Abstract
Evidence from longitudinal cohort studies demonstrates that wheezing which begins in early life and continues into the school age years generally persists into adulthood. This persistent wheezing is associated with lung function deficits and airways hyperresponsiveness that appear to be established in the first few years of life. Allergic sensitization early in life, early life infection with rhinovirus, or colonization with any of a number of bacteria have been associated with increased risk of persistent wheeze. Early life, whether in utero or in the first few years of life, presents a window of vulnerability during which airway injury results in persistent airways dysfunction. Available data futher suggest that a second such window of vulnerability may be present in the preadolescent and adolescent years. Lung function growth patterns established by age 6 generally continue into early to mid-adulthood, typically leaving groups of individuals with wheezing that persists into or relapses in adulthood with mean FEV1 about 10% predicted lower than their peers who do not wheeze. Subgroups of patients with persistent asthma, however, may have progressive declines in lung function, and enter adulthood with even lower lung function. The concern exists that these deficits in lung function apparent in early adulthood may put individuals at risk for the later development of chronic obstructive pulmonary disease.
Keywords: Asthma, Wheezing, Lung function, Airways hyperresponsiveness, Atopy, Outcomes, Infancy, Childhood, Early onset
Introduction
In recent years, evidence has accumulated that much of chronic persistent asthma begins in childhood, and that respiratory outcomes later in life can be traced to events in early life (1,2). Through longitudinal studies, various patterns of wheezing have been brought to light, with symptoms emerging at various times in childhood. In several of these studies, respiratory data collection began in mid to late childhood with retrospective determination of early life events (3–19). In two, outcomes to mid adulthood have been determined (3–9), and one has concentrated exclusively on a large number of subjects with diagnosed asthma (12–16). In other longitudinal studies, subjects have been followed from birth, allowing for prospective assessment of the development of patterns of wheeze (20–42); however, most of these cohorts have been assembled fairly recently, with follow-up to early adulthood reported in only two (20–22, 28, 29, 32). Strikingly, the findings from these longitudinal studies from birth have helped to explain patterns of lung function and clinical outcomes observed in those with data collection beginning in mid to late childhood.
The purpose of this review is to summarize what is known about the outcomes of early onset wheezing, with special emphasis on lung function outcomes. Our working definition of “early onset wheezing” was wheezing that begins during infancy or the preschool years. The papers included in this review were identified through a search of the PubMed database using a variety of search terms in combination including wheeze, asthma, early onset, longitudinal, birth cohort, lung function, and outcomes. Additional references were identified from citations in the papers that were reviewed, as well as from further PubMed searches using author names or study titles as keywords. The studies selected for review included those with data collected during childhood, as well as those with long-term followup, with special focus on serial lung-function data. Shorter-term studies that appeared to fill in knowledge gaps were also included. Although there is still much to be learned, our review of the literature suggests that indeed the seeds of chronic airways dysfunction are sown in early life.
Prospective cohorts enrolled from childhood
Several long-term studies following cohorts of children into adulthood have yielded insights into the natural history and outcomes of childhood asthma. The Melbourne Asthma Study has followed a group of children from a 1957 birth cohort, from which several groups of children with wheezing and a control group without wheezing were randomly selected at age 7, and an additional group with severe wheezing at age 10 was added (3,4). Five groups were followed longitudinally: the control group, a group with “mild wheezy bronchitis” (< 5 episodes of wheezing associated with respiratory tract infection [RTI]), a group with “wheezy bronchitis” (5 or more wheezy RTIs), a group with asthma (wheeze unassociated with RTI), and a group with “severe asthma” (persistent symptoms, barrel chest deformity, FEV1/FVC of 50% or less). Of interest, the authors note that the severe asthma group were characterized by onset of symptoms before age 3. The last published examinations of this cohort occurred in 1999 and 2003, both of which detailed data from the subjects at age 42 years (4, 5). Of the original wheezy bronchitis group, 60% were wheeze free and only 5% had persistent asthma. Of the original asthma group, 70% still had symptoms as did 90% of the severe asthma group, almost half of whom had persistent wheeze. As shown in Table 1, lung function as assessed by FEV1 and FEV1/FVC was no different in the wheezy bronchitis groups as compared with the control group. Subjects who had childhood asthma had about a 10% reduction in FEV1 and 5% reduction in FEV1/FVC relative to those who did not have childhood asthma, and the lowest lung function indices (20% reduction in FEV1 and 10% reduction in FEV1/FVC) were exhibited by those in the severe asthma group. The magnitude of the differences between the groups was well established by age 10 and despite report of ongoing symptoms, did not worsen through age 42. Very few of the subjects received anti-inflammatory therapy. The probability of severe asthma in adult life was increased in the presence of other atopic disease or skin prick test (SPT) reactivity to house-dust mite (HDM) or rye grass. The lessons from this study are clear. Outcomes were generally quite good in children with small numbers of wheezing episodes associated with viral illness; however, frequent and severe symptoms in childhood were associated with a high probability of their continuing into adult life. Furthermore, lower levels of lung function in children with persistent asthma were already present by ages 7–10 years, without further relative decline to mid-adulthood. (4)
Table 1.
Lung function outcomes in early to mid adulthood grouped by wheezing/asthma groups in several population cohorts followed from birth or childhood. The FEV1 data from reference 8 were expressed in the original paper as percent of the mean of their controls, and are listed here as such. The FEV1/FVC data were similarly expressed in the original paper but based on their data from Table 4 (8), the actual ratios of the groups were calculated and are presented here.
| Study | Reference | Subjects | Age | FEV1 | FEV1/FVC |
|---|---|---|---|---|---|
| Melbourne Asthma Study | 5 | Asthma age 7 | 42 | 95% predicted* | 75%* |
| 5 | Severe asthma | 42 | 85% predicted* | 70%* | |
| 5 | Wheezy bronchitis age 7 | 42 | 102% predicted | 79% | |
| 5 | Control | 42 | 104% predicted | 80% | |
| British National Child Development Study | 8 | Wheeze at 35; onset 0–7 | 35 | 90.1% control mean* | 77%* |
| 8 | Wheeze at 35; onset 8–16 | 35 | 90.9%* control mean | 76%* | |
| 8 | Wheeze at 35; onset 17–23 | 35 | 92.9% control mean* | 78%* | |
| 8 | Wheeze at 35; wheeze onset 24–35 | 35 | 97.4% control mean | 81% | |
| 8 | No wheeze at 35; wheeze onset 0–7 | 35 | 97.8% control mean | 81% | |
| 8 | No wheeze at 35; wheeze onset 8–16 | 35 | 99.8% control mean | 81% | |
| 8 | No wheeze at 35; wheeze onset 17–23 | 35 | 97.4% control mean | 81% | |
| 8 | No wheeze at 35; wheeze onset 24–35 | 35 | 96.2% control mean | 80% | |
| 8 | Control (never wheeze) | 35 | 82% | ||
| Dunedin Multidisciplinary Health and Development Study | 10 | Persistent wheeze from onset | 26 | 96.6% predicted | 78% |
| 10 | Relapse | 26 | 95.7% predicted | 79.1% | |
| 10 | Never wheezed predicted** | 26 | 105.6% predicted | 83.7%** | |
| Tucson CRS | 29 | Chronic asthma | 22 | −0.37 (Z-score)*** | |
| 29 | Newly diagnosed | 22 | −0.49 (Z-score)**** | ||
| 29 | Inactive | 22 | 0.09 (Z-score) | ||
| 29 | No asthma | 22 | 0.10 (Z-score) |
p<0.001 compared with controls,
p<0.001 for the trend,
p=0.004 compared with no asthma,
p=0.005 compared with no asthma
The British National Child Development Study followed all children born in England, Scotland and Wales during the week of March 3–9, 1958, as well as immigrants sharing the same birth week. A nested substudy examined the development and natural history of asthma and atopic diseases. The cohort was followed at ages 7, 11, 16, 23, 33 and 42 years at which time they were asked about wheezing and asthma, as well as hay fever, allergic rhinitis and eczema. A subgroup of subjects underwent spirometry at ages 35 and 45, and an additional subgroup had total and specific IgE to dust, cat and grass measured at age 45. By age 7, 18.3% of the subjects had experienced wheezing. One half of these children were still wheezing at age 7 but by age 11, two thirds were wheeze free (6). Strikingly, among those who had no reports of wheezing at the ages 23 and 33 surveys, those who had a history of childhood wheeze were significantly more likely to wheeze at 42 than those without such a history, an association which remained significant after adjustment for smoking history, atopy, gender and total IgE (7). The prebronchodilator spirometry data from the subjects at age 35 are shown in Table 1; active wheezing at age 35 coupled with wheeze onset prior to age 23 was associated with up to 10% reduction in FEV1 and 5–6% reduction in FEV1/FVC (8). Early life wheezing prior to ages 7 or 16 did not impact on the rate of decline in lung function at ages 35–45 relative to those who did not have this history of early life wheeze (9). Thus, adding to the findings from the Melbourne Asthma Study, this study confirmed that while many with childhood wheezing have good outcomes, much wheezing in adult life, even that which may appear to be new-onset wheeze, is associated with antecedent wheeze in childhood. Furthermore, this study provided additional evidence that lung function patterns are established prior to adulthood and that those with persistent or relapsing wheeze enter adulthood with relative deficits in lung function.
The Dunedin Multidisciplinary Health and Development Study enrolled the 1,139 children born in Dunedin, New Zealand between April 1972 and March 1973. Also nested in this study was a substudy looking at asthma symptoms, lung function, and atopy. Questionnaires were administered at 2–5 year intervals between ages 9 and 26, airways hyperresponsiveness (AHR) to methacholine was assessed 5 times between ages 9 and 21 years, atopy assessed at ages 11, 13 and 21 years, and bronchodilator responsiveness at 18 and 26 years. Respiratory events between birth and age 9 were assessed retrospectively at age 9. Of the original sample, 613 (59% of the original cohort) provided complete respiratory data. In 14.5% of subjects, wheezing persisted either from age 9 or from the time of the first wheeze, while 12.4% had a remission followed by a relapse by age 26. Increased AHR (methacholine PC20 ≤ 8 mg/ml) or >10% bronchodilator responsiveness at age 9 were associated with a 4-fold increased odds of persistence and a 7-fold increased odds of relapse of wheezing by age 26. Positive SPT to HDM at age 13 was associated with a 2-fold odds of persistence and relapse. Additionally, smoking at age 21 and female gender were each associated with a 2-fold odds of persistence of wheeze. Younger age at onset of wheezing was associated with wheezing relapse at age 26 years. Subjects with persistent wheezing and relapse had consistently lower FEV1/FVC than did those who never reported wheezing, but the slopes for the change in FEV1/FVC did not differ between the wheezing groups and those who did not report wheezing. As shown in Table 1, at age 26, subjects with persistent or relapsed wheezing exhibited about a 10% reduction in FEV1 and 5% reduction in FEV1/FVC in comparison to those who had never wheezed (10). Thus, the Dunedin study reiterated the finding from the Melbourne and the British studies that, while much childhood wheezing appears to remit, some wheezing, particularly that associated with airways hyperresponsiveness, atopy or smoking, persists into or relapses in early adulthood. Furthermore, this study provided yet more evidence for the concept that lung function patterns are established by school age, and that those with persistent or relapsing wheezing enter adulthood with lung function deficits.
Risk factors for hospital admission for asthma were also examined in the Dunedin cohort. Subjects with more allergy as assessed by greater number of SPT positives, positives to HDM, cat, higher total IgE and greater eosinophilia were at greater risk for hospitalization. Subjects with earlier age at onset (mean 4.3 years), higher frequency of symptoms, lower lung function at age 9 (mean FEV1 92% predicted vs 98%; mean FEV1/FVC 83% vs 88%), and a greater likelihood of AHR at age 9 were at increased risk. Subjects with multiple admissions had even earlier age at onset (mean 2.7 years) more allergy and greater lung function decrements at age 9 than those with only one admission (mean FEV1 86% predicted vs 94%; mean FEV1/FVC 78% vs 84%). The slopes for change in FEV1/FVC were not different between hospitalization groups. Of note, the biggest impact of age of onset on hospitalizations was noted in those admissions occurring at age 9 or earlier (11).
The Childhood Asthma Management Program (CAMP) began as a multicenter, randomized, double masked clinical trial to compare the long-term safety and efficacy of three approaches to the management of mild to moderate childhood asthma: budesonide and albuterol, nedocromil and albuterol, and placebo and albuterol. The study enrolled 1041 children all with mild or moderate asthma, ages 5–12 years, the treatment phase was 4–6 years, followed by a 5 year follow-up observational phase at the end of which 941 (90%) of the original cohort were still being followed. Longer duration of disease prior to enrollment, ascertained retrospectively at the outset of the study, was associated with lower pre- and post-bronchodilator FEV1 (mean prebronchodilator FEV1 93.9% predicted with −0.907% predicted/year of duration; mean postbronchodilator FEV1 102.8% predicted with −0.625% predicted/year of duration), FEV1/FVC (mean prebronchodilator FEV1/FVC 79.7% with −0.729% /year of duration), and methacholine AHR (mean PC20 2.08 mg/ml, mean ln PC20 0.11 with −0.05/year of duration, indicating a greater degree of hyperresponsiveness with longer duration). Additionally, longer asthma duration was associated with more use of asthma medications prior to enrollment, a greater likelihood of being assessed as having moderately severe asthma, exercise-induced symptoms, and higher asthma symptom scores during the run-in phase. While several markers of atopy (serum IgE, number of positive SPT, peripheral blood eosinophilia) were also associated with disease duration, they did not confound the relationship between disease duration and lung function, although male gender and markers of atopy were associated with lower lung function (12). The link between asthma duration and asthma severity as assessed by lung function and symptoms, may have been mediated by increased methacholine responsiveness, but the associations were modest at best (13).
Immediately following the treatment phase of the study, the CAMP investigators noted that 25.6% of the participants experienced a 1% or more reduction in post-bronchodilator FEV1 per year over the course of that phase of the study, which was unaffected by the treatment assignment (mean FEV1 97.4% predicted vs 105.7% predicted)(14). Those who experienced this reduction in lung function were younger, smaller, male, and had an asthma diagnosis made at an earlier age, but had a shorter duration of disease, less atopy (defined by SPT responsiveness and serum IgE), better lung function at the outset, and lower percentage of hospitalization than those who did not experience this drop. On the other hand, among those who did experience this reduction in lung function, better baseline FEV1 predicted a less steep slope of decline. Despite the lower degree of atopy at the outset of the study, subjects with more rapid decline of lung function did exhibit more sputum eosinophils after the washout from the treatment phase of the study (mean 4.6% vs 2.1%)(14).
Followed beyond the end of the treatment phase and into late adolescence, CAMP participants exhibited progressively increased magnitude of airways obstruction relative to a historical longitudinal cohort of children without asthma (most consistently exhibited by progressive reductions in FEV1/FVC; by age 18, male CAMP subjects had a mean FEV1/FVC 76.5% vs 86.3% in the non-asthma cohort; the ratio in the CAMP females was 79.4% vs 89.5%) but without indication of the impact of age at onset or duration of disease on this outcome (15). As Martinez points out, though, in the CAMP population, much of the difference in FEV1/FVC relative to nonasthmatics was already present by age 6 (1). Remission of asthma in the CAMP cohort was infrequent (6%) and associated with milder disease, less atopy, better lung function (mean prebronchodilator FEV1 95.9% vs 91.3%; mean FEV1/FVC 82.6% vs 77.9%) and less AHR (geometric mean methacholine FEV1 PC 20 1.8 mg/ml vs 0.8 mg/ml) at entry into the study (16).
The Childhood Asthma Study was a smaller study of subjects with moderate to severe persistent asthma who were initially enrolled in a study of immunotherapy for childhood atopic asthma. The study population initially consisted of 121 subjects age 5–12 years at randomization, of whom 85 were reevaluated as young adults (ages 17–30 years). Of these 85 subjects, 13 (15.3%) were in remission with mean prebronchodilator FEV1 94.3% predicted, 19 (22.4%) had mild intermittent disease with mean FEV1 91.9% predicted, 12 (14.1%) had mild persistent disease with mean FEV1 87.9% predicted, 25 (29.4%) had moderate persistent disease with mean FEV1 79.6% predicted, and 16 (18.8%) had severe persistent disease with mean FEV1 66% predicted. Bronchodilator reversibility was greatest in those with moderate and severe persistent disease, yet their FEV1 did not normalize. Although they exhibited significant atopy, subjects who went into remission had lower mean IgE (412 vs 1136 vs 968), and a lower number of positive SPT (7 vs 9 vs 10) than did their peers with intermittent and persistent asthma at followup. Additionally, subjects who went into remission used fewer asthma medications during the initial trial than did their peers who exhibited ongoing disease. Of note, lung function and methacholine responsiveness at initial randomization did not predict remission; all three groups had randomization mean FEV1 below where one would expect for a healthy normal school-age child (range 86.7–93.2% predicted) and were severely hyperresponsive (mean PC20 range 0.22–0.14) (17). Strikingly, 48% of the subjects in the study exhibited abnormal lung function as adults, and of the subgroup that consented to a steroid trial, three fourths failed to improve. Longer duration of asthma, heightened methacholine responsiveness in childhood (PC20 0.11 vs 0.18 mg/ml), and premature birth at ≤ 34 weeks gestation were associated with these abnormalities (18).
Considered collectively, these studies and others (19) demonstrate that wheezing illness in childhood, particularly more persistent disease, is associated with ongoing persistence into adulthood, or, in the case of those that go into remission, an increased likelihood of relapse in young to mid adulthood. This disease pattern is associated with atopy, and aggravated by smoking. Finally, lung function changes associated with this disease are established before mid to late childhood, and wheezing that persists from childhood into adulthood or that relapses in adulthood is consistently associated with at least a 10% reduction in percent predicted FEV1 in these adults, relative to those who never wheeze. The rate of decline in lung function on the whole is similar between groups with wheeze and groups that remain asymptomatic, though there may be groups of asthmatics with progressive declines in lung function. Although the data are inconsistent, these studies suggest that earlier onset in childhood are associated with more severe and persistent disease and lower lung function.
Prospective birth cohorts
One of the major drawbacks in studies beginning in mid to late childhood in which asthma onset is determined retrospectively is the lack of accuracy and precision in the detection of the timing of the first symptoms of asthma. An additional issue is the high probability of ascertainment bias; namely that subjects with more severe disease are likely to be overrepresented amongst those whose parents report earlier onset of asthma. Also recall bias can be a major limitation in studies relating clinical phenotype and/or lung function to early life events because those who remain ill are more likely have parents who would remember that they were ill as infants or toddlers. To address this, several longitudinal cohort studies have been carried out in which subjects have been followed prospectively from birth, allowing for prospective evaluation of the development of asthma during infancy and childhood and the impact of early onset disease on long-term outcomes.
The earliest prospective observational birth cohort is the Tucson Children’s Respiratory Study (CRS) (20,21). Offspring of parents enrolled in a large health maintenance organization were recruited shortly after birth between May 1980 and October 1984, and a total of 1246 newborns were initially included. The cohort reflected a general population sample, and was not specifically selected for a history of asthma or atopy in the family. Questionnaires were obtained at baseline from the parents detailing their histories of respiratory illness, smoking habits and education. The children were seen by their primary care pediatricians whenever they developed signs and symptoms of lower respiratory tract illness (LRTI), and the pediatricians in turn recorded detailed histories and physical examinations to characterize these illnesses. In addition, the parents completed questionnaires when the children were ages 1.6 and 6 years in which they gave further wheezing history, as well as histories of rhinitis and eczema. Serum IgE was ascertained in cord blood (n=750), as well as in specimens obtained at 9 months (n=672) and 6 years (n=460) of life. Skin prick testing was done at age 6 (n=629). In addition, in 125 subjects, pulmonary function testing was assessed at age 2.4 months, before the occurrence of any LRTI, by obtaining partial expiratory flow-volume curves through chest compression. The maximal flow at functional residual capacity (V’maxFRC) was used as the marker to reflect the size of the intrapulmonary airways. At age 6, partial flow volume curves were obtained in 526 subjects, and V’maxFRC again calculated. At age 6 years, 826 children had complete data on LRTI in the first 3 years along with completed questionnaires at that time. At age six years, subjects were designated as having never wheezed (n=403, 51.5% of the total), those with at least one wheezing LRTI in the first three years of life but no wheezing at age 6 (transient early wheezing; n=147, 19.9%), those who had no wheezing LRTI in the first three years of life but who wheezed at age 6 (late onset wheezing; n=112, 15%), and those who had at least one wheezing LRTI by age 3 and had wheezing at age 6 (persistent wheezing; n=100, 13.7%) (18). Transient early wheezing was associated with maternal smoking. Late onset wheezing was associated with maternal asthma, male gender and early onset (year 1) rhinitis. At age 6, elevated serum IgE and SPT reactivity were more frequent in this group than in transient early or never wheezers. Persistent wheezing was associated with maternal asthma, increased frequency of wheezing and likelihood of being given a diagnosis of asthma, higher serum IgE at 9 months and 6 years, and SPT reactivity at age 6. As depicted in the figure, prior to the onset of wheezing, subjects with transient early wheeze had V’max FRC levels that were lower than those of their peers in all other groups (who did not differ from each other). By age 6, these transient early wheezers still had lower V’max FRC than those who had never wheezed, but those in the persistent wheeze group had the lowest lung function of all of the groups, suggesting that relative to their peers, their airways grew more slowly. The lung function in the late onset wheezers did not significantly differ from those who did not wheeze. The data thus suggested that those with more frequent persistent disease begin with symptoms very early in life and have an atopic tendency that is expressed in the first year of life perhaps during a critical time of rapid lung growth. Although their airways are of normal caliber at birth, there is evidence of loss of lung function (reduced growth in airway conductance) relative to their peers by age 6, consistent with the other studies demonstrating that the lung function changes in persistent asthma were already established by mid childhood (22).
Data from four-year-old subjects participating in a prospective birth cohort study in Antwerp Belgium who underwent lung function by forced oscillation at age 4 support the findings from the Tucson CRS cohort. Those children with transient early wheezing and those with persistent wheezing had airways resistance that was higher than those who had never wheezed. Those with persistent wheezing had a greater degree of atopy and a better response to bronchodilator (23). Lung function from infancy was not reported in that cohort.
In contrast to the Tucson CRS data, it is important also to mention that some studies from birth have reported low lung function shortly after birth as a risk factor for asthma in later childhood (24, 25). One group of studies deserves particular mention. Investigators in Perth Australia recruited a cohort of 243 infants before birth, and obtained lung function and AHR data at 1 month, 6 months and 12 months of age with follow up to 11 years of age. The lung function index measured was V’max FRC, which was carried out with the same technique as that used in Tucson. In that cohort, decreased V’max FRC at 1 month (Z score −0.59 adjusted standard deviations from the mean) was associated with persistent but not with transient wheeze. Again, the highest risk of an asthma diagnosis at age 11 was associated with the persistent wheeze group. It was not clear why the pulmonary function findings in Perth diverged from those in Tucson (25). In other cohorts, transient early wheezing has been associated with normal lung function in mid childhood, findings that also deviate from those in the Tucson CRS cohort (26). As shown in Table 2, the degree of AHR to histamine at 1 month did not differ between those with and without wheeze at age 11. Throughout the first year of life, all of the subjects in the Perth cohort gradually became less responsive to histamine, but those who did not wheeze at age 11 lost their responsiveness to a much greater extent than did those who wheezed at age 11. This difference was significant at 12 months of age and was labeled by the investigators, “persistence of increased airway responsiveness” (27).
Table 2.
Changes in airway hyperresponsiveness in infancy grouped by wheezing outcomes at age 11, from the Perth cohort (27). Table constructed from the data in the body of the paper.
| Wheezing at age 11 | No wheezing at age 11 | p | |
|---|---|---|---|
| Histamine PC40 at 1 month in mg/ml | 0.69 | 0.89 | 0.274 |
| Histamine PC40 at 6 months in mg/ml | 0.90 | 1.68 | 0.162 |
| Histamine PC40 at 12 months in mg/ml | 1.67 | 3.12 | 0.016 |
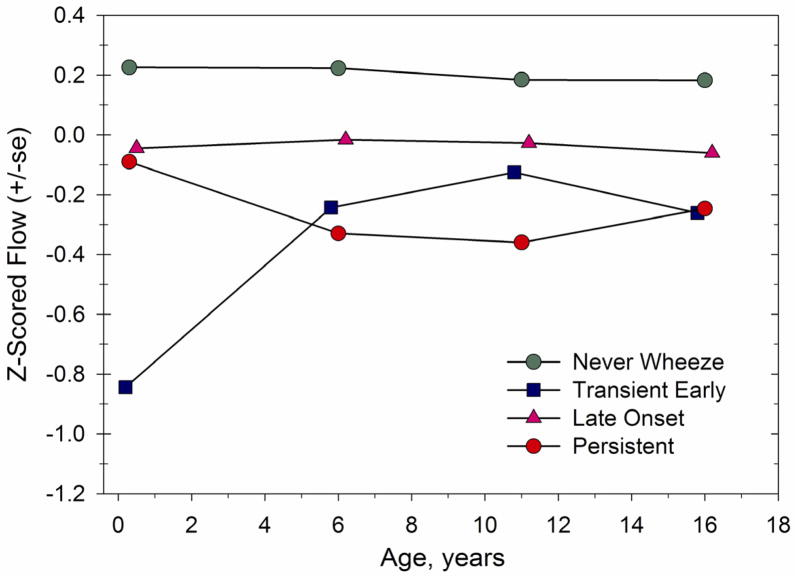
As regards wheezing in adolescence and early adulthood, the birth cohort studies have demonstrated findings similar to those cohorts begun in later childhood. Wheezing patterns established in the first 6 years of life were found to persist at age 16 in the Tucson CRS cohort (28). Thus, those who were wheezing at age 6 were more likely to be having continued wheezing at age 16. Furthermore, the increased prevalence of atopy in the 6 year-old subjects with wheezing also persisted into adolescence. These patterns were persistent regardless of whether they had begun before or after age 3. Strikingly, the lung function patterns established by age 6 persisted to age 16; ie: transient early wheezers and persistent wheezers continued to have lung function which was diminished relative to their peers with late onset wheeze or no wheeze in the first 6 years of life (Figure). Late onset wheezers had lung function which was slightly but not significantly diminished compared with that of never wheezers (28).
The CRS cohort was reassessed at age 22 with an emphasis on diagnosed asthma and wheeze. By age 22, 30% of the cohort had at one point in their lives received a physician diagnosis of asthma, 21.3% of the cohort had active asthma, and 19.2% reported wheeze without a formal asthma diagnosis. Subjects with wheezing at age 6, whether persistent or late onset, smokers, those with parental asthma, those sensitized to Alternaria, and those with low V’max FRC or hyperresponsiveness to cold air at age 6 were more likely to have current asthma or wheeze at age 22. Asthmatic subjects at age 22 were grouped into the categories of newly diagnosed asthma (no report of asthma between ages 2 and 16 but a diagnosis and symptoms at age 22), inactive asthma (diagnosis and symptoms between ages 2 and 16 but no symptoms at 22) and chronic asthma (diagnosis and symptoms between 2 and 16 and symptoms at 22). As shown in Table 1, lung function as assessed by FEV1/FVC was reduced in both newly diagnosed and chronic asthma. Subjects with chronic or inactive asthma at age 22 were grouped into three categories relative to their age at diagnosis: 2–6 years, 8–11 years and 13–16 years. After adjustment for asthma status and gender, age at diagnosis was significantly and linearly related to FEV1/FVC; i.e. later diagnosis was associated with better lung function. Newly diagnosed asthma at age 22 was associated with female gender, parental asthma, late onset and persistent wheezing at age 6, low lung function (lowest quartile of V’maxFRC) and cold air hyperresponsiveness at age 6. Interestingly, FEV1/FVC in newly diagnosed asthma was consistent with that in asthma of those with a past diagnosis who had been diagnosed earliest. In over 63% of cases of newly diagnosed asthma at age 22, episodes of wheezing had occurred during the first three years of life or were reported by the parents by age 6. While newly diagnosed asthma was associated with SPT positivity at age 22, it was not associated with sensitization at age 6. Thus, airway dysfunction was present early in life but allergic sensitization came later in this group of subjects. Interestingly, bronchodilator response was reduced in subjects with newly diagnosed asthma, but not in those with chronic disease, and this, coupled with the relatively low FEV1/FVC suggests the potential for a progressive airways dysfunction in this group (29).
More recently, studies have been underway of larger birth cohorts, and a statistical technique called latent class analysis has been applied to the data to ascertain patterns of early life wheeze. Based on the pattern of responses over time to questions about wheezing, subjects are assigned to a number of phenotypes called latent classes (30). Through this type of analysis on large numbers of subjects, additional patterns of early life wheeze have emerged. In the ALSPAC study, examining 6,265 subjects born in Avon UK, six phenotypes were identified, and in the PIAMA study examining 2,810 subjects from multiple centers in the Netherlands, five phenotypes emerged (30,31). The one newly characterized phenotype in common between both studies is the intermediate onset phenotype, characterized by little to no wheeze until 18–24 months, then a rise to high prevalence by 42–48 months. The subjects in both studies have been followed to 8 years. In both cohorts, intermediate onset wheeze was associated with a diagnosis of asthma by age 8 as were persistent wheeze and late onset wheeze. Intermediate onset, along with late onset and persistent wheeze, were all associated with atopy. At 8 years, decrements in lung function were noted in both studies. In the ALSPAC cohort, the greatest decrements in FEV1 were seen in the intermediate onset and persistent wheeze groups, along with those in a sixth group, prolonged early wheeze, while AHR was highest in the intermediate and late onset phenotypes (30). In the PIAMA cohort, the greatest deficit in FEV1 at age 8 was in the persistent wheeze group, while increased AHR was seen in the persistent, intermediate onset and late onset phenotypes (31). The ongoing prevalence of wheeze at age 7.5–8 years was higher among the persistent wheezing and intermediate onset wheezing groups in ALSPAC than in PIAMA. Longer term outcomes of the intermediate onset phenotype await ongoing followup of these cohorts. Also, of note, early life lung function is not reported for these cohorts.
Numerous longitudinal studies have established early life allergic sensitization, particularly to perennial allergens, as a major risk factor for persistence of wheeze (32,33,34,35,36,37,38). Latent class analysis on a population-based birth cohort in Manchester, England demonstrated an association between early sensitization to multiple allergens, persistent wheeze, asthma prevalence and severity (39).
In addition to allergic sensitization, data are emerging suggesting that early-life colonization or infection with specific organisms is associated with persistent wheeze and asthma. While early life virus-induced wheezing is common early in the development of asthma, data from a population in Madison Wisconsin at increased risk for asthma suggests that, of all of the respiratory viruses, rhinovirus wheezing infections presenting in the outpatient setting in infancy and early childhood are the most significant predictors of subsequent asthma and reduced lung function (age 8 mean FEV1 96% predicted in those with early rhinoviral wheezing vs 102% in those without) (40) . Lung function was not measured in this cohort prior to the onset of illness, so it is unclear whether diminished lung function is a cause rather than an effect of the wheezing associated with these rhinoviral infections (36,40). Fecal colonization with Clostridium difficile at age 1 month was associated with asthma, wheeze and atopy at age 6–7 years (41). In infants born to mothers with asthma, colonization at 1 month of age with S. Pneumoniae, M. Catarrhalis, H. Influenzae, or a combination of these organisms was associated with persistent wheezing, acute severe wheeze, asthma and bronchodilator reversibility at age 5 (42).
Discussion
The available data agree on the major point that wheeze that begins in early life and persists beyond the third year of age generally persists into adulthood. In those with what appears to be a new onset of wheeze in adulthood, the likelihood is high that there was an antecedent wheeze in childhood. Those who wheeze in early life are much more likely to persist if they have concomitant atopy. Relapsing wheezing is also more likely in the presence of atopy; furthermore, asthma which is newly diagnosed in early adulthood is associated with new sensitization during adolescence. Smoking and female gender likewise increase the risk of wheeze relapse. There is some disagreement among the studies as to whether individuals with persistent wheeze start life with normal or reduced lung function, but general agreement that by school age the lung function patterns are established and track in parallel through at least early to mid adulthood, with persistent wheezers and relapsers demonstrating ongoing decrements in lung function along with heightened AHR.
The etiology of asthma is poorly understood. As the disease we call asthma reflects a number of different wheezing phenotypes, it is likely that there is also a heterogeneity of etiologies. Given the multiplicity of findings in early onset persistent wheezing, ie: some born with normal lung function, and some with abnormal lung function, it is probable that even early life persistent wheezing reflects a heterogeneity of phenotypes with differing etiologies.
In those with persistent wheeze, airways dysfunction develops either prior to birth or shortly thereafter. The data from the Tucson CRS suggest that changes occur between birth and age 6. The added data from Antwerp suggest that these changes are already evident by age 4. Indeed, reticular basement membrane thickening and eosinophilic inflammation have been documented in preschoolers ages 1–3 with chronic wheezing (43) and have been documented in individuals as young as 10 months of age (44). While the data from Perth and Tucson diverge with regard to initial lung function in those with persistent wheeze, both studies point to events that play out in the first year of life, setting the stage for persistent wheezing. In the Tucson cohort, total IgE, which was not elevated in cord blood, was elevated by 9 months in persistent wheezers. Furthermore, those with persistent wheeze had diminished interferon-gamma production in mitogen-stimulated peripheral blood mononuclear cells obtained at 9 months of age (45). Finally a relative eosinophilia accompanied the first wheezy LRTI in those with persistent wheezing (46). In the Perth cohort, the persistence of increased AHR did not become apparent until 12 months of age. While the Perth investigators did not note increased atopy at the 12 month mark, they examined skin test reactivity rather than IgE (27). Thus, considering the Tucson and Perth cohorts collectively, developments occurred in the first year of life affecting airway function and atopy, which appeared to set the stage for persistent wheezing.
Although atopy and airway dysfunction appear to both set the stage for the development of persistent wheeze, they do not appear to be controlled by the same genes (47,48). It is possible that when atopy develops at a particular window of vulnerability in the growth of the airway, perhaps in conjunction with specific microbial illnesses or colonizations, persistent wheeze and asthma ensue. In most individuals, this window of vulnerability appears to manifest itself in the first year of life. Considering the individuals in the Tucson CRS cohort with new onset asthma at age 22 who were sensitized late, it is intriguing to consider that new sensitization during adolescence, a time of rapid growth velocity of the airways (15) may have also occurred during a second window of vulnerability leading to the relatively late development of asthma. The association of asthma in teenage and early adult years with active smoking (10,29,49) and the association of new onset adolescent wheezing, increased peak flow variability and albuterol responsiveness independent of atopy in girls who become overweight or obese during the school years (50) lend further credence to the concept of a second window of vulnerability during late childhood and adolescence for airway injury to occur and wheeze and asthma ensue. Finally, childhood onset asthma as distinct from later onset asthma appears to be associated with its own unique genes (49, 51). The risk of wheeze and asthma sequelae from airway injuries at particular windows of vulnerability may therefore be genetically determined, a concept supported by recent evidence of genetic variants associated with early onset asthma, low lung function, and heightened disease severity (51).
Most of the studies suggest that once lung function patterns are established in individuals with wheezing by early school age years, further losses relative to their non-wheezing peers do not occur in most patients. However, the finding of a roughly 10% decrement in FEV1 and 5% decrement in FEV1/FVC during early to mid adulthood in those with persistent or relapsed wheezing is strikingly consistent among the studies. Although the mean values of FEV1 in these groups fall within the range of what clinicians would consider “normal,” one may speculate that since they do not attain maximal lung function, as those with persistent or relapsing wheezing age, they will be more likely to manifest severe chronic airways disease as they drop their lung function below the threshold for COPD (1). It is important also to consider the progressive loss of lung function noted in participants in the CAMP study, which were not noted in the longitudinal population based studies (14,15). Although the participants in CAMP had mild to moderate persistent asthma, they still manifested disease which was much more severe than those in the population-based cohorts. Individuals with severe persistent asthma may lose lung function at an even more accelerated pace, as evidenced especially by those subjects in the Childhood Asthma Study who entered adult life with mean FEV1 of 66% predicted (17). Thus, one may further speculate that smaller subgroups of individuals with persistent asthma may indeed be at heightened risk for development of even more severe COPD, possibly due to coinheritance of genes coding for severe, early onset asthma, severe AHR, and severe atopy.
Although our understanding of the natural history of asthma has advanced considerably over the past 30 years, there is still much to be learned. Cohort sizes have been relatively small and the emergence of new wheezing phenotypes from larger cohorts illustrates the tremendous value of larger sample sizes. Pooling of data such as that occurring in the GA2LEN initiative (52) should help but lack of standardization of study protocols can hamper the effort. Subjects who are most at risk, namely inner-city minorities, are woefully underrepresented in longitudinal cohort studies, though a new study is examining the development of asthma in these groups as well (53). Asthma that is clinically significant is not very frequent in population studies, thus necessitating very large cohorts such as exist in the United States National Children’s Study (54) to detect and have power to examine risk factors for severe disease. There is a need to gather more data on in-utero exposures. Additionally, pre-morbid lung function data are relatively sparse and difficult to obtain. New techniques, such as the lung clearance index, which measures the washout of inert gas and detects airways obstruction, may facilitate obtaining data on larger numbers of infants, which can then be followed through the preschool years, childhood, adolescence and adulthood (55). Finally, existing cohorts that were assembled at birth or during childhood have been followed only to mid adulthood; thus any association of their childhood disease with later COPD remains a matter of speculation.
In summary, individuals with persistent and relapsing wheezing in early to mid adulthood exhibit abnormalities in lung function, which have their roots in infancy and childhood. Furthermore, it would appear that there are two postnatal windows of vulnerability during which the growing airway is at risk for injury leading to wheeze and asthma: during the first few years, in combination with allergic sensitization and possibly infection, and during adolescence, in combination also with allergic sensitization, but also with weight gain and smoking. The primary prevention of early onset persistent wheeze and asthma rests upon an improved understanding of events leading to its inception. Realizing that goal will require large collaborative efforts on a global scale. The rewards of such efforts should impact countless lifetimes.
Figure 1.
Cross-sectional z scores of height-adjusted maximal expiratory flows at ages 2.4 mo, 6, 11 and 16 years for the preschool wheezing groups in the Tucson Children’s Respiratory Study. The z score indicates how many standard deviations from the mean an individual’s value lies. This figure illustrates that those with persistent wheezing begin life with flows that do not differ from their peers with late-onset wheeze or those who never wheeze, but by age 6 demonstrate decrements in flow relative to these other groups. In contrast, those with transient early wheezing demonstrate early and persistent reductions in flow. Taken from Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, et al. Outcome of asthma and wheezing in the first 6 years of life. Follow-up through adolescence. Am J Respir Crit Care Med 2005;172:1253–8 (28). Official Journal of the American Thoracic Society. Reprinted with permission of the American Thoracic Society. Copyright (C) 2005 American Thoracic Society.
Key Concepts.
Early onset wheeze with atopy is associated with lung function deficit in adolescence/early adulthood.
Atopy with high IgE, eosinophilia, or skin prick test positivity is associated with increased risk of persistent wheeze.
Airway infection in early life with rhinovirus or colonization with S. Pneumoniae, M. Catarrhalis, H. Influenzae are associated with persistent wheeze and asthma.
Most deficits in lung function appear to be established as early as birth, but certainly within the first 6 years of life.
About 25% of patients with clinically diagnosed asthma are at risk for accelerated declines in lung function.
Adolescence may represent a second window of vulnerability for the development of persistent wheeze and asthma.
Acknowledgments
Supported by NIH grants HL-09H112, HL-056177 and AI-042268
The authors thank Debra Stern for her assistance with the figure and Brenda Lambert for her assistance with manuscript formatting and submission.
Abbreviations
- COPD
chronic obstructive pulmonary disease
- LRTI
lower respiratory tract illness
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- SPT
skin prick test
- HDM
house-dust mite
- IgE
immunoglobulin E
- AHR
airways hyperresponsiveness
- CAMP
Childhood Asthma Management Program
- CRS
(Tucson) Children’s Respiratory Study
- V’max FRC
maximal flow at functional residual capacity
- ALSPAC
Avon Longitudinal Study of Parents and Children
- PIAMA
(Dutch) Prevention and Incidence of Asthma and Mite Allergy study
- GA2LEN
Global Allergy and Asthma European Network
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martinez FD. The origins of asthma and chronic obstructive pulmonary disease in early life. Proc Am Thorac Soc. 2009;6:272–7. doi: 10.1513/pats.200808-092RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez FD. New insights into the natural history of asthma: primary prevention on the horizon. J Allergy Clin Immunol. 2011;128:939–45. doi: 10.1016/j.jaci.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams HE, McNicol KN. Prevalence, natural history and relationship of wheezy bronchitis and asthma in children. An epidemiological study. BMJ. 1969;4:321–5. doi: 10.1136/bmj.4.5679.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phelan PD, Robertson CF, Olinsky A. The Melbourne Asthma Study: 1964–1999. J Allergy Clin Immunol. 2002;109:189–94. doi: 10.1067/mai.2002.120951. [DOI] [PubMed] [Google Scholar]
- 5.Horak E, Lanigan A, Roberts M, Welsh L, Wilson J, Carlin JB, et al. Longitudinal study of childhood wheezy bronchitis and asthma: outcome at age 42. BMJ. 2003;326:422–3. doi: 10.1136/bmj.326.7386.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strachan DP, Butland BK, Anderson HR. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ. 1996;312:1195–9. doi: 10.1136/bmj.312.7040.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butland BK, Strachan DP. Asthma onset and relapse in adult life: the British 1958 birth cohort study. Ann Allergy Asthma Immunol. 2007;98:337–43. doi: 10.1016/S1081-1206(10)60879-4. [DOI] [PubMed] [Google Scholar]
- 8.Strachan DP, Griffiths M, Johnston IDA, Anderson HR. Ventilatory function in British adults after asthma or wheezing illness at ages 0–35. Am J Respir Crit Care Med. 1996;154:1629–35. doi: 10.1164/ajrccm.154.6.8970346. [DOI] [PubMed] [Google Scholar]
- 9.Marossy AE, Strachan DP, Rudnicka AR, Anderson HR. Childhood chest illness and the rate of decline of adult lung function between ages 35 and 45 years. Am J Respir Crit Care Med. 2007;175:355–9. doi: 10.1164/rccm.200607-1023OC. [DOI] [PubMed] [Google Scholar]
- 10.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, et al. A longitudinal, population-based cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–22. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen F, Taylor DR, Flannery EM, Cowan JO, Greene JM, Herbison GP, Sears MR. Risk factors for hospital admission for asthma from childhood to young adulthood: a longitudinal population study. J Allergy Clin Immunol. 2002;110:220–7. doi: 10.1067/mai.2002.125295. [DOI] [PubMed] [Google Scholar]
- 12.Zeiger RS, Dawson C, Weiss S CAMP Research Group. Relationships between duration of asthma and asthma severity among children in the Childhood Asthma Management Program (CAMP) J Allergy Clin Immunol. 1999:376–87. doi: 10.1016/s0091-6749(99)70460-4. [DOI] [PubMed] [Google Scholar]
- 13.Weiss ST, Van Natta ML, Zeiger RS CAMP Research Group. Relationships between increased airway responsiveness and asthma severity in the Childhood Asthma Management Program. Am J Respir Crit Care Med. 2000;162:50–6. doi: 10.1164/ajrccm.162.1.9811005. [DOI] [PubMed] [Google Scholar]
- 14.Covar RA, Spahn JD, Murphy JR, Szefler SJ CAMP Research Group. Progression of asthma measured by lung function in the Childhood Asthma Management Program. Am J Respir Crit Care Med. 2004;170:234–41. doi: 10.1164/rccm.200308-1174OC. [DOI] [PubMed] [Google Scholar]
- 15.Strunk RC, Weiss ST, Yates KP, Tonascia J, Zeiger RS, Szefler SJ CAMP Research Group. Mild to moderate asthma affects lung growth in children and adolescents. J Allergy Clin Immunol. 2006;118:1040–7. doi: 10.1016/j.jaci.2006.07.053. [DOI] [PubMed] [Google Scholar]
- 16.Covar RA, Strunk R, Zeiger RS, Wilson LA, Liu AH, Weiss S, et al. Predictors of remitting, periodic, and persistent childhood asthma. J Allergy Clin Immunol. 2010;125:359–66. doi: 10.1016/j.jaci.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Limb SL, Brown KC, Wood RA, Wise RA, Eggleston PA, Tonascia J, et al. Adult asthma severity in individuals with a history of childhood asthma. J Allergy Clin Immunol. 2005;115:61–6. doi: 10.1016/j.jaci.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 18.Limb SL, Brown KC, Wood RA, Wise RA, Eggleston PA, Tonascia J, Adkinson NF. Irreversible lung function deficits in young adults with a history of childhood asthma. J Allergy Clin Immunol. 2005;116:1213–9. doi: 10.1016/j.jaci.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Strachan D, Gerritsen J. Long-term outcome of early childhood wheezing: population data. Eur Resp J. 1996;9(Suppl21):42s–47s. [PubMed] [Google Scholar]
- 20.Taussig LM, Wright AL, Morgan WJ, Harrison HR, Ray CG. The Tucson Children’s Respiratory Study. I. Design and implementation of a prospective study of acute and chronic respiratory illness in children. Am J Epidemiol. 1989;129:1219–31. doi: 10.1093/oxfordjournals.aje.a115242. [DOI] [PubMed] [Google Scholar]
- 21.Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson Children’s Respiratory Study: 1980 to present. J Allergy Clin Immunol. 2003;111:661–75. doi: 10.1067/mai.2003.162. [DOI] [PubMed] [Google Scholar]
- 22.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ Group Health Medical Associates. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 23.Oostveen E, Dom S, Desager K, Hagendorens M, De Backer W, Weyler J. Lung function and bronchodilator response in 4-year-old children with different wheezing phenotypes. Eur Resp J. 2010;35:865–72. doi: 10.1183/09031936.00023409. [DOI] [PubMed] [Google Scholar]
- 24.Haland G, Lodrup Carlsen KC, Sandvik L, Devulapalli CS, Munthe-Kaas MC, Pettersen M, et al. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med. 2006;355:1682–9. doi: 10.1056/NEJMoa052885. [DOI] [PubMed] [Google Scholar]
- 25.Turner SW, Palmer LJ, Rye PJ, Gibson NA, Judge PK, Cox M, et al. The relationship between infant airway function, childhood airway responsiveness and asthma. Am J Respir Crit Care Med. 2004;169:921–7. doi: 10.1164/rccm.200307-891OC. [DOI] [PubMed] [Google Scholar]
- 26.Lau S, Illi S, Sommerfeld C, Niggemann B, Volkel K, Madloch C, et al. Transient early wheeze is not associated with impaired lung function in 7-yr-old children. Eur Respir J. 2003;21:834–41. doi: 10.1183/09031936.03.00037203. [DOI] [PubMed] [Google Scholar]
- 27.Turner SW, Young S, Goldblatt J, Landau LI, LeSouef PN. Childhood asthma and increased airway responsiveness. A relationship that begins in infancy. Am J Respir Crit Care Med. 2009;179:98–104. doi: 10.1164/rccm.200805-804OC. [DOI] [PubMed] [Google Scholar]
- 28.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, et al. Outcome of asthma and wheezing in the first 6 years of life, follow-up through adolescence. Am J Respir Crit Care Med. 2005;172:1253–8. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD. Wheezing and bronchial hyperresponsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372:1058–64. doi: 10.1016/S0140-6736(08)61447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson J, Granell R, Heron J, Sherriff A, Simpson A, Woodcock A, et al. Associations of wheezing phenotypes in the first six years of life with atopy, lung function and airway responsiveness in mid–childhood. Thorax. 2008;63:974–80. doi: 10.1136/thx.2007.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savenije OE, Granell R, Caudri D, Koppelman GH, Smit HA, Wijga A, et al. Comparison of childhood wheezing phenotypes in 2 birth cohorts: ALSPAC and PIAMA. J Allergy Clin Immunol. 2011;127:1505–12. doi: 10.1016/j.jaci.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Rhodes HL, Thomas P, Sporik R, Holgate ST, Cogswell JJ. A birth cohort study of subjects at risk for atopy. Twenty-two-year follow-up of wheeze and atopic status. Am J Respir Crit Care Med. 2002;165:176–80. doi: 10.1164/ajrccm.165.2.2104032. [DOI] [PubMed] [Google Scholar]
- 33.Kurukulaaratchy RJ, Matthews S, Holgate ST, Arshad SH. Predicting persistent wheeze among children who wheeze during early life. Eur Respir J. 2003;22:767–71. doi: 10.1183/09031936.03.00005903. [DOI] [PubMed] [Google Scholar]
- 34.Illi S, von Mutius E, Lau S, Niggemann B, Gruber C, Wahn U MAS group. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–70. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 35.Torrent M, Sunyer J, Garcia R, Harris J, Iturriaga MV, Puig C, et al. Early-life allergen exposure and atopy, asthma, and wheeze up to 6 years of age. Am J Respir Crit Care Med. 2007;176:446–53. doi: 10.1164/rccm.200607-916OC. [DOI] [PubMed] [Google Scholar]
- 36.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus infections in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lodge CJ, Lowe AJ, Gurrin LC, Hill DJ, Hosking CS, Khalafzai RU, et al. House dust mite sensitization in toddlers predicts current wheeze at age 12 years. J Allergy Clin Immunol. 2011;128:782–8. doi: 10.1016/j.jaci.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 38.Kusel MMH, Kebadze T, Johnston SL, Holt PG, Sly PD. Febrile respiratory illnesses in infancy & atopy are risk factors for persistent asthma and wheeze. Eur Respir J. 2011 Sep 20; doi: 10.1183/09031936.00193310. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 39.Simpson A, Tan VYF, Winn J, Svensen M, Bishop CM, Heckerman DE, et al. Beyond atopy. Multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med. 2010;181:1200–6. doi: 10.1164/rccm.200907-1101OC. [DOI] [PubMed] [Google Scholar]
- 40.Guilbert TW, Singh AM, Danov Z, Evans MD, Jackson DJ, Burton R, et al. Decreased lung function after preschool wheezing rhinovirus illnesses in children at risk to develop asthma. J Allergy Clin Immunol. 2011;128:532–8. doi: 10.1016/j.jaci.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Nimwegen FA, Penders J, Stobberingh EE, Postma DS, Koppelman GH, Kerkhof M, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol. 2011;128:948–55. doi: 10.1016/j.jaci.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 42.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–95. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 43.Saglani S, Payne DN, Zhu J, Wang Z, Nicholson AG, Bush A, Jeffery PK. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med. 2007;176:858–64. doi: 10.1164/rccm.200702-212OC. [DOI] [PubMed] [Google Scholar]
- 44.Saglani S, Malmstrom K, Pelkonen AS, Malmberg LP, Lindahl H, Kajosaari M, et al. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med. 2005;171:722–7. doi: 10.1164/rccm.200410-1404OC. [DOI] [PubMed] [Google Scholar]
- 45.Stern DA, Guerra S, Halonen M, Wright AL, Martinez FD. Low IFN-γ production in the first year of life as a predictor of wheeze during childhood. J Allergy Clin Immunol. 2007;120:835–41. doi: 10.1016/j.jaci.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 46.Martinez FD, Stern DA, Wright AL, Taussig LM, Halonen M. Differential immune responses to acute lower respiratory illness in early life and subsequent development of persistent wheezing and asthma. J Allergy Clin Immunol. 1998;102:915–20. doi: 10.1016/s0091-6749(98)70328-8. [DOI] [PubMed] [Google Scholar]
- 47.Bisgaard H, Bonnelykke K, Sleiman PMA, Brasholt M, Chawes B, Kreiner-Moller E, et al. Chromosome 17q21 gene variants are associated with asthma and exacerbations but not atopy in early childhood. Am J Respir Crit Care Med. 2009;179:179–85. doi: 10.1164/rccm.200809-1436OC. [DOI] [PubMed] [Google Scholar]
- 48.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–21. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Avila L, Soto-Martinez ME, Soto-Quiros ME, Celedon JC. Asthma, current wheezing, and tobacco use among adolescents and young adults in Costa Rica. J Asthma. 2005;42:543–7. doi: 10.1080/02770900500214791. [DOI] [PubMed] [Google Scholar]
- 50.Castro-Rodriguez JA, Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Increased incidence of asthmalike symptoms in girls who become overweight or obese during the school years. Am J Respir Crit Care Med. 2001;163:1344–9. doi: 10.1164/ajrccm.163.6.2006140. [DOI] [PubMed] [Google Scholar]
- 51.Forno E, Lasky-Su J, Himes B, Howrylak J, Ramsey C, Brehm J, et al. Genome-wide association study of the age of onset of childhood asthma. J Allergy Clin Immunol. 2012 doi: 10.1016/j.jaci.2012.03.020. e pub 4 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keil T, Kulig M, Simpson A, Custovic A, Wickman M, Kull I, et al. European birth cohort studies on asthma and atopic diseases: II. Comparison of outcomes and exposures – a GA2LEN initiative. Allergy. 2006;61:1104–11. doi: 10.1111/j.1398-9995.2006.01167.x. [DOI] [PubMed] [Google Scholar]
- 53.Gern JE, Visness CM, Gergen PJ, Wood RA, Bloomberg GR, O’Connor GT, et al. The Urban Environment and Childhood Asthma (URECA) birth cohort study: design, methods, and study population. BMC Pulmonary Medicine. 2009;9:17. doi: 10.1186/1471-2466-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Landrigan PJ, Transande L, Thorpe LE, Gwynn C, Lioy PJ, D’Alton ME, et al. The Natoinal Children’s Study: A 21-year prospective study of 100000 American children. Pediatrics. 2006;118:2173–86. doi: 10.1542/peds.2006-0360. [DOI] [PubMed] [Google Scholar]
- 55.Robinson PD, Goldman MD, Gustafsson PM. Inert gas washout: theoretical background and clinical utility in respiratory disease. Respiration. 2009;78:339–55. doi: 10.1159/000225373. [DOI] [PubMed] [Google Scholar]