Abstract
A recent resurgence in basic and applied research on photosynthesis has been driven in part by recognition that fulfilling future food and energy requirements will necessitate improvements in crop carbon-fixation efficiencies. Photosynthesis in traditional terrestrial crops is being reexamined in light of molecular strategies employed by photosynthetic microbes to enhance the activity of the Calvin cycle. Synthetic biology is well-situated to provide original approaches for compartmentalizing and enhancing photosynthetic reactions in a species independent manner. Furthermore, the elucidation of alternative carbon-fixation routes distinct from the Calvin cycle raises possibilities that alternative pathways and organisms can be utilized to fix atmospheric carbon dioxide into useful materials.
Introduction
Autotrophic CO2 fixation underlies nearly all biological processes on Earth and has generated reservoirs of prehistorically-fixed carbon, which are currently utilized to satisfy >80% of global energy demand. Concerns of sustainability, indirect costs, and geopolitical ramifications of such fossil fuel reserves are motivating research on alternative, renewable energy resources. Solar-driven energy technologies are attractive solutions, particularly as the average incident solar flux (>120,000 TW) exceeds the energy demand of all nations (~15 TW) by nearly 4 orders of magnitude. While some physical processes for solar capture (e.g. photovoltaic cells) will likely continue to possess higher efficiencies of energy capture, biological pathways that use solar energy to fix CO2 are of particular importance for their capacity for self-renewal and energy storage within chemical bonds compatible with existing infrastructure (8). In this brief review, we provide an overview of recent developments towards improving canonical photosynthetic processes as well as emerging ideas for engineering novel autotrophic, CO2-fixation pathways into industrially-tractable organisms.
Improving Calvin Cycle Efficiencies
Carbon fixation through the reductive pentose phosphate pathway (Calvin cycle; Fig. 1) is the most biologically abundant and economically-relevant pathway, and has therefore received the vast majority of scientific attention (41). While many plant species have been bred for centuries to improve their agricultural or economic value, these approaches have typically yielded varieties with a higher % of biomass diverted towards a given product (e.g. edible seed or fruit), rather than higher photosynthetic efficiencies. Only within the last couple of decades, following improvements in plant transformation techniques (50), has the focus of crop enhancement begun to shift towards novel strategies for enhancing rates of total biomass fixation and/or photosynthetic efficiencies (Table 1).
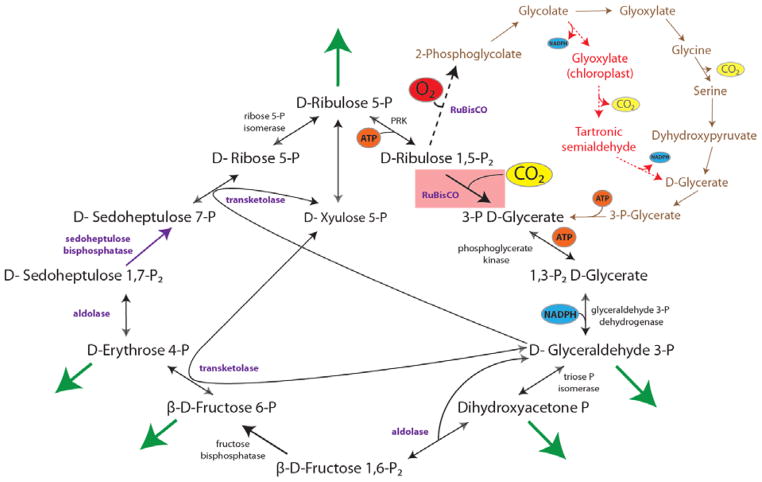
Figure 1. The reductive pentose phosphate pathway (Calvin cycle) and regulatory control points.
Schematic of the enzymes and intermediates of the Calvin cycle. The carboxylating step involving Ribulose 1, 5-bisphosphate carboxylase oxygenase (RuBisCO) is highlighted in red, with the inorganic carbon source (carbon dioxide) highlighted in yellow. Enzymes with known or suspected rate-limiting control over Calvin cycle activity under some natural conditions are shown in bold with purple text. The oxygenase reaction of RuBisCO is displayed (dashed arrow) and the canonical plant phosphoglycolate-processing photorespiratory pathway is shown in brown. An alternative, chloroplast-specific pathway engineered into plants (23) is shown in dashed red arrows. Reactions requiring or generating ATP (orange circles) or NADPH (blue circles) are designated (although in some organisms, NADH may be substituted for NADPH in some reactions). Entry or exit of carbon dioxide (yellow circles) or oxygen (red) is denoted. Major exit points for intermediates of the Calvin cycle are designated by green arrows.
Table 1.
Strategies towards enhanced carbon fixation
| Target | Relevant pathway(s) | Modification | Approach | Refs |
|---|---|---|---|---|
| Light-gathering efficiency | All light-dependent carbon fixation pathways | Reduced antenna size | Chlorophyll/pigment synthesis knockout or downregulation | (35, 42) |
| Expand wavelength usage | Heterologous expression of alternative chlorophylls and phycobilins | (11, 50) | ||
| Carbon concentrating mechanisms | CO2-utilizing pathways | Carboxysome | Expression of carboxysomes in chloroplasts | (9) |
| Novel scaffold design | Construction of novel subcellular compartments using synthetic RNA or protein scaffolds | (12, 14) | ||
| Alternative and synthetic carbon fixation pathways | Alternative carboxylating enzymes | Use of carboxylating enzymes dependent on bicarbonate in alkaline environments | (4, 15) | |
| Carboxylating reactions | Calvin Cycle | Faster RuBisCO activity | Heterologous expression of high-activity, low specficity RuBisCOs in CO2-rich environments | (45, 50) |
| 3-HOP, HB/HP | Decreased oxygen sensitivity | Reconstruction of naturally oxygen-tolerant carbon-fixation pathways in industrially-relevant species | (4, 10, 48) | |
| Synthetic carbon fixation pathway | Improved specificity & activity | Use of non-canonical carboxylating enzymes (e.g. PEP carboxylase, pyruvate carboxylase) | (3) | |
| Electro-synthesis | Anaerobic carbon-fixation pathways | Acceptance of electrons from cathode | Expression of outer membrane electron donor/acceptor complexes | (22, 29, 36) |
The Calvin cycle utilizes a notoriously inefficient carboxylating enzyme, RuBisCO, featuring sluggish catalytic rates and an oxygenation reaction that forms toxic phosphoglycolate, which must be routed through photorespiratory pathways, releasing previously fixed carbon (Fig. 1). Consequently, reengineering RuBisCO has been a primary objective for enhancing photosynthesis (45), although most of these efforts have met with limited success. Part of the problem lies in an intrinsic trade-off between RuBisCO’s selectivity and velocity that restricts the activity of these enzymes (43); engineering a faster enzyme leads to photorespiratory efficiency losses. However, RuBisCO is widely conserved across many species and some natural variants of RuBisCO are slightly more effective than others (e.g. from red algae species), so metagenomic surveys of natural RuBisCO diversity may identify superior enzymes more effectively than rational engineering approaches (2, 43). High-throughput characterization and utilization of RuBisCO homologs would be aided by recent developments in the in vitro expression of RuBisCO (28), genome-level chloroplast reengineering techniques (37), as well as the demonstration of successfully-assembled heterologous RuBisCO in plant chloroplasts (46). Together, these strategies could also be used to engineer cultivars expressing RuBisCOs with higher velocity and less selectivity, which would be preferable in CO2-rich environments – such as are found inside bioreactors or under the currently rising atmospheric CO2 levels (50).
To date, the most successful engineering approaches to increase rates of carbon fixation in autotrophs have not involved modification of RuBisCO itself, but of neighboring pathways and enzymes. For example, the Calvin cycle is sensitive to heat stress; at extreme temperatures this is an intrinsic function of instabilities in metabolic intermediates (6), but mild heat stress also reduces pathway throughput, partially due to the thermolability of RuBisCO activase (40). Expression of thermostable forms of RuBisCO activase in Arabidopsis partially bypassed this restraint, leading to increases in biomass accumulation under moderate temperature stress (25). Rerouting phosphoglycolate produced from RuBisCO’s oxygenase activity through expression of either of two bacterially-derived bypasses (glycolate-to-glycerate (23) Fig. 1; or glycolate-to-malate (32)) in Arabidopsis chloroplasts has also resulted in higher CO2 fixation rates and biomass accumulation, perhaps as a result of the reduced energy requirement of this pathway (23).
Apart from RuBisCO, Metabolic Control Analysis and characterization of mutants with down regulated Calvin cycle genes indicate that other enzymes share control of flux through the Calvin cycle(38, 41, 49)(Fig. 1). Indeed, overexpression of sedoheptulase 1,7-bisphosphatase has improved photosynthetic rates, biomass accumulation and/or stress tolerance in tobacco and rice plants (reviewed in (38, 41, 49)). A more complicated picture emerges upon increased expression of other enzymes thought to control the Calvin cycle (transketolase, aldolase), which lead to decreased photosynthesis and less robust plant growth (38), perhaps because of unbalanced exit of Calvin cycle intermediates. Finally, there are some intriguing indications that the Calvin cycle itself may not always be the limiting factor for carbon fixation; downstream ‘metabolic sinks’ utilizing triose phosphate intermediates in plants (26, 41) and photosynthetic microbes (13) appear to restrict carbon-fixation rates under certain conditions. Ultimately, deeper insight into the regulation of Calvin cycle intermediates is critical to balance all enzymatic activities for more efficient photosynthesis. Calvin cycle regulation can be inferred through computer-assisted analysis of dynamic isotope labeling measurements (47), and while such measurements are currently rare for photobiological pathways, considerable gains in photosynthetic models could be achieved through the use of this technique in algae and cyanobacteria (41).
Other improvements in carbon fixation can be realized by altering the way that photosynthetic organisms process abiotic environmental factors upstream of the Calvin cycle. For example, photosynthetic organisms have been evolutionarily selected to compete for limited light; consequently the absorptive capacity of light-gathering antenna can exceed metabolic capacity even under moderate illumination. This leads to shedding of excess energy in the form of heat, fluorescence, and the generation of reactive oxygen species, as well as an unnecessary shading of leaves and cells lower in the light column (50). Minimizing excess light absorption is expected to provide the most benefit in dense cultures of photosynthetic microbes where light penetration is shallow, although plants could also benefit from a reduced need to engage photoinhibitory pathways under high-intensity light. Chlorophyll antenna size has been reduced in the green algae Chlamydomonas using a variety of methods to down regulate chlorophyll synthesis (5, 31, 35). The resulting strains transmit more light and are less sensitive to photoinhibition, leading to increased overall culture productivities at higher irradiances.
The concentration of inorganic carbon is another important abiotic factor influencing carbon fixation, and photosynthetic organisms have developed a variety of carbon concentrating strategies that raise the effective availability of CO2 for the Calvin cycle over ambient levels (16, 24, 33, 39). Generally, these strategies compartmentalize RuBisCO near sites of CO2 generation, thereby increasing substrate availability and suppressing RuBisCO’s undesirable oxygenation side-reaction. In algae, subcellular compartments are used to concentrate carbon-fixing machinery (e.g. carboxysomes and pyrenoids (16, 39)), while C4 plants specialize ‘carbon capture’ and ‘carbon fixation’ reactions across different tissue types (19). So far, success importing C4-like carbon assimilation into C3 plants has been limited, in part because of the reliance of C4 metabolism on underlying plant anatomical features (50). While expressing a cyanobacterial carbonic anhydrase in tobacco does increase photosynthetic rates under specific conditions (27), more ambitious approaches exporting cyanobacterial or algal carbon concentrating mechanisms may yield further gains.
Synthetic biology offers novel engineering strategies for improving abiotic response pathways in photosynthetic organisms. For example, exporting effective carbon-concentrating mechanisms from cyanobacteria into plant chloroplasts is a more attractive possibility following the demonstration that functional carboxysomes are assembled and packaged when heterologously-expressed in E. coli (9). Alternatively, synthetic protein- and RNA-based scaffolds could be developed to allow for de-novo construction of subcellular carbon-concentrating compartments (12, 14, 17). Approaches to modify light-responsiveness are suggested by synthetic light-responsive transcriptional circuits constructed in E. coli that utilize cyanobacterial proteins to differentiate between different light qualities and activate distinct response pathways (42). Design of similar circuits in photosynthetic organisms could allow researchers to coordinately express orthogonal pathways alongside endogenous, ‘photoacclimation’ regulatory networks (34) to exert influence over the production of light-harvesting pigments, without sacrificing the natural dynamic range important for photosynthetic growth under variable environmental conditions (1).
Alternative Natural Carbon Fixation Pathways
Five alternative autotrophic strategies have been elucidated that could be applied to effectively fix carbon instead of the Calvin cycle (Fig. 2). Existing reviews on these alternative carbon fixation cycles provide an excellent discussion of the timeline of their discovery, evolutionary history (6, 7, 18, 30), and ecological distribution (20). Four of the five alternative pathways are similar in that they incorporate inorganic carbon into existing carbon backbones and utilize acetyl-CoA/succinyl-CoA cycles to fix carbon; the enzymes used in these cycles also partially overlap one another (Fig. 2). A sixth carbon-fixation route, the reductive acetyl-CoA pathway, is evolutionarily ancient and phylogenetically wide-spread, but is not discussed herein due to its strict anaerobic requirements and complex metallo-chemistry that would complicate its use outside of the acetogenic microbes in which it is found (but see (4, 6) for comprehensive reviews). Although many of the carboxylating reactions in these cycles are expected to be partially rate-limiting (3, 4, 10)(Fig. 2), additional features of each pathway imply unique advantages and drawbacks relative to the Calvin cycle for future attempts to harness them for biotechnological applications (3, 6, 48).
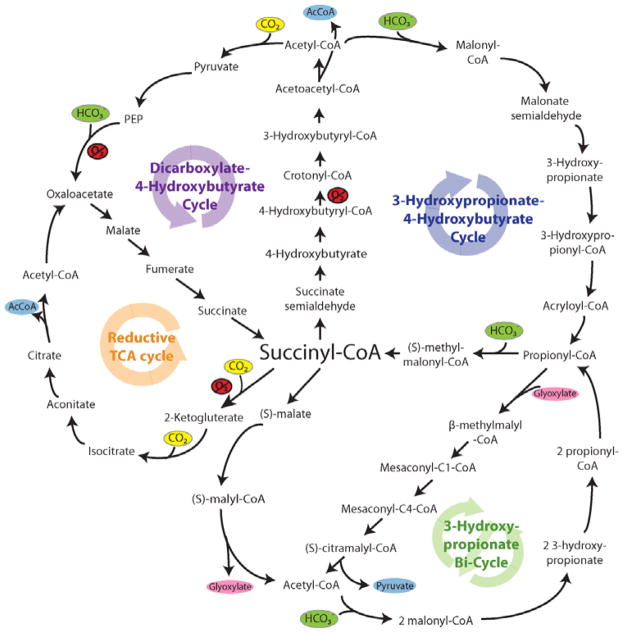
Figure 2. Alternative natural carbon fixation pathways.
Schematic of the enzymes and intermediates of 4 of the 5 alternative (non-Calvin cycle), natural carbon fixation pathways utilized by autotrophic and chemolithotrophic microorganisms. All pathways use a common strategy of cycling between succinyl-CoA (center) and acetyl-CoA (circumference) in generate C2 or C3 carbon products which exit the cycle (shown in blue; acetyl-CoA, pyruvate). These pathways partially overlap one another with regard to metabolic intermediates and enzyme usage, allowing them to be diagramed as ‘spokes’ on a circle, centered on succinyl-CoA. The carboxylating steps are indicated by the incorporation of carbon dioxide (yellow circles) or bicarbonate (green circles). Reactions requiring enzymes or co-factors with partial or complete oxygen sensitivity are indicated in red (O2), although some alternative oxygen-resistant enzymes exist for some of these reactions (4).
One way to assess the potential of different carbon-fixation pathways is through analysis of the theoretical requirements for energy (ATP) and reducing equivalents needed to fix a molecule of carbon. Thermodynamic modeling of alternative carbon-fixation cycles has been used to predict energy demand for biomass synthesis (10) and maximal pathway specific activities (defined as metabolic flux per mg of total enzymes) across a range of physiological conditions (3). These calculations can only predict optimal ‘upper-limits’ of pathway efficiency, nonetheless some interesting features of alternative carbon-fixation pathways become apparent using these approaches. For example, analyses indicate that alternative carbon-fixation pathways have comparable or superior theoretical efficiencies and lower ATP requirements per carbon fixed relative to the Calvin cycle (4, 10).
Another feature to consider in alternative carbon-fixation pathways is the capacity of some carboxylating enzymes to utilize bicarbonate rather than dissolved carbon dioxide. The concentration of dissolved carbon dioxide is independent of pH whereas bicarbonate becomes preferentially more soluble at pH >7. This means that pathways that make use of bicarbonate and operate in organisms that grow in alkaline environments (common in many cyanobacteria and eukaryotic algae), would not need to rely as heavily upon carbon concentrating mechanisms to increase substrate concentrations, even at ambient CO2 levels. In this context, the 3-hydroxypropionate bicycle (3-HOP) is a particularly interesting pathway because its carboxylating enzymes, acetyl-CoA carboxylase and propionyl-CoA carboxylase, both use bicarbonate as a substrate. Furthermore, 3-HOP enzymes are insensitive to oxygen and do not catalyze oxygenation reactions like those of RuBisCO (48), which could ultimately allow higher productivities even though the 3-HOP energy requirement and pathway specific activity is similar to the Calvin cycle (3, 4, 10). Since the alternative carbon-fixation pathways are naturally found in unusual environments/organisms, they may need to be reconstructed within more canonical laboratory organisms before their full potential can be assessed.
Carbon Fixation through Synthetic Pathways
Digital curation of known enzymatic functions provides an expanding list (>5700 currently in the KEGG database) of biological reactions that can be mined to create novel biochemical pathways in silico. Coupled with more effective DNA cloning and synthesis methods (see Tian’s review in this issue), possibilities arise for construction of metabolic pathways that are not naturally found in one organism. Known carboxylating enzymes were recently analyzed in context with all other annotated biological reactions to identify novel pathways for fixing carbon that would also be feasible when considering thermodynamic constraints (3). Many new pathways were suggested, the most intriguing of which revolve around the use of phosphoenol-pyruvate (PEP) carboxylase or pyruvate carboxylase in a manner analogous to the first steps of carbon concentrating metabolism performed in the mesophyll cells of C4-plants. However, instead of the eventual re-release of carbon dioxide (as is carried out in the bundle-sheath cells of C4 plants; (19)), metabolic bypasses composed of 8 or more enzymes would be used to convert malate to pyruvate without the loss of fixed carbon (3). These synthetic pathways are particularly attractive because of the favorable properties of the carboxylating enzymes that would theoretically allow much higher rates and/or efficiencies of carbon capture.
In several cases, the feasibility of using alternative carbon fixation pathways (natural or synthetic) might be heavily influenced by the source of reducing equivalents used to reduce inorganic carbon. In oxygenic photosynthesis, low-potential electrons come from a photosystem-catalyzed water-splitting reaction that generates oxygen, whereas a number of the most efficient natural and theoretical carbon-fixing pathways contain oxygen-sensitive enzymes incompatible with the use of water as an electron donor (Fig. 2). While other reducing agents (e.g. gaseous hydrogen) are typically linked to the activity of these anaerobic pathways in nature, providing these reducing agents in scaled microbial reactors could present significant technical/cost barriers. One possibility is the use of electrodes to produce compounds (e.g. H2, formate) or reduce soluble mediators that can donate electrons to bacteria grown near the cathode (21, 44). More recent strategies have involved the identification and/or engineering of microbes capable of directly accepting electrons from a cathode to generate useful compounds by ‘electrosynthesis’ (Box 1). Identification of economically-feasible processes that could provide reducing equivalents would greatly increase the potential for using alternative carbon fixation pathways that depend on oxygen-sensitive enzymes (10).
Box 1. Electrosynthesis.
While most research on interfacing microbes with electrodes has been carried out to analyze the capacity of microbes to provide electrical current, there is emerging evidence that a subset of microbes can also directly accept electrons from a charged surface (29). This property would allow electrical current to be transformed into more stable and transportable forms and could be particularly attractive in the context of renewable energy sources that generate intermittent power. Presently, very few microbes are known to be capable of accepting electrons in this manner. Surprisingly, those acetogens which have demonstrated natural ability to directly accept electrons from a cathode appear to utilize these reducing equivalents very efficiently (~80%) to fix carbon dioxide, although the rates of electron transfer in these pilot studies were extremely slow (36). The partial reconstruction of Shewanella’s electron-donating machinery in E. coli (22) suggests that other transmembrane electron pathways might be expressed to allow industrially-relevant organisms to accept electrons. If this could be achieved, efficient carbon fixation pathways (e.g. rTCA) could be continuously driven using reducing equivalents from the electrode while maintaining anoxic conditions. While a promising concept, the feasibility of scaled electrosynthesis will ultimately depend on the ability to construct cathodes with large surface areas as well as a much more detailed understanding of extracellular electron transfer pathways.
Conclusions and future directions
Biological carbon fixation pathways represent a means to capitalize on renewable solar energy to generate transportable fuels and other valuable commodities. Increased capacity to modify photosynthetic organisms coupled with synthetic biological approaches allow for new opportunities to radically re-engineer more efficient photosynthesis and novel carbon-fixation pathways (Table 1). It is important to note that a number of the engineering strategies outlined are compatible with one another, such that the most effective solutions may utilize a combination of cross-species and synthetic constructs in order to approach the theoretical limits for biological capture of solar power.
Highlights.
Biological carbon-fixation is an important part of energy sustainability efforts.
Calvin cycle improvement to arise by engineering neglected rate-limiting pathways.
Synthetic biology offers new approaches to improve photosynthetic efficiencies.
Alternative/synthetic carbon fixation routes are emerging as industrially-relevant.
Acknowledgments
We would like to thank members of the Silver Lab for valuable comments and assistance with the manuscript, especially Patrick Boyle, Wade Hicks, Matt Mattozzi and Colby Stoddard. Authors acknowledge funding from the National Institute of General Medical Sciences, Award Number F32GM093516, Army Research Office Award No W911NF-09-1-0226, DOE ARPA-E Award #DE-0000079 Cooperative Agreement, and the Wyss Institute for Biologically Inspired Engineering.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Athanasiou K, Dyson BC, Webster RE, Johnson GN. Dynamic acclimation of photosynthesis increases plant fitness in changing environments. Plant Physiol. 2010;152:366–373. doi: 10.1104/pp.109.149351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badger MR, Bek EJ. Multiple Rubisco forms in proteobacteria: their functional significance in relation to CO2 acquisition by the CBB cycle. J Exp Bot. 2008;59:1525–1541. doi: 10.1093/jxb/erm297. [DOI] [PubMed] [Google Scholar]
- 3**.Bar-Even A, Noor E, Lewis NE, Milo R. Design and analysis of synthetic carbon fixation pathways. Proc Natl Acad Sci U S A. 2010;107:8889–8894. doi: 10.1073/pnas.0907176107. Demonstration of a novel approach of constructing and analyzing the thermodynamic feasibility of synthetic carbon-fixation pathways in silico by mining databases of known enzymatic functions. Supported by an excellent and comprehensive Supplement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bar-Even A, Noor E, Milo R. A survey of carbon fixation pathways through a quantitative lens. J Exp Bot. 2011 doi: 10.1093/jxb/err417. [DOI] [PubMed] [Google Scholar]
- 5.Beckmann J, Lehr F, Finazzi G, Hankamer B, Posten C, Wobbe L, Kruse O. Improvement of light to biomass conversion by de-regulation of light-harvesting protein translation in Chlamydomonas reinhardtii. J Biotechnol. 2009;142:70–77. doi: 10.1016/j.jbiotec.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 6**.Berg IA. Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Appl Environ Microbiol. 2011;77:1925–1936. doi: 10.1128/AEM.02473-10. An excellent and accessible review of the natural carbon-fixation pathways. Particularly informative with regard to the distinct ecological niches where alternative carbon fixation strategies are superior to the Calvin cycle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg IA, Kockelkorn D, Ramos-Vera WH, Say RF, Zarzycki J, Hugler M, Alber BE, Fuchs G. Autotrophic carbon fixation in archaea. Nat Rev Microbiol. 2010;8:447–460. doi: 10.1038/nrmicro2365. [DOI] [PubMed] [Google Scholar]
- 8*.Blankenship RE, Tiede DM, Barber J, Brudvig GW, Fleming G, Ghirardi M, Gunner MR, Junge W, Kramer DM, Melis A, Moore TA, Moser CC, Nocera DG, Nozik AJ, Ort DR, Parson WW, Prince RC, Sayre RT. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science. 2011;332:805–809. doi: 10.1126/science.1200165. This meta-analysis of solar capture techniques (both physical and biological) is a fair treatment of the potential (and problems) for renewable solar energy technologies. [DOI] [PubMed] [Google Scholar]
- 9.Bonacci W, Teng PK, Afonso B, Niederholtmeyer H, Grob P, Silver PA, Savage DF. Modularity of a carbon-fixing protein organelle. Proc Natl Acad Sci U S A. 2012;109:478–483. doi: 10.1073/pnas.1108557109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyle NR, Morgan JA. Computation of metabolic fluxes and efficiencies for biological carbon dioxide fixation. Metab Eng. 2011;13:150–158. doi: 10.1016/j.ymben.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Chen M, Blankenship RE. Expanding the solar spectrum used by photosynthesis. Trends Plant Sci. 2011;16:427–431. doi: 10.1016/j.tplants.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Delebecque CJ, Lindner AB, Silver PA, Aldaye FA. Organization of intracellular reactions with rationally designed RNA assemblies. Science. 2011;333:470–474. doi: 10.1126/science.1206938. [DOI] [PubMed] [Google Scholar]
- 13.Ducat DC, Avelar-Rivas JA, Way JC, Silver PA. Re-routing Carbon-flux to Enhance Photosynthetic Productivity. Appl Environ Microbiol. 2012 doi: 10.1128/AEM.07901-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dueber JE, Wu GC, Malmirchegini GR, Moon TS, Petzold CJ, Ullal AV, Prather KL, Keasling JD. Synthetic protein scaffolds provide modular control over metabolic flux. Nat Biotechnol. 2009;27:753–759. doi: 10.1038/nbt.1557. [DOI] [PubMed] [Google Scholar]
- 15.Erb TJ. Carboxylases in natural and synthetic microbial pathways. Appl Environ Microbiol. 2011;77:8466–8477. doi: 10.1128/AEM.05702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espie GS, Kimber MS. Carboxysomes: cyanobacterial RubisCO comes in small packages. Photosynth Res. 2011;109:7–20. doi: 10.1007/s11120-011-9656-y. [DOI] [PubMed] [Google Scholar]
- 17.Fleishman SJ, Whitehead TA, Ekiert DC, Dreyfus C, Corn JE, Strauch EM, Wilson IA, Baker D. Computational design of proteins targeting the conserved stem region of influenza hemagglutinin. Science. 2011;332:816–821. doi: 10.1126/science.1202617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuchs G. Alternative pathways of carbon dioxide fixation: insights into the early evolution of life? Annu Rev Microbiol. 2011;65:631–658. doi: 10.1146/annurev-micro-090110-102801. [DOI] [PubMed] [Google Scholar]
- 19.Furbank RT. Evolution of the C(4) photosynthetic mechanism: are there really three C(4) acid decarboxylation types? J Exp Bot. 2011;62:3103–3108. doi: 10.1093/jxb/err080. [DOI] [PubMed] [Google Scholar]
- 20.Hugler M, Sievert SM. Beyond the Calvin cycle: autotrophic carbon fixation in the ocean. Ann Rev Mar Sci. 2011;3:261–289. doi: 10.1146/annurev-marine-120709-142712. [DOI] [PubMed] [Google Scholar]
- 21.Innocent B, Liaigre D, Pasquier D, Ropital F, Léger JM, Kokoh K. Electro-reduction of carbon dioxide to formate on lead electrode in aqueous medium. Journal of Applied Electrochemistry. 2009;39:227–232. [Google Scholar]
- 22.Jensen HM, Albers AE, Malley KR, Londer YY, Cohen BE, Helms BA, Weigele P, Groves JT, Ajo-Franklin CM. Engineering of a synthetic electron conduit in living cells. Proc Natl Acad Sci U S A. 2010;107:19213–19218. doi: 10.1073/pnas.1009645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kebeish R, Niessen M, Thiruveedhi K, Bari R, Hirsch HJ, Rosenkranz R, Stabler N, Schonfeld B, Kreuzaler F, Peterhansel C. Chloroplastic photorespiratory bypass increases photosynthesis and biomass production in Arabidopsis thaliana. Nat Biotechnol. 2007;25:593–599. doi: 10.1038/nbt1299. [DOI] [PubMed] [Google Scholar]
- 24.Kerfeld CA, Heinhorst S, Cannon GC. Bacterial microcompartments. Annu Rev Microbiol. 2010;64:391–408. doi: 10.1146/annurev.micro.112408.134211. [DOI] [PubMed] [Google Scholar]
- 25.Kurek I, Chang TK, Bertain SM, Madrigal A, Liu L, Lassner MW, Zhu G. Enhanced Thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. Plant Cell. 2007;19:3230–3241. doi: 10.1105/tpc.107.054171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leakey AD, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Exp Bot. 2009;60:2859–2876. doi: 10.1093/jxb/erp096. [DOI] [PubMed] [Google Scholar]
- 27.Lieman-Hurwitz J, Rachmilevitch S, Mittler R, Marcus Y, Kaplan A. Enhanced photosynthesis and growth of transgenic plants that express ictB, a gene involved in HCO3− accumulation in cyanobacteria. Plant Biotechnol J. 2003;1:43–50. doi: 10.1046/j.1467-7652.2003.00003.x. [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Young AL, Starling-Windhof A, Bracher A, Saschenbrecker S, Rao BV, Rao KV, Berninghausen O, Mielke T, Hartl FU, Beckmann R, Hayer-Hartl M. Coupled chaperone action in folding and assembly of hexadecameric Rubisco. Nature. 2010;463:197–202. doi: 10.1038/nature08651. [DOI] [PubMed] [Google Scholar]
- 29.Lovley DR, Nevin KP. A shift in the current: new applications and concepts for microbe-electrode electron exchange. Curr Opin Biotechnol. 2011;22:441–448. doi: 10.1016/j.copbio.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 30*.Martin WF. Hydrogen, metals, bifurcating electrons, and proton gradients: The early evolution of biological energy conservation. FEBS Lett. 2011 doi: 10.1016/j.febslet.2011.09.031. Excellent discussion of alternative natural carbon fixation pathways within the context of the evolution of early life forms. [DOI] [PubMed] [Google Scholar]
- 31.Masuda T, Tanaka A, Melis A. Chlorophyll antenna size adjustments by irradiance in Dunaliella salina involve coordinate regulation of chlorophyll a oxygenase (CAO) and Lhcb gene expression. Plant Mol Biol. 2003;51:757–771. doi: 10.1023/a:1022545118212. [DOI] [PubMed] [Google Scholar]
- 32.Maurino VGKDE, Flügge, Ulf-ingo, Koln DE. Means for Improving Agrobiological Traits in a Plant by Providiing a Plant Cell Comprising in its Chloroplasts Enzymatic Activities for Converting Glycolate Into Malate. United States: 2011. [Google Scholar]
- 33.Minic Z, Thongbam PD. The biological deep sea hydrothermal vent as a model to study carbon dioxide capturing enzymes. Mar Drugs. 2011;9:719–738. doi: 10.3390/md9050719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muramatsu M, Hihara Y. Acclimation to high-light conditions in cyanobacteria: from gene expression to physiological responses. J Plant Res. 2012;125:11–39. doi: 10.1007/s10265-011-0454-6. [DOI] [PubMed] [Google Scholar]
- 35.Mussgnug JH, Thomas-Hall S, Rupprecht J, Foo A, Klassen V, McDowall A, Schenk PM, Kruse O, Hankamer B. Engineering photosynthetic light capture: impacts on improved solar energy to biomass conversion. Plant Biotechnol J. 2007;5:802–814. doi: 10.1111/j.1467-7652.2007.00285.x. [DOI] [PubMed] [Google Scholar]
- 36.Nevin KP, Woodard TL, Franks AE, Summers ZM, Lovley DR. Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. MBio. 2010:1. doi: 10.1128/mBio.00103-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Neill BM, Mikkelson KL, Gutierrez NM, Cunningham JL, Wolff KL, Szyjka SJ, Yohn CB, Redding KE, Mendez MJ. An exogenous chloroplast genome for complex sequence manipulation in algae. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raines CA. Increasing photosynthetic carbon assimilation in C3 plants to improve crop yield: current and future strategies. Plant Physiol. 2011;155:36–42. doi: 10.1104/pp.110.168559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reinfelder JR. Carbon concentrating mechanisms in eukaryotic marine phytoplankton. Ann Rev Mar Sci. 2011;3:291–315. doi: 10.1146/annurev-marine-120709-142720. [DOI] [PubMed] [Google Scholar]
- 40.Salvucci ME, DeRidder BP, Portis AR., Jr Effect of activase level and isoform on the thermotolerance of photosynthesis in Arabidopsis. J Exp Bot. 2006;57:3793–3799. doi: 10.1093/jxb/erl140. [DOI] [PubMed] [Google Scholar]
- 41*.Stitt M, Lunn J, Usadel B. Arabidopsis and primary photosynthetic metabolism - more than the icing on the cake. Plant J. 2010;61:1067–1091. doi: 10.1111/j.1365-313X.2010.04142.x. A solid overview of plant photosynthetic research with focus on flux analysis methods and discussion of the Calvin cycle in the context of multiple control points. [DOI] [PubMed] [Google Scholar]
- 42.Tabor JJ, Levskaya A, Voigt CA. Multichromatic control of gene expression in Escherichia coli. J Mol Biol. 2011;405:315–324. doi: 10.1016/j.jmb.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tcherkez GG, Farquhar GD, Andrews TJ. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc Natl Acad Sci U S A. 2006;103:7246–7251. doi: 10.1073/pnas.0600605103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thrash JC, Coates JD. Review: Direct and indirect electrical stimulation of microbial metabolism. Environ Sci Technol. 2008;42:3921–3931. doi: 10.1021/es702668w. [DOI] [PubMed] [Google Scholar]
- 45.Whitney SM, Houtz RL, Alonso H. Advancing our understanding and capacity to engineer nature’s CO2-sequestering enzyme, Rubisco. Plant Physiol. 2011;155:27–35. doi: 10.1104/pp.110.164814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitney SM, Kane HJ, Houtz RL, Sharwood RE. Rubisco oligomers composed of linked small and large subunits assemble in tobacco plastids and have higher affinities for CO2 and O2. Plant Physiol. 2009;149:1887–1895. doi: 10.1104/pp.109.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young JD, Shastri AA, Stephanopoulos G, Morgan JA. Mapping photoautotrophic metabolism with isotopically nonstationary (13)C flux analysis. Metab Eng. 2011;13:656–665. doi: 10.1016/j.ymben.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zarzycki J, Brecht V, Muller M, Fuchs G. Identifying the missing steps of the autotrophic 3-hydroxypropionate CO2 fixation cycle in Chloroflexus aurantiacus. Proc Natl Acad Sci U S A. 2009;106:21317–21322. doi: 10.1073/pnas.0908356106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu XG, de Sturler E, Long SP. Optimizing the distribution of resources between enzymes of carbon metabolism can dramatically increase photosynthetic rate: a numerical simulation using an evolutionary algorithm. Plant Physiol. 2007;145:513–526. doi: 10.1104/pp.107.103713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Zhu XG, Long SP, Ort DR. Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol. 2010;61:235–261. doi: 10.1146/annurev-arplant-042809-112206. A comprehensive review of recent efforts to improve plant photosynthetic processes and the potential gains to by achieved through different engineering strategies. [DOI] [PubMed] [Google Scholar]