, the increased hypertrophy observed in our study in males is in contrast to a previous study from our laboratory that did not observe a sex-specific effect of ISO treatment (47). This difference could be due to the effects of phytoestrogens contained in the standard rodent diet. Multiple studies have shown an attenuation of pathologic hypertrophy in male mice eating a diet containing phytoestrogens (48, 49).
Our transcriptional analysis, shown in Fig. 4, suggests that ISO-induced pathologic hypertrophy is induced through different pathways in males vs. females and that this difference is not related to estrogen signaling and does not correlate with the amount of hypertrophy. Also, we show a profoundly higher baseline level of important progrowth transcription factors, NKX2.5 and GATA-4, in females compared with males. The mechanisms and implications of this result are currently under investigation.
Aromatase ablation does not change the hypertrophic response in males.
The importance of endogenous estrogens in modulating cardiac hypertrophy in males is unclear. ERβ deletion increased myocyte hypertrophy in male mice in response to pressure overload in one study (9). However, another study showed decreased hypertrophy in ERβKO males after pressure overload (8). No change in hypertrophy after pressure overload was seen in male ERαKO mice (8). Male mice lacking another ER, GPER, developed hypertrophy and display reduced cardiac function (50). However, although GPER is clearly important in the heart, its status as an ER remains controversial (51, 52). We did not see any effects on cardiac function, hypertrophy, or hypertrophic signaling at baseline or in response to ISO in male ArKO mice. Our results strongly suggest that estrogens do not play an important role in the male heart. It is possible that ERs have an important functional role in the male heart that does not require estrogen binding. Some evidence for this comes from reported effects on signaling by ER independently of estrogens (19).
Physiologic hypertrophy
Sex differences in the hypertrophic response of the heart to exercise have been published by multiple groups, with females developing more hypertrophy than males, but is unclear whether estrogens mediate this sex difference (28, 53). Thus, we hypothesized that the deprivation of estrogens would decrease cardiac hypertrophy in response to exercise. However, we did not see the expected sex difference in cardiac growth in response to exercise in WT males and females. We speculate that this apparent discrepancy between our results and previously published data on sex differences in physiologic hypertrophy is a result of our mice being raised on a phytoestrogen-free diet (casein based), whereas the published studies used mice fed the traditional phytoestrogen-rich soy diet. In fact, our lab previously published a study showing that changing the diet from animals fed a soy diet to a casein-based diet reduces or eliminates the sex difference in mice with genetic heart disease (27). Furthermore, ArKO female mice fed the standard rodent chow have partial rescue of uterine phenotypes compared with animals fed a soy-free diet (25).
Despite their high levels of hypertrophic kinases, ArKO females did not display a greater hypertrophic response to wheel running. Because AKT activation has been implicated in physiologic hypertrophy, we measured pAKT/AKT levels after 7 d of running. Interestingly, pAKT was induced by exercise in WT but not ArKO females (data not shown). The level of pAKT/AKT in WT females after running was equivalent to the ArKO sedentary females. Thus, it seems the ArKO females already had sufficient activated AKT for physiologic hypertrophy. The pathways that are induced by wheel-running in ArKO mice remain to be elucidated.
Relevance to human health
The benefits and risks of hormone replacement therapy remain unresolved, especially with respect to the cardiovascular effects of combined administration of conjugated equine estrogens and progesterone (54). Importantly, our studies directly address the possible negative cardiovascular effect of use of aromatase inhibitors to treat breast cancer (55) and as a prophylactic treatment to prevent breast cancer in healthy postmenopausal women (56). Our results show a direct and dramatic effect of complete aromatase inhibition on baseline cardiac function and pathologic cardiac hypertrophy in females. In addition, we found that many important hypertrophic signaling pathways are up-regulated at baseline in ArKO mice. Whether the same responses would be seen in postmenopausal women treated with aromatase inhibitors remains an unanswered question. Although trials with aromatase inhibitors did not show a significant increase in cardiovascular events (57), none of these trials monitored cardiac performance before and after treatment. In light of the data presented here, it would be prudent to monitor cardiac function before, during, and after aromatase inhibitor treatment. Given the wealth of literature on the modulation of cardiac function by estrogens, it seems reasonable to assume that long-term aromatase inhibition would affect cardiac function, especially given the possibility of underlying heart disease.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health Grant HL50560 (to L.A.L.) and by the American Heart Association Postdoctoral Fellowship 11POST7780011 (to P.A.H.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AKT
- Protein kinase B
- ANF
- atrial natriuretic factor
- ArKO
- aromatase KO
- CaMK II
- Ca2+/calmodulin-dependent protein kinase II
- ER
- estrogen receptor
- FAK
- focal adhesion kinase
- GATA-4
- GATA-binding protein 4
- GPER
- G protein-coupled ER
- GSK
- glycogen synthase kinase
- ISO
- isoproterenol
- KO
- knockout
- LVIDd
- LVID at diastole
- LVIDs
- left ventricular internal dimension at systole
- MCIP
- modulatory calcineurin-interacting protein
- NFATc3
- nuclear factor of activated T-cells, cytoplasmic 3
- Nkx2.5
- NK2 homeobox 5
- p
- phospho
- SERCA
- sarcoplasmic reticulum Ca2+-ATPase
- STAT
- signal transducer and activator of transcription
- WT
- wild type.
References
- 1. Bhupathy P, Haines CD, Leinwand LA. 2010. Influence of sex hormones and phytoestrogens on heart disease in men and women. Womens Health 6:77–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rohini A, Agrawal N, Koyani CN, Singh R. 2010. Molecular targets and regulators of cardiac hypertrophy. Pharmacol Res 61:269–280 [DOI] [PubMed] [Google Scholar]
- 3. Arias-Loza PA, Jazbutyte V, Pelzer T. 2008. Genetic and pharmacologic strategies to determine the function of estrogen receptor α and estrogen receptor β in cardiovascular system. Gend Med 5(Suppl A):S34–S45 [DOI] [PubMed] [Google Scholar]
- 4. Donaldson C, Eder S, Baker C, Aronovitz MJ, Weiss AD, Hall-Porter M, Wang F, Ackerman A, Karas RH, Molkentin JD, Patten RD. 2009. Estrogen attenuates left ventricular and cardiomyocyte hypertrophy by an estrogen receptor-dependent pathway that increases calcineurin degradation. Circ Res 104:265–275, 211p following 275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brower GL, Gardner JD, Janicki JS. 2003. Gender mediated cardiac protection from adverse ventricular remodeling is abolished by ovariectomy. Mol Cell Biochem 251:89–95 [PubMed] [Google Scholar]
- 6. Thireau J, Aimond F, Poisson D, Zhang B, Bruneval P, Eder V, Richard S, Babuty D. 2010. New insights into sexual dimorphism during progression of heart failure and rhythm disorders. Endocrinology 151:1837–1845 [DOI] [PubMed] [Google Scholar]
- 7. Dent MR, Tappia PS, Dhalla NS. 2010. Gender differences in cardiac dysfunction and remodeling due to volume overload. J Card Fail 16:439–449 [DOI] [PubMed] [Google Scholar]
- 8. Skavdahl M, Steenbergen C, Clark J, Myers P, Demianenko T, Mao L, Rockman HA, Korach KS, Murphy E. 2005. Estrogen receptor-β mediates male-female differences in the development of pressure overload hypertrophy. Am J Physiol Heart Circ Physiol 288:H469–H476 [DOI] [PubMed] [Google Scholar]
- 9. Fliegner D, Schubert C, Penkalla A, Witt H, Kararigas G, Kararigas G, Dworatzek E, Staub E, Martus P, Ruiz Noppinger P, Kintscher U, Gustafsson JA, Regitz-Zagrosek V. 2010. Female sex and estrogen receptor-β attenuate cardiac remodeling and apoptosis in pressure overload. Am J Physiol Regul Integr Comp Physiol 298:R1597–R1606 [DOI] [PubMed] [Google Scholar]
- 10. Pedram A, Razandi M, Lubahn D, Liu J, Vannan M, Levin ER. 2008. Estrogen inhibits cardiac hypertrophy: role of estrogen receptor-β to inhibit calcineurin. Endocrinology 149:3361–3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhuiyan MS, Shioda N, Fukunaga K. 2007. Ovariectomy augments pressure overload-induced hypertrophy associated with changes in Akt and nitric oxide synthase signaling pathways in female rats. Am J Physiol Endocrinol Metab 293:E1606–E1614 [DOI] [PubMed] [Google Scholar]
- 12. Satoh M, Matter CM, Ogita H, Takeshita K, Wang CY, Dorn GW, 2nd, Liao JK. 2007. Inhibition of apoptosis-regulated signaling kinase-1 and prevention of congestive heart failure by estrogen. Circulation 115:3197–3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kili A, Javadov S, Karmazyn M. 2009. Estrogen exerts concentration-dependent pro-and anti-hypertrophic effects on adult cultured ventricular myocytes. Role of NHE-1 in estrogen-induced hypertrophy. J Mol Cell Cardiol 46:360–369 [DOI] [PubMed] [Google Scholar]
- 14. Xin HB, Senbonmatsu T, Cheng DS, Wang YX, Copello JA, Ji GJ, Collier ML, Deng KY, Jeyakumar LH, Magnuson MA, Inagami T, Kotlikoff MI, Fleischer S. 2002. Oestrogen protects FKBP12.6 null mice from cardiac hypertrophy. Nature 416:334–338 [DOI] [PubMed] [Google Scholar]
- 15. Thum T, Borlak J. 2002. Testosterone, cytochrome P450, and cardiac hypertrophy. FASEB J 16:1537–1549 [DOI] [PubMed] [Google Scholar]
- 16. Arias-Loza PA, Hu K, Frantz S, Dienesch C, Bayer B, Wu R, Ertl G, Pelzer T. 2011. Medroxyprogesterone acetate aggravates oxidative stress and left ventricular dysfunction in rats with chronic myocardial infarction. Toxicol Pathol 39:867–878 [DOI] [PubMed] [Google Scholar]
- 17. Rissman EF, Wersinger SR, Taylor JA, Lubahn DB. 1997. Estrogen receptor function as revealed by knockout studies: neuroendocrine and behavioral aspects. Horm Behav 31:232–243 [DOI] [PubMed] [Google Scholar]
- 18. Morani A, Barros RP, Imamov O, Hultenby K, Arner A, Warner M, Gustafsson JA. 2006. Lung dysfunction causes systemic hypoxia in estrogen receptor β knockout (ERβ−/−) mice. Proc Natl Acad Sci USA 103:7165–7169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qin X, Wang XH, Yang ZH, Ding LH, Xu XJ, Cheng L, Niu C, Sun HW, Zhang H, Ye QN. 2008. Repression of NFAT3 transcriptional activity by estrogen receptors. Cell Mol Life Sci 65:2752–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Honda S, Harada N, Ito S, Takagi Y, Maeda S. 1998. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem Biophys Res Commun 252:445–449 [DOI] [PubMed] [Google Scholar]
- 21. Boon WC, Horne MK. 2011. Aromatase and its inhibition in behaviour, obsessive compulsive disorder and parkinsonism. Steroids 76:816–819 [DOI] [PubMed] [Google Scholar]
- 22. Britt KL, Stanton PG, Misso M, Simpson ER, Findlay JK. 2004. The effects of estrogen on the expression of genes underlying the differentiation of somatic cells in the murine gonad. Endocrinology 145:3950–3960 [DOI] [PubMed] [Google Scholar]
- 23. Bell JR, Mellor KM, Wollermann AC, Ip WT, Reichelt ME, Meachem SJ, Simpson ER, Delbridge LM. 2011. Aromatase deficiency confers paradoxical postischemic cardioprotection. Endocrinology 152:4937–4947 [DOI] [PubMed] [Google Scholar]
- 24. Patten RD, Pourati I, Aronovitz MJ, Alsheikh-Ali A, Eder S, Force T, Mendelsohn ME, Karas RH. 2008. 17β-Estradiol differentially affects left ventricular and cardiomyocyte hypertrophy following myocardial infarction and pressure overload. J Card Fail 14:245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Britt KL, Simpson ER, Findlay JK. 2005. Effects of phytoestrogens on the ovarian and pituitary phenotypes of estrogen-deficient female aromatase knockout mice. Menopause 12:174–185 [DOI] [PubMed] [Google Scholar]
- 26. Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. 2001. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol 90:1900–1908 [DOI] [PubMed] [Google Scholar]
- 27. Stauffer BL, Konhilas JP, Luczak ED, Leinwand LA. 2006. Soy diet worsens heart disease in mice. J Clin Invest 116:209–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Konhilas JP, Maass AH, Luckey SW, Stauffer BL, Olson EN, Leinwand LA. 2004. Sex modifies exercise and cardiac adaptation in mice. Am J Physiol Heart Circ Physiol 287:H2768–H2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr, Bers DM, Brown JH. 2003. The δC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res 92:912–919 [DOI] [PubMed] [Google Scholar]
- 30. Wilkins BJ, Molkentin JD. 2004. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun 322:1178–1191 [DOI] [PubMed] [Google Scholar]
- 31. Fahrbach SE, Meisel RL, Pfaff DW. 1985. Preoptic implants of estradiol increase wheel running but not the open field activity of female rats. Physiol Behav 35:985–992 [DOI] [PubMed] [Google Scholar]
- 32. Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. 2003. Estrogen increases locomotor activity in mice through estrogen receptor α: specificity for the type of activity. Endocrinology 144:230–239 [DOI] [PubMed] [Google Scholar]
- 33. Pelzer T, Loza PA, Hu K, Bayer B, Dienesch C, Calvillo L, Couse JF, Korach KS, Neyses L, Ertl G. 2005. Increased mortality and aggravation of heart failure in estrogen receptor-β knockout mice after myocardial infarction. Circulation 111:1492–1498 [DOI] [PubMed] [Google Scholar]
- 34. Babiker FA, Lips D, Meyer R, Delvaux E, Zandberg P, Janssen B, van Eys G, Grohé C, Doevendans PA. 2006. Estrogen receptor β protects the murine heart against left ventricular hypertrophy. Arterioscler Thromb Vasc Biol 26:1524–1530 [DOI] [PubMed] [Google Scholar]
- 35. Scheuer J, Malhotra A, Schaible TF, Capasso J. 1987. Effects of gonadectomy and hormonal replacement on rat hearts. Circ Res 61:12–19 [DOI] [PubMed] [Google Scholar]
- 36. Paigel AS, Ribeiro RF, Jr, Fernandes AA, Targueta GP, Vassallo DV, Stefanon I. 2011. Myocardial contractility is preserved early but reduced late after ovariectomy in young female rats. Reprod Biol Endocrinol 9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pedram A, Razandi M, O'Mahony F, Lubahn D, Levin ER. 2010. Estrogen receptor-β prevents cardiac fibrosis. Mol Endocrinol 24:2152–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murphy E, Lagranha C, Deschamps A, Kohr M, Nguyen T, Wong R, Sun J, Steenbergen C. 2011. Mechanism of cardioprotection: what can we learn from females? Pediatr Cardiol 32:354–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ikeda Y, Aihara K, Akaike M, Sato T, Ishikawa K, Ise T, Yagi S, Iwase T, Ueda Y, Yoshida S, Azuma H, Walsh K, Tamaki T, Kato S, Matsumoto T. 2010. Androgen receptor counteracts Doxorubicin-induced cardiotoxicity in male mice. Mol Endocrinol 24:1338–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Altamirano F, Oyarce C, Silva P, Toyos M, Wilson C, Lavandero S, Uhlén P, Estrada M. 2009. Testosterone induces cardiomyocyte hypertrophy through mammalian target of rapamycin complex 1 pathway. J Endocrinol 202:299–307 [DOI] [PubMed] [Google Scholar]
- 41. Huang C, Gu H, Zhang W, Herrmann JL, Wang M. 2010. Testosterone-down-regulated Akt pathway during cardiac ischemia/reperfusion: a mechanism involving BAD, Bcl-2 and FOXO3a. J Surg Res 164:e1–e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Svartberg J, von Mühlen D, Schirmer H, Barrett-Connor E, Sundfjord J, Jorde R. 2004. Association of endogenous testosterone with blood pressure and left ventricular mass in men. The Tromso study. Eur J Endocrinol 150:65–71 [DOI] [PubMed] [Google Scholar]
- 43. Osadchii OE. 2007. Cardiac hypertrophy induced by sustained β-adrenoreceptor activation: pathophysiological aspects. Heart Fail Rev 12:66–86 [DOI] [PubMed] [Google Scholar]
- 44. Bupha-Intr T, Wattanapermpool J. 2006. Regulatory role of ovarian sex hormones in calcium uptake activity of cardiac sarcoplasmic reticulum. Am J Physiol Heart Circ Physiol 291:H1101–H1108 [DOI] [PubMed] [Google Scholar]
- 45. Liu CG, Xu KQ, Xu X, Huang JJ, Xiao JC, Zhang JP, Song HP. 2007. 17β-Oestradiol regulates the expression of Na+/K+-ATPase β1-subunit, sarcoplasmic reticulum Ca2+-ATPase and carbonic anhydrase iv in H9C2 cells. Clin Exp Pharmacol Physiol 34:998–1004 [DOI] [PubMed] [Google Scholar]
- 46. Yan S, Chen Y, Dong M, Song W, Belcher SM, Wang HS. 2011. Bisphenol A and 17β-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS One 6:e25455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maass AH, Ikeda K, Oberdorf-Maass S, Maier SK, Leinwand LA. 2004. Hypertrophy, fibrosis, and sudden cardiac death in response to pathological stimuli in mice with mutations in cardiac troponin T. Circulation 110:2102–2109 [DOI] [PubMed] [Google Scholar]
- 48. Cox KB, Liu J, Tian L, Barnes S, Yang Q, Wood PA. 2009. Cardiac hypertrophy in mice with long-chain acyl-CoA dehydrogenase or very long-chain acyl-CoA dehydrogenase deficiency. Lab Invest 89:1348–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li J, Xie ZZ, Tang YB. 2010. Genistein prevents myocardial hypertrophy in 2-kidney 1-clip renal hypertensive rats by restoring eNOS pathway. Pharmacology 86:240–248 [DOI] [PubMed] [Google Scholar]
- 50. Delbeck M, Golz S, Vonk R, Janssen W, Hucho T, Isensee J, Schäfer S, Otto C. 2011. Impaired left-ventricular cardiac function in male GPR30-deficient mice. Mol Med Report 4:37–40 [DOI] [PubMed] [Google Scholar]
- 51. Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, Wang ZY. 2010. Involvement of estrogen receptor variant ER-α36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol 24:709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Langer G, Bader B, Meoli L, Isensee J, Delbeck M, Noppinger PR, Otto C. 2010. A critical review of fundamental controversies in the field of GPR30 research. Steroids 75:603–610 [DOI] [PubMed] [Google Scholar]
- 53. Foryst-Ludwig A, Kreissl MC, Sprang C, Thalke B, Böhm C, Benz V, Gürgen D, Dragun D, Schubert C, Mai K, Stawowy P, Spranger J, Regitz-Zagrosek V, Unger T, Kintscher U. 2011. Sex differences in physiological cardiac hypertrophy are associated with exercise-mediated changes in energy substrate availability. Am J Physiol Heart Circ Physiol 301:H115–H122 [DOI] [PubMed] [Google Scholar]
- 54. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. 2002. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women's health initiative randomized controlled trial. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- 55. Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, Buyse M, Baum M, Buzdar A, Colleoni M, Coombes C, Snowdon C, Gnant M, Jakesz R, Kaufmann M, Boccardo F, Godwin J, Davies C, Peto R. 2010. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 28:509–518 [DOI] [PubMed] [Google Scholar]
- 56. Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, McTiernan A, Robbins J, Johnson KC, Martin LW, Winquist E, Sarto GE, Garber JE, Fabian CJ, Pujol P, Maunsell E, Farmer P, Gelmon KA, Tu D, Richardson H. 2011. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 364:2381–2391 [DOI] [PubMed] [Google Scholar]
- 57. Ewer MS, Glück S. 2009. A woman's heart: the impact of adjuvant endocrine therapy on cardiovascular health. Cancer 115:1813–1826 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.