Abstract
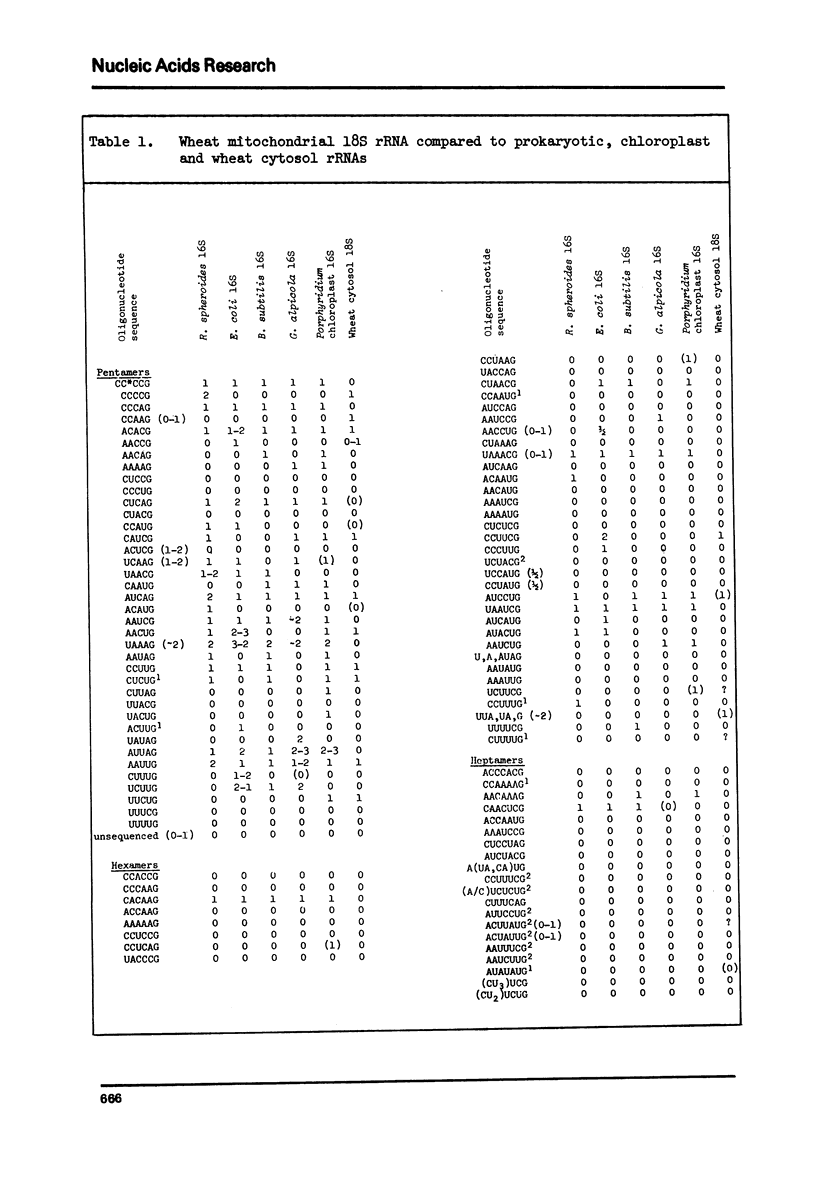
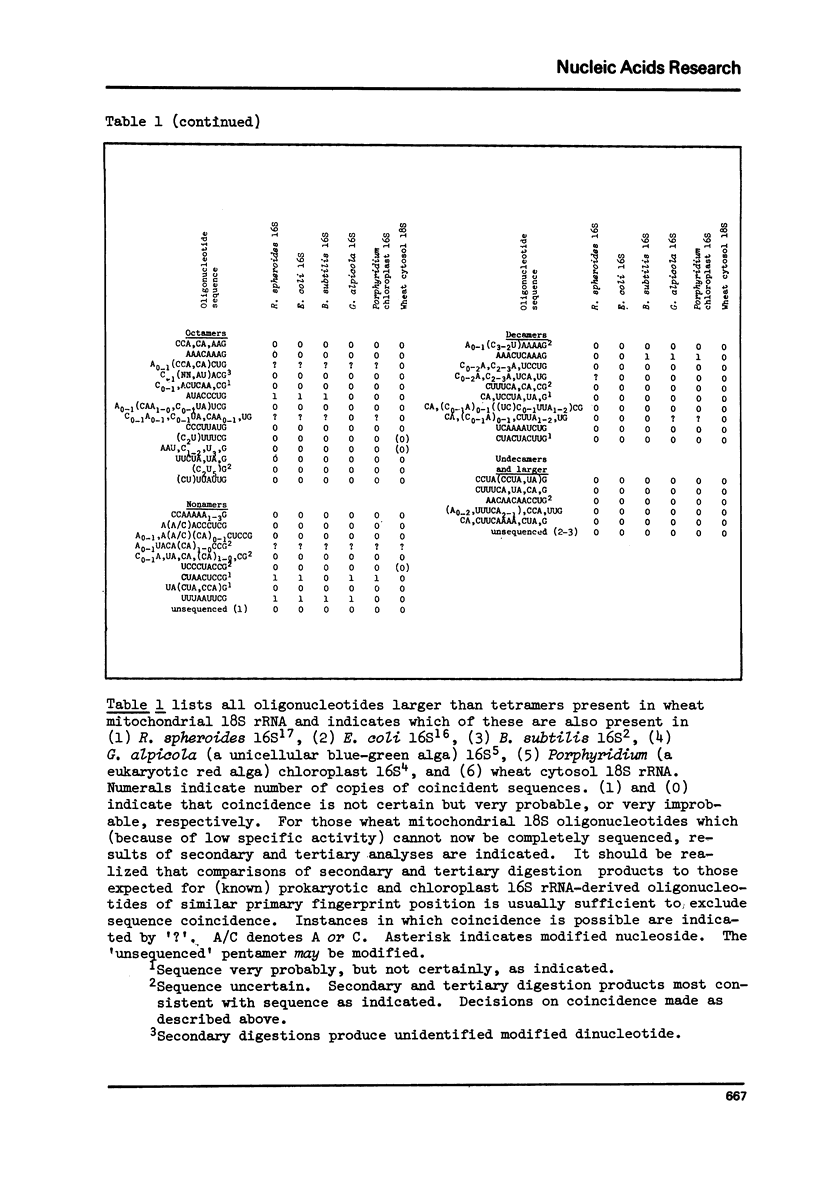
We present a catalog of sequences of oligonucleotides produced by T1 ribonuclease digestion of 32P-labeled small-ribosomal-subunit RNA ("18S rRNA) isolated from purified wheat embryo mitochondria. This catalog is compared to catalogs published for prokaryotic and chloroplast 16S rRNAs and to preliminary results for wheat cytosol 18S rRNA. These comparisons indicate that: (1) wheat mitochondrial 18S rRNA is clearly prokaryotic in nature, showing significantly more sequence homology with 16S rRNAs than can be expected to arise by chance (p less than 0.000001); (2) shared oligonucleotide sequences include an especially high proportion of those identified as conserved in the evolution of prokaryotic rRNAs; and (3) wheat embryo mitochondrial and cytosol 18S rRNAs retain no more, and perhaps less, than the minimum sequence homology detectable by this sensitive method. These results argue in favor of an endosymbiotic origin for mitochondria.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonen L., Doolittle W. F. On the prokaryotic nature of red algal chloroplasts. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2310–2314. doi: 10.1073/pnas.72.6.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen L., Doolittle W. F. Partial sequences of 16S rRNA and the phylogeny of blue-green algae and chloroplasts. Nature. 1976 Jun 24;261(5562):669–673. doi: 10.1038/261669a0. [DOI] [PubMed] [Google Scholar]
- Cavalier-tsmith T. The origin of nuclei and of eukaryotic cells. Nature. 1975 Aug 7;256(5517):463–468. doi: 10.1038/256463a0. [DOI] [PubMed] [Google Scholar]
- Cunningham R. S., Bonen L., Doolittle W. F., Gray M. W. Unique species of 5S, 18S, and 26S ribosomal RNA in wheat mitochondria. FEBS Lett. 1976 Oct 15;69(1):116–122. doi: 10.1016/0014-5793(76)80666-7. [DOI] [PubMed] [Google Scholar]
- Lau R. Y., Kennedy T. D., Lane B. G. Wheat-embryo ribonucleates. III. Modified nucleotide constituents in each of the 5.8S, 18S and 26S ribonucleates. Can J Biochem. 1974 Dec;52(12):1110–1123. doi: 10.1139/o74-155. [DOI] [PubMed] [Google Scholar]
- Pechman K. J., Woese C. R. Characterization of the primary structural homology between the 16s ribosomal RNAs of Escherichia coli and Bacillus megaterium by oligomer cataloging. J Mol Evol. 1972;1(3):230–240. doi: 10.1007/BF01660242. [DOI] [PubMed] [Google Scholar]
- Raff R. A., Mahler H. R. The symbiont that never was: an inquiry into the evolutionary origin of the mitochondrion. Symp Soc Exp Biol. 1975;(29):41–92. [PMC free article] [PubMed] [Google Scholar]
- Raven P. H. A multiple origin for plastids and mitochondria. Science. 1970 Aug 14;169(3946):641–646. doi: 10.1126/science.169.3946.641. [DOI] [PubMed] [Google Scholar]
- Sanders J. P., Heyting C., Borst P. The organization of genes in yeast mitochondrial DNA. I. The genes for large and small ribosomal RNA are far apart. Biochem Biophys Res Commun. 1975 Jul 22;65(2):699–707. doi: 10.1016/s0006-291x(75)80202-6. [DOI] [PubMed] [Google Scholar]
- Uchida T., Bonen L., Schaup H. W., Lewis B. J., Zablen L., Woese C. The use of ribonuclease U2 in RNA sequence determination. Some corrections in the catalog of oligomers produced by ribonuclease T1 digestion of Escherichia coli 16S ribosomal RNA. J Mol Evol. 1974 Feb 28;3(1):63–77. doi: 10.1007/BF01795977. [DOI] [PubMed] [Google Scholar]
- Uzzell T., Spolsky C. Mitochondria and plastids as endosymbionts: a revival of special creation? Am Sci. 1974 May-Jun;62(3):334–343. [PubMed] [Google Scholar]
- Woese C. R., Fox G. E., Zablen L., Uchida T., Bonen L., Pechman K., Lewis B. J., Stahl D. Conservation of primary structure in 16S ribosomal RNA. Nature. 1975 Mar 6;254(5495):83–86. doi: 10.1038/254083a0. [DOI] [PubMed] [Google Scholar]
- Woese C., Sogin M., Stahl D., Lewis B. J., Bonen L. A comparison of the 16S ribosomal RNAs from mesophilic and thermophilic bacilli: some modifications in the Sanger method for RNA sequencing. J Mol Evol. 1976 Apr 9;7(3):197–213. doi: 10.1007/BF01731489. [DOI] [PubMed] [Google Scholar]
- Zablen L. B., Kissil M. S., Woese C. R., Buetow D. E. Phylogenetic origin of the chloroplast and prokaryotic nature of its ribosomal RNA. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2418–2422. doi: 10.1073/pnas.72.6.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablen L., Woese C. R. Procaryote phylogeny IV: concerning the phylogenetic status of a photosynthetic bacterium. J Mol Evol. 1975 Jun 9;5(1):25–34. doi: 10.1007/BF01732011. [DOI] [PubMed] [Google Scholar]


