Abstract
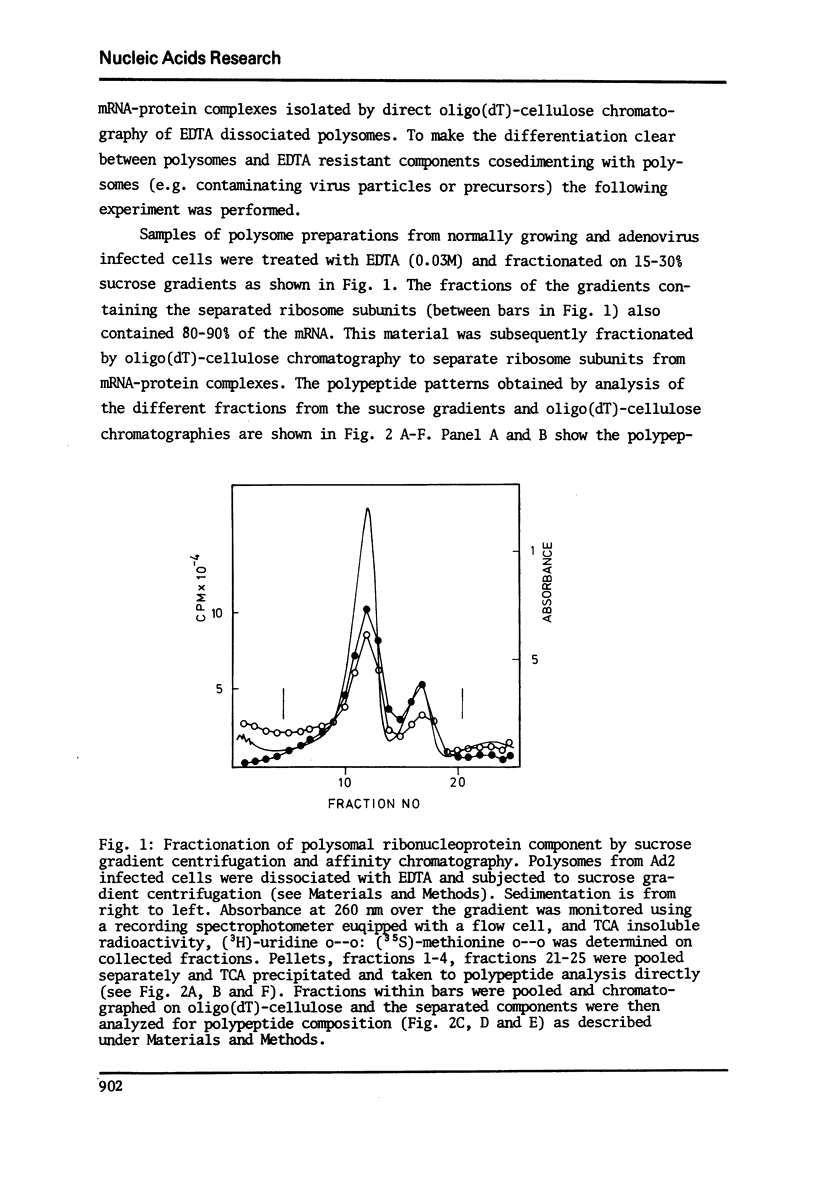
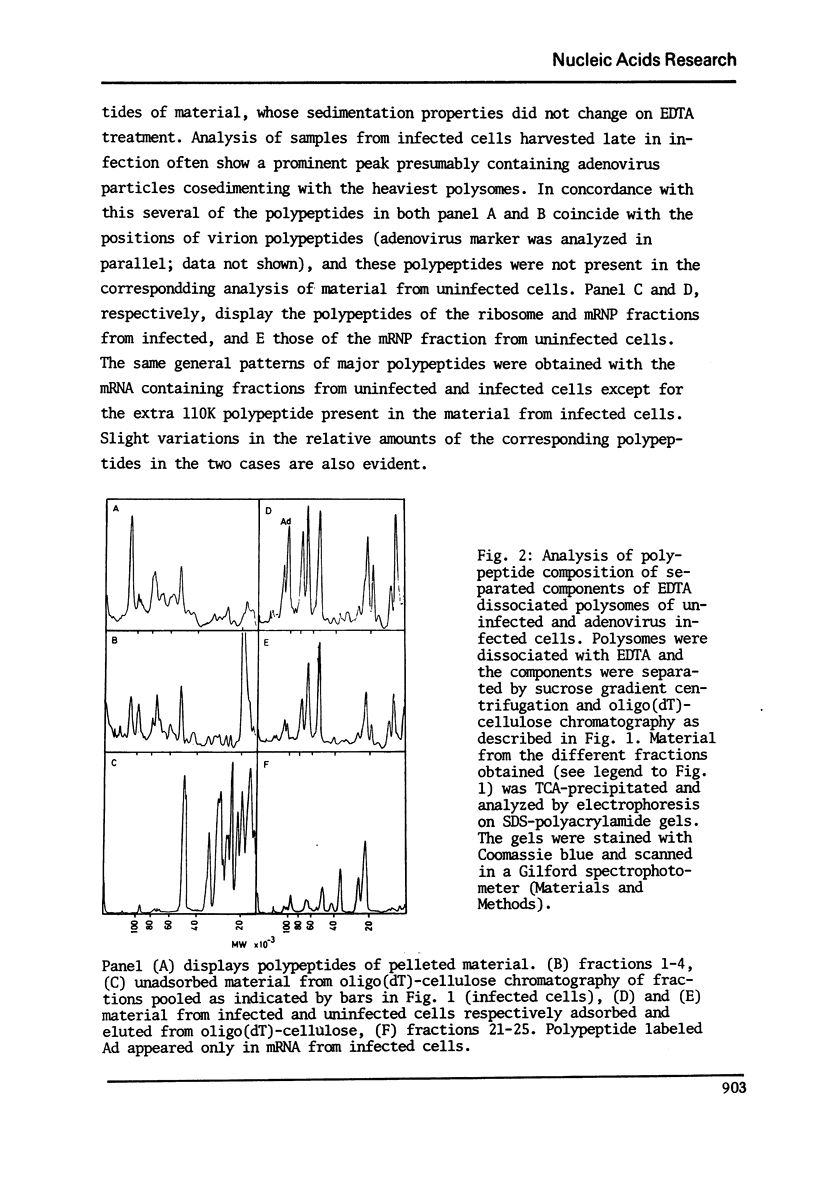
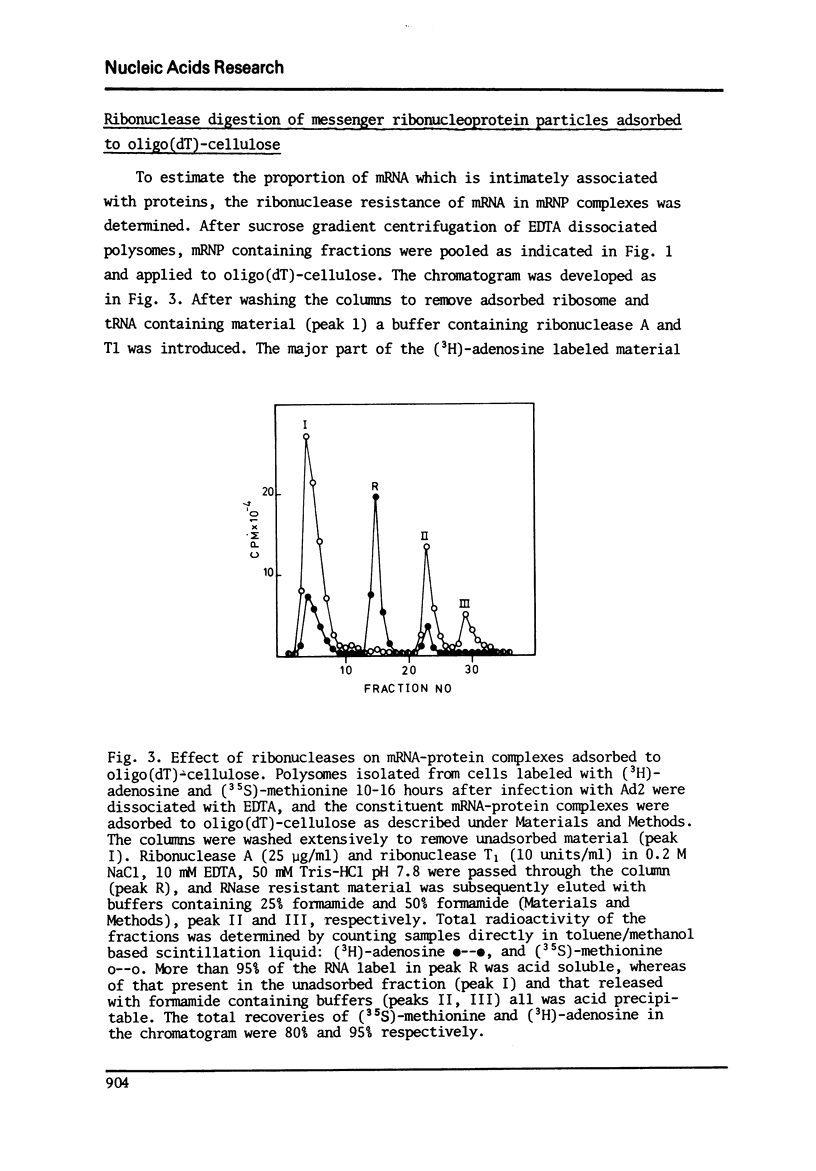
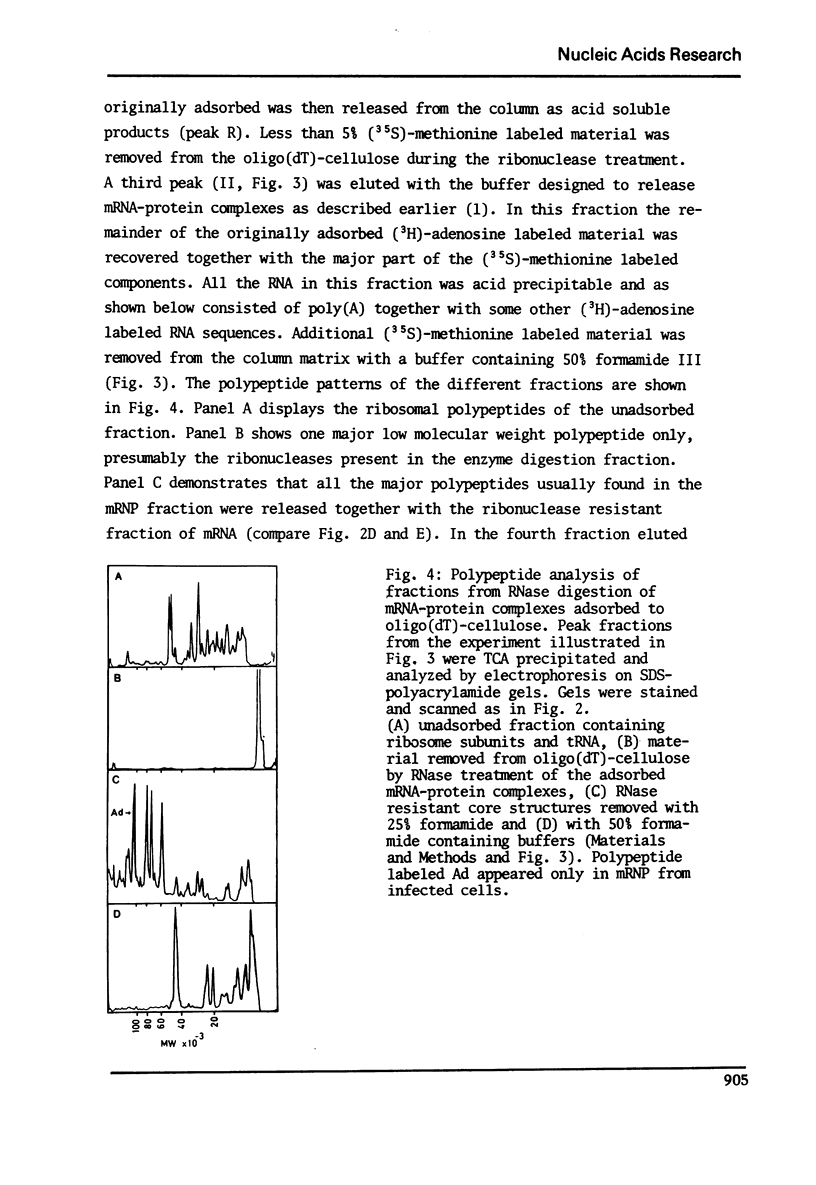
In a previous report we described the use of oligo(dT)-cellulose for the isolation of mRNA-protein complexes from EDTA-dissociated polysomes extracted from normally growing or adenovirus infected KB-cells (I). Experiments presented here provide evidence that proteins involved in these complexes bind specifically to mRNA since: a) the proteins and mRNA cosediment through sucrose gradients, b) they adsorb and elute from oligo(dT)-cellulose together, and c) analysis of the products from ribonuclease digestion experiments show that the poly (A) end and a separate small fraction of the mRNA are resistant to the enzymes and attached to protein.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Darnell J. E. Biogenesis and characterization of histone messenger RNA in HeLa cells. J Mol Biol. 1972 Jun 28;67(3):397–406. doi: 10.1016/0022-2836(72)90458-5. [DOI] [PubMed] [Google Scholar]
- Ajtkhozhin M. A., Akhanov A. U. Release of mRNP-particles of the informosome type from polyribosomes of higher plant embryos. FEBS Lett. 1974 May 1;41(2):275–279. doi: 10.1016/0014-5793(74)81228-7. [DOI] [PubMed] [Google Scholar]
- Auerbach S., Pederson T. Phosphorylation of messenger RNA-bound proteins in HeLa cells. Biochem Biophys Res Commun. 1975 Mar 3;63(1):149–156. doi: 10.1016/s0006-291x(75)80023-4. [DOI] [PubMed] [Google Scholar]
- Barrieux A., Ingraham H. A., David D. N., Rosenfeld M. G. Isolation of messenger-like ribonucleoproteins. Biochemistry. 1975 May 6;14(9):1815–1821. doi: 10.1021/bi00680a002. [DOI] [PubMed] [Google Scholar]
- Blobel G. A protein of molecular weight 78,000 bound to the polyadenylate region of eukaryotic messenger RNAs. Proc Natl Acad Sci U S A. 1973 Mar;70(3):924–928. doi: 10.1073/pnas.70.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan R. N., Hayashi M. Two proteins are bound to most species of polysomal mRNA. Nat New Biol. 1973 Aug 29;244(139):271–274. doi: 10.1038/newbio244271a0. [DOI] [PubMed] [Google Scholar]
- Egly J. M., Johnson B. C., Stricker C., Mandel P., Kempf J., Pfleger N., Girardot J. M. Newly phosphorylated proteins associated with cytoplasmic dRNA. FEBS Lett. 1972 May 1;22(2):181–184. doi: 10.1016/0014-5793(72)80039-5. [DOI] [PubMed] [Google Scholar]
- Ernst V., Arnstein H. R. Synthesis of alpha- and beta-globin directed by messenger ribonucleoprotein from rabbit reticulocytes. Biochim Biophys Acta. 1975 Jan 20;378(2):251–259. doi: 10.1016/0005-2787(75)90113-6. [DOI] [PubMed] [Google Scholar]
- Falvey A. K., Staehelin T. Structure and function of mammalian ribosomes. I. Isolation and characterization of active liver ribosomal subunits. J Mol Biol. 1970 Oct 14;53(1):1–19. doi: 10.1016/0022-2836(70)90042-2. [DOI] [PubMed] [Google Scholar]
- Favre A., Morel C., Scherrer K. The secondary structure and poly(A) content of globin messenger RNA as a pure RNA and in polyribosome-derived ribonucleoprotein complexes. Eur J Biochem. 1975 Sep 1;57(1):147–157. doi: 10.1111/j.1432-1033.1975.tb02285.x. [DOI] [PubMed] [Google Scholar]
- Hendrick D., Schwarz W., Pitzel S., Tiedemann H. Messenger ribonucleoprotein-directed globin synthesis in an embryonic brain cell-free system. Biochim Biophys Acta. 1974 Mar 27;340(3):278–284. doi: 10.1016/0005-2787(74)90273-1. [DOI] [PubMed] [Google Scholar]
- Henshaw E. C. Messenger RNA in rat liver polyribosomes: evidence that it exists as ribonucleoprotein particles. J Mol Biol. 1968 Sep 28;36(3):401–411. doi: 10.1016/0022-2836(68)90164-2. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Bonner J. Preparation, molecular weight, base composition, and secondary structure of giant nuclear ribonucleic acid. Biochemistry. 1973 Jun 5;12(12):2330–2338. doi: 10.1021/bi00736a023. [DOI] [PubMed] [Google Scholar]
- Irwin D., Kumar A., Malt R. A. Messenger ribonucleoprotein complexes isolated with oligo(dT)-cellulose chromatography from kidney polysomes. Cell. 1975 Feb;4(2):157–165. doi: 10.1016/0092-8674(75)90122-1. [DOI] [PubMed] [Google Scholar]
- Jeffery W. R., Brawerman G. Association of the polyadenylate segment of messenger RNA with other polynucleotide sequences in mouse sarcoma 180 polyribosomes. Biochemistry. 1975 Jul 29;14(15):3445–3451. doi: 10.1021/bi00686a024. [DOI] [PubMed] [Google Scholar]
- Kisselev O. I., Gaitskhoki B. S., Klimov N. A. Messenger RNA-containing ribonucleoprotein from mitochondrial polyribosomes of rat liver. Mol Biol Rep. 1975 Jul;2(2):143–149. doi: 10.1007/BF00357545. [DOI] [PubMed] [Google Scholar]
- Kumar A., Lindberg U. Characterization of messenger ribonucleoprotein and messenger RNA from KB cells. Proc Natl Acad Sci U S A. 1972 Mar;69(3):681–685. doi: 10.1073/pnas.69.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Pederson T. Comparison of proteins bound to heterogeneous nuclear RNA and messenger RNA in HeLa cells. J Mol Biol. 1975 Aug 15;96(3):353–365. doi: 10.1016/0022-2836(75)90165-5. [DOI] [PubMed] [Google Scholar]
- Kwan S. W., Brawerman G. A particle associated with the polyadenylate segment in mammalian messenger RNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3247–3250. doi: 10.1073/pnas.69.11.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham H., Darnell J. E. Entrance of mRNA into HeLa cell cytoplasm in puromycin-treated cells. J Mol Biol. 1965 Nov;14(1):13–22. doi: 10.1016/s0022-2836(65)80225-x. [DOI] [PubMed] [Google Scholar]
- Lawford G. R. The effect of incubation with puromycin on the dissociation of rat liver ribosomes into active subunits. Biochem Biophys Res Commun. 1969 Sep 24;37(1):143–150. doi: 10.1016/0006-291x(69)90892-4. [DOI] [PubMed] [Google Scholar]
- Lebleu B., Marbaix G., Huez G., Temmerman J., Burny A., Chantrenne H. Characterization of the messenger ribonucleoprotein released from reticulocyte polyribosomes by EDTA treatment. Eur J Biochem. 1971 Mar 11;19(2):264–269. doi: 10.1111/j.1432-1033.1971.tb01313.x. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Anderson C. W., Baum P. R., Gesteland R. F. Mapping of late adenovirus genes by cell-free translation of RNA selected by hybridization to specific DNA fragments. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1344–1348. doi: 10.1073/pnas.72.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U., Persson T. Isolation of mRNA from KB-cells by affinity chromatography on polyuridylic acid covalently linked to Sepharose. Eur J Biochem. 1972 Dec 4;31(2):246–254. doi: 10.1111/j.1432-1033.1972.tb02527.x. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Persson T., Philipson L. Isolation and characterization of adenovirus messenger ribonucleic acid in productive infection. J Virol. 1972 Nov;10(5):909–919. doi: 10.1128/jvi.10.5.909-919.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U., Sundquist B. Isolation of messenger ribonucleoproteins from mammalian cells. J Mol Biol. 1974 Jun 25;86(2):451–468. doi: 10.1016/0022-2836(74)90030-8. [DOI] [PubMed] [Google Scholar]
- Lingrel J. B., Lockard R. E., Jones R. F., Burr H. E., Holder J. W. Biologically active messenger-RNA for hemoglobin. Ser Haematol. 1971;4(3):37–69. [PubMed] [Google Scholar]
- Morel C., Gander E. S., Herzberg M., Dubochet J., Scherrer K. The duck-globin messenger-ribonucleoprotein complex. Resistance to high ionic strength, particle gel electrophoresis, composition and visualisation by dark-field electron microscopy. Eur J Biochem. 1973 Jul 16;36(2):455–464. doi: 10.1111/j.1432-1033.1973.tb02930.x. [DOI] [PubMed] [Google Scholar]
- Morel Carlos, Kayibanda Boniface, Scherrer Klaus. Proteins associated with globin messenger RNA in avian erythroblasts: Isolation and comparison with the proteins bound to nuclear messenger-likie RNA. FEBS Lett. 1971 Oct 15;18(1):84–88. doi: 10.1016/0014-5793(71)80413-1. [DOI] [PubMed] [Google Scholar]
- Nudel U., Lebleu B., Zehavi-Willner T., Revel M. Mesenger ribonucleoprotein and initiation factors in rabbit-reticulocyte polyribosomes. Eur J Biochem. 1973 Mar 1;33(2):314–322. doi: 10.1111/j.1432-1033.1973.tb02685.x. [DOI] [PubMed] [Google Scholar]
- Obrig T. G., Antonoff R. S., Kirwin K. S., Ferguson J. J., Jr The binding of adenosine-3',5'-monophosphate by messenger ribonucleoprotein-like particles. Biochem Biophys Res Commun. 1975 Sep 2;66(1):437–443. doi: 10.1016/s0006-291x(75)80347-0. [DOI] [PubMed] [Google Scholar]
- Olsnes S. Further studies on the protein bound to the messenger RNA in mammalian polysomes. Eur J Biochem. 1971 Dec 10;23(3):557–563. doi: 10.1111/j.1432-1033.1971.tb01654.x. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA-protein complexes and newly synthesized ribosomal subunits: analysis of free particles and components of polyribosomes. J Mol Biol. 1968 Jul 14;35(1):37–59. doi: 10.1016/s0022-2836(68)80035-x. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. Sequence at the 3' end of globin mRNA shows homology with immunoglobulin light chain mRNA. Nature. 1974 Nov 29;252(5482):359–362. doi: 10.1038/252359a0. [DOI] [PubMed] [Google Scholar]
- Saborio J. L., Koch G. Reversible inhibition of protein synthesis in HeLa cells by dimethylsulfoxide. J Biol Chem. 1973 Dec 25;248(24):8343–8347. [PubMed] [Google Scholar]
- Saborio J. L., Pong S. S., Koch G. Selective and reversible inhibition of initiation of protein synthesis in mammalian cells. J Mol Biol. 1974 May 15;85(2):195–211. doi: 10.1016/0022-2836(74)90360-x. [DOI] [PubMed] [Google Scholar]
- Salditt-Georgieff M., Jelinek W., Darnell J. E., Furuichi Y., Morgan M., Shatkin A. Methyl labeling of HeLa cell hnRNA: a comparison with mRNA. Cell. 1976 Feb;7(2):227–237. doi: 10.1016/0092-8674(76)90022-2. [DOI] [PubMed] [Google Scholar]
- Sampson J., Mathews M. B., Osborn M., Borghetti A. F. Hemoglobin messenger ribonucleic acid translation in cell-free systems from rat and mouse liver and Landschutz ascites cells. Biochemistry. 1972 Sep 12;11(19):3636–3640. doi: 10.1021/bi00769a022. [DOI] [PubMed] [Google Scholar]
- Schochetman G., Perry R. P. Characterization of the messenger RNA released from L cell polyribosomes as a result of temperature shock. J Mol Biol. 1972 Feb 14;63(3):577–590. doi: 10.1016/0022-2836(72)90449-4. [DOI] [PubMed] [Google Scholar]
- Sundquist B., Persson T. Effect of homopolyribonucleotides on messenger ribonucleoprotein particles. Nucleic Acids Res. 1977 Apr;4(4):917–928. doi: 10.1093/nar/4.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Marel P., Tasseron-de Jong J. G., Bosch L. The proteins associated with mRNA from uninfected and adenovirus type 5-infected KB cells. FEBS Lett. 1975 Mar 1;51(1):330–334. doi: 10.1016/0014-5793(75)80919-7. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN E. F. Polysomal site of protein synthesis in HeLa cells. Biochem Biophys Res Commun. 1963 May 22;11:301–306. doi: 10.1016/0006-291x(63)90561-8. [DOI] [PubMed] [Google Scholar]


