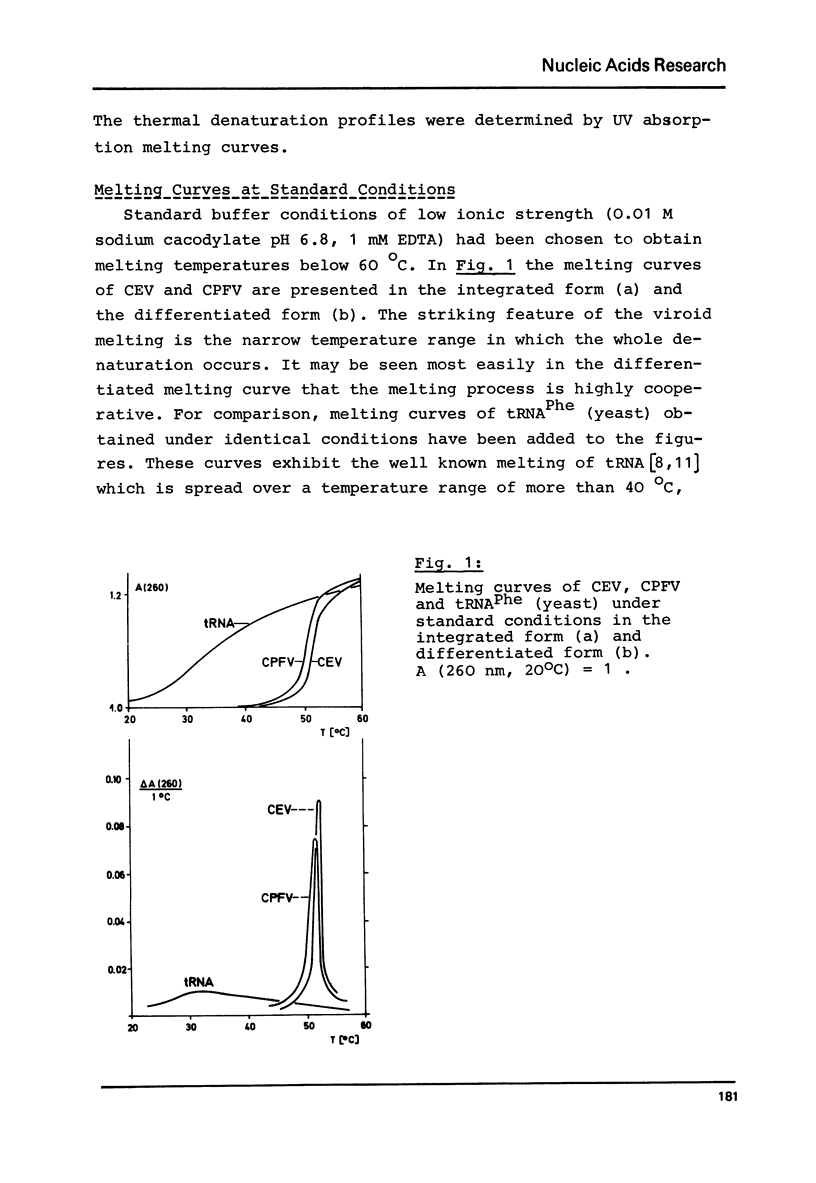
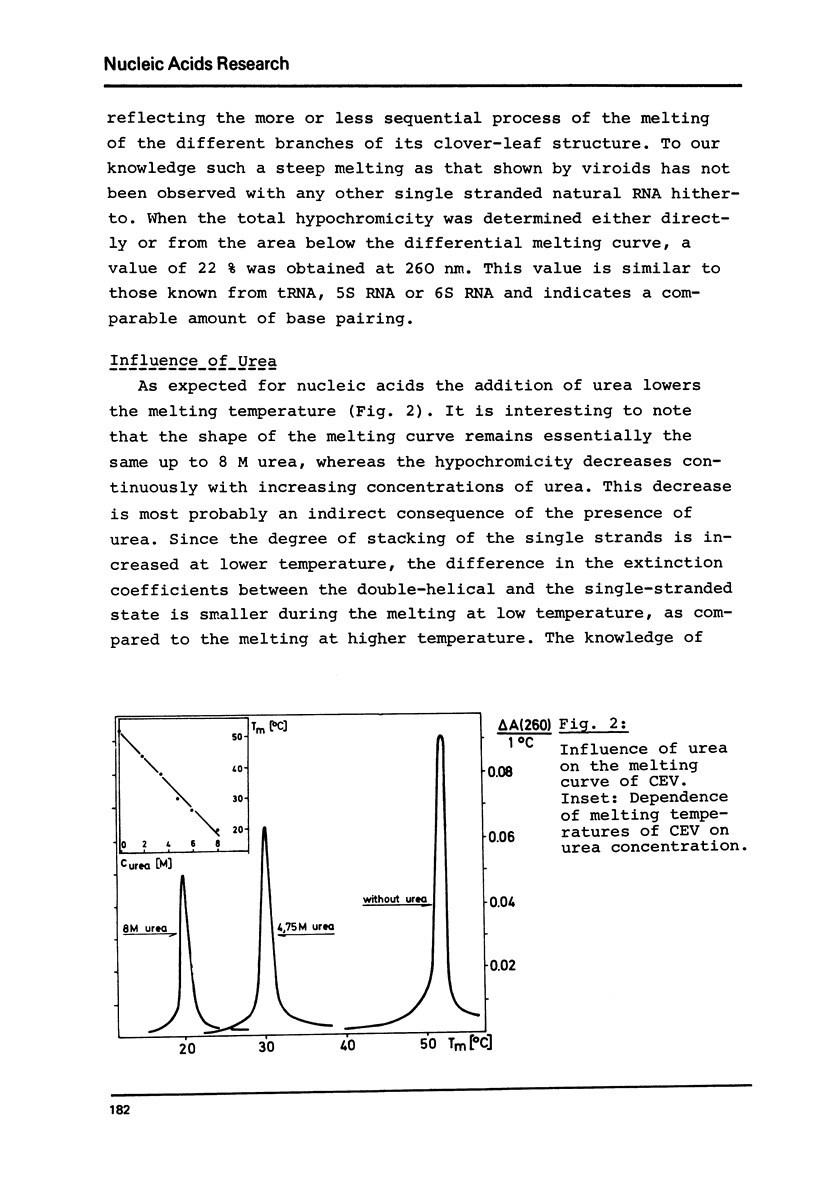
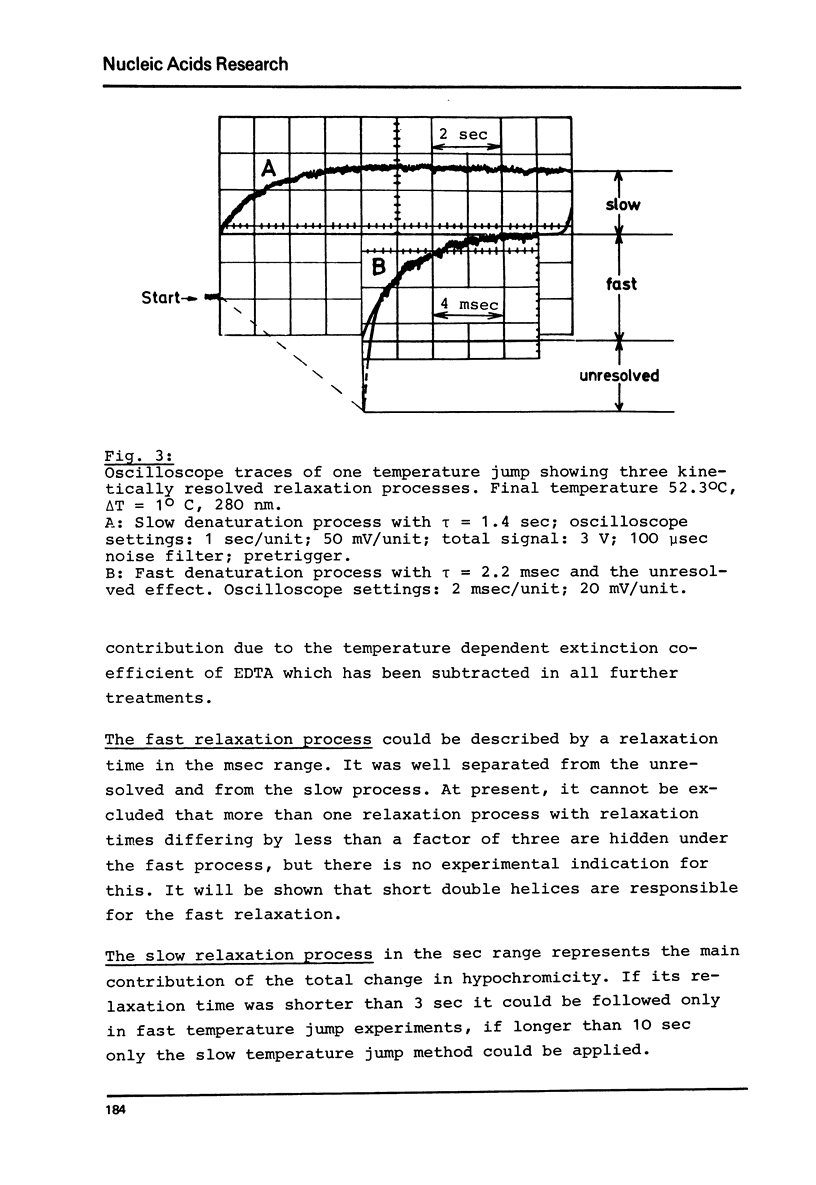
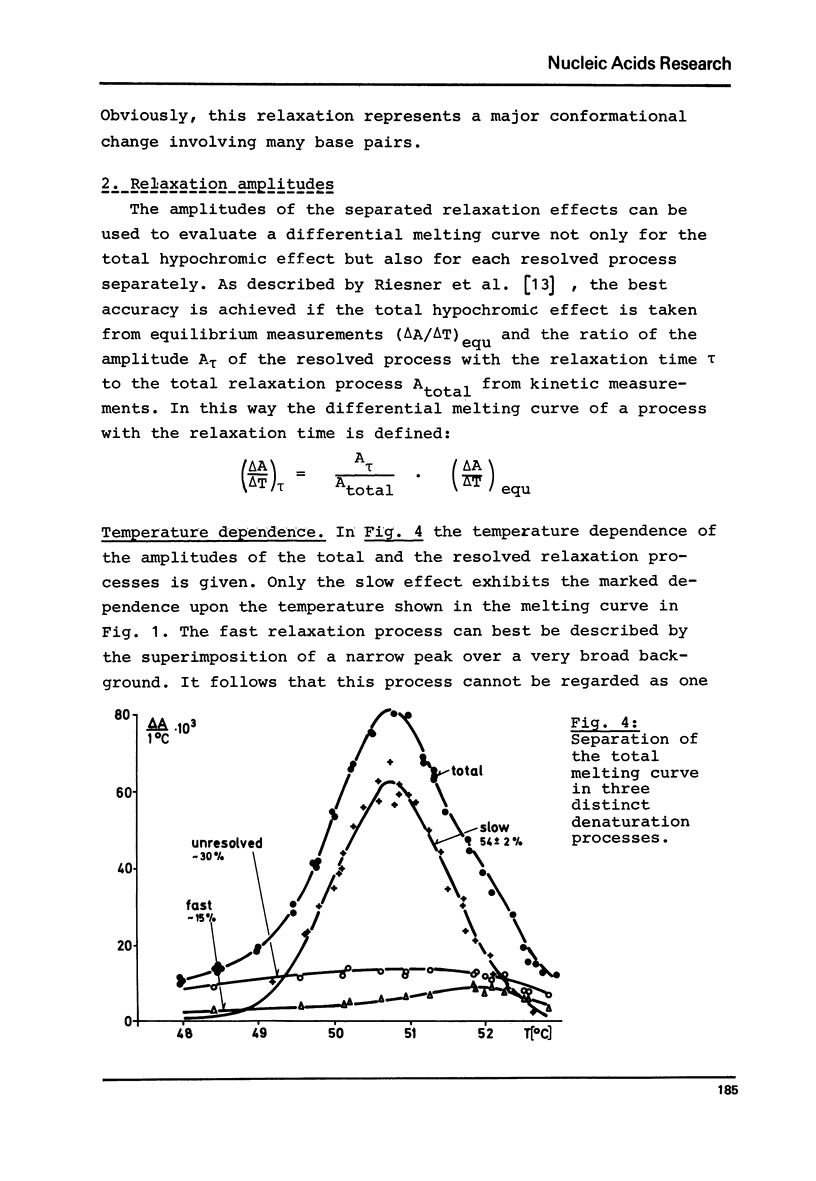
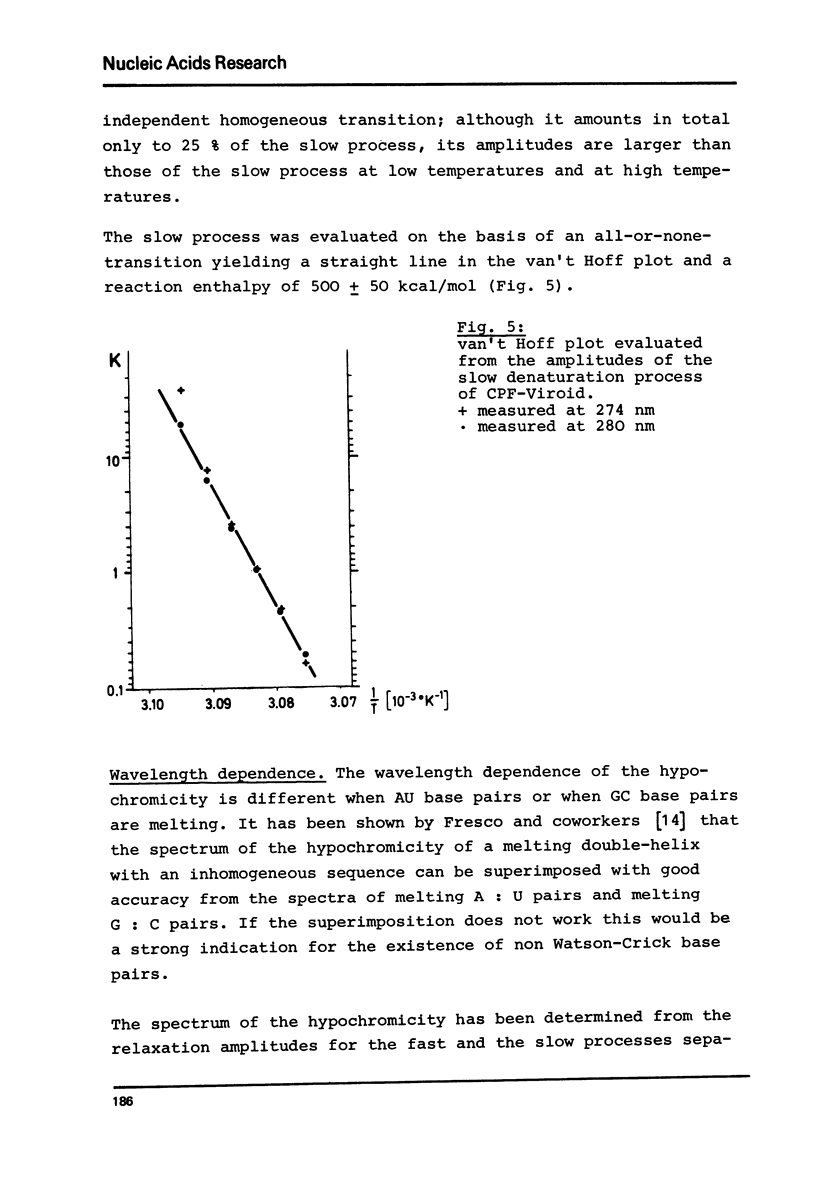
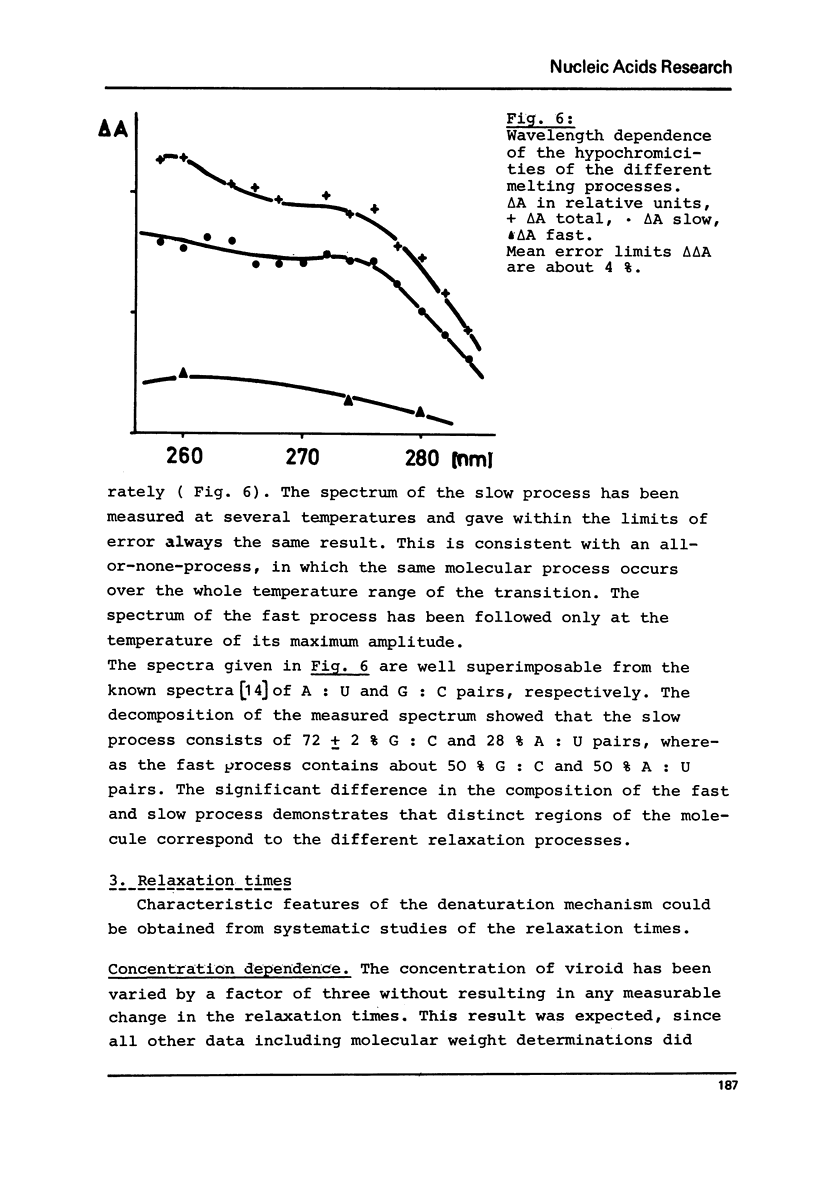
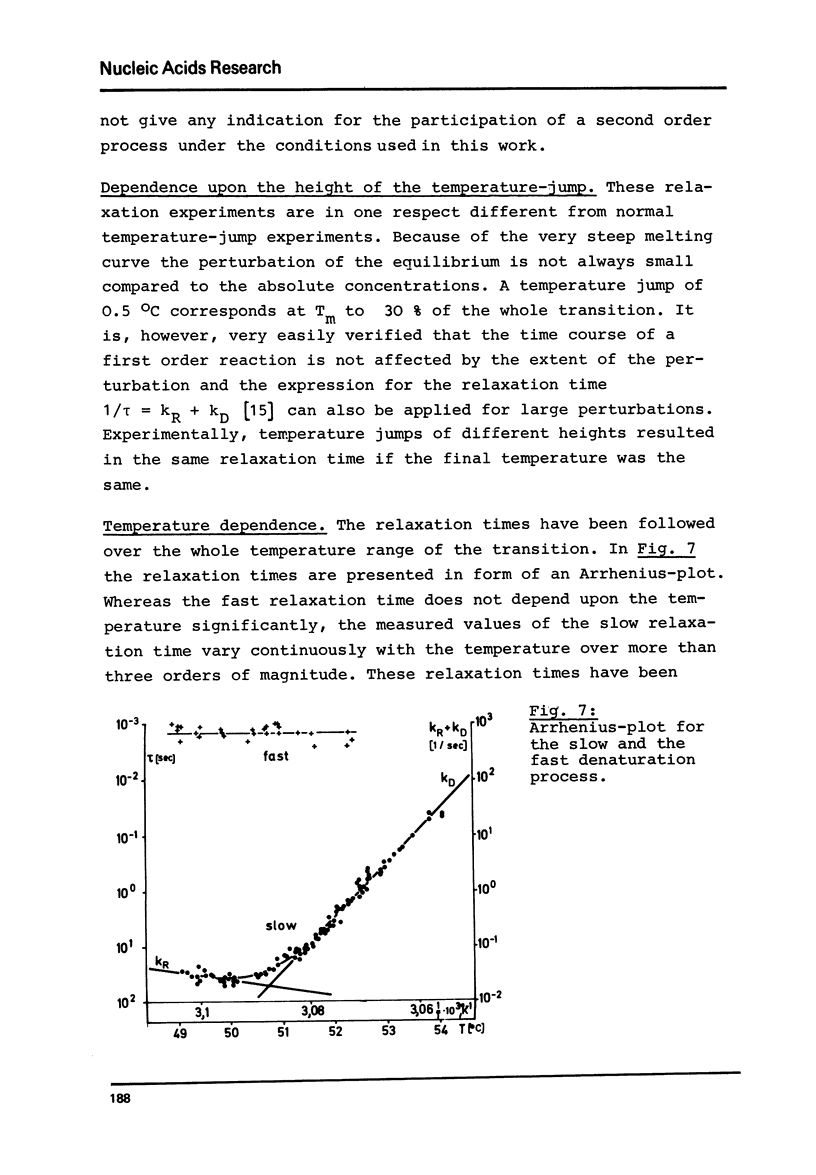
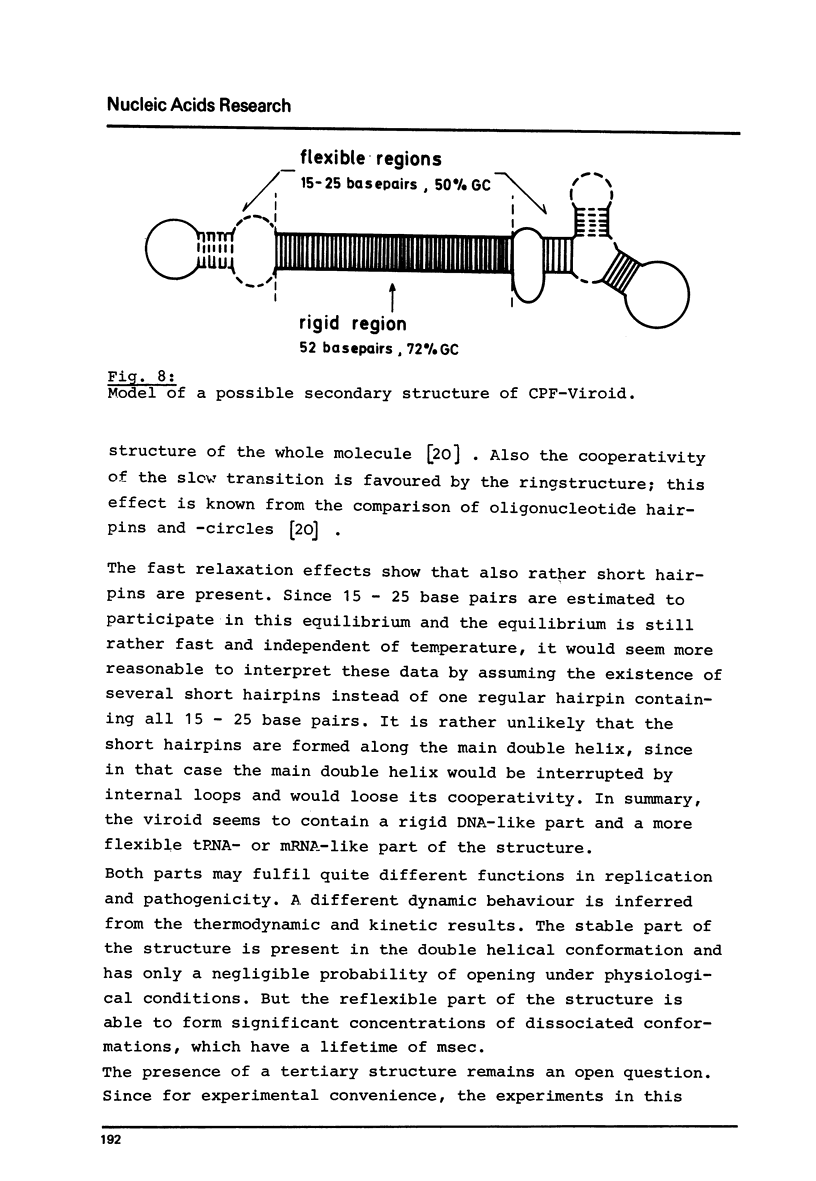
Abstract
Viroids are uncoated infectious RNA molecules (MW 107 000-127 000) known as pathogens of certain higher plants. Thermodynamic and kinetic studies were carried out on highly purified viroid preparations by applying UV-absorption melting analysis and temperature jump methods. The thermal denaturation of viroids is characterized by high thermal stability, high cooperativity and a high degree of base pairing. Two relaxation processes could be resolved; a process in the sec range could be evaluated as an independent all-or-none-transition with the following properties: reaction enthalpy= 550 kcal/mol, activation enthalpy of the dissociation = 470 kcal/mol; G : C content = 72 %. These data indicate the existence of an uninterrupted double helix of 52 base pairs. A process in the msec range involves 15 - 25 base pairs which are most probably distributed over several short double helical stretches. A tentative model for the secondary structure of viroids isproposed and the possible functional implications of their physicochemical properties are discussed.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biebricher C. K., Orgel L. E. An RNA that multiplies indefinitely with DNA-dependent RNA polymerase: selection from a random copolymer. Proc Natl Acad Sci U S A. 1973 Mar;70(3):934–938. doi: 10.1073/pnas.70.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borer P. N., Dengler B., Tinoco I., Jr, Uhlenbeck O. C. Stability of ribonucleic acid double-stranded helices. J Mol Biol. 1974 Jul 15;86(4):843–853. doi: 10.1016/0022-2836(74)90357-x. [DOI] [PubMed] [Google Scholar]
- Coutts S. M., Riesner D., Römer R., Rabl C. R., Maass G. Kinetics of conformational changes in tRNA Phe (yeast) as studied by the fluorescence of the Y-base and of formycin substituted for the 3'-terminal adenine. Biophys Chem. 1975 Oct;3(4):275–289. doi: 10.1016/0301-4622(75)80020-2. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Viroids: the smallest known agents of infectious disease. Annu Rev Microbiol. 1974;28(0):23–39. doi: 10.1146/annurev.mi.28.100174.000323. [DOI] [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. II. Small hairpin loops. J Mol Biol. 1973 Feb 5;73(4):497–511. doi: 10.1016/0022-2836(73)90096-x. [DOI] [PubMed] [Google Scholar]
- Mills D. R., Kramer F. R., Spiegelman S. Complete nucleotide sequence of a replicating RNA molecule. Science. 1973 Jun 1;180(4089):916–927. doi: 10.1126/science.180.4089.916. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Einfache Temperatursprung-Methode im Sekunden-bis Stundenbereich und die reversible denaturierung von Chymotrypsin. Eur J Biochem. 1968 Apr;4(3):373–377. doi: 10.1111/j.1432-1033.1968.tb00221.x. [DOI] [PubMed] [Google Scholar]
- Pörchke D. The nature of stacking interations in polynucleotides. Molecular states in Oligo- and polyribocytidylic acids by relaxation analysis. Biochemistry. 1976 Apr 6;15(7):1495–1499. doi: 10.1021/bi00652a021. [DOI] [PubMed] [Google Scholar]
- Pörschke D., Eigen M. Co-operative non-enzymic base recognition. 3. Kinetics of the helix-coil transition of the oligoribouridylic--oligoriboadenylic acid system and of oligoriboadenylic acid alone at acidic pH. J Mol Biol. 1971 Dec 14;62(2):361–381. doi: 10.1016/0022-2836(71)90433-5. [DOI] [PubMed] [Google Scholar]
- Riesner D., Pingoud A., Boehme D., Peters F., Maass G. Distinct steps in the specific binding of tRNA to aminoacyl-tRNA synthetase. Temperature-jump studies on the serine-specific system from yeast and the tyrosine-specific system from Escherichia coli. Eur J Biochem. 1976 Sep;68(1):71–80. doi: 10.1111/j.1432-1033.1976.tb10765.x. [DOI] [PubMed] [Google Scholar]
- Riesner D., Römer R., Maass G. Kinetic study of the three conformational transitions of alanine specific transfer RNA from yeast. Eur J Biochem. 1970 Jul;15(1):85–91. doi: 10.1111/j.1432-1033.1970.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Römer R., Riesner D., Coutts S. M., Maass G. The coupling of conformational transitions in alanine specific transfer ribonucleic acid from yeast studied by a modified differential absorption technique. Eur J Biochem. 1970 Jul;15(1):77–84. doi: 10.1111/j.1432-1033.1970.tb00978.x. [DOI] [PubMed] [Google Scholar]
- Scheffler I. E., Elson E. L., Baldwin R. L. Helix formation by d(TA) oligomers. II. Analysis of the helix-coli transitions of linear and circular oligomers. J Mol Biol. 1970 Feb 28;48(1):145–171. doi: 10.1016/0022-2836(70)90225-1. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Morris T. J., Weathers L. G., Rodorf B. F., Kearns D. R. Physical properties of a minimal infectious RNA(viroik) associated with the exocortis disease. Virology. 1975 Jan;63(1):160–167. doi: 10.1016/0042-6822(75)90381-5. [DOI] [PubMed] [Google Scholar]


