Abstract
The G protein α-subunit Gsα mediates receptor-stimulated cAMP generation. Heterozygous inactivating Gsα mutations on the maternal allele result in obesity primarily due to reduced energy expenditure in Albright hereditary osteodystrophy patients and in mice. We previously showed that mice with central nervous system (CNS)-specific Gsα deletion on the maternal allele (mBrGs KO) also develop severe obesity with reduced energy expenditure and that Gsα is primarily expressed from the maternal allele in the paraventricular nucleus (PVN) of the hypothalamus, an important site of energy balance regulation. We now generated mice with PVN-specific Gsα deficiency by mating Single-minded 1-cre and Gsα-floxed mice. Homozygous Gsα deletion produced early lethality. Heterozygotes with maternal Gsα deletion (mPVNGsKO) also developed obesity and had small reductions in energy expenditure. However, this effect was much milder than that found in mBrGsKO mice and was more prominent in males. We previously showed mBrGsKO mice to have significant reductions in melanocortin receptor agonist-stimulated energy expenditure and now show that mBrGsKO mice have impaired cold-induced brown adipose tissue stimulation. In contrast, these effects were absent in mPVNGsKO mice. mPVNGsKO mice also had minimal effects on glucose metabolism as compared with mBrGsKO mice. Consistent with the presence of Gsα imprinting, paternal heterozygotes showed no changes in energy or glucose metabolism. These results indicate that although Gsα deficiency in PVN partially contributes to the metabolic phenotype resulting from maternal Gsα mutations, Gsα imprinting in other CNS regions is also important in mediating the CNS effects of Gsα mutations on energy and glucose metabolism.
Monogenic obesity disorders provide important clues about genes and mechanisms involved in energy and glucose metabolism that may be altered in more common forms of obesity. One such disorder is Albright hereditary osteodystrophy (AHO), which is caused by heterozygous inactivating mutations of a ubiquitously expressed G protein α-subunit (Gsα) that couples hormone, neurotransmitter, and other receptors to stimulation of adenylyl cyclase and generation of intracellular cAMP (1). Although skeletal and other physical abnormalities are found in all AHO patients, multihormone resistance and obesity only develop in patients in which the mutation is on the maternal allele (2). These parent-of-origin effects are caused by tissue-specific Gsα imprinting, an epigenetic phenomenon in which Gsα is preferentially expressed from the maternal allele in certain tissues. In tissues affected by Gsα imprinting, mutation of the active maternal Gsα allele leads to severe Gsα deficiency, whereas mutation of the inactive paternal allele has little effect on Gsα expression.
Similar to what is observed in humans, maternal (but not paternal) germline Gsα mutations also lead to obesity, as well as insulin-resistant diabetes, in mice (3). Obesity in maternal Gsα mice (E1m−) is due primarily to reduced sympathetic nervous system activity (SNA) and energy expenditure with no primary effect on food intake (3). These findings are likely relevant to humans because AHO patients with maternal Gsα mutations were reported to have very low serum norepinephrine levels, which is a marker of SNA (4), and weight gain in the absence of hyperphagia was well documented in one AHO patient (5). The importance of reduced SNA and energy expenditure in the development of the metabolic phenotype was confirmed by the observation that obesity and insulin-resistant diabetes developed in mice with central nervous system (CNS)-specific Gsα deletion on the maternal allele (mBrGs KO) but not in mice with CNS-specific paternal Gsα deletion (pBrGsKO) (6).
Melanocortins, such as α-MSH, act in the CNS via Gsα-coupled receptors (primarily MC4R) to promote negative energy balance by inhibiting food intake and stimulating SNA and energy expenditure (7, 8). MC4R mutations are the most common cause of human monogenic obesity (9) and also lead to obesity in mice (10), in both cases the result of both hyperphagia and reduced SNA and energy expenditure. MC4R pathways also regulate glucose metabolism and insulin sensitivity (11, 12). Unlike normal mice, MC4RKO mice do not acutely inhibit food intake or stimulate energy expenditure in response to the MC3/4R agonist melanotan II (MTII) (13). In contrast, mBrGsKO mice inhibit food intake normally in response to MTII, but their ability to stimulate energy expenditure in response to MTII is impaired (6).
MC4R receptors are expressed in many CNS regions, including regions known to be involved in regulation of energy balance such as the paraventricular nucleus (PVN) of the hypothalamus (14, 15). MC4R receptors in PVN neurons are activated by proopiomelanocortin neurons releasing α-MSH and inhibited by neuropeptide Y/agouti-related peptide neurons, both of which originate in the arcuate nucleus of the hypothalamus (16–17). Interestingly, reexpression of MC4R in PVN of MC4RKO mice leads to reversal of hyperphagia and partially reverses obesity but has little effect on the reduced energy expenditure (18). We showed that Gsα is imprinted in PVN (6), and therefore maternal Gsα deletion in this region may be important in the pathogenesis of obesity in AHO patients. In the present study we use Single minded 1 (Sim1)-cre mice, which express cre recombinase in PVN and a few other CNS sites, to generate mice with PVN-specific Gsα deletion (18). We show that maternal Gsα deletion in PVN partially contributes to obesity and reduced energy expenditure resulting from maternal Gsα mutations, although other CNS regions are also important in mediating the effect of Gsα mutation on energy expenditure. Moreover, our results indicate that melanocortin effects on food intake in PVN are mediated via Gsα-independent pathways.
Materials and Methods
Animals
Female mice with loxP sites surrounding Gsα exon 1 (E1fl/fl, E1fl/+) (3) were mated with male Sim1-cre mice [gift from B. Lowell (Beth Israel Deaconess Medical Center)], in which cre recombinase is strongly expressed in PVN, supraoptic nucleus, nucleus of the lateral olfactory tract, and posterior hypothalamus, with more scattered expression in other brain regions (18), to generate mPVNGsKO mice. pPVNGsKO mice were generated with reciprocal crosses. mBrGsKO and pBrGsKO mice were generated using Nestin-cre as previously described (6). All mice were maintained on a Black Swiss background. E1+/+or E1fl/+:cre− littermates were used as controls. Mice were genotyped by PCR as previously described (3). Animals were maintained on a 12-h light, 12-h dark cycle (0600 h/1800 h) and standard pellet diet (NIH-07, 5% fat by weight). All experiments were performed on 12- to 16-wk-old mice unless otherwise indicated. Studies were approved by the NIDDK Animal Care and Use Committee.
Food intake, body composition, and metabolism
Body composition, energy expenditure, activity levels, and food intake were measured as previously described (6). Energy expenditure was determined over a 24-h period by indirect calorimetry using a four-chamber Oxymax system (Columbus Instruments, Columbus, OH). Mice were acclimated for 1 d in the metabolic chamber at room temperature before measurements. Measurements at both 21–23 C and 30 C were performed in mice that were housed at ambient room temperature. For measurements at 30 C the temperature in the chamber was raised to 30 C in the morning, and measurements were begun 1 h after raising the temperature. Resting energy expenditure (REE) was determined as the means of points measured when the animal was not ambulating. Day (light cycle) was 0600 h to 1800 h and night (dark cycle) was 1800h to 0600 h.
MTII injections
Single-caged mice received 200 μg MTII ip (Sigma, St. Louis, MO) or vehicle (PBS, 100 μl ip) 30 min before lights out, and food intake was measured for 3.5 h in the dark. For energy expenditure, mice were placed in indirect calorimetry chambers for 24 h at 30 C and received MTII (10 μg/g ip) the following day at 1200 h. Resting O2 consumption was measured for 3 h before and 3 h after injection, excluding the first hour after injection.
Blood pressure, heart rate, and body temperature
Blood pressure and heart rate were measured with a BP-2000 Specimen platform (Visitech, Newtown, PA). Core body temperature was measured with a TH-5 rectal probe (Thermalet, Houston, TX) inserted 1 cm deep at room temperature and hourly during 4 C exposure in mice caged individually without bedding. Food and water were provided ad libitum.
Biochemical assays
RIA kits were used to measure serum insulin, leptin, adiponectin, and free T4 (Linco Research, Inc., Billerica, MA), T3 (Siemens, Deerfield, IL), and corticosterone levels (MP Biomedicals, Solon, OH). Free fatty acids and triglycerides were measured with reagents from Roche (Branchburg, NJ) and Thermo Scientific (Waltham, MA), respectively. Serum glucose levels were measured with a Glucometer Elite (Bayer Corp., Tarrytown, NJ). For glucose and insulin tolerance tests, overnight-fasted mice received glucose (2 mg/g ip) or insulin (Humulin, 0.75 mIU/g ip) and glucose was measured from tail blood. Brown adipose tissue (BAT) catecholamine levels were measured as previously described (6).
RNA analysis
Quantitative in situ hybridization of brain slices using sense and antisense Gsα exon 1 mRNA probes was performed as previously described (6). Quantitative RT-PCR was performed as previously described (19).
Statistical analysis
Data are expressed as mean ± sem. Statistical significance was determined by two-tailed unpaired t test or ANOVA with differences considered significant at P < 0.05.
Results
Generation of mice with Gsα haploinsufficiency in PVN
Mice with heterozygous maternal or paternal allele Gsα deletion in PVN (mPVNGsKO or pPVNGsKO) were generated by reciprocal crosses of Gsα-floxed mice (E1fl/fl, E1fl/+) with Sim1-cre mice. The number of mutants at weaning was consistent with expected Mendelian ratios. Complete loss of Gsα in PVN may be embryonically lethal, because breeding between heterozygotes (E1fl/+:Sim1-cre+) did not generate any homozygotes (E1fl/fl:Sim1-cre+) in 18 litters (143 offspring).
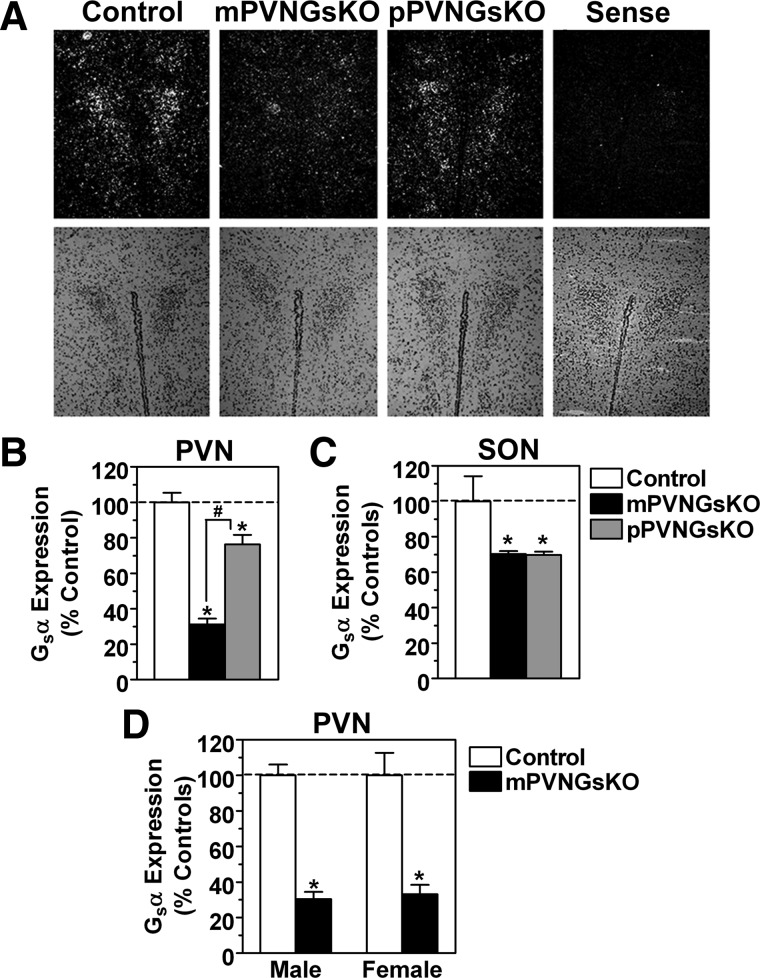
Based on in situ hybridization, Gsα expression was more markedly reduced in mPVNGsKO mice (31% of control) than in pPVNGsKO mice (76% of control) (Fig. 1, A and B), similar to previous findings in both germline and brain-specific Gsα knockout mice (6). Gsα expression was similarly reduced in PVN of male and female mPVNGsKO mice (Fig. 1D). It is interesting to note that PVN Gsα expression in maternal germline Gsα knockout mice (E1m−) was reduced to even lower levels (∼16% of control) (6), which may reflect the additional loss of Gsα expression in nonneuronal PVN supporting cells in E1m− mice or possibly that recombination by Sim-cre is not fully efficient. Gsα expression was similarly reduced in the supraoptic nucleus of mPVNGsKO and pPVNGsKO mice (both 70 ± 2% of control) (Fig 1C), consistent with lack of Gsα imprinting in this region. Gsα expression was unaffected in other brain areas (cerebral cortex, cerebellum; data not shown).
Fig. 1.
Gsα expression in PVNGsKO mice. A, In situ hybridization with antisense Gsα-specific probe in PVN of control, mPVNGsKO, and pPVNGsKO mice. Sense indicates the result of a control mouse using sense Gsα (dark field, upper panels; hematoxylin and eosin, lower panels). B and C, Quantitation of Gsα expression in PVN (panel B) and supraoptic nucleus (SON, panel C) by in situ hybridization in control (open bars), mPVNGsKO (solid bars), and pPVNGsKO mice (gray bars) (expressed as % control, n = 9–24/group). D, Quantitation of in situ hybridization for PVN Gsα expression in male and female mPVNGsKO mice and their controls. *, P < 0.05 vs. control; #, P < 0.05 vs. mPVNGsKO by one-way ANOVA.
mPVNGsKO mice have mild changes in body weight and composition
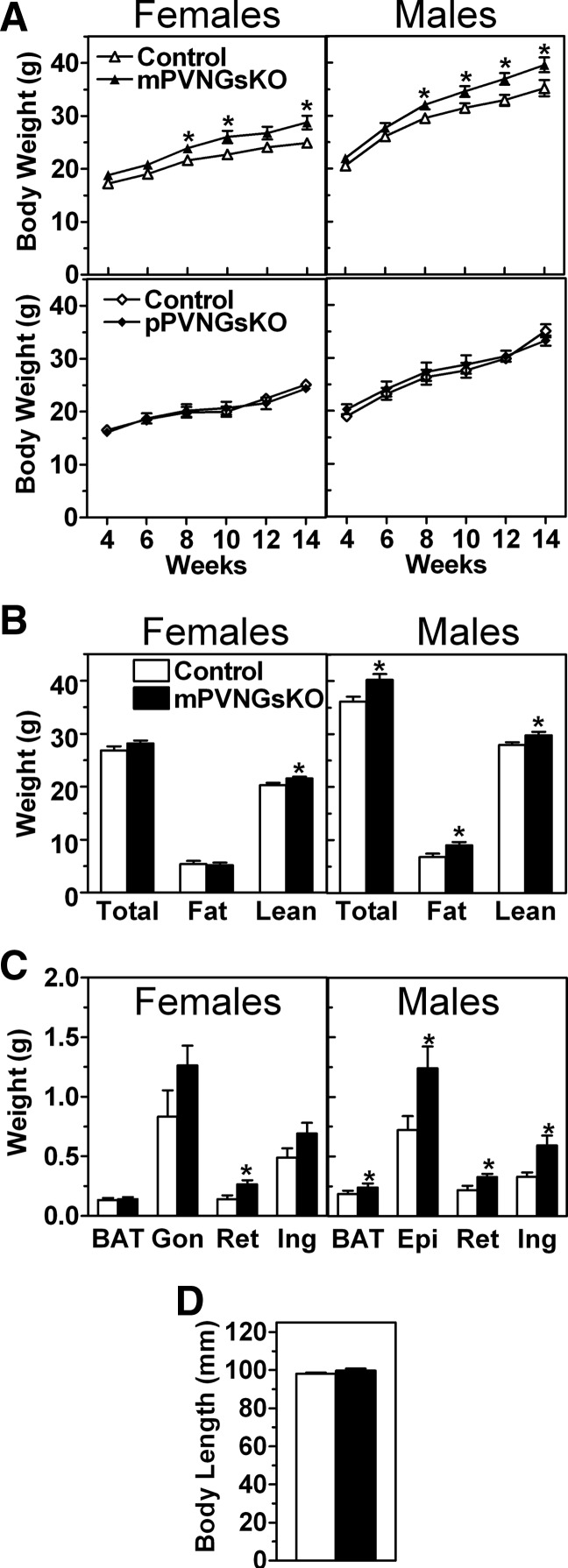
Male mPVNGsKO mice gained more weight than control mice starting at 6 wk of age (Fig. 2A), with an associated increase in both fat mass (Fig. 2B) and brown and white adipose tissue (BAT, WAT) pad weights (Fig. 2C). Consistent with these findings, male mPVNGsKO mice had increased serum triglyceride levels and tended to have higher leptin levels (Table 1). Similar to what was shown previously in mBrGsKO mice (6), the increased weight of mPVNGsKO mice was not due to an increase in body length (Fig. 2D). Female mPVNGsKO mice also gained more weight than controls, although the effect was very small (Fig. 2A) This small change in body weight was associated with a small but significant increase in lean mass with no detectable change in fat mass as measured by NMR (Fig. 2B) or in serum triglyceride or leptin levels (Table 1). However WAT pad weights tended to be slightly higher in female mPVNGsKO mice, with a statistically significant increase in the retroperitoneal WAT pad (Fig. 2C).
Fig. 2.
Effect of PVN-specific Gsα deficiency on body weight and composition. A, Body weight vs. age of female (left panels) and male (right panels) mPVNGsKO (upper panels) and pPVNGsKO (lower panels) mice and their respective control littermates (n = 9–18/group). B, Body composition of 12- to 16-wk-old female (left) and male (right) mPVNGsKO mice and their respective controls (n = 11–13/group) C, Fat pad weights in 6- to 8-month-old female (left panel) and male (right panel) mPVNGsKO mice and their controls (n = 6–8/group; BAT, interscapular BAT; Gon, gonadal; Ret, retroperitoneal; Ing, inguinal; Epi, epididymal). D, Body length in male mPVNGsKO mice and controls (n = 13–17/group). *, P < 0.05 vs. control by t test.
Table 1.
Serum chemistries in mPVNGsKO, pPVNGsKO, and control mice
| Male |
Female |
Male |
Female |
|||||
|---|---|---|---|---|---|---|---|---|
| mControl | mPVNGsKO | mControl | mPVNGsKO | pControl | pPVNGsKO | pControl | pPVNGsKO | |
| Glucose (fast; mg/dl) | 112 ± 17 | 126 ± 18 | 82 ± 4 | 90 ± 5 | 66 ± 1 | 63 ± 3 | 55 ± 2 | 58 ± 5 |
| Glucose (fed; mg/dl) | 223 ± 17 | 243 ± 29 | 175 ± 13 | 179 ± 16 | 264 ± 18 | 304 ± 32 | 195 ± 21 | 186 ± 18 |
| Insulin (fed; ng/ml) | 4.4 ± 0.9 | 12.5 ± 3.1a | 1.0 ± 0.3 | 1.2 ± 0.3 | 3.9 ± 1.3 | 4.2 ± 1.5 | 1.5 ± 0.6 | 1.1 ± 0.2 |
| FFA (mm) | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.2 | 0.7 ± 0.1 | 0.7 ± 0.1 |
| TG (mg/dl) | 274 ± 22 | 395 ± 34a | 134 ± 27 | 141 ± 25 | 233 ± 49 | 276 ± 36 | 211 ± 42 | 118 ± 16 |
| Leptin (ng/ml) | 10.9 ± 1.8 | 14.8 ± 1.0 | 9.9 ± 2.1 | 11.0 ± 1.6 | 12.5 ± 1.0 | 16.1 ± 2.0 | 11.2 ± 1.3 | 12.0 ± 1.5 |
| T3 (ng/ml) | 1.72 ± 0.08 | 1.80 ± 0.10 | ND | ND | ND | ND | ND | ND |
| Free T4 (μg/dl) | 4.22 ± 0.35 | 3.95 ± 0.26 | ND | ND | ND | ND | ND | ND |
| Corticosterone (ng/ml) | 312 ± 37 | 200 ± 45 | ND | ND | ND | ND | ND | ND |
Data are presented as mean ± sem; n = 6–14/group. TG, Triglyceride; FFA, free fatty acid; ND, not done.
, P < 0.05.
Body weight gain in mPVNGsKO mice was much less than the body weight gain that we previously reported in mBrGsKO mice (6). At 14 wk of age, male mBrGsKO mice were 32% heavier than their controls (46.5 ± 0.8 vs. 35.4 ± 0.8 g) (6), whereas male mPVNGsKO mice were only 13% heavier than controls (39.6 ± 1.4 vs. 35.2 ± 1.6 g). Similarly, female mBrGsKO mice were 37% heavier than their controls (37.1 ± 1.5 vs. 27.1 ± 1.7 g) (6), whereas female mPVNGsKO mice were 16% heavier than controls (28.8 ± 1.3 vs. 24.9 ± 0.8 g). These differences in adiposity between mBrGsKO and mPVNGsKO are still present in mice that are being bred simultaneously in our laboratory. Consistent with the previously reported parent-of-origin effect in BrGsKO mice, pPVNGsKO mice had normal growth rate, fat mass, and serum triglyceride and leptin levels (Fig. 2A, lower panel, Table 1, and data not shown). Similar to what was previously reported for mBrGsKO mice (6), there were no differences in serum free fatty acid, corticosterone, and thyroid hormone levels between mutants and controls (Table 1).
mPVNGsKO mice have minimal changes in energy balance
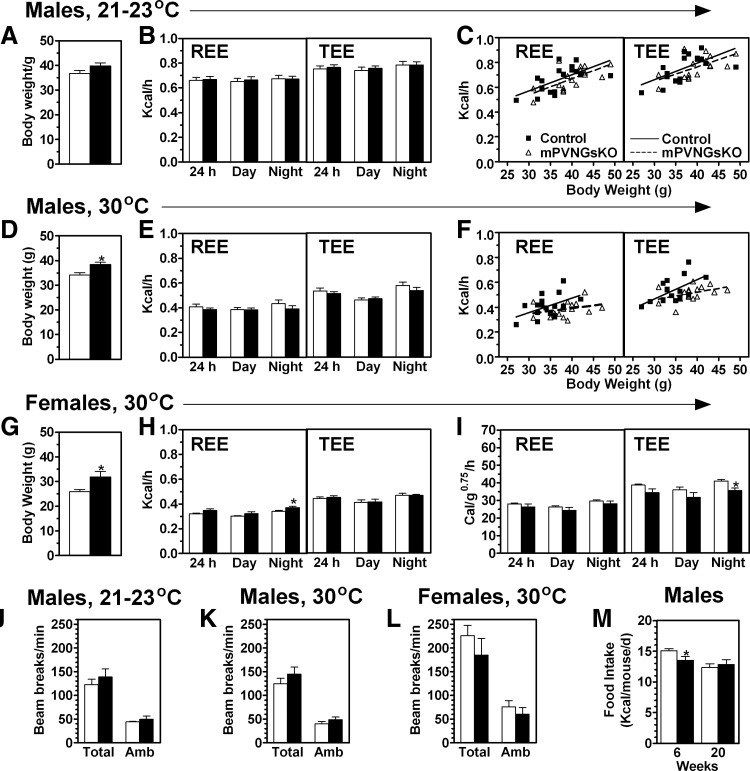
We previously showed that male and female mBrGsKO mice have significantly reduced resting and total energy expenditure rates (REE and TEE, respectively) normalized by body weight when measured at room temperature (23 C) (6). Mean REE and TEE rates were unaffected in male mPVNGsKO mice at 23 C (Fig. 3, A and B), and linear regressions showed mPVNGsKO and control mice to have similar relationships between REE and TEE vs. body weight (Fig. 3C). In contrast, we have previously shown mBrGsKO mice to have a rightward shift in the TEE regression line at 23 C (6). When mice raised and housed at room temperature had the same measurements performed at 30 C (thermoneutrality), both REE and TEE tended to be slightly lower in mPVNGsKO mice, particularly at night, although the differences were not significant (Fig. 3, D and E). Linear regressions showed both groups to have similar REE and TEE at lower body weights, with a tendency for REE and TEE to be lower in mPVNGsKO mice at higher body weights (Fig. 3F). Female mPVNGsKO and control mice had similar absolute REE and TEE rates at 30 C (Fig. 3, G and H). Females also had no differences in REE when normalized by body weight, but TEE tended to be lower over 24 h, and the differences were significant during the night (Fig. 3I). Whereas mBrGsKO mice were shown previously to have reduced activity levels (6), physical activity levels were unaffected in male and female mPVNGsKO mice (Fig. 3, J and K). Respiratory exchange ratios (vCO2/cO2) were also unaffected in male and female mPVNGsKO mice (data not shown). Male mPVNGsKO mice had no increase in food intake compared with controls at either 6 or 20 wk of age (Fig. 3M). Overall, these results suggest that the small increase in adiposity in mPVNGsKO mice (particularly in males), is most likely due to small reductions in energy expenditure, although these effects are much more subtle than the changes observed in E1m− (3) and mBrGsKO mice (6).
Fig. 3.
Energy balance studies in mPVNGsKO mice. A–C, Body weight (panel A), REE and TEE, respectively (panel B), and linear regressions of 24 h REE and TEE vs. body weight (panel C; control, filled squares, solid lines; mPVNGsKO: open triangles, dashed lines) measured at room temperature (21–23 C) in 12- to 16-wk-old male control and mPVNGsKO mice (n = 17–18/group). D–F, Body weight (panel D), absolute REE and TEE (panel E) and (F) linear regressions of 24 h REE and TEE vs. body weight (panel F) measured at 30 C in 12- to 16-wk-old male control and mPVNGsKO mice (n = 17/group). G–I, Body weight (panel G), absolute REE and TEE (panel H), and normalized REE and TEE (panel I) measured at 30 C in 16- to 18-wk-old female control and mPVNGsKO mice (n = 6/group). Absolute and normalized REE and TEE are shown as mean data measured over a 24-h period as well as during the day and night periods. J–L, Total and ambulatory (Amb) activity measured over 24 h in males at room temperature (panel J), males at 30 C (panel K), and females at 30 C (panel L) during the metabolic studies shown in panels A–I. M, Daily food intake in male mPVNGsKO mice and controls at 6 and 20 wk (n = 6–9/group). *, P < 0.05 vs. control by t test.
Melanocortin actions and thermogenesis remain relatively intact in mPVNGsKO mice
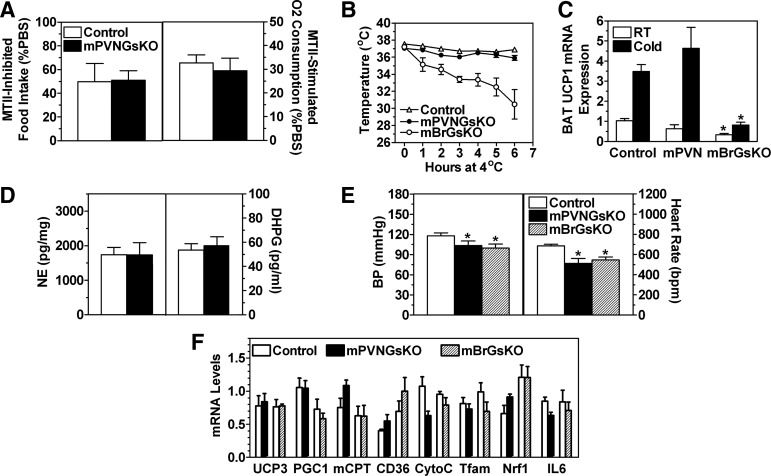
We previously showed that the melanocortin MC3/4R agonist MTII is able to inhibit food intake normally in mBrGsKO mice, whereas its ability to increase energy expenditure is impaired in these mice (6). In contrast, the ability of MTII to acutely inhibit food intake and stimulate REE was unaffected in mPVNGsKO mice (Fig. 4A), suggesting that reduced Gsα expression in PVN does not account for the impaired MTII-stimulated energy expenditure response observed in mBrGsKO mice.
Fig. 4.
Effect of PVN-specific Gsα deficiency on thermogenesis and cold tolerance. A, Food intake measured for 3.5 h after 200 μg MTII ip (left; expressed as % as that consumed after PBS injection) and % increase in energy expenditure after 10 μg/g MTII ip (right) in 5-month-old male mPVNGsKO and control mice (n = 6–10/group). B, Rectal temperature in 8- to 10-month-old mPVNGsKO, mBrGsKO, and control mice during exposure to 4 C environment (n = 5–7/group). C, BAT UCP1 mRNA expression in the same three groups of mice at room temperature (RT) and after 6 h of cold (4 C) exposure (n = 5–7/group). D, BAT norepinephrine (NE, left) and dihydroxyphenylglycol (DHPG, right) concentrations in male mPVNGsKO (solid bars) and control mice (open bars) (n = 6–8/group). E, Systolic blood pressure (left) and heart rate (right) in male mPVNGsKO, mBrGsKO, and control mice (n = 6–8/group). F, Gene expression in quadriceps muscles from mPVNGsKO and mBrGsKO mice and their respective control littermates (n = 4–6/group). PGC1, PPARγ coactivator 1α; mCPT, mitochondrial carnitine-palmitoyltransferase; CytoC, cytochrome C; Tfam, transcription factor A mitochondrial; Nrf1, nuclear respiratory factor 1. *, P < 0.05 vs. control by t test.
We also compared the cold-induced thermogenic response in mBrGsKO and mPVNGsKO mice. This response is mediated by sympathetic nervous system (SNS) stimulation of BAT in response to cold exposure, which induces uncoupling protein 1 (UCP1) expression and lipid oxidation to generate heat and maintain body temperature, a process that is MC4R dependent (20). mBrGsKO mice had a clear impairment in cold-induced BAT activation, as they had a lower temperature at room temperature (mBrGsKO 37.2 ± 0.2 vs. control 37.7 ± 0.2 C; P < 0.05), and could not maintain their body temperature at 4 C (Fig. 4B). Consistent with this, they also had significantly reduced BAT UCP1 mRNA expression at baseline with poor induction after 6 h of cold exposure (Fig. 4C). In contrast, mPVNGsKO mice maintained normal body temperatures at baseline and at 4 C (Fig. 4B) and had normal baseline and cold-induced BAT UCP1 mRNA levels (Fig. 4C). Consistent with differential effects on SNA in BAT of mBrGsKO and mPVNGsKO mice, mBrGsKO mice were previously shown to have reduced levels of norepinephrine and its metabolite dihydroxyphenylglycol in BAT (6), whereas these levels were unaffected in mPVNGsKO mice (Fig. 4D). In addition, BAT PGC-1α mRNA levels were reduced by more than 50% in mBrGsKO mice but were unaffected in mPVNGsKO mice (data not shown). These results indicate that PVN-specific Gsα deficiency does not account for the impaired cold-induced SNS activation of BAT observed in mBrGsKO mice. These differential effects on SNS activation may be tissue specific, because both blood pressure and heart rate measured simultaneously in mBrGsKO and mPVNGsKO were similarly reduced in both groups of mice (Fig. 4E). The results for mBrGsKO mice are consistent with prior published results showing reduced blood pressure and heart rate in a separate cohort of mBrGsKO mice (6). We also found no differences in expression of genes involved in fatty acid oxidation or mitochondrial function in skeletal muscle of either mBrGsKO or mPVNGsKO mice while on regular diet (Fig. 4F).
Abnormal glucose metabolism in mPVNGsKO mice
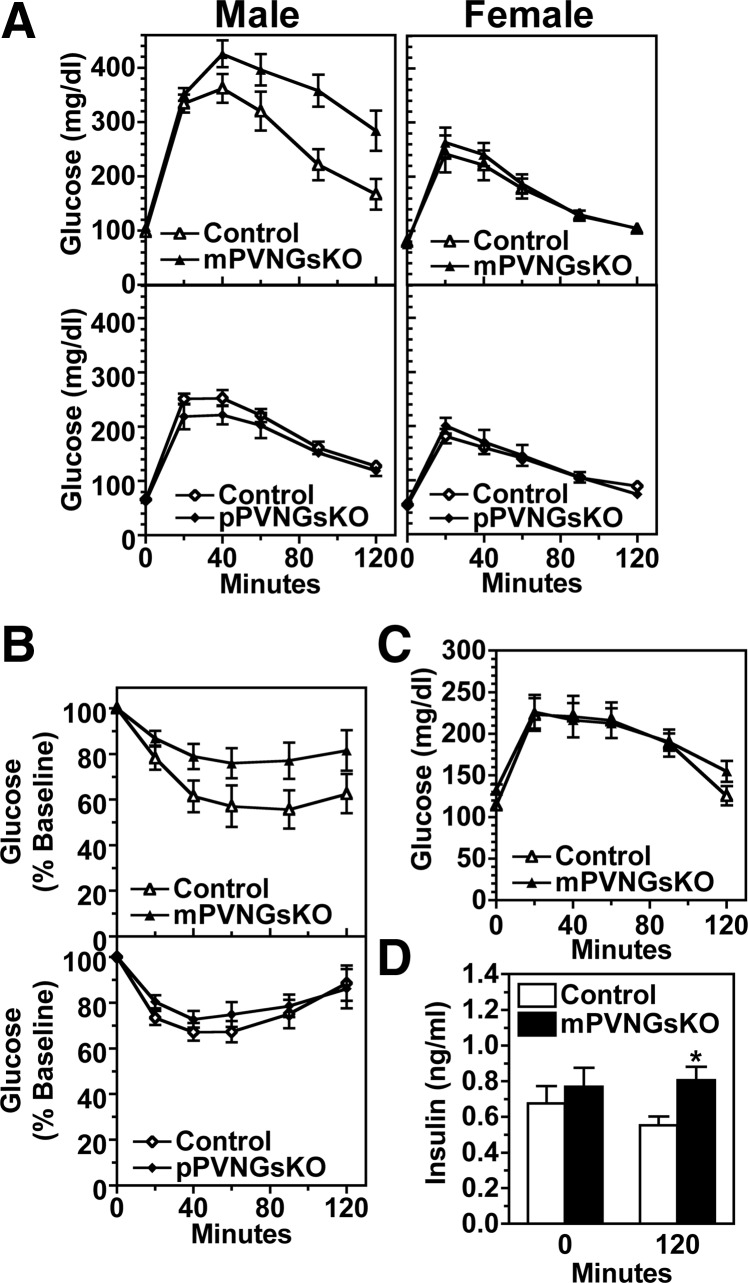
Both fed and fasted blood glucose levels were similar in mPVNGsKO and control mice (Table 1). Fed insulin levels were significantly higher in male mPVNGsKO mice but were unaffected in female mutants (Table 1). Glucose and insulin tolerance tests showed that 12 wk-old male mPVNGsKO mice developed glucose intolerance and insulin resistance (Fig. 5, A and B). However, this effect was sex-specific, because female mPVNGsKO mice maintained normal glucose tolerance (Fig 5A), which may reflect sex-specific differences in adiposity and other metabolic parameters. Similar to what we previously observed in pBrGsKO mice (6), pPVNGsKO mice showed no abnormalities in glucose metabolism (Fig. 5A and Table 1), consistent with a parent-of-origin effect of PVN-specific Gsα mutation on glucose metabolism. Whereas we showed previously that mBrGsKO mice developed glucose intolerance and hyperinsulinemia before the development of obesity (6 wk) (6), 6 wk-old male mPVNGsKO had normal glucose tolerance and baseline insulin levels, although insulin levels were mildly elevated at 120 min after glucose injection (Fig. 5, C and D). These findings suggest that, in contrast to whole-brain maternal Gsα deletion, maternal Gsα deletion limited to PVN does not significantly affect glucose metabolism independent of its effect on obesity.
Fig. 5.
Effect of PVN-specific Gsα deficiency on glucose metabolism. A, Glucose tolerance tests in 12-wk-old male (left panels) and female (right panels) mPVNGsKO (upper panels) and pPVNGsKO mice (lower panels), and their respective controls (n = 5–9/group). B, Insulin tolerance tests in 16- to 20-wk-old male mPVNGsKO (upper panel) and pPVNGsKO mice (lower panel) and their respective controls (n = 11–14/group). C, Glucose tolerance test in 6-wk-old male mPVNGsKO and control mice (n = 10–12/group). D, Serum insulin levels at baseline (time 0) and 120 min after injection during the glucose tolerance test shown in panel C. *, P < 0.05 vs. control by t test.
Discussion
In the present study, we investigated the metabolic consequences of PVN-specific Gsα deficiency. Because a truly PVN-specific cre-expressing mouse is unavailable, we used Sim1-cre mice in which cre is strongly expressed in PVN, supraoptic nucleus, nucleus of the lateral olfactory tract, and posterior hypothalamus, and to a lesser extent in other brain regions (18). In situ hybridization in mPVNGsKO and pPVNGsKO mice confirmed Gsα imprinting with preferential expression from the maternal allele in PVN. The presence of mild obesity and glucose intolerance in mPVNGsKO, but not pPVNGsKO, mice provides evidence that PVN-specific Gsα deficiency may contribute to the parent-of-origin effects of Gsα mutation on energy balance in mice (and possibly in AHO patients). The metabolic effects are more prominent in male mPVNGsKO mice, but this does not appear to be due to differences in Gsα expression because Gsα expression in PVN was similarly reduced in male and female mPVNGsKO mice. We have ruled out a role of Gsα deficiency in the supraoptic nucleus in the parent-of-origin-specific metabolic phenotype because Gsα expression in this region was similarly reduced in mPVNGsKO and pPVNGsKO mice. However, we cannot rule out the possibility that Gsα deficiency in Sim1 neurons located at other sites (e.g. nucleus of the lateral olfactory tract, posterior hypothalamus) may contribute to the mild phenotypic changes that we observed.
MC4R mutations result in several physiological consequences, including hyperphagia, reduced SNA and energy expenditure, increased body length, and abnormal glucose metabolism (9–10, 21–22). Like mBrGsKO mice, mPVNGsKO mice did not have all the features seen with MC4R mutations, such as hyperphagia and increased linear growth, and maintained a normal anorexic response to a melanocortin agonist. This may be relevant to humans as obesity in the absence of hyperphagia was documented in a child with a maternal Gsα mutation (5). Consistent with our findings, PVN-specific deletion of cAMP-response element binding protein 1, a downstream target of Gsα, also leads to obesity with reduced energy expenditure in the absence of hyperphagia (23). PVN is an important site for regulation of food intake by MC4R (15, 18). However, our results suggest that this response in PVN is not Gsα mediated. Interestingly, mutation of Sim1, a relatively PVN-specific gene, in humans and mice results in obesity due to hyperphagia and increased body length with little effect on energy expenditure or glucose metabolism (24–27), MC4RKO-related effects opposite to those seen with Gsα deficiency. In addition, opposite to what is seen with Gsα mutations in mBrGsKO mice, Sim1 mutations lead to an impaired anorexic response to MTII, with no effect on MTII stimulation of energy intake (26). MC4R has also been shown to couple to the G protein family Gq/11α, which activates phospholipase C, protein kinase C, and intracellular inositol triphosphate/calcium (28), and Gq/11α has been shown to mediate MC4R/phospholipase C/calcium signaling in hypothalamic neurons (29). We recently observed that PVN-specific deletion of Gq/11α using the same Sim1-cre line used in this study leads to massive obesity with hyperphagia (Li, Y., M. Chen, and L.S. Weinstein, manuscript in preparation), which along with the results presented in this study, implicate Gq/11α rather than Gsα as the G protein that mediates the effect of MC4R on food intake in PVN.
Although mPVNGsKO mice showed increased adiposity and reduced energy expenditure (particularly in males), these effects were much less severe than in mBrGsKO (6), implicating Gsα deficiency in CNS regions outside of PVN as important contributors to the metabolic phenotype associated with maternal Gsα mutations. Moreover, whereas mBrGsKO mice have impaired stimulation of energy expenditure by a melanocortin agonist (6), we found no such impairment in mPVNGsKO mice. This may not be totally surprising given that restoration of MC4R in PVN of MC4RKO mice was shown to have little effect on restoring normal energy expenditure rates (18). We cannot fully rule out the possibility that we do not see a more severe phenotype in mPVNGsKO mice due to incomplete Gsα deletion in the PVN. However the differences in the loss of PVN Gsα expression between mPVNGsKO and mBrGsKO mice are very small (70 vs. 84%) given the drastic difference in phenotype between the two models and are most likely explained by the fact that Gsα expression is also lost in nonneuronal cells in mBrGsKO mice in addition to the PVN neurons, which express Sim1.
Another thermogenic response that is differentially affected in mBrGsKO and mPVNGsKO mice is cold-induced activation of BAT, a thermogenic response mediated by MC4R stimulation of SNA (20, 30), being impaired in mBrGsKO but not mPVNGsKO mice. Whereas metabolic responses involving MC4R and SNA are differentially affected in mBrGsKO and mPVNGsKO mice, both heart rate and blood pressure were similarly reduced in both groups of mice, which may be expected given that the PVN is an important site for MC4R activation of SNS pathways that control cardiovascular function (32–33). Whereas MC4R pathways also regulate the hypothalamic-pituitary-thyroid axis by stimulating TSH-releasing peptide expression in PVN neurons (34), we previously showed the thyroid hormone status to be unaffected in maternal germline GsαKO (E1m−) (3) and mBrGsKO mice (6), and thyroid hormone levels were also unaffected in mPVNGsKO mice.
Our findings of differential effects on energy expenditure and thermogenesis in mBrGsKO and mPVNGsKO mice implicate CNS regions other than PVN as sites where Gsα is involved in stimulation of energy expenditure. Because Gsα mutations affect energy metabolism only when the mutation is on the maternal allele, it is likely that Gsα is imprinted in other regions involved in energy regulation. In fact, MC4R receptors are widely expressed in the CNS (14, 15). We have already shown that Gsα is not imprinted in the nucleus of the solitary tract (6). Other candidates include the rostral raphe pallidus area in the brain stem, which was shown to be important for melanocortin activation of BAT thermogenesis (35, 36), and the intermediolateral column of the spinal cord, which contains the sympathetic preganglionic neurons that receive inputs from the rostral raphe pallidus and PVN (37). Whether Gsα is imprinted and/or contributes to regulation of energy metabolism in these regions needs to be further elucidated. Given that nestin-cre (used to generate mBrGsKO mice) is also expressed in the peripheral nervous system, it is also possible that Gsα deficiency in the peripheral nervous system may contribute to the metabolic defects in mBrGsKO mice.
Central melanocortin signaling also directly regulates insulin secretion and sensitivity on peripheral tissues (11, 12). Both MC4RKO and mBrGsKO mice develop glucose intolerance and hyperinsulinemia before the onset of obesity (6, 12) whereas mPVNGsKO mice had normal glucose tolerance and insulin levels before the onset of obesity. Male mPVNGsKO mice developed glucose tolerance and insulin resistance at a later age after the onset of obesity, but this was not observed in females, perhaps because they had milder obesity than males. It therefore seems likely that the independent effect of MC4R signaling on peripheral glucose metabolism is also mediated by Gsα at one or more CNS sites outside of the PVN.
In summary, our results show that central melanocortins regulate energy balance through both Gsα-dependent and -independent pathways. Gsα mediates the activation of energy expenditure and thermogenesis by melanocortins at multiple CNS sites, including PVN, and these regions contribute to the parent-of-origin effects of Gsα mutations on energy metabolism.
Acknowledgments
We thank J. Wang, W. Jou, and T. Chanturiya (National institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD); and K. Pacak and H. Turkova (Eunice Shriver National Institute of Child Health and Human Development, Bethesda, MD) for technical assistance and B. Lowell (Beth Israel-Deaconess Medical Center, Boston, MA) for Sim1-cre mice.
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AHO
- Albright hereditary osteodystrophy
- BAT
- brown adipose tissue
- CNS
- central nervous system
- MTII
- melanotan II
- PVN
- paraventricular nucleus
- REE
- resting energy expenditure
- SNA
- sympathetic nervous system activity
- SNS
- sympathetic nervous system
- TEE
- total energy expenditure
- UCP1
- uncoupling protein 1
- WAT
- white adipose tissue.
References
- 1. Weinstein LS, Chen M, Xie T, Liu J. 2006. Genetic diseases associated with heterotrimeric G proteins. Trends Pharmacol Sci 27:260–266 [DOI] [PubMed] [Google Scholar]
- 2. Long DN, McGuire S, Levine MA, Weinstein LS, Germain-Lee EL. 2007. Body mass index differences in pseudohypoparathyroidism type 1a versus pseudopseudohypoparathyroidism may implicate paternal imprinting of Gαs in the development of human obesity. J Clin Endocrinol Metab 92:1073–1079 [DOI] [PubMed] [Google Scholar]
- 3. Chen M, Gavrilova O, Liu J, Xie T, Deng C, Nguyen AT, Nackers LM, Lorenzo J, Shen L, Weinstein LS. 2005. Alternative Gnas gene products have opposite effects on glucose and lipid metabolism. Proc Natl Acad Sci USA 102:7386–7391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carel JC, Le Stunff C, Condamine L, Mallet E, Chaussain JL, Adnot P, Garabédian M, Bougnères P. 1999. Resistance to the lipolytic action of epinephrine: a new feature of protein Gs deficiency. J Clin Endocrinol Metab 84:4127–4131 [DOI] [PubMed] [Google Scholar]
- 5. Dekelbab BH, Aughton DJ, Levine MA. 2009. Pseudohypoparathyroidism type 1A and morbid obesity in infancy. Endocr Pract 15:249–253 [DOI] [PubMed] [Google Scholar]
- 6. Chen M, Wang J, Dickerson KE, Kelleher J, Xie T, Gupta D, Lai EW, Pacak K, Gavrilova O, Weinstein LS. 2009. Central nervous system imprinting of the G protein Gsα and its role in metabolic regulation. Cell Metab 9:548–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butler AA, Cone RD. 2002. The melanocortin receptors: lessons from knockout models. Neuropeptides 36:77–84 [DOI] [PubMed] [Google Scholar]
- 8. Nogueíras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schürmann A, Joost HG, Hammond C, Hui DY, Woods SC, Rahmouni K, Butler AA, Farooqi IS, O'Rahilly S, Rohner-Jeanrenaud F, Tschöp MH. 2007. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest 117:3475–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. 2003. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 348:1085–1095 [DOI] [PubMed] [Google Scholar]
- 10. Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. 1997. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88:131–141 [DOI] [PubMed] [Google Scholar]
- 11. Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L. 2001. Central melanocortin receptors regulate insulin action. J Clin Invest 108:1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fan W, Dinulescu DM, Butler AA, Zhou J, Marks DL, Cone RD. 2000. The central melanocortin system can directly regulate serum insulin levels. Endocrinology 141:3072–3079 [DOI] [PubMed] [Google Scholar]
- 13. Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, Burn P, Palmiter RD. 1999. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet 21:119–122 [DOI] [PubMed] [Google Scholar]
- 14. Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. 1994. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol 8:1298–1308 [DOI] [PubMed] [Google Scholar]
- 15. Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. 2003. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol 457:213–235 [DOI] [PubMed] [Google Scholar]
- 16. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. 2006. Central nervous system control of food intake and body weight. Nature 443:289–295 [DOI] [PubMed] [Google Scholar]
- 17. Cone RD. 2005. Anatomy and regulation of the central melanocortin system. Nat Neurosci 8:571–578 [DOI] [PubMed] [Google Scholar]
- 18. Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. 2005. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123:493–505 [DOI] [PubMed] [Google Scholar]
- 19. Chen M, Gavrilova O, Zhao WQ, Nguyen A, Lorenzo J, Shen L, Nackers L, Pack S, Jou W, Weinstein LS. 2005. Increased glucose tolerance and reduced adiposity in the absence of fasting hypoglycemia in mice with liver-specific Gsα deficiency. J Clin Invest 115:3217–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Voss-Andreae A, Murphy JG, Ellacott KL, Stuart RC, Nillni EA, Cone RD, Fan W. 2007. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology 148:1550–1560 [DOI] [PubMed] [Google Scholar]
- 21. Butler AA, Cone RD. 2003. Knockout studies defining different roles for melanocortin receptors in energy homeostasis. Ann NY Acad Sci 994:240–245 [DOI] [PubMed] [Google Scholar]
- 22. Weide K, Christ N, Moar KM, Arens J, Hinney A, Mercer JG, Eiden S, Schmidt I. 2003. Hyperphagia, not hypometabolism, causes early onset obesity in melanocortin-4 receptor knockout mice. Physiol Genomics 13:47–56 [DOI] [PubMed] [Google Scholar]
- 23. Chiappini F, Cunha LL, Harris JC, Hollenberg AN. 2011. Lack of cAMP-response element-binding protein 1 in the hypothalamus causes obesity. J Biol Chem 286:8094–8105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Michaud JL, Boucher F, Melnyk A, Gauthíer F, Goshu E, Lévy E, Mitchell GA, Himms-Hagen J, Fan CM. 2001. Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum Mol Genet 10:1465–1473 [DOI] [PubMed] [Google Scholar]
- 25. Holder JL, Jr, Butte NF, Zinn AR. 2000. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum Mol Genet 9:101–108 [DOI] [PubMed] [Google Scholar]
- 26. Kublaoui BM, Holder JL, Jr, Gemelli T, Zinn AR. 2006. Sim1 haploinsufficiency impairs melanocortin-mediated anorexia and activation of paraventricular nucleus neurons. Mol Endocrinol 20:2483–2492 [DOI] [PubMed] [Google Scholar]
- 27. Kublaouí BM, Holder JL, Jr, Tolson KP, Gemelli T, Zinn AR. 2006. SIM1 overexpression partially rescues agouti yellow and diet-induced obesity by normalizing food intake. Endocrinology 147:4542–4549 [DOI] [PubMed] [Google Scholar]
- 28. Peters MF, Scott CW. 2009. Evaluating cellular impedance assays for detection of GPCR pleiotropic signaling and functional selectivity. J Biomol Screen 14:246–255 [DOI] [PubMed] [Google Scholar]
- 29. Newman EA, Chai BX, Zhang W, Li JY, Ammori JB, Mulholland MW. 2006. Activation of the melanocortin-4 receptor mobilizes intracellular free calcium in immortalized hypothalamic neurons. J Surg Res 132:201–207 [DOI] [PubMed] [Google Scholar]
- 30. Fan W, Voss-Andreae A, Cao WH, Morrison SF. 2005. Regulation of thermogenesis by the central melanocortin system. Peptides 26:1800–1813 [DOI] [PubMed] [Google Scholar]
- 31. Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. 2001. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci 4:605–611 [DOI] [PubMed] [Google Scholar]
- 32. Ward KR, Bardgett JF, Wolfgang L, Stocker SD. 2011. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension 57:435–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martin DS, Haywood JR. 1992. Sympathetic nervous system activation by glutamate injections into the paraventricular nucleus. Brain Res 577:261–267 [DOI] [PubMed] [Google Scholar]
- 34. Harris M, Aschkenasi C, Elias CF, Chandrankunnel A, Nillni EA, Bjøorbaek C, Elmquist JK, Flier JS, Hollenberg AN. 2001. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J Clin Invest 107:111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fan W, Morrison SF, Cao WH, Yu P. 2007. Thermogenesis activated by central melanocortin signaling is dependent on neurons in the rostral raphe pallidus (rRPa) area. Brain Res 1179:61–69 [DOI] [PubMed] [Google Scholar]
- 36. Nakamura K, Matsumura K, Kobayashi S, Kaneko T. 2005. Sympathetic premotor neurons mediating thermoregulatory functions. Neurosci Res 51:1–8 [DOI] [PubMed] [Google Scholar]
- 37. Loewy AD. 1981. Raphe pallidus and raphe obscurus projections to the intermediolateral cell column in the rat. Brain Res 222:129–133 [DOI] [PubMed] [Google Scholar]