Abstract
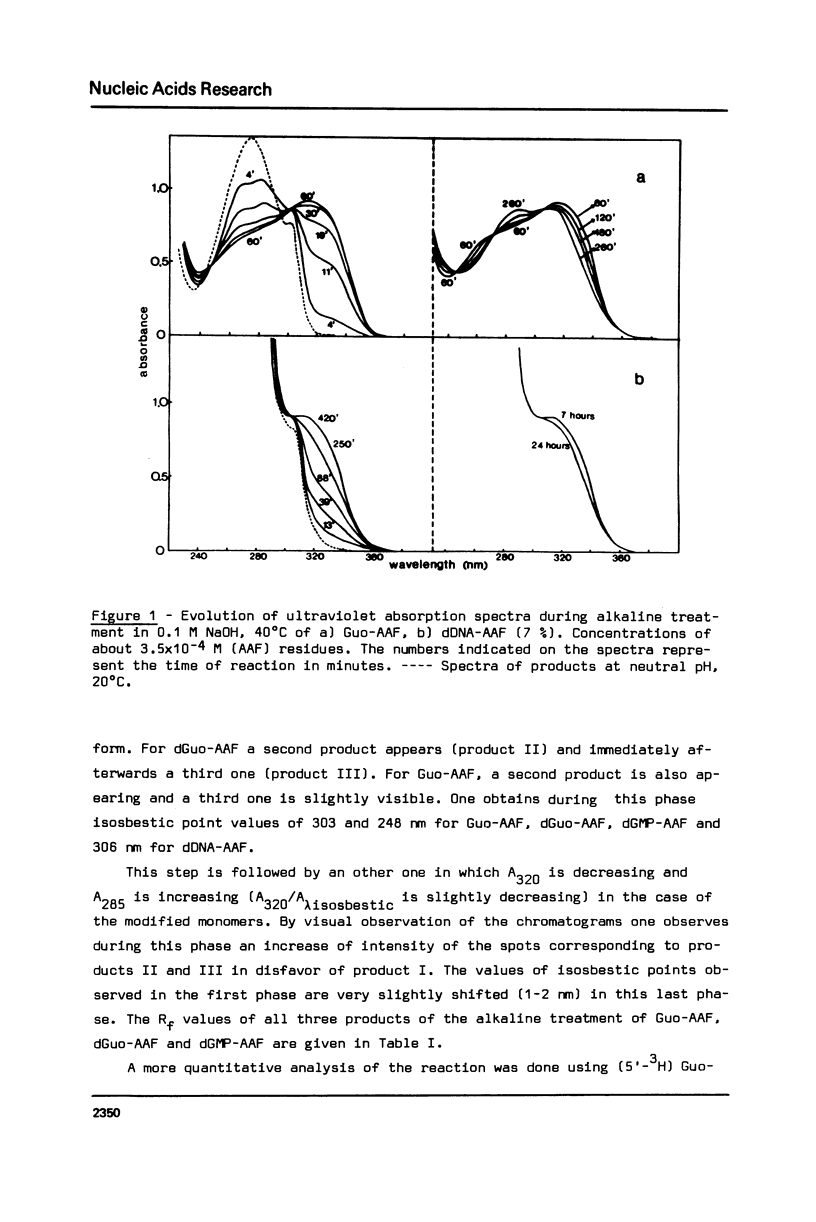
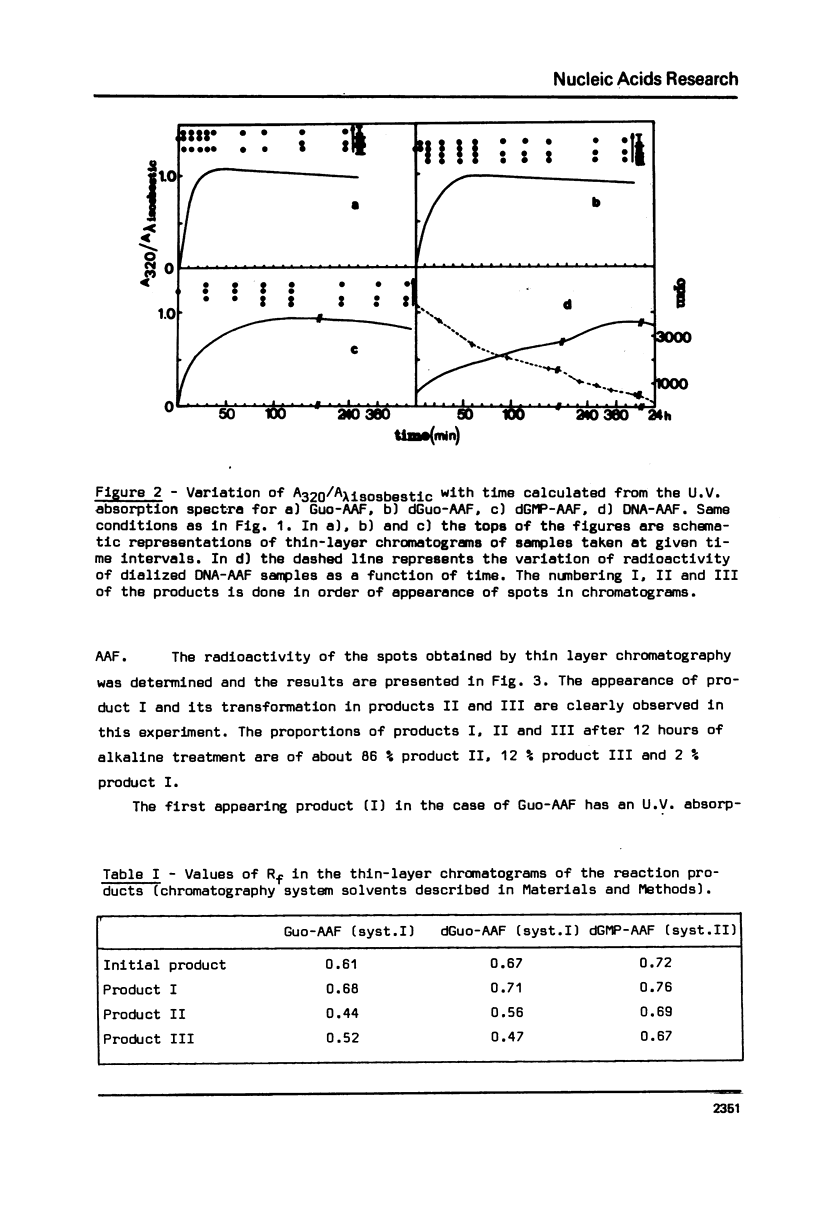
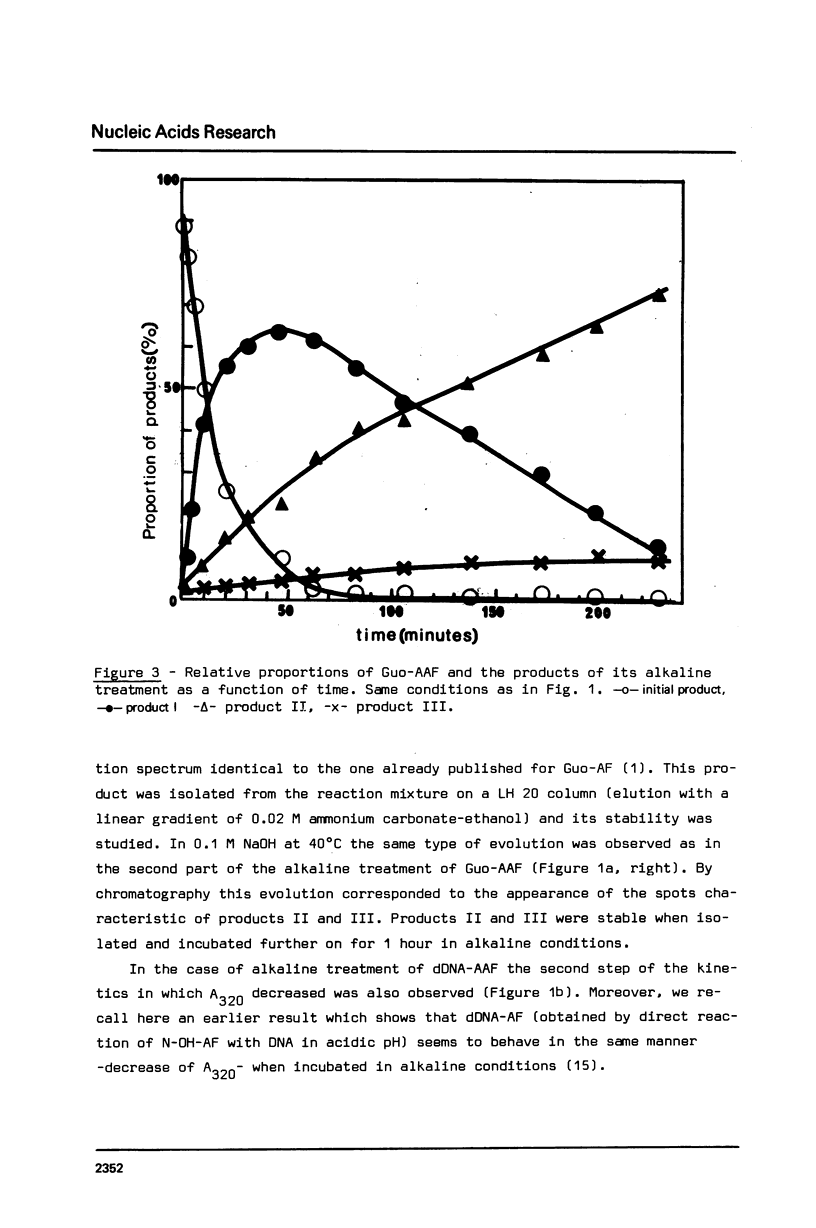
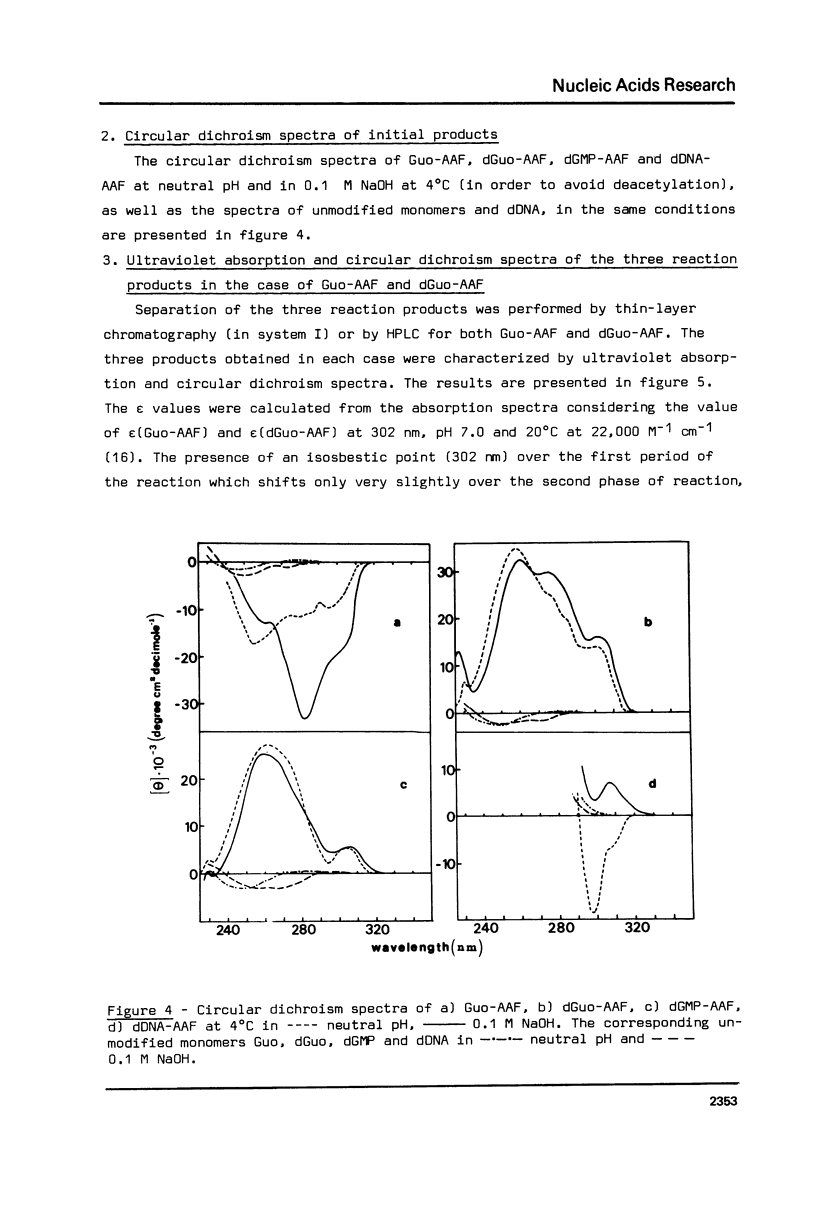
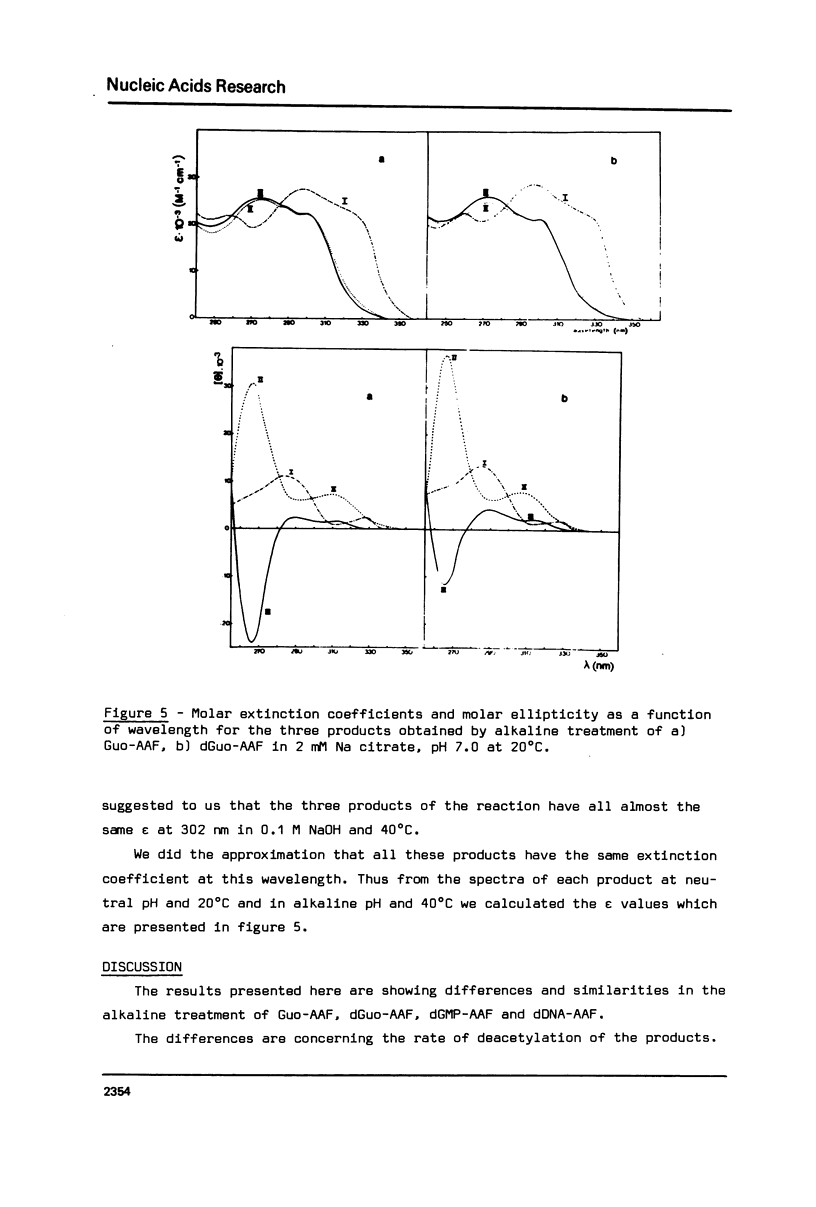
The alkaline treatment of Guo, dGuo, dGMP and denatured DNA modified by N-acetoxyacetylaminofluorene (N-AcO-AAF) was performed in 0.1 M NaOH at 40 degrees C. The kinetics of the reaction were followed by ultraviolet absorption and by chromatographic methods and were found different for the four products under study. Circular dichroism spectra show differences in the environment of acetylaminofluorene residue in these products. The alkaline treatment of Guo-AAF (and dGuo-AAF) leads to the formation of three products. These products were separated by thin layer chromatography and by HPLC and were characterized by spectroscopic methods. One is the already known unstable Guo-AF (and respectively dGuo-AF) (1). The other two products are relatively stable products of the transformation of Guo-AF (or dGuo-AF). These last ones present almost identical ultraviolet absorption spectra, but very different circular dichroism spectra.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Gurney E. G., Miller J. A., Bartsch H. Carcinogens as frameshift mutagens: metabolites and derivatives of 2-acetylaminofluorene and other aromatic amine carcinogens. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3128–3132. doi: 10.1073/pnas.69.11.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R. P. Arylamidation and arylation by the carcinogen N-2-fluorenylacetamide: a sensitive and rapid radiochemical assay. Anal Biochem. 1978 Dec;91(2):663–673. doi: 10.1016/0003-2697(78)90553-5. [DOI] [PubMed] [Google Scholar]
- Guigues M., Leng M. Reactivity of antibodies to guanosine modified by the carcinogen N-acetoxy-N-2-acetylaminofluorene. Nucleic Acids Res. 1979 Feb;6(2):733–744. doi: 10.1093/nar/6.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving C. C. Enzymatic deacetylation of N-hydroxy-2-acetylaminofluorene by liver microsomes. Cancer Res. 1966 Jul;26(7):1390–1396. [PubMed] [Google Scholar]
- Irving C. C., Veazey R. A. Persistent binding of 2-acetylaminofluorene to rat liver DNA in vivo and consideration of the mechanism of binding of N-hydroxy-2-acetylaminofluorene to rat liver nucleic acids. Cancer Res. 1969 Oct;29(10):1799–1804. [PubMed] [Google Scholar]
- Kapuler A. M., Michelson A. M. The reaction of the carcinogen N-acetoxy-2-acetyl-aminofluorene with DNA and other polynucleotides and its stereochemical implications. Biochim Biophys Acta. 1971 Mar 25;232(3):436–450. doi: 10.1016/0005-2787(71)90598-3. [DOI] [PubMed] [Google Scholar]
- King C. M., Phillips B. Instability of fluorenylamine-substituted polynucleotides: loss of carcinogen and production of an altered nucleic acid. Chem Biol Interact. 1970 Oct;2(3):267–271. doi: 10.1016/0009-2797(70)90030-x. [DOI] [PubMed] [Google Scholar]
- King C. M., Phillips B. N-hydroxy-2-fluorenylacetamide. Reaction of the carcinogen with guanosine, ribonucleic acid, deoxyribonucleic acid, and protein following enzymatic deacetylation or esterification. J Biol Chem. 1969 Nov 25;244(22):6209–6216. [PubMed] [Google Scholar]
- Kriek E. Carcinogenesis by aromatic amines. Biochim Biophys Acta. 1974 Sep 9;355(2):177–203. doi: 10.1016/0304-419x(74)90003-1. [DOI] [PubMed] [Google Scholar]
- Kriek E., Miller J. A., Juhl U., Miller E. C. 8-(N-2-fluorenylacetamido)guanosine, an arylamidation reaction product of guanosine and the carcinogen N-acetoxy-N-2-fluorenylacetamide in neutral solution. Biochemistry. 1967 Jan;6(1):177–182. doi: 10.1021/bi00853a029. [DOI] [PubMed] [Google Scholar]
- Kriek E. On the interaction of N-2-fluorenylhydroxylamine with nucleic acids in vitro. Biochem Biophys Res Commun. 1965 Sep 22;20(6):793–799. doi: 10.1016/0006-291x(65)90088-4. [DOI] [PubMed] [Google Scholar]
- Kriek E. On the mechanism of action of carcinogenic aromatic amines. I. Binding of 2-acetylaminofluorene and N-hydroxy-2-acetylaminofluorene to rat-liver nucleic acids in vivo. Chem Biol Interact. 1969 Oct;1(1):3–17. doi: 10.1016/0009-2797(69)90015-5. [DOI] [PubMed] [Google Scholar]
- Kriek E. Persistent binding of a new reaction product of the carcinogen N-hydroxy-N-2-acetylaminofluorene with guanine in rat liver DNA in vivo. Cancer Res. 1972 Oct;32(10):2042–2048. [PubMed] [Google Scholar]
- Miller E. C. Some current perspectives on chemical carcinogenesis in humans and experimental animals: Presidential Address. Cancer Res. 1978 Jun;38(6):1479–1496. [PubMed] [Google Scholar]
- Sakai S., Reinhold C. E., Wirth P. J., Thorgeirsson S. S. Mechanism of in vitro mutagenic activation and covalent binding of N-hydroxy-2-acetylaminofluorene in isolated liver cell nuclei from rat and mouse. Cancer Res. 1978 Jul;38(7):2058–2067. [PubMed] [Google Scholar]
- Schut H. A., Thorgeirsson S. S. In vitro metabolism and mutagenic activation of 2-acetylaminofluorene by subcellular liver fractions from Cotton rats. Cancer Res. 1978 Aug;38(8):2501–2507. [PubMed] [Google Scholar]
- Spodheim-Maurizot M., Saint-Ruf G., Leng M. Conformational changes induced in DNA by in vitro reaction with N-hydroxy-N-2-aminofluorene. Nucleic Acids Res. 1979 Apr;6(4):1683–1694. doi: 10.1093/nar/6.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout D. L., Becker F. F. Metabolism of 2-aminofluorene and 2-acetylaminofluorene to mutagens by rat hepatocyte nuclei. Cancer Res. 1979 Apr;39(4):1168–1173. [PubMed] [Google Scholar]
- Westra J. G., Kriek E., Hittenhausen H. Identification of the persistently bound form of the carcinogen N-acetyl-2-aminofluorene to rat liver DNA in vivo. Chem Biol Interact. 1976 Oct 2;15(2):149–164. doi: 10.1016/0009-2797(76)90160-5. [DOI] [PubMed] [Google Scholar]


