Abstract
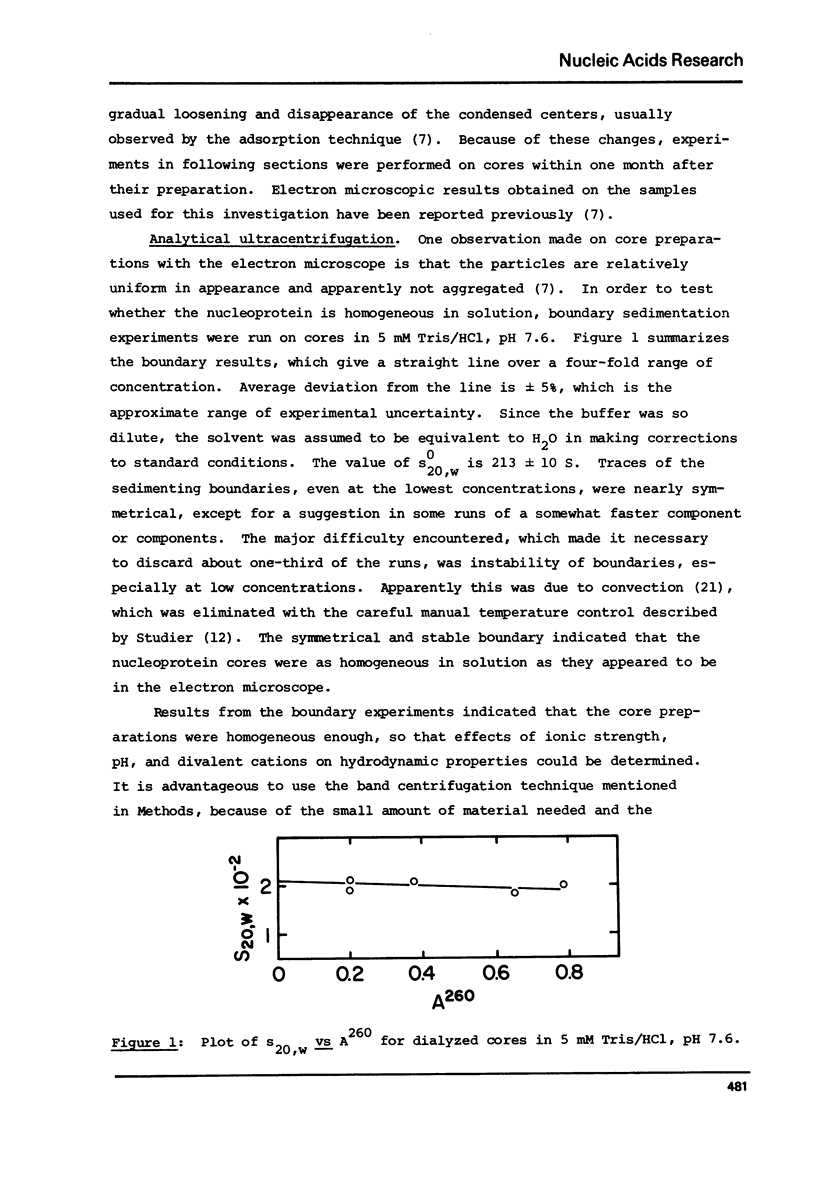
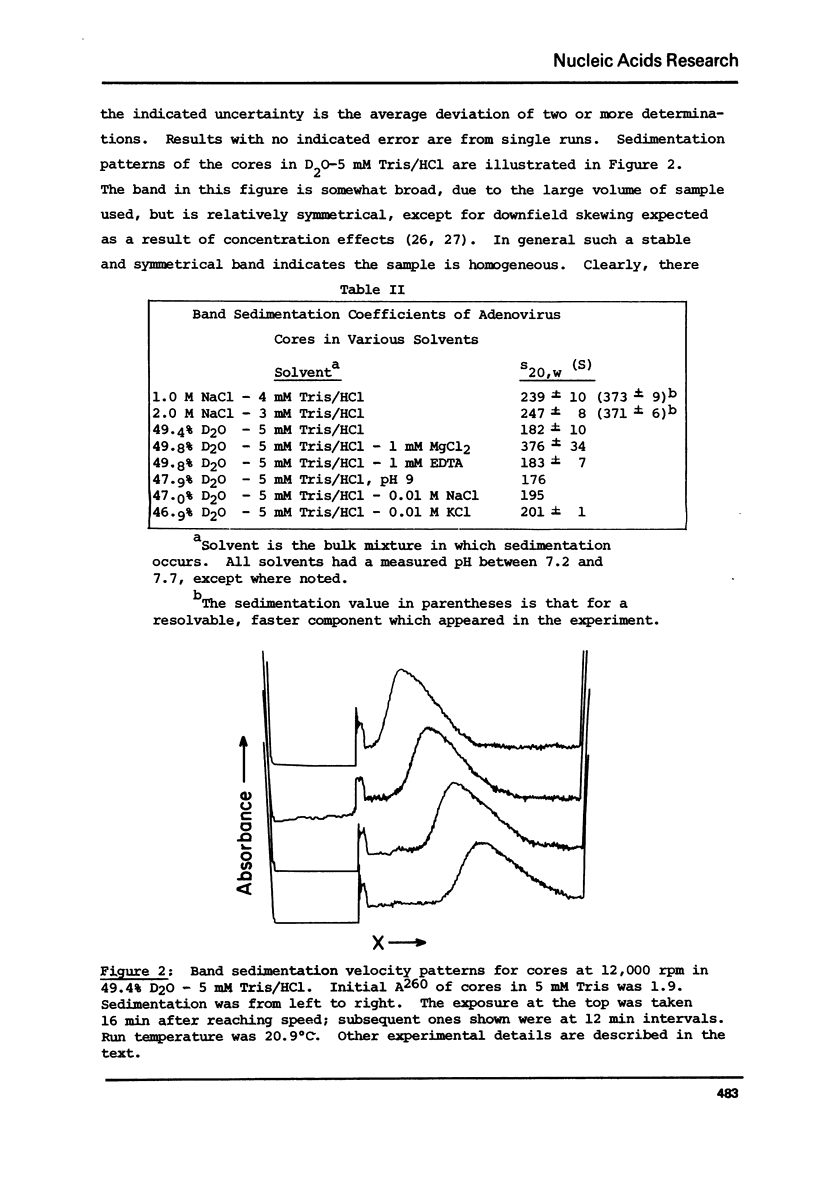
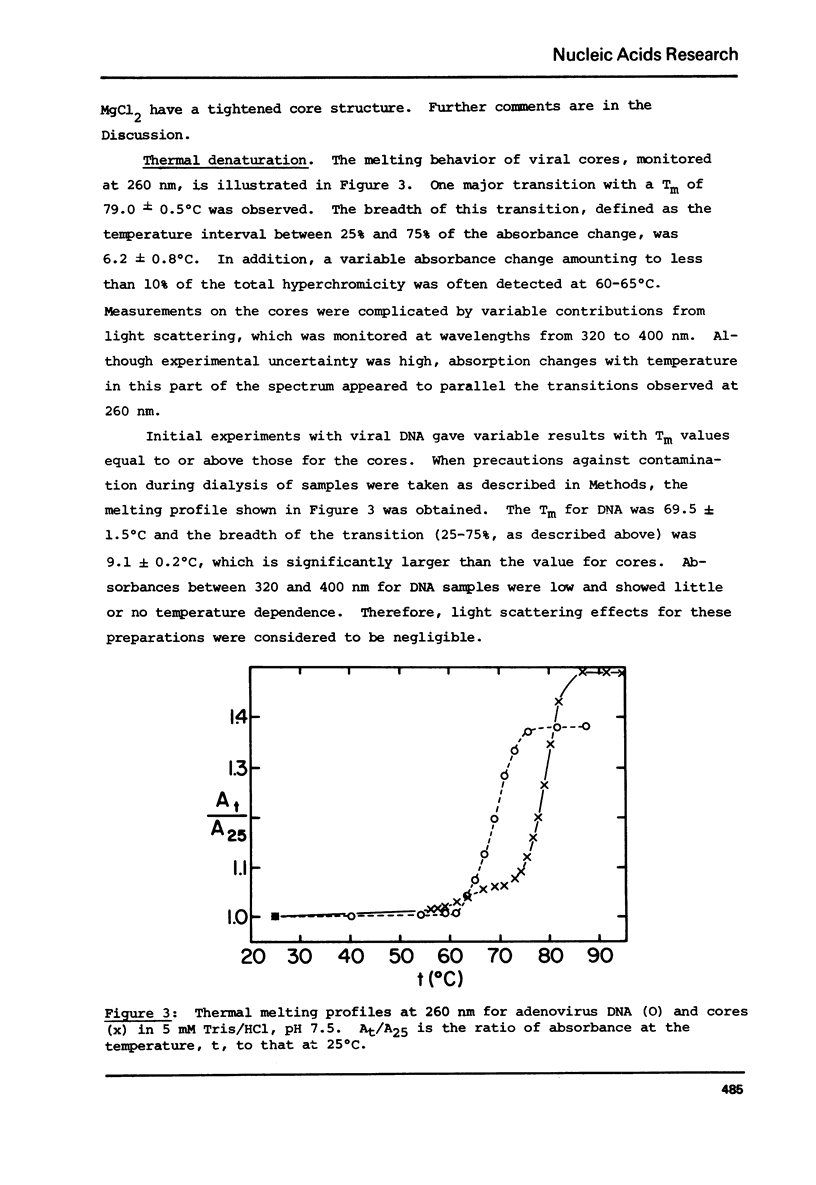
Analytical ultracentrifugation, thermal denaturation, and electron microscopy have been used to study nucleoprotein core particles, obtained from disrupted type 5 adenovirus and partially purified on glycerol density gradients. Electron microscopy at low salt concentrations has shown that the cores are homogeneous particles with characteristic structures, which vary with conditions of observation from a fairly loose network of fibers to a highly condensed, compact particle. Sedimentation measurements in the analytical ultracentrifuge, both by boundary and by band techniques, show that the cores are relatively homogeneous in solution and have sedimentation coefficients near 185 S at low salt concentrations, about 243 S in 1 or 2 M NaCl, and 376 S in 1 mM MgCl2. Correlation of sedimentation data with electron microscopic observations suggests that the 185 S particle has a loose, fibrous structure, while the faster species are more highly condensed particles. The melting temperature of the cores in 5 mM Tris/HCl is 79 degrees C, which is 10 degrees C higher than the Tm for purified, viral DNA. This indicates that the protein enhances the stability of DNA in the nucleoprotein complex.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. T., Westphal M., Burlingham B. T., Winterhoff U., Doerfler W. Structure and composition of the adenovirus type 2 core. J Virol. 1975 Aug;16(2):366–387. doi: 10.1128/jvi.16.2.366-387.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corden J., Engelking H. M., Pearson G. D. Chromatin-like organization of the adenovirus chromosome. Proc Natl Acad Sci U S A. 1976 Feb;73(2):401–404. doi: 10.1073/pnas.73.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELSENFELD G., SANDEEN G. The dispersion of the hyperchromic effect in thermally induced transitions of nucleic acids. J Mol Biol. 1962 Dec;5:587–610. doi: 10.1016/s0022-2836(62)80088-6. [DOI] [PubMed] [Google Scholar]
- GREEN M. Studies on the biosynthesis of viral DNA. Cold Spring Harb Symp Quant Biol. 1962;27:219–235. doi: 10.1101/sqb.1962.027.001.022. [DOI] [PubMed] [Google Scholar]
- Jensen R. H., Chalkley R. The physical state of nucleohistone under physiological ionic strength. The effect of interaction with free nucleic acids. Biochemistry. 1968 Dec;7(12):4388–4395. doi: 10.1021/bi00852a035. [DOI] [PubMed] [Google Scholar]
- Kates J., Beeson J. Ribonucleic acid synthesis in vaccinia virus. I. The mechanism of synthesis and release of RNA in vaccinia cores. J Mol Biol. 1970 May 28;50(1):1–18. doi: 10.1016/0022-2836(70)90100-2. [DOI] [PubMed] [Google Scholar]
- Lewis E. A., DeBuysere M. S., Rees A. W. Configuration of unsheared nucleohistone. Effects of ionic strength and of histone F1 removal. Biochemistry. 1976 Jan 13;15(1):186–192. doi: 10.1021/bi00646a029. [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Harpst J. A., Russell W. C. Electron microscopy of adenovirus cores. J Gen Virol. 1975 Jul;28(1):49–58. doi: 10.1099/0022-1317-28-1-49. [DOI] [PubMed] [Google Scholar]
- Olins D. E., Olins A. L., Von Hippel P. H. Model nucleoprotein complexes: studies on the interaction of cationic homopolypeptides with DNA. J Mol Biol. 1967 Mar 14;24(2):157–176. doi: 10.1016/0022-2836(67)90324-5. [DOI] [PubMed] [Google Scholar]
- Olins D. E., Olins A. L., Von Hippel P. H. On the structure and stability of DNA-protamine and DNA-polypeptide complexes. J Mol Biol. 1968 Apr 14;33(1):265–281. doi: 10.1016/0022-2836(68)90293-3. [DOI] [PubMed] [Google Scholar]
- Pereira H. G., Skehel J. J. Spontaneous and tryptic degradation of virus particles and structural components of adenoviruses. J Gen Virol. 1971 Jul;12(1):13–24. doi: 10.1099/0022-1317-12-1-13. [DOI] [PubMed] [Google Scholar]
- Raukas E., Kooli K. Selection of optimal set of wavelengths for analysis of hyperchromic spectra. Anal Biochem. 1975 Jun;66(2):545–555. doi: 10.1016/0003-2697(75)90622-3. [DOI] [PubMed] [Google Scholar]
- Russell W. C. Adenovirus-specified proteins. In: strategy of the viral genome. Ciba Found Symp. 1971:335–353. [PubMed] [Google Scholar]
- Russell W. C., McIntosh K., Skehel J. J. The preparation and properties of adenovirus cores. J Gen Virol. 1971 Apr;11(1):35–46. doi: 10.1099/0022-1317-11-1-35. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Valentine R. C., Pereira H. G. The effect of heat on the anatomy of the adenovirus. J Gen Virol. 1967 Oct;1(4):509–522. doi: 10.1099/0022-1317-1-4-509. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schlesinger R. W. Adenoviruses: the nature of the virion and of controlling factors in productive or abortive infection and tumorigenesis. Adv Virus Res. 1969;14:1–61. doi: 10.1016/s0065-3527(08)60556-4. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., BRUNER R., KENT R., WEIGLE J. Band-centrifugation of macromolecules and viruses in self-generating density gradients. Proc Natl Acad Sci U S A. 1963 Jun;49:902–910. doi: 10.1073/pnas.49.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine R. C., Pereira H. G. Antigens and structure of the adenovirus. J Mol Biol. 1965 Aug;13(1):13–20. doi: 10.1016/s0022-2836(65)80076-6. [DOI] [PubMed] [Google Scholar]
- van der Eb A. J., van Kesteren L. W., van Bruggen E. F. Structural properties of adenovirus DNA's. Biochim Biophys Acta. 1969 Jun 17;182(2):530–541. doi: 10.1016/0005-2787(69)90205-6. [DOI] [PubMed] [Google Scholar]


