Abstract
Background
Prostate cancer mortality rates in the US declined by over 40% between 1991 and 2005. The impact of changes in primary treatment and adjuvant and neoadjuvant hormonal therapy on this decline is unknown.
Methods
Application of three independently developed models of prostate cancer natural history and disease detection under common assumptions about treatment patterns, treatment efficacy, and survival in the population. Primary treatment patterns are from the Surveillance, Epidemiology and End Results registry and hormonal therapy frequencies are from the CaPSURE database; treatment efficacies are based on estimates from randomized trials and comparative effectiveness studies of treatment alternatives. The models project prostate cancer mortality without PSA screening and in the presence and absence of treatment benefit. Impact of primary treatment is expressed as a fraction of the difference between observed mortality and projected mortality in the absence of treatment benefit.
Results
The three models project that changes in treatment explain 22–33% of the mortality decline by 2005. These contributions are accounted for mostly by surgery and radiation therapy, which increased in frequency until the 1990s; hormonal therapies contributed little to the mortality decline by 2005. Assuming that treatment benefit is less for older men, changes in treatment explain only 16–23% of the mortality decline by 2005.
Conclusions
Changes in primary treatment explain a minority of the observed decline in prostate cancer mortality. The remainder of the decline is likely due to other interventions, such as PSA screening and advances in the treatment of recurrent and progressive disease.
Keywords: Computer simulation, mortality, prostatectomy, prostatic neoplasms, radiotherapy, surveillance
1. Introduction
Since the early 1990s we have witnessed a spectacular decline in prostate cancer mortality in the US. Between 1991 and 2005 alone, prostate cancer mortality declined by 42% from 103 to 60 deaths per 100,000 men aged 50–84 y. This remarkable success story coincided with dramatic changes in the control of the disease: the widespread adoption of prostate-specific antigen (PSA) screening beginning around 1987, advances in treatment of early stage tumors, and changes in the detection and treatment of recurrent and progressive disease.
Because of the simultaneous dissemination of PSA screening and changes in treatment, a clear explanation for the drop in prostate cancer deaths has been elusive. In a 2003 editorial titled “The Prostate Cancer Conundrum,” Albertsen questioned the relative roles of primary surgery and adjuvant hormonal therapy for localized disease in explaining the mortality trends.1 Rates of surgery surged in the 1980s following the development of nerve-sparing techniques for radical prostatectomy. Randomized trial results indicate that radical prostatectomy improves disease-specific survival relative to watchful waiting, with a 38% reduction in the risk of prostate cancer death.2 Hormonal therapy, previously reserved for men with advanced cancers, is particularly efficacious when used in combination with external-beam radiation therapy, and its use dramatically increased in the mid- to late 1990s.3
The role of PSA screening in explaining the drop in disease-specific deaths has also been questioned4 but has not been conclusively determined. Long-awaited results from two large prostate cancer screening trials failed to convincingly establish screening benefit, with the European trial showing a 20% lower disease-specific mortality rate in the screening arm over a median of 9 years5 and the US trial showing no difference between the control and screening arms after 7 years of complete follow-up.6 However, it is generally recognized that since men on the control arm of the US trial received “usual care,” which included routine screening,7 the results should be interpreted as a comparison between moderate and high screening intensities.8
The Cancer Intervention and Surveillance Modeling Network (CISNET) prostate group was formed to quantify the relative contributions of screening and treatment changes to the mortality declines. Previously, CISNET prostate models were used to show that early detection due to screening could account for approximately 45–70% of the decline in prostate cancer mortality under a “stage-shift” mechanism for screening benefit. The stage-shift mechanism specifies that disease shifted to an earlier stage by screening enjoys a corresponding improvement in disease-specific survival. This mechanism is a central motivator underlying all cancer screening studies; however, the extent to which it holds is not known conclusively in the case of prostate cancer.
In this article, we take a different approach and quantify the fraction of the mortality decline plausibly due to treatment changes among men with non-metastatic disease. To do this, we model the dissemination and benefits of first-line treatment (radical prostatectomy and radiation therapy alone or in combination with hormonal therapy) and project their impact on mortality in the absence of screening. The results are informative about the likely role of treatment changes in explaining prostate cancer mortality declines. In addition, they are suggestive of a potential role for screening and/or other practice changes, such as treatment for recurrent or progressive cancer.
2. Methods
2.1 The CISNET paradigm
The CISNET approach is, at its core, a model of disease natural history, representing the individual experience of disease onset and progression, diagnosis, and death in the absence of any interventions of interest. Interventions, such as screening and/or treatment, are then superimposed based on analyses of patterns of care in the population and on known efficacy from randomized trials or assumed mechanisms of benefit.
In the present setting the models first produce projections of prostate cancer mortality in the absence of screening and treatment among cases diagnosed from 1975 because limited population-representative data are available before 1975 to inform the natural history models. By “absence of treatment” we mean in the absence of treatment benefit, as all projections under this setting assume that primary treatment interventions are not beneficial (i.e., the hazard ratio for disease-specific survival equals 1.0 relative to conservative management). The models also project mortality in the presence of treatment but in the absence of screening, i.e., assuming that stage and grade distributions at diagnosis would have remained as observed in the pre-PSA era. We project mortality in the absence of screening because projections in the presence of screening would rely on an assumed survival benefit of screening, a benefit with greater uncertainty than the benefits of primary treatments.
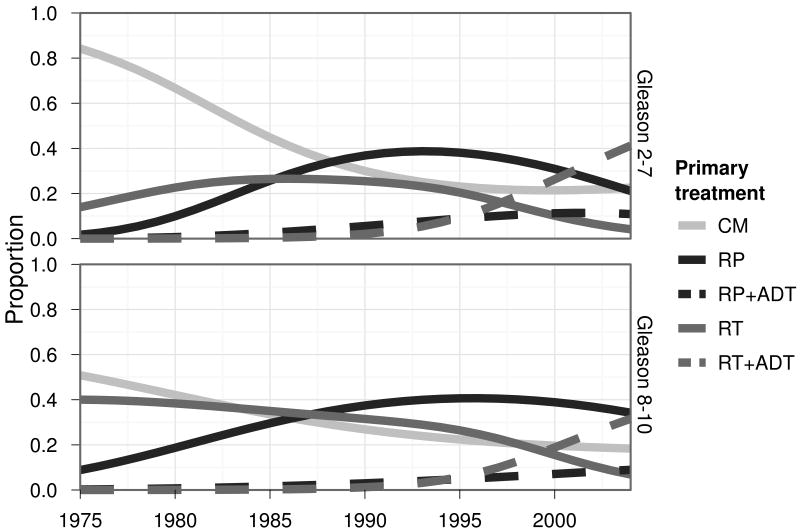
The impact of treatment occurs through changes in treatment distributions (Figure 1) as well as through treatment benefit (treatment-specific hazard ratios for disease-specific survival that are less than 1.0 relative to conservative management). We use the terms “in the presence of treatment” and “in the presence of changes in treatment” interchangeably. By comparing the mortality projections in the presence and absence of treatment with observed disease-specific mortality trends, we can quantify the fraction of the mortality decline associated with treatment. For example, if a projection in the presence of treatment lies half way between our projection in the absence of treatment and observed mortality, we would conclude that treatment alone (due to treatment benefit and changes in treatment patterns) accounts for approximately 50% of the observed mortality decline.
Figure 1.
Primary treatment dissemination patterns in the US.
Footnote to Figure 1. RP is radical prostatectomy and is defined by SEER codes 50, 58, 60, and 68 prior to 1997 and 50 and 70 beginning in 1998. RT is radiation therapy and is defined by SEER categories beam radiation, radioactive implants, radioisotopes, combination of beam with implants or isotopes, or radiation with method or source unspecified. ADT is androgen deprivation therapy from CaPSURE.
The CISNET prostate working group consists of three groups developing independent models of prostate cancer natural history informed by common information on patterns of screening, disease incidence, and other-cause mortality. Each natural history model is different, but the models are each calibrated using age-, year-, stage-, and grade-specific prostate cancer incidence from the Surveillance, Epidemiology, and End Results (SEER) program before and after the introduction of PSA screening. The calibrated natural history models are then combined with common information on treatment patterns and disease-specific survival to project prostate cancer mortality under plausible assumptions about treatment efficacy.
In the next section we briefly describe each natural history model and the calibration methods used. We then detail survival modeling procedures, our data sources, and assumptions regarding treatment efficacy.
2.2 Model structures
Detailed descriptions of individual models and a joint report comparing the models are available at http://cisnet.cancer.gov/prostate/profiles.html.
2.2.1 The FHCRC model
In the Fred Hutchinson Cancer Research Center (FHCRC) model, the risk of disease onset, and associated Gleason grade category which is fixed at onset, depends on age. Disease progresses from localized to metastatic stages and from latent to symptomatic states based on risks that depend on grade-specific PSA levels. Distributions of PSA growth rates were estimated using longitudinal PSA measurements from men in the control arm of the Prostate Cancer Prevention Trial.9 Given individual PSA trajectories and natural histories, PSA screening patterns,10 biopsy compliance frequencies observed in the US-based Prostate, Lung, Colorectal, and Ovarian cancer screening trial,11, 12 and trends in biopsy sensitivity in the population,13 risks of transitioning from one state to the next were estimated using maximum likelihood to obtain parameter estimates that best reproduce SEER incidence.14
2.2.2 The MISCAN model
In the Erasmus University Medical Center MIcrosimulation SCreening ANalysis (MISCAN) prostate model, cancer development is modeled as a semi-Markov process governing transitions from one state to the next. In addition to the healthy state, there are 18 states in the natural history of prostate cancer that are derived from combinations of clinical T (T1, T2, and T3) and M (M0 and M1) stages in the TNM staging system and Gleason grade (well, moderately, and poorly differentiated). Cancers in each state may be clinically diagnosed or detected by a PSA test and subsequent biopsy, the probability of which is combined in a single sensitivity parameter that is state-specific. Model parameters (progression rates between states and test sensitivities) were estimated using data from the Rotterdam section of the ERSPC.15, 16 For calibration to the US situation, we re-estimated the test sensitivity parameters and estimated an additional stage-specific risk of clinical diagnosis to capture different pre-PSA disease diagnosis patterns in the US as compared with Europe. US-specific estimates for the parameters were obtained by calibrating the model to the observed age-specific incidence and age-specific metastatic stage distribution using maximum likelihood.17
2.2.3 The UMICH model
The University of Michigan (UMICH) natural history model consists of disease-free, pre-clinical, and clinical states. An analytic formulation first estimates age- and year-specific disease incidence based on PSA screening patterns,10 assuming parametric distributions for age at onset and for time from onset to diagnosis, and increasing test sensitivity with time since onset. As in the MISCAN model, test sensitivity reflects both the diagnostic properties of the test itself and the frequency and sensitivity of any subsequent biopsy. Parameters are estimated by averaging over these distributions and calibrating the resulting marginal incidence against observed incidence.18 Next, disease stage (SEER local-regional or distant) and grade category (Gleason score 2–7 or 8–10) at diagnosis are estimated based on time from onset to diagnosis and mode of detection (screen or clinical) via a multinomial logistic model.19 Maximum likelihood estimation of the joint model of age-specific incidence trends and stage/grade distributions informs the distributions of these clinical characteristics.
2.4 Modeling survival
In the absence of treatment, all three models generate disease-specific survival based on SEER data among men diagnosed just prior to the PSA era, during the calendar interval 1983–1986. A Poisson regression model is fit to the disease-specific survival frequencies, censoring deaths due to other causes and adjusting for age, stage, and grade at diagnosis and initial treatment (radical prostatectomy, radiation therapy, both, or neither). Then the fitted survival curve for men receiving neither treatment is used to predict disease-specific survival times under no screening and no initial therapy. Relative to local-regional survival, trends in survival for distant stage disease have remained fairly constant over time.20
2.5 Treatment dissemination and efficacy
Dissemination of treatment is modeled based on two data sources. Trends in primary treatments—radical prostatectomy (RP) and radiation therapy (RT)—are based on data from SEER, which records first cancer-directed therapy received. Trends in receipt of adjuvant or neoadjuvant androgen deprivation therapy (ADT), also called hormonal therapy, are based on the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) database.3, 21 CaPSURE was initiated in 1995 to document community trends in prostate cancer practice patterns, epidemiology, and outcomes. It is a longitudinal, observational database accruing data from 40 urologic practice sites over its history. There are currently over 14,000 men enrolled in CaPSURE. CaPSURE collects approximately 1,000 clinical and patient-reported variables. Clinical information is collected by the treating urologist at baseline and with each follow-up visit. Figure 1 documents trends in primary treatment by Gleason category.
We model five initial treatment courses for local-regional disease: conservative management (CM), RP, RP+ADT, RT, and RT+ADT. These treatments are modeled because they represent the predominant treatment interventions used to treat prostate cancer, because their utilization has changed over time, and because there is quantitative evidence regarding efficacy available from randomized trials. We grouped three-dimensional conformal beam external beam radiotherapy, intensity modulated radiation therapy, and low- and high-dose interstitial brachytherapy into a single RT. This was based on a recent comparative effectiveness review from the Agency for Healthcare Research and Quality's Evidence-Based Practice Center at Tufts University which found no studies reporting a significant difference in overall survival or biochemical failure among the various forms of radiation.22 Unfortunately, there are no randomized comparisons of all treatments; consequently, we integrate evidence from several sources.
A brief summary of comparative effectiveness results for these treatments is as follows. There is evidence that RP is more efficacious than CM with a relative risk of 0.62.2 There are no clinical trials directly comparing RP with RT; however, comparative effectiveness studies adjusting for case mix and progression risk consistently show a benefit for RP over RT with two recent studies producing adjusted relative risks of 0.45 and 0.47 for the endpoint of disease-specific mortality.23, 24 There is evidence from clinical trials that RT+ADT is more efficacious than RT alone25-27 and evidence from a recent comparative effectiveness study that RT+ADT is similar to RP (relative risk = 1.14 for RT+ADT relative to RP, p = 0.6124). For a recent review see Wilt et al.28
Based on these studies, we assume a hazard ratio of 0.62 for RP relative to CM and for RP+ADT relative to CM and apply this to prostate cancer-specific survival for untreated cases. However, to make the relative benefit of RT alone consistent with published studies would either require RT alone to be almost without benefit or RT+ADT to be far superior than RP. Therefore our assumed benefit range for RT compromises, reflecting lower benefit than either RP or RT+ADT, but not so low as to make RT completely ineffective. We also assume a time-varying relative risk associated with RT relative to CM to reflect the improvement in the efficacy of RT as more intense dose-delivery regimens evolved. Specifically, we assume the hazard ratio for RT relative to CM improved linearly from 0.9 in 1990 to 0.7 or 0.8 in 1995 and remained constant thereafter. This assumption implies a relative risk for RP versus RT that is either 0.62/0.8 ≈ 0.77 or 0.62/0.7 ≈ 0.89 after 1995. To reflect an even greater relative benefit of RP relative to RT alone, consistent with recent comparative effectiveness studies,23, 24 we also conduct a high-efficacy sensitivity experiment in which we use the more efficacious assumption for RT (hazard ratio 0.7 relative to CM after 1995) and lower the hazard ratio for RP relative to CM to 0.4, implying a relative risk for RP versus RT of 0.4/0.7 ≈ 0.57 after 1995.
We also consider age-specific hazard ratios for all curative treatments, based on the finding from the Scandinavian trial that RP was more beneficial in younger than in older men. Specifically, we consider hazard ratios for RP, RP+ADT, and RT+ADT relative to CM of 0.49 for men aged 50–64 y at diagnosis and 0.83 for men aged 65–84 y at diagnosis.2 Corresponding hazard ratios for RT among men aged 50–64 y at diagnosis improve from 0.9 in 1990 to 0.49×0.70/0.62 ≈ 0.55 and to 0.49×0.80/0.62 ≈ 0.63 in 1995 remaining constant thereafter, while the hazard ratio for RT is constant at 0.9 for men aged 65–84 y at diagnosis for all years. In other words, we preserve the benefits of RT relative to RP within each age group. These four basic assumption sets (not including the high-efficacy sensitivity experiment) are summarized in Table 1.
Table 1.
Assumed hazard ratios for prostate cancer survival for primary and hormonal treatments relative to conservative management.
| Efficacy assumptions | RP, RP+ADT, and RT+ADT | RT by 1995 | ||
|---|---|---|---|---|
|
|
|
|||
| 50–64 y | 65–84 y | 50–64 y | 65–84 y | |
| Assumption set 1 | 0.62 | 0.62 | 0.70 | 0.70 |
| Assumption set 2 | 0.62 | 0.62 | 0.80 | 0.80 |
| Assumption set 3 | 0.49 | 0.83 | 0.55 | 0.90 |
| Assumption set 4 | 0.49 | 0.83 | 0.63 | 0.90 |
Notes: RP is radical prostatectomy, RT is radiation therapy, ADT is androgen deprivation therapy. All-age and age-specific hazard ratios for RP, RP+ADT, and RT+ADT are from Bill-Axelson et al.2 with similarity between RP and RT+ADT based on Cooperberg et al.23; we assume RP+RT is similar to RP. The all-age hazard ratio for RT is 0.90 until 1990, improves linearly to 0.70 (Assumption set 1) or 0.80 (Assumption set 2) in 1995, and remains constant thereafter. The age-specific hazard ratio for RT for men aged 50–64 y at diagnosis is 0.90 until 1990, improves linearly to 0.55 (Assumption set 3) or 0.63 (Assumption set 4) in 1995, and remains constant thereafter; the hazard ratio for RT for men aged 65–84 y at diagnosis is a constant 0.90 across calendar years.
Finally, we consider two additional sensitivity experiments, both using the more efficacious assumptions for RP (hazard ratio 0.62 relative to CM for all ages) and RT (hazard ratio 0.7 relative to CM after 1995). The first uses the UMICH model and allows prostate cancer incidence to continue its pre-PSA increase in the absence of screening, lowering the both distant stage incidence and prostate cancer mortality. The second uses the FHCRC model and assumes that all cases reported as receiving conservative management in SEER actually received radiation therapy as an extreme correction for possible underreporting of radiation therapy in SEER registries.29
3. Results
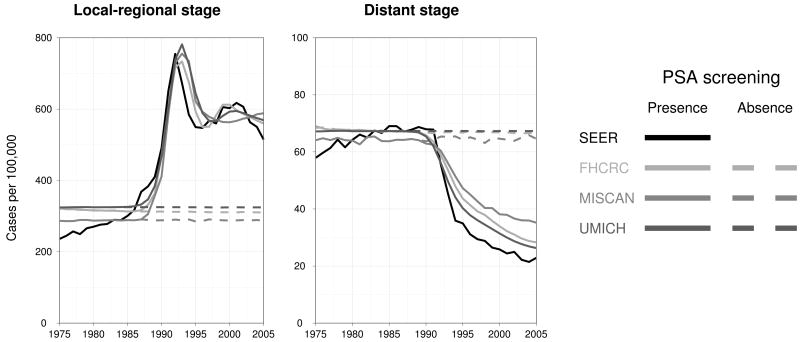
Age-adjusted prostate cancer incidence trends observed in the SEER registries are shown by stage in Figure 2. Also shown are corresponding incidence trends projected by the three models. The model projections replicate key features of the trends in local-regional incidence, including the rapid escalation in the late 1980s, the peak and initial decline in the early 1990s, and the stabilization at a higher level in the late 1990s. There is greater variability across models in the distant stage trends, though all models reproduce the scale of pre-PSA incidence and the rapid decline in the mid- to late 1990s. We present incidence projections in the presence of PSA screening to show how well the calibrated natural history models perform relative to observed incidence. Based on these calibrated natural history models, under common assumptions about treatment and survival, we project prostate cancer mortality in the absence of PSA screening.
Figure 2.
Age-adjusted SEER (black) and CISNET model-projected prostate cancer incidence in the presence (solid grays) and absence (dashed grays) of PSA screening.
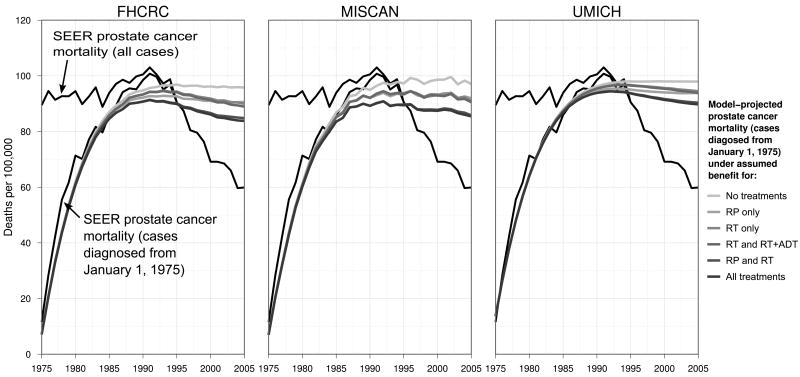
Age-adjusted mortality projections allowing benefit for each treatment based on assumption set 1 are presented in Figure 3. All models reproduce the accumulation of prostate cancer deaths by 1985 and slightly underestimate the peak in 1991. All models project a more-or less constant continuation of mortality in the absence of treatment benefit and a modest decrease in the presence of treatment benefit. By 2005, mortality projections allowing benefit for all treatments represent up to one-third of the difference between mortality projected in the absence of treatment benefit and observed mortality.
Figure 3.
Age-adjusted SEER (black line) and CISNET model-projected (gray lines) prostate cancer mortality among cases diagnosed from January 1, 1975, under no primary treatment benefit or a combination of primary treatments. Model projections are based on assumption set 1. For comparison, the figure also shows SEER prostate cancer mortality among all cases.
Table 2 provides a quantitative summary of the estimated contribution of each treatment to the observed mortality decline. The three models generally agree that changes in RP and RT each played a role in the mortality decline. Under assumption set 1, RP explains 11–14%, RT explains 9–16%, and ADT explains 1–3% of the mortality decline relative to projected mortality in the absence of treatment benefit. Impacts are smaller when treatment is less beneficial for older men; under assumption set 4, corresponding impacts are 10–12%, 5–7%, and 1–3%. Changes in treatment explain from 22–33% to 16–23% across the four sets of assumed efficacy levels.
Table 2.
Projected prostate cancer mortality rates per 100,000 men aged 50–84 y in 2005 by model, assumption about primary and hormonal treatment benefit, and treatment allowing benefit. The corresponding mortality rate from SEER was 59.9 in 2005.
| Model | None | RP | RT | RT+ADT | RP+RT | RP+RT+ADT | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FHCRC | |||||||||||
| Set 1 | 95.8 | 90.6 | (14.4) | 90.0 | (16.2) | 88.9 | (19.0) | 84.8 | (30.5) | 83.8 | (33.4) |
| Set 2 | 95.8 | 90.6 | (14.4) | 91.9 | (10.7) | 89.5 | (17.4) | 86.8 | (25.1) | 84.4 | (31.8) |
| Set 3 | 95.8 | 91.3 | (12.4) | 92.8 | (8.2) | 91.8 | (10.9) | 88.4 | (20.7) | 87.4 | (23.4) |
| Set 4 | 95.8 | 91.3 | (12.4) | 93.1 | (7.4) | 92.0 | (10.5) | 88.7 | (19.8) | 87.5 | (23.0) |
| MISCAN | |||||||||||
| Set 1 | 97.1 | 92.0 | (13.7) | 90.9 | (16.6) | 90.5 | (17.7) | 85.8 | (30.3) | 85.4 | (31.3) |
| Set 2 | 97.1 | 92.0 | (13.7) | 93.0 | (10.9) | 91.5 | (15.0) | 88.0 | (24.6) | 86.4 | (28.7) |
| Set 3 | 97.1 | 94.4 | (7.3) | 94.2 | (7.8) | 93.8 | (8.8) | 91.5 | (15.1) | 91.1 | (16.2) |
| Set 4 | 97.1 | 94.4 | (7.3) | 94.4 | (7.3) | 93.9 | (8.7) | 91.6 | (14.7) | 91.1 | (16.0) |
| UMICH | |||||||||||
| Set 1 | 98.0 | 93.8 | (10.8) | 94.5 | (9.1) | 93.8 | (10.9) | 90.4 | (20.0) | 89.7 | (21.6) |
| Set 2 | 98.0 | 93.8 | (10.8) | 95.6 | (6.3) | 94.3 | (9.6) | 91.5 | (17.0) | 90.1 | (20.6) |
| Set 3 | 98.0 | 94.1 | (10.1) | 95.9 | (5.3) | 95.4 | (6.8) | 92.0 | (15.6) | 91.5 | (16.9) |
| Set 4 | 98.0 | 94.1 | (10.1) | 96.1 | (4.9) | 95.4 | (6.7) | 92.2 | (15.1) | 91.6 | (16.7) |
Notes: Percent declines relative to the difference between mortality projected under no treatment benefit and SEER observed mortality are shown in parentheses. RP is radical prostatectomy, RT is radiation therapy, ADT is androgen deprivation therapy.
Despite conceptual differences across models about how prostate cancer develops and progresses, the models provide consistent results concerning the contributions of treatment to the difference between observed mortality in the year 2005 and the mortality that would have been expected in the absence of advances in treatment. Specifically, the models project that prostate cancer mortality would have stabilized at just under 100 deaths per 100,000 men aged 50–84 y in the absence of treatment benefit. Our computation of the percent of the mortality decline explained by treatment trends in the year 2005 indicates a significant role for primary treatment, with treatment alone explaining up to one-third of the difference between the observed mortality rate in the year 2005 and the rate projected in the absence of treatment benefit.
Under our high-efficacy sensitivity experiment, changes in treatment still explained only about half (range across the three models 42–53%) of the decline in mortality by 2005. Allowing prostate cancer incidence to continue its pre-PSA increase in the absence of screening, the UMICH model projects that changes in treatment explained 30% rather than 22% of the decline in mortality by 2005. And assuming all cases reported as receiving conservative management in SEER actually received radiation therapy, the FHCRC model projects that changes in treatment explained 46% rather than 33% of the decline in mortality by 2005.
Thus, we conclude that advances in primary treatment likely played an important role in the dramatic drop in prostate cancer mortality observed since the early 1990s. However, changes in primary treatment alone do not explain the majority of the mortality decline.
Discussion
The decline in prostate cancer mortality that began in the early 1990s has been striking and sustained. Between 1994 and 2005, prostate cancer deaths dropped by an average rate of 4.1% per year and they are still declining.
The present study uses comparative modeling to investigate one of the most plausible explanations for the mortality decline, i.e., changes in primary treatment, with the goal of shedding light also on the potential roles of screening and other interventions. Our results indicate that treatment explains a non-trivial fraction of the drop in disease-specific deaths, but the majority of the decline is likely explained by other factors such as screening or improvements in disease management after primary therapy. For example, with almost all patients being monitored with PSA after diagnosis, metastatic or potentially metastatic tumors are being re-treated considerably earlier.30 Salvage treatments given at biochemical failure have been associated with significant improvements in disease-specific survival.31 These changes in secondary disease management may have been primarily responsible for the early decline in mortality; based on recent screening trial results, we would not expect to see a substantial decline in mortality as early as was observed due to screening alone.
Our results rest on several key assumptions. First, each natural history model makes different assumptions about disease onset, progression, and diagnosis in the absence of screening. As a result, the three models project three estimates for the fraction of the mortality decline explained by treatment. We find that our conclusions are robust even given this inter-model uncertainty. Second, all models assume that disease incidence would have remained constant at pre-PSA levels after 1987. A sensitivity experiment found that our conclusions are robust even if disease incidence would have continued its increasing trend. Third, all models assume that baseline (in the absence of screening or treatment) prostate cancer survival remained constant in the PSA era. Even if this survival improved over time, perhaps due to advances in treating recurrent disease, this would have little impact on our results because it would imply similar relative differences between projected mortality rates in the presence and absence of changes in treatment.
Our study uses data from a variety of sources which are subject to limitations. Although SEER is the most authoritative resource for information on disease incidence and survival in the US, we note again that estimates of prostate cancer survival in the absence of screening are not available in the PSA era. We also use SEER data on the first course of cancer-directed therapy to estimate the frequencies of radical prostatectomy and radiation therapy. A sensitivity experiment found that our conclusions are robust even if all cases recorded as receiving conservative management in SEER actually received radiation therapy. Finally, our treatment efficacy estimates, which are based on the most rigorous and up-to-date results from randomized trials and comparative effectiveness studies, are still subject to moderate uncertainty. Our sensitivity experiments found that our conclusions are robust even assuming that radical prostatectomy primarily benefits younger men and/or assuming that improvements in radiation technology achieved efficacy similar to radical prostatectomy.
The models use estimates of the efficacy of radical prostatectomy relative to conservative management from the benchmark Scandinavian randomized controlled trial.2 A recent observational study32 compared Medicare patients in the US who did and did not receive radical prostatectomy. After adjusting for selection, the study found an advantage for surgery, even among older men, who were not found to benefit significantly in the Scandinavian trial. If surgery is more efficacious in the US, then our results may be somewhat conservative since changes in the frequency of radical prostatectomy are associated with an important portion of the decline in mortality associated with primary treatment.
In conclusion, the results of this modeling study clearly identify a role for primary treatment changes in US prostate cancer mortality declines, but a large fraction of the decline is left unexplained. This clearly suggests a role for PSA screening, but it also indicates that we should not assume that screening is as effective as suggested by the overall drop in prostate cancer mortality observed in the PSA era. Indeed, there is a clear role for primary treatment change and possibly advances in treatment for recurrent or progressive disease, and there may be a synergy with earlier detection due to screening. Further modeling studies will investigate the extent to which screening and treatment jointly explain the mortality decline and will also highlight the role of other interventions such as advances in disease management for recurrent and metastatic disease.
Acknowledgments
Funding sources: This research was supported by Award Number U01CA88160 from the National Cancer Institute and Award Number U01CA157224 from the National Cancer Institute and the Centers for Disease Control. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, the National Institutes of Health, or the Centers for Disease Control.
Footnotes
Financial disclosures: None.
Contributor Information
Ruth Etzioni, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, USA.
Roman Gulati, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, USA.
Alex Tsodikov, Department of Biostatistics, University of Michigan, USA.
Elisabeth M Wever, Department of Public Health, Erasmus University Medical Center, The Netherlands.
David F Penson, Department of Urologic Surgery, Vanderbilt University Medical Center, USA.
Eveline AM Heijnsdijk, Department of Public Health, Erasmus University Medical Center, The Netherlands.
Jeffrey Katcher, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, USA.
Gerrit Draisma, Department of Public Health, Erasmus University Medical Center, The Netherlands.
Eric J Feuer, Division of Cancer Control and Population Sciences, National Cancer Institute, USA.
Harry J de Koning, Department of Public Health, Erasmus University Medical Center, The Netherlands.
Angela B Mariotto, Division of Cancer Control and Population Sciences, National Cancer Institute, USA.
References
- 1.Albertsen PC. The prostate cancer conundrum. J Natl Cancer Inst. 2003;95(13):930–1. doi: 10.1093/jnci/95.13.930. [DOI] [PubMed] [Google Scholar]
- 2.Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364(18):1708–17. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 3.Cooperberg MR, Grossfeld GD, Lubeck DP, Carroll PR. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst. 2003;95(13):981–9. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hankey BF, Feuer EJ, Clegg LX, et al. Cancer surveillance series: interpreting trends in prostate cancer--part I: Evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst. 1999;91(12):1017–24. doi: 10.1093/jnci/91.12.1017. [DOI] [PubMed] [Google Scholar]
- 5.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 6.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinsky PF, Black A, Kramer BS, Miller A, Prorok PC, Berg C. Assessing contamination and compliance in the prostate component of the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial. Clin Trials. 2010;7(4):303–11. doi: 10.1177/1740774510374091. [DOI] [PubMed] [Google Scholar]
- 8.Berg CD. The Prostate, Lung, Colorectal and Ovarian cancer screening trial: The prostate cancer screening results in context. Acta Oncol. 2011;50(1):12–7. doi: 10.3109/0284186X.2010.531283. [DOI] [PubMed] [Google Scholar]
- 9.Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: Results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98(8):529–34. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 10.Mariotto A, Etzioni R, Krapcho M, Feuer EJ. Reconstructing prostate-specific antigen (PSA) testing patterns among black and white men in the US from Medicare claims and the National Health Interview Survey. Cancer. 2007;109(9):1877–86. doi: 10.1002/cncr.22607. [DOI] [PubMed] [Google Scholar]
- 11.Pinsky PF, Crawford ED, Kramer BS, et al. Repeat prostate biopsy in the prostate, lung, colorectal and ovarian cancer screening trial. BJU Int. 2007;99(4):775–9. doi: 10.1111/j.1464-410X.2007.06708.x. [DOI] [PubMed] [Google Scholar]
- 12.Grubb RL, 3rd, Pinsky PF, Greenlee RT, et al. Prostate cancer screening in the Prostate, Lung, Colorectal and Ovarian cancer screening trial: update on findings from the initial four rounds of screening in a randomized trial. BJU Int. 2008;102(11):1524–30. doi: 10.1111/j.1464-410X.2008.08214.x. [DOI] [PubMed] [Google Scholar]
- 13.Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control. 2008;19(2):175–81. doi: 10.1007/s10552-007-9083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulati R, Inoue L, Katcher J, Hazelton W, Etzioni R. Calibrating disease progression models using population data: a critical precursor to policy development in cancer control. Biostatistics. 2010;11(4):707–19. doi: 10.1093/biostatistics/kxq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: Estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95(12):868–78. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 16.Draisma G, Postma R, Schröder FH, van der Kwast TH, de Koning HJ. Gleason score, age and screening: modeling dedifferentiation in prostate cancer. Int J Cancer. 2006;119(10):2366–71. doi: 10.1002/ijc.22158. [DOI] [PubMed] [Google Scholar]
- 17.Wever EM, Draisma G, Heijnsdijk EA, et al. Prostate-specific antigen screening in the United States vs in the European Randomized Study of Screening for Prostate Cancer-Rotterdam. J Natl Cancer Inst. 2010;102(5):352–5. doi: 10.1093/jnci/djp533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsodikov A, Szabo A, Wegelin J. A population model of prostate cancer incidence. Stat in Med. 2006;25(16):2846–66. doi: 10.1002/sim.2257. [DOI] [PubMed] [Google Scholar]
- 19.Chefo S, Tsodikov A. Stage-specific cancer incidence: An artificially mixed multinomial logit model. Stat Med. 2009;28(15):2054–76. doi: 10.1002/sim.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cetin K, Beebe-Dimmer JL, Fryzek JP, Markus R, Carducci MA. Recent time trends in the epidemiology of stage IV prostate cancer in the United States: analysis of data from the Surveillance, Epidemiology, and End Results Program. Urology. 2010;75(6):1396–404. doi: 10.1016/j.urology.2009.07.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lubeck DP, Litwin MS, Henning JM, et al. The CaPSURE database: A methodology for clinical practice and research in prostate cancer. Urology. 1996;48(5):773–7. doi: 10.1016/s0090-4295(96)00226-9. [DOI] [PubMed] [Google Scholar]
- 22.Bannuru RR, Dvorak T, Obadan N, et al. Comparative evaluation of radiation treatments for clinically localized prostate cancer: an updated systematic review. Ann Intern Med. 155(3):171–8. doi: 10.7326/0003-4819-155-3-201108020-00347. [DOI] [PubMed] [Google Scholar]
- 23.Cooperberg MR, Vickers AJ, Broering JM, Carroll PR. Comparative risk-adjusted mortality outcomes after primary surgery, radiotherapy, or androgen-deprivation therapy for localized prostate cancer. Cancer. 2010;116(22):5226–34. doi: 10.1002/cncr.25456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boorjian SA, Karnes RJ, Viterbo R, et al. Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer. 2011;117(13):2883–91. doi: 10.1002/cncr.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): A phase III randomised trial. Lancet. 2002;360(9327):103–6. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 26.D'Amico AV, Chen MH, Roehl KA, Catalona WJ. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351(2):125–35. doi: 10.1056/NEJMoa032975. [DOI] [PubMed] [Google Scholar]
- 27.Denham JW, Steigler A, Lamb DS, et al. Short-term androgen deprivation and radiotherapy for locally advanced prostate cancer: results from the Trans-Tasman Radiation Oncology Group 96. 01 randomised controlled trial Lancet Oncol. 2005;6(11):841–50. doi: 10.1016/S1470-2045(05)70348-X. [DOI] [PubMed] [Google Scholar]
- 28.Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148(6):435–48. doi: 10.7326/0003-4819-148-6-200803180-00209. [DOI] [PubMed] [Google Scholar]
- 29.Jagsi R, Abrahamse P, Hawley ST, Graff JJ, Hamilton AS, Katz SJ. Underascertainment of radiotherapy receipt in Surveillance, Epidemiology, and End Results registry data. Cancer. 118(2):333–41. doi: 10.1002/cncr.26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. Jama. 1999;281(17):1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 31.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299(23):2760–9. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadley J, Yabroff KR, Barrett MJ, Penson DF, Saigal CS, Potosky AL. Comparative effectiveness of prostate cancer treatments: evaluating statistical adjustments for confounding in observational data. J Natl Cancer Inst. 102(23):1780–93. doi: 10.1093/jnci/djq393. [DOI] [PMC free article] [PubMed] [Google Scholar]