Abstract
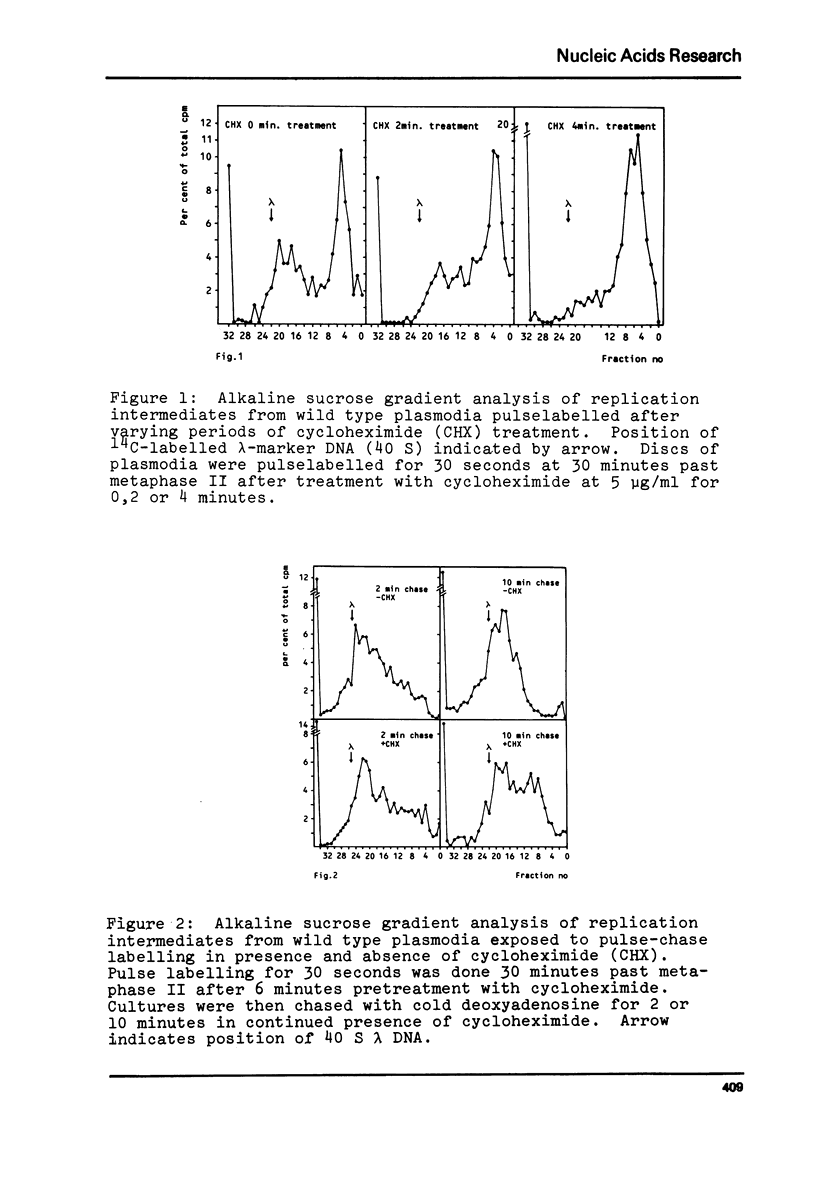
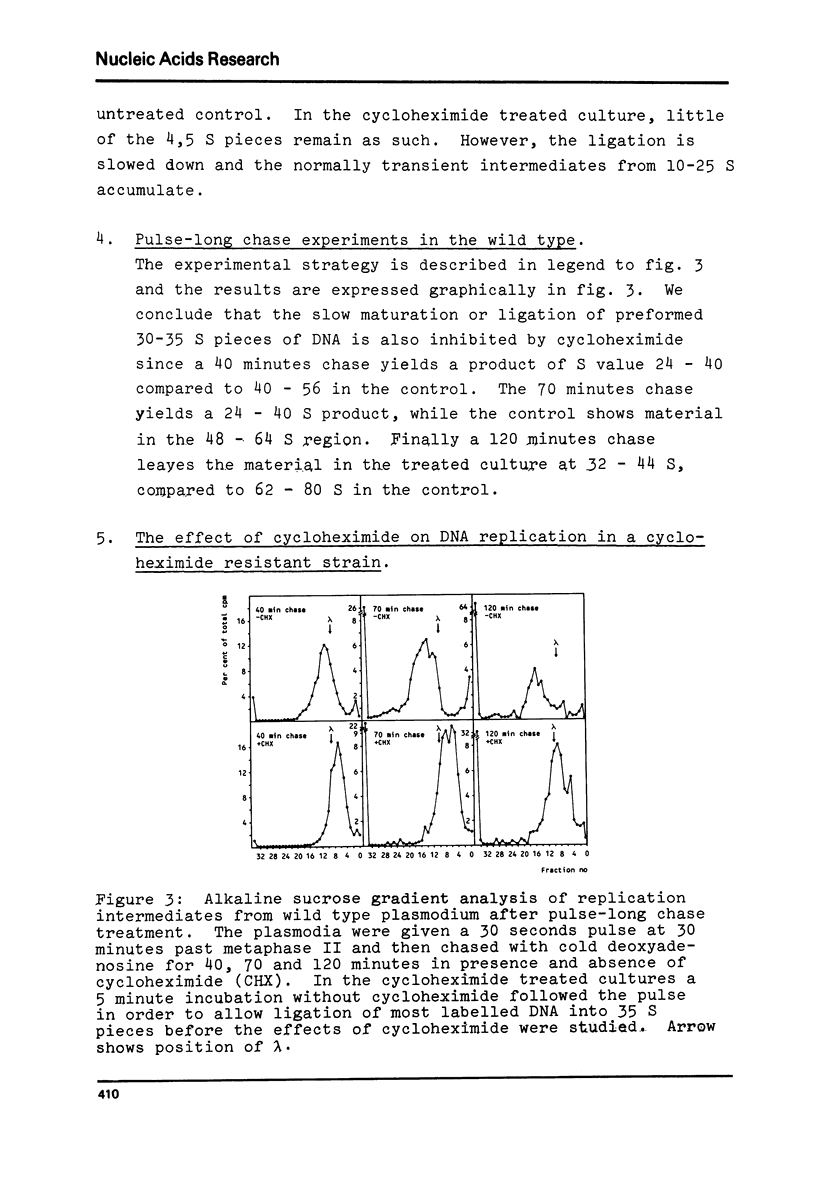
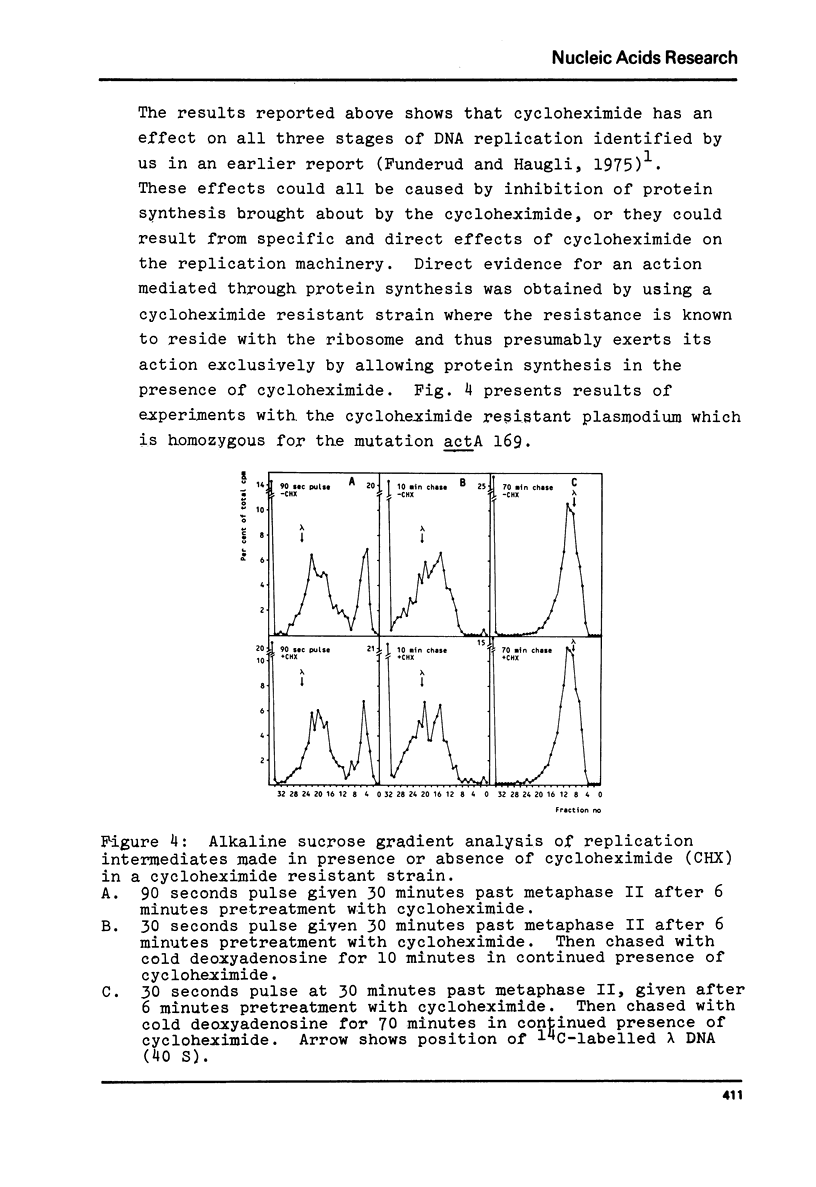
Synchronous plasmodia of cycloheximide-sensitive and cycloheximide-resistant strains of Physarum polycephalum were labelled with 3[H]-deoxyadenosine in pulse and pulse-chase experiments in presence and absence of cycloheximide. The replication products were studied with alkaline sucrose gradient sedimentation analysis. We show that the action of cycloheximide on DNA replication in Physarum is mediated through the ribosome, since the ribosomally located resistance also makes the plasmodial DNA replication refractile to the action of cycloheximide. Cycloheximide caused inhibition of three stages in DNA replication in the wild type: first, the formation of primary replication units ("Okazaki" size fragments), secondly, the ligation of primary units into secondary ("Replicon" size) units and thirdly, the ligation of secondary units into mature DNA.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bersier D., Braun R. Effect of cycloheximide on pools of deoxyribonucleoside triphosphates. Exp Cell Res. 1974 Mar 15;84(1):436–440. doi: 10.1016/0014-4827(74)90427-3. [DOI] [PubMed] [Google Scholar]
- Cummins J. E., Rusch H. P. Limited DNA synthesis in the absence of protein synthesis in Physarum polycephalum. J Cell Biol. 1966 Dec;31(3):577–583. doi: 10.1083/jcb.31.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. H., Littman S. R., Evans T. E., Brewer E. N. Effects of cycloheximide on thymidine metabolism and on DNA strand elongation in physarum polycephalum. J Mol Biol. 1976 Jun 14;104(1):169–184. doi: 10.1016/0022-2836(76)90007-3. [DOI] [PubMed] [Google Scholar]
- Gautschi J. R., Kern R. M. DNA replication in mammalian cells in the presence of cycloheximide. Exp Cell Res. 1973 Jul;80(1):15–26. doi: 10.1016/0014-4827(73)90270-x. [DOI] [PubMed] [Google Scholar]
- Gautschi J. R. Letter: Effects of puromycin on DNA chain elongation in mammalian cells. J Mol Biol. 1974 Mar 25;84(1):223–229. doi: 10.1016/0022-2836(74)90224-1. [DOI] [PubMed] [Google Scholar]
- Haugli F. B., Dove W. F., Jimenez A. Genetics and biochemistry of cycloheximide resistance in Physarum polycephalum. Mol Gen Genet. 1972;118(2):97–107. doi: 10.1007/BF00267081. [DOI] [PubMed] [Google Scholar]
- KERRIDGE D. The effect of actidione and other antifungal agents on nucleic acid and protein synthesis in Saccharomyces carlsbergensis. J Gen Microbiol. 1958 Dec;19(3):497–506. doi: 10.1099/00221287-19-3-497. [DOI] [PubMed] [Google Scholar]
- Muldoon J. J., Evans T. E., Nygaard O. F., Evans H. H. Control of DNA replication by protein synthesis at defined times during the S period in Physarum polycephalum. Biochim Biophys Acta. 1971 Oct 14;247(2):310–321. doi: 10.1016/0005-2787(71)90679-4. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Holtzer H. Fine control of DNA synthesis in developing chick red blood cells. J Mol Biol. 1972 Apr 28;66(1):13–35. doi: 10.1016/s0022-2836(72)80003-2. [DOI] [PubMed] [Google Scholar]
- Werner D., Maier G. Deficiency of joining of Okazaki-type fragments in absence of cellular protein synthesis. Eur J Biochem. 1975 Jun;54(2):351–358. doi: 10.1111/j.1432-1033.1975.tb04145.x. [DOI] [PubMed] [Google Scholar]
- Werry P. A., Wanka F. The effect of cycloheximide on the synthesis of major and satellite DNA components in Physarum polycephalum. Biochim Biophys Acta. 1972 Dec 6;287(2):232–235. doi: 10.1016/0005-2787(72)90372-3. [DOI] [PubMed] [Google Scholar]


