Abstract
Root tips of many plant species release a number of border, or border-like, cells that are thought to play a major role in the protection of root meristem. However, little is currently known on the structure and function of the cell wall components of such root cells. Here, we investigate the sugar composition of the cell wall of the root cap in two species: pea (Pisum sativum), which makes border cells, and Brassica napus, which makes border-like cells. We find that the cell walls are highly enriched in arabinose and galactose, two major residues of arabinogalactan proteins. We confirm the presence of arabinogalactan protein epitopes on root cap cell walls using immunofluorescence microscopy. We then focused on these proteoglycans by analyzing their carbohydrate moieties, linkages, and electrophoretic characteristics. The data reveal (1) significant structural differences between B. napus and pea root cap arabinogalactan proteins and (2) a cross-link between these proteoglycans and pectic polysaccharides. Finally, we assessed the impact of root cap arabinogalactan proteins on the behavior of zoospores of Aphanomyces euteiches, an oomycetous pathogen of pea roots. We find that although the arabinogalactan proteins of both species induce encystment and prevent germination, the effects of both species are similar. However, the arabinogalactan protein fraction from pea attracts zoospores far more effectively than that from B. napus. This suggests that root arabinogalactan proteins are involved in the control of early infection of roots and highlights a novel role for these proteoglycans in root-microbe interactions.
Plant roots are highly sensitive to environmental factors such as moisture gradients, minerals, and soil microorganisms, and they are able to modify their growth to optimize whole plant health (Barlow, 2003). The capacity of the root system to explore the soil in response to abiotic and biotic stimuli depends to a considerable extent on the apical region of the root, termed the root cap. This small and seemingly insignificant part of the root is essential for plant survival and development (Barlow, 2003; Wen et al., 2007). The root cap cells originate from the root cap meristem. As the cells progress and differentiate during cap development, they become involved in specific functions, including gravity sensing, hydrotropism, and the synthesis of exudates (Vicré et al., 1998; Roy et al., 2002; Borsics et al., 2007; Ponce et al., 2008). In most plant species, such as pea (Pisum sativum) and cotton (Gossypium hirsutum), cells at the periphery of the root cap become detached as individual living cells, called root border cells (Hawes et al., 2003; Knox et al., 2007). The sloughing of thousands of root cap cells into the rhizosphere is not only important in assisting the growing root to penetrate the soil but also provides a sheath of living cells surrounding the vulnerable root tip, protecting it against pathogens and abiotic stresses (Gunawardena and Hawes, 2002; Wen et al., 2009).
Root cap development and border cell detachment are genetically regulated processes, and the number of cells produced is conserved for species belonging to the same family (Hawes and Lin, 1990; Brigham et al., 1998; Hawes et al., 2003). Root tips of plants belonging to the Brassicaceae family, such as the model plant Arabidopsis (Arabidopsis thaliana), do not produce “classical” border cells but they do release related cells, termed “border-like cells” (Vicré et al., 2005). Root caps from Arabidopsis or Brassica napus release clumps of cells that remain attached to each other and to the root tips and do not disperse individually, as border cells do (Driouich et al., 2007). In view of their similarities and differences, these were termed border-like cells. While border cells have been well studied in many species, such as pea (Hawes et al., 2000; Gunawardena and Hawes, 2002, Cannesan et al., 2011), there is relatively little information available so far regarding border-like cells because of their recent discovery (Vicré et al., 2005; Driouich et al., 2007, 2010; Durand et al., 2009).
It is known that the border-like cells from Arabidopsis are specifically enriched in arabinogalactan protein epitopes (Vicré et al., 2005). Arabinogalactan proteins are highly glycosylated proteins of the cell wall also found in the plasma membrane and root secretions (Bacic et al., 1986; Moody et al., 1988; Gaspar et al., 2001; Schildknecht et al., 2004; Seifert and Roberts, 2007; Driouich and Baskin, 2008; Durand et al., 2009; Ma et al., 2010). These extracellular proteoglycans are structurally complex macromolecules due to the large branched-glycan chains, containing up to 98% carbohydrate mainly O-linked to the hydroxy Pro residues of the protein backbone (Ellis et al., 2010). They consist primarily of (1→3)-β-galactan and (1→6)-β-linked galactan chains with (1→3,1→6)-linked branched points (Seifert and Roberts, 2007). l-Ara is the second most abundant type of sugar residue found in arabinogalactan proteins. Other “minor” sugars, such as d-GlcA, l-Rha, l-Fuc, and d-Xyl, can also be present in the side chains carried by the (1→3)-β-galactan backbone. Most arabinogalactan proteins have the ability to bind a class of synthetic phenylazo dyes, such as the β-glucosyl Yariv (β-GlcY) reagent that has been widely used for their precipitation and subsequent purification (Ding and Zhu, 1997). Arabinogalactan proteins are temporally and spatially regulated during plant development and are involved in many processes, including expansion, cell-cell interaction, and pollen tube guidance (Cheung et al., 1993; Majewska-Sawka and Nothnagel, 2000; Nguema-Ona et al., 2006, 2007; Seifert and Roberts, 2007; Driouich and Baskin, 2008).
Here, we have characterized cell walls from root caps, border cells, and border-like cells of pea, the model plant for studying border cell function (Hawes et al., 2000), and B. napus, a species that produces border-like cells (Driouich et al., 2007). We found that root caps and border-like cells/border cells contain significant amounts of arabinogalactan proteins. Furthermore, we have taken advantage of the pathosystem pea-Aphanomyces euteiches to investigate the effects of arabinogalactan proteins from root caps on zoospore behavior and development. Common root rot due to the oomycete A. euteiches is considered the major destructive soil-borne disease of pea, whereas, on the contrary, root-infecting oomycetes are not naturally occurring pathogens of B. napus crops. We found that arabinogalactan proteins are involved in early root infection. We also show that these proteoglycans selectively induce chemotaxis, zoospore encystment, and a significant inhibition of cyst germination. These findings provide evidence for a previously uncharacterized role of arabinogalactan proteins in root-zoospore interaction.
RESULTS
Formation of Border-Like Cells in B. napus and Border Cells in Pea
While pea is known to release large numbers of individual root border cells (Hawes et al., 2000; Cannesan et al., 2011), B. napus produced border-like cells that do not disperse into solution and remain attached to each other in layers, as described previously for Arabidopsis (Fig. 1). Border-like cells first appeared in 2-d-old seedlings but were few in number. By 6 d, their number increased to reach 370 ± 115 cells per root tip, corresponding to a root length of 89 ± 10 mm (means ± sd; n = 30 roots). If the roots are grown through soil, border-like cells also remain closely attached to the root cap, but when water is added they radiate outward (Supplemental Fig. S1). As the presence of mucilage at the root tip could not be detected visually in bright-field microscopy, we used ruthenium red, a dye that stains acidic polymers (e.g. pectin) known to be generally present in root exudates. The dye revealed quite abundant mucilage associated with border cells of pea but not with the border-like cells produced by B. napus (Fig. 1, E and F). This result is consistent with the previous observation made with Arabidopsis border-like cells, which have been shown to be covered by a thin layer of mucilage only (Durand et al., 2009). In B. napus, border-like cells were strongly stained by calcein-AM (Supplemental Fig. S2), which indicates that these cells were still viable at the time of their detachment from the root cap, as shown previously for Arabidopsis (Vicré et al., 2005).
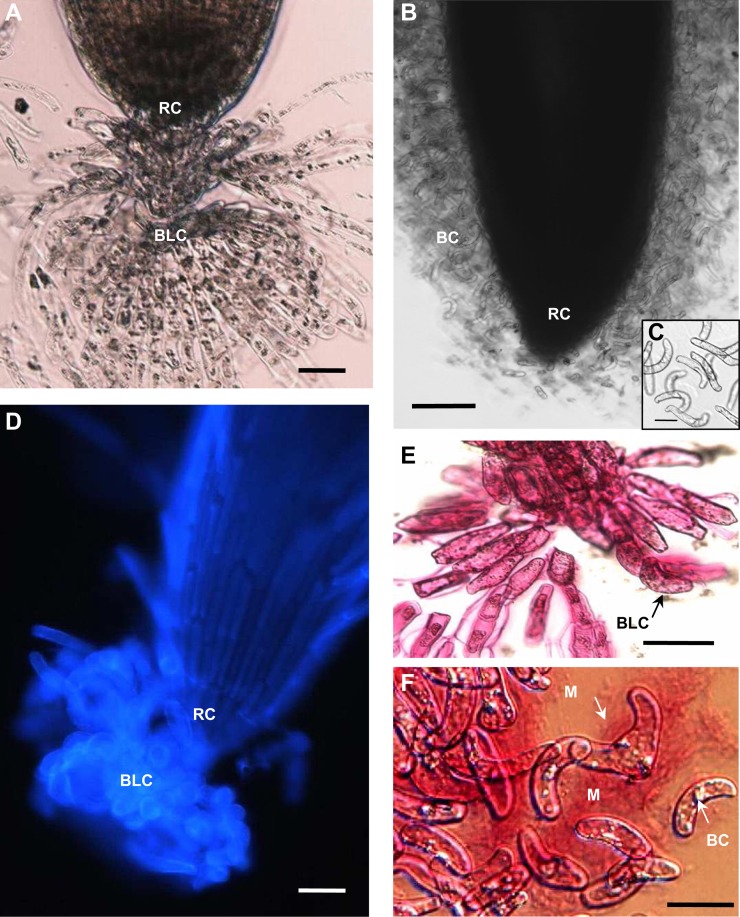
Figure 1.
Histochemical characterization of root border and border-like cells. A, Bright-field micrograph of a B. napus root apex. Border-like cells radiate out into the medium while remaining attached to the root cap. B, Bright-field micrograph of a pea root tip placed in water and releasing a large number of border cells. C, Bright-field micrograph of border cells from pea showing that they are dispersed individually in solution. D, Fluorescence micrograph of a B. napus root apex stained with calcofluor showing a block of border-like cells clamped to the root cap. E and F, Bright-field micrographs of the border-like cells of B. napus (E) and the border cells of pea (F) stained with ruthenium red. Note the abundance of stained material (mucilage) surrounding pea border cells but not around the border-like cells. BC, Border cells; BLC, border-like cells; M, mucilage; RC, root cap. Bars = 20 µm (A and D), 50 µm (B), and 10 µm (C, E, and F). [See online article for color version of this figure.]
Monosaccharide Composition of B. napus and Pea Root Cap Cell Walls
Among monosaccharides of noncellulosic cell wall polysaccharides, Ara was the most abundant (Table I). GalUA and Gal were also plentiful. The abundance of the monosaccharides was similar in both species, except that Gal was comparatively more abundant in B. napus. In contrast, Glc was the more abundant in pea. However, variability in Glc content must be considered with caution, because this monomer may arise from hemicelluloses as well as from contaminating starch. Rha was present at similar levels in both species and at half or less of the amount of Gal. Taken together, the monosaccharide composition suggests that, along with the traditional wall components (hemicellulose, pectin), the root cap contains arabinogalactan proteins in quite significant amounts. Indeed, the presence of these proteoglycans in the cell wall material was confirmed by a strong positive reaction with the β-GlcY reagent (Supplemental Fig. S3).
Table I. Monosaccharide composition of cell wall extracts from B. napus and pea root caps including border cells and border-like cells.
The values give averages ± se of three independent experiments.
| Sugar | B. napus | Pea |
|---|---|---|
| mol % | ||
| Ara | 28 ± 3.2 | 29.5 ± 3.5 |
| Gal | 21 ± 2.8 | 12 ± 1.8 |
| GalUA | 15 ± 2.3 | 14 ± 1.9 |
| Xyl | 14 ± 2 | 13 ± 1.8 |
| Glc | 7 ± 3.2 | 16 ± 2.4 |
| Man | 6 ± 1 | 5 ± 0.6 |
| Fuc | 1 ± 0.2 | 1 ± 0.3 |
| GlcA | 1 ± 0.4 | 1 ± 0.2 |
Distribution of Arabinogalactan Protein Epitopes in the Cell Walls of Border and Border-Like Cells
To further test whether arabinogalactan proteins are present in the cell walls of border and border-like cells, we stained roots with a panel of monoclonal antibodies that have been widely used for immunocytochemical studies of the distribution of these proteoglycans (Supplemental Table S1; Pennell et al., 1989; Knox et al., 1990; Dolan et al., 1995; Andème-Onzighi et al., 2002). These antibodies strongly stained the border-like cells of B. napus (Fig. 2). Likewise, the border cells of pea were strongly stained (Fig. 2; data not shown). No labeling was observed in control roots when no primary antibody was used (Supplemental Fig. S4). Insofar as each of these three antisera recognizes distinct polysaccharide epitopes, the common staining pattern supports the conclusion that the cell surfaces of root border and border-like cells contain accessible arabinogalactan proteins.
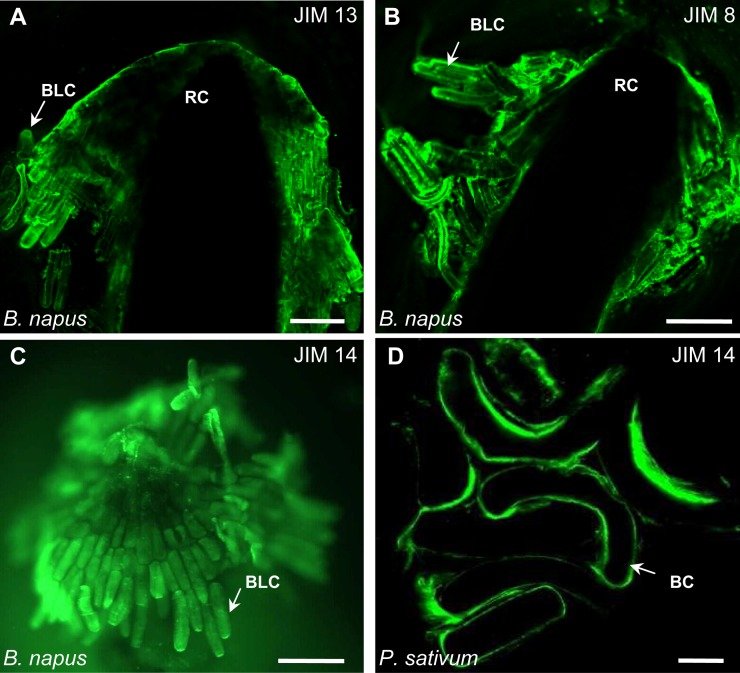
Figure 2.
Fluorescence micrographs of root apices and border cells/border-like cells immunostained with monoclonal antibodies recognizing arabinogalactan protein epitopes. A to C, B. napus border-like cells immunolabeled with JIM13 (A), JIM8 (B), and JIM14 (C). D, Pea border cells immunostained with JIM14. Note that the cell surfaces are strongly labeled with all the antibodies. BC, Border cells; BLC, border-like cells; RC, root cap. Bars = 20 µm.
Biochemical Analysis of the β-GlcY Reagent-Precipitable Material
Most arabinogalactan proteins specifically precipitate in the presence of the β-GlcY reagent (Yariv et al., 1967; Popper, 2011). To characterize the carbohydrate composition of arabinogalactan proteins in root cap cells, we subjected the material precipitated by β-GlcY to compositional analysis following the procedure of York et al. (1985). In general, the overall monosaccharide content of this fraction was similar in B. napus and pea (Table II). As expected, the precipitated material was enriched in Gal and Ara. Together, these two residues accounted for 60% of the monosaccharide constituents in B. napus and nearly 48% in pea. Interestingly, in B. napus, Gal itself constituted nearly 40% of the monosaccharides, giving rise to an Ara-to-Gal ratio of 0.62; in contrast, that ratio was 0.78 in pea, which is typical of arabinogalactan protein composition (Thude and Classen, 2005; Maurer et al., 2010). It should be noted that the ratio of Ara to Gal in pea root arabinogalactan proteins is 2.5, which is distinct from that of border cell arabinogalactan proteins (Supplemental Table S2). This finding suggests that arabinogalactan protein sugar composition from root cap/border cells is quite different from that of the whole root in pea. As expected, Man, Fuc, and Glc were present in only minor amounts. Surprisingly, there was a considerable amount of Xyl, GalUA, and, in pea, Rha, results that suggest the presence of pectic polysaccharides in the precipitated fraction. Anion-exchange chromatography was performed as an attempt to separate arabinogalactan proteins from potential contaminating pectins (Supplemental Fig. S5). However, both arabinogalactan proteins and pectins bound to the column and were coeluted with 0.05 to 0.2 m NaCl. Fractionation of the β-GlcY precipitate by anion-exchange chromatography failed to clearly separate arabinogalactan proteins from pectins. Therefore, based on these analyses, it is likely that pectins detected in the β-GlcY precipitate are covalently bound to arabinogalactan proteins rather than being coprecipitated with them.
Table II. Monosaccharide composition of β-GlcY precipitate obtained from B. napus and pea root caps including border cells and border-like cells.
The β-GlcY precipitate represents the soluble fraction of the cell surface that is either arabinogalactan proteins or covalently/strongly associated with arabinogalactan proteins. The values indicate averages ± se of three independent experiments.
| Sugar | B. napus | Pea |
|---|---|---|
| mol % | ||
| Gal | 37 ± 3.9 | 27 ± 3 |
| Ara | 23 ± 2.6 | 21 ± 2.3 |
| GalUA | 16 ± 1.9 | 18 ± 1.9 |
| Xyl | 15 ± 2 | 15 ± 2 |
| Rha | 2 ± 0.4 | 7.5 ± 1.2 |
| Man | 4 ± 0.5 | 2 ± 0.2 |
| GlcA | 1 ± 0.3 | 4 ± 0.5 |
| Glc | 1 ± 0.2 | 3.5 ± 0.7 |
| Fuc | 1 ± 0.1 | 2 ± 0.3 |
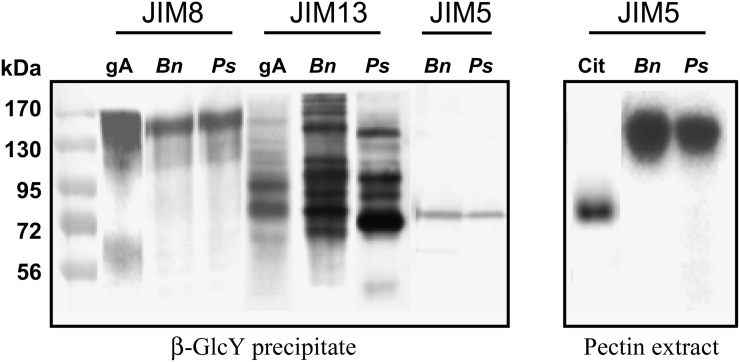
To check for the occurrence of pectin in the precipitates, we blotted the fraction and probed it with monoclonal antisera against pectic epitopes. The results are summarized in Table III. The precipitates from both species reacted strongly with JIM5 and JIM7, implying the presence of homogalacturonan. To provide additional information on the presence of pectin, the β-GlcY-precipitated fraction was separated by SDS-PAGE, blotted, and probed with anti-arabinogalactan protein and anti-pectin antisera (Fig. 3). In both species, JIM8 reacted with a smear of high-Mr material, roughly coincident with the gum arabic standard, whereas JIM13 reacted with bands throughout the gel, and the pattern of recognized bands was different among the two species and gum arabic. In addition, JIM13 antibody reacted very strongly with two bands (100 and 140 kD) in the root cap/border cell material from pea that are not detected in pea root extract (Supplemental Fig. S6). These data suggest that arabinogalactan protein populations are different between root cap/border cells and the rest of the root in pea. Such a resolution of multiple bands of arabinogalactan proteins with JIM13 is unusual, as probing with anti-arabinogalactan protein monoclonal antibodies on western blots generally reveals a smear of material (Jauh and Lord, 1996; Šamaj et al., 2000; Lu et al., 2001). However, the presence of sharp bands does not represent a distinguishing feature of the root cap/border cells, as this occurs also in the whole root of B. napus and pea (Supplemental Fig. S6). Such a pattern of labeling is also found in the root extract of radish (Raphanus sativus), a Brassicaceae species like Arabidopsis that also produces border-like cells. In contrast, the anti-homogalacturonan antiserum, JIM5, detected a single sharp band, running at about 85 kD and equally reactive in both species. In parallel, pectin fractions were extracted with ammonium oxalate, separated by SDS-PAGE, blotted, and probed with JIM5. This antibody recognized a diffuse band of high Mr that was possibly more abundant in B. napus. This range does not coincide with the single band detected with this antibody in the β-GlcY precipitate. Based on these data, we conclude that the homogalacturonan in the β-GlcY fraction was cross-linked to one or more arabinogalactan proteins rather than precipitated directly by the β-GlcY reagent.
Table III. Summary of immunodot binding assays on the β-GlcY precipitate isolated from root caps (including border cells and border-like cells) of B. napus and pea.
−, ±, and + refer to negative, moderate, and highly positive labeling, respectively. Control samples were lime pectin (LM) and gum arabic (GA).
| Antibody | Epitope | Reference | B. napus | Pea | Controls |
|
|---|---|---|---|---|---|---|
| LM | GA | |||||
| JIM5 | Partially methyl-esterified epitope of homogalacturonan/deesterified homogalacturonan | Willats et al. (2000); Clausen et al. (2003) | + | + | + | − |
| JIM7 | Partially methyl-esterified epitope of homogalacturonan | Willats et al. (2000); Clausen et al. (2003) | + | + | + | − |
| LM6 | (1→5)-α-l-Arabinan (may also bind to arabinogalactan proteins) | Willats et al. (1998) | + | + | + | ± |
| LM8 | Xylogalacturonan-associated epitope | Willats et al. (2004) | + | + | ± | − |
| JIM13 | β-d-Glcρ A-(1→3)-α-d-Galρ A-(1→2)-l-Rha | Yates et al. (1996) | + | + | − | + |
| JIM8 | Carbohydrate portion of arabinogalactan proteins | Knox et al. (1991) | + | + | − | + |
| JIM14 | Carbohydrate portion of arabinogalactan proteins | Knox et al. (1991) | + | + | − | + |
Figure 3.
SDS-PAGE analysis of selected fractions extracted from root cap cell walls, which include border and border-like cells. The β-GlcY precipitate (15 µg) and extracted pectin (15 µg) were run on gels, blotted, and probed with antisera recognizing arabinogalactan proteins (JIM8 and JIM13) and pectin (JIM5). Molecular mass is indicated in kD on the left. Bn and Ps indicate material from B. napus and pea, respectively. Cit, Commercial citrus pectin; gA, gum arabic.
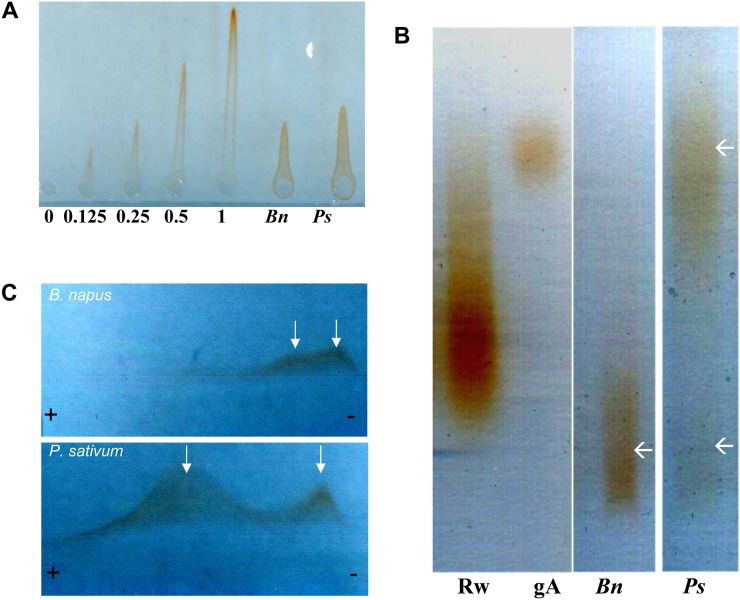
The amount of material present in the Yariv reagent precipitate was quantified by rocket gel electrophoresis (Fig. 4A). Based on the gum arabic standard, root caps were estimated to contain 0.20 ± 0.03 mg g−1 dry weight of putative arabinogalactan protein. Interestingly, electrophoresis performed on agarose gels revealed different populations of arabinogalactan proteins in the two species (Fig. 4B). In pea, the material was predominantly high Mr (approximately the overlapping of the gum arabic standard) and contained lesser amounts of material at low Mr; in B. napus, only low-Mr material was detected. This distinction of the putative arabinogalactan protein populations based on Mr and/or electrical charge density was confirmed by cross electrophoresis with the β-GlcY reagent (Fig. 4C). Here again, the material from pea shows peaks at both high and low Mr, whereas that from B. napus had little if any high-Mr material and the low-Mr material had a broad distribution, possibly subdivided into two main populations (Fig. 4C, arrows). Evidently the putative arabinogalactan protein fraction from the two root caps differs qualitatively.
Figure 4.
Characterization of the β-GlcY precipitate using native electrophoresis methods. A, Rocket gel electrophoresis run on an agarose gel containing β-GlcY reagent. Numbers below the rockets give the amount of gum arabic loaded (mg) and from which the concentration of Yariv-positive material in the root cap fractions was estimated. Bn and Ps indicate material from B. napus and pea, respectively. B, Linear arabinogalactan protein profiles revealed by agarose gel electrophoresis followed by staining with β-GlcY reagent. gA, Twenty micrograms of gum arabic; Rw, 10 µg of red wine arabinogalactan proteins. Different populations of arabinogalactan proteins are indicated by white arrows. C, Two-dimensional arabinogalactan protein profile revealed by cross electrophoresis followed by staining with β-GlcY reagent. Different populations of arabinogalactan proteins are indicated by white arrows. [See online article for color version of this figure.]
For the final analytical method, we characterized sugar linkage based on methanolysis, as described in “Materials and Methods.” Gal was 3-, 6-, and 3,6-linked as well as terminal, consistent with the branched galactan backbone for the arabinogalactan chains (Table IV). Ara was mostly in the furanose form and either 5-linked or terminal, results that are typical of arabinogalactan proteins from other sources (Tryfona et al., 2010). In addition, appreciable amounts of 2- and 2,4-2-linked Rha and 4-linked Xyl were present as well as smaller amounts of terminal Fuc, terminal Xyl, and 4-Man, indicating a possible occurrence of rhamnogalacturonan I, xylan, and mannan in the precipitate. Comparing the two species, pea had substantially more 3-Gal and less 3,6-Gal. A difference in the extent of branching within the galactan backbone might be related to the differences in electrophoretic mobility observed under native conditions (Fig. 4).
Table IV. Linkage analysis of the β-GlcY precipitate isolated from root caps including border cells and border-like cells of B. napus and pea.
The β-GlcY precipitate represents the soluble fraction of the cell surface that is either arabinogalactan proteins or covalently/strongly associated with arabinogalactan proteins. T, Terminal.
| Linkage | B. napus | Pea |
|---|---|---|
| mol % | ||
| T-Araf | 9 | 3 |
| T-Arap | 1 | 3 |
| 5-Araf | 15 | 7 |
| 2-Rha | 1 | 6 |
| 2,4-Rha | 9 | 3 |
| T-Fuc | 4 | 3 |
| T-Xyl | 3 | 3 |
| 4-Xyl | 8 | 10 |
| T-Gal | 8 | 9 |
| 3-Gal | 12 | 33 |
| 6-Gal | 11 | 7 |
| 3,6-Gal | 14 | 3 |
| 4-Man | 4 | 9 |
In summary, our analysis shows that β-GlcY precipitates material from the root cap cell walls of the two species that contains a considerable quantity of arabinogalactan protein. The material appeared to differ qualitatively between the species, particularly in its native electrophoretic mobility. Subsequently, we will refer to the β-GlcY precipitated fraction as “arabinogalactan protein,” even though we recognize that it may contain additional polysaccharides.
Effect of Arabinogalactan Proteins on the Behavior of A. euteiches Zoospores
The oomycete A. euteiches is responsible for common root rot, a destructive soil-borne disease of pea. In contrast, oomycetes causing such root diseases in B. napus are rare if not entirely absent. Therefore, we compared the arabinogalactan protein fractions isolated from the two species on the behavior of A. euteiches zoospores. Infection by oomycetes begins with zoospore attraction based on chemotaxis, followed by encystment (which corresponds to a loss of motility as the flagella are detached), and subsequently cyst germination to initiate an infection (Hardham and Suzaki, 1986; Tyler, 2002). We analyzed each in turn.
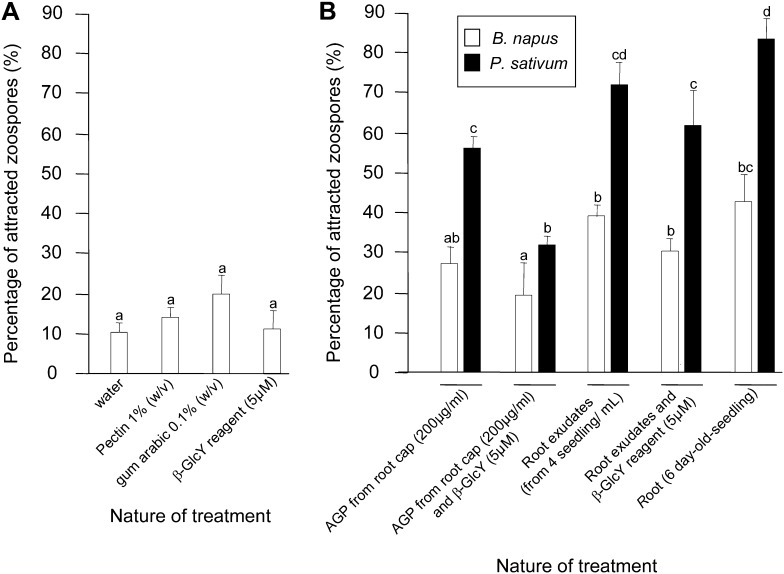
We began by assaying chemotaxis (Fig. 5). When placed in a drop of water and observed using the microscope, zoospores of A. euteiches move randomly without any preferential direction; in contrast, when an attractant is placed at one edge of the drop, the zoospores move more uniformly in the direction of the drop. We assayed this quantitatively as the percentage of zoospores moving toward the test compound over 4-h observation windows. In tests of purified compounds, neither commercial citrus pectin, gum arabic, nor β-GlcY reagent significantly attracted the zoospores (Fig. 5A). When an intact root or total root exudates were used as the test compound, zoospores were strongly attracted (Fig. 5B). The effect was much larger for pea compared with B. napus, and the effect of the whole root was moderately stronger than the whole extract. Interestingly, the purified arabinogalactan protein fraction was almost exactly as effective as the total root exudates. The chemotactic response was sensitive to arabinogalactan proteins at a concentration of 50 µg mL−1 or greater (Supplemental Fig. S7). To determine whether arabinogalactan proteins themselves were responsible for the attraction, the exudate and the fraction were each treated with β-GlcY reagent. Although the total exudate retained considerable activity after this treatment, the arabinogalactan protein fraction was rendered little more attractive than water. All of these trends were similar with respect to the large activity in pea and the modest activity of B. napus.
Figure 5.
Chemotactic response of A. euteiches zoospores to various substances over a period of 4 h. A, Experiments with standards. Gum arabic was used at 0.1% (w/v), citrus pectin at 1% (w/v), and β-GlcY at 5 µm. B, Experiments with plant-derived material. The root exudate represents material collected from four roots. Bars plot means ± se of three replicates, based on 200 zoospores per replicate. AGP, Arabinogalactan proteins.
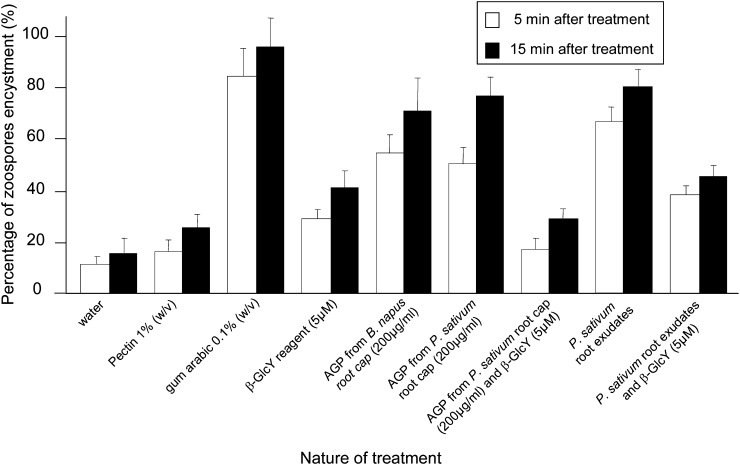
We next assayed the encystment of zoospores. When A. euteiches zoospores are suspended in water, approximately 10% of them encyst within 5 min, and the rest encyst slowly over several hours (Supplemental Fig. S8). Therefore, to assay the effect of test compounds, we quantified the percentage of encysted spores at 5 and 15 min after suspension (Fig. 6). Gum arabic has been previously reported to induce the encystment of A. euteiches (Deacon and Saxena, 1998) and was extremely effective under our conditions, causing nearly 100% encystment by 15 min; in contrast, treatment with citrus pectin was not distinguishable from water. Although not as effective as gum arabic, exudates from pea roots and the arabinogalactan protein fractions from both pea and B. napus strongly stimulated encystment to about the same extent. This is in contrast to chemotaxis, which was stimulated far more effectively by the arabinogalactan protein fraction from pea than from B. napus. The encystment assay was sensitive to arabinogalactan proteins at a concentration of 20 µg mL−1 or greater but was not dose dependent (Supplemental Fig. S9). The encystment activity was due to an appreciable extent to arabinogalactan protein, as indicated by treatment with β-GlcY being able to reduce the activity, particularly for the purified arabinogalactan protein, which was almost completely inactivated.
Figure 6.
Encystment of zoospores as a function of various treatments. Concentrations of substances used are as for Figure 5. Bars plot means ± se of three replicates, based on 200 zoospores per replicate. AGP, Arabinogalactan proteins.
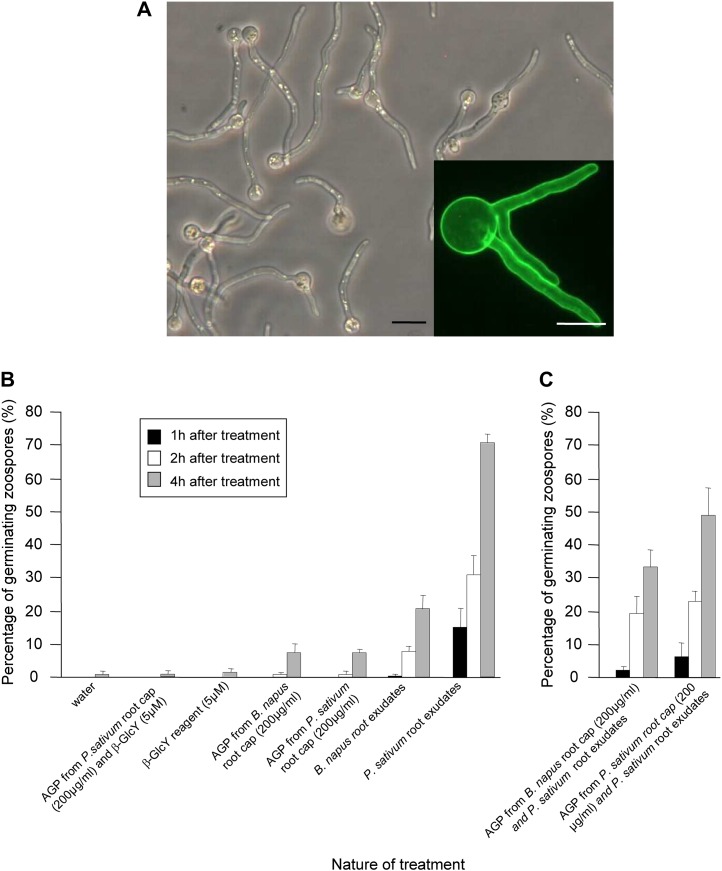
Finally, we assayed germination (Fig. 7; Supplemental Fig. S10). There was almost no germination in water, even up to 4 h after suspending spores. Root exudates strongly stimulated germination (Fig. 7A), and exudates from pea were approximately three times more effective than those from B. napus (Fig. 7B). However, in this assay, the arabinogalactan protein fraction was ineffective and, in fact, appeared to be inhibitory. This was seen in 24-h treatments, where there was modest germination of spores incubated in water but almost none for those incubated in arabinogalactan protein fractions from either species (data not shown). Additionally, the effectiveness of the pea root exudate was decreased when it was mixed with the arabinogalactan protein fraction, and the fraction from pea was more inhibitory than the fraction from B. napus (Fig. 7).
Figure 7.
Germination of encysted zoospores as a function of various treatments. Concentrations of substances are as for Figure 5. A, Micrographs showing the germination of zoospore cysts into pea root exudates. Bar = 10 µm. The inset shows a germinating zoospore stained with fluorescein isothiocyanate-wheat germ agglutinin. Bar = 5 µm. B, Effects of exudates and the arabinogalactan protein fraction on germination. C, Effects of pea exudate and arabinogalactan protein fractions given together. Zoospores were first encysted in the presence of arabinogalactan protein for 5 min before being additionally treated with pea root exudates for the indicated times. Note that the presence of arabinogalactan protein partially inhibits the stimulatory effect of the exudate on germination. Bars plot means ± se of three replicates, based on at least 200 zoospores per replicate. AGP, Arabinogalactan proteins. [See online article for color version of this figure.]
DISCUSSION
In this study, we investigated the structural characteristics of root cap arabinogalactan proteins from pea, a species that produces border cells (Hawes et al., 2000), and B. napus, a species that produces border-like cells (Driouich et al., 2007). We also assessed the effects of these proteoglycans on the development of A. euteiches, an oomycetous pathogen that causes root rot disease of pea.
Arabinogalactan proteins are heterogeneous proteoglycans characterized by a highly complex and diverse carbohydrate moiety. The glycomodule of arabinogalactan proteins varies between plant species and organs and is developmentally regulated during plant growth (Pennell et al., 1991; Dolan et al., 1995). An indication for the production of arabinogalactan proteins by pea roots has been made previously by several authors, and Ara and Gal have been reported as major sugar residues in root-secreted slime (Bacic et al., 1986; Moody et al., 1988; Knee et al., 2001; Xie et al., 2012). Furthermore, a pea border cell-specific β-galactosidase has been reported to be required for pea root growth, and although the endogenous substrate of this enzyme has not been determined, arabinogalactan proteins were proposed as candidates (Wen et al., 2008). Also, arabinogalactan protein epitopes were found to be highly expressed in Arabidopsis border-like cells (van Hengel and Roberts 2002; Vicré et al., 2005). We show that root caps as well as border cells and border-like cells contain significant amounts of arabinogalactan protein and that these proteoglycans have distinct compositional features depending on the species. Interestingly, our data support the conclusion that the structure of arabinogalactan proteins may differ quite significantly between the root cap (including root border cells) and the root proper. In addition to Ara and Gal residues, the isolated arabinogalactan protein fractions from both species contained substantial amounts of other sugars, including GalUA and Xyl, neither of which is a major constituent of arabinogalactan proteins. Our data suggest that these sugars are related to the occurrence of pectic polysaccharides, such as homogalacturonan, rhamnogalacturonan I, or xylogalacturonan, and we hypothesize that these polymers are part of pectin-arabinogalactan protein complexes present within the cell wall of root cap cells. Such an association between arabinogalactan proteins and pectin has been reported previously in cell wall extracts from carrot (Daucus carota) cell cultures, tap root, and hop (Humulus lupulus; Oosterveld et al., 2002; Immerzeel et al., 2006). Although homogalacturonan domains have been implicated in arabinogalactan protein-pectin interactions, the exact linkage type and the residues involved remain to be determined.
In addition to homogalacturonan, our arabinogalactan protein fractions seem to contain xylogalacturonan (Table III). Xylogalacturonan is a pectic polysaccharide that also has homogalacturonan domains. Xylogalacturonan epitopes are recognized by LM8 and have been reported previously to be abundant in root cap and border cells (Willats et al., 2004; Durand et al., 2009), but the function of this polysaccharide in such cells is not fully known, although it has been proposed to make cell walls resistant to degradation by pectinases secreted by pathogens (Jensen et al., 2008). Indeed, it has been reported that substitution of galacturonan domains with Xyl makes the polymer more resistant to digestion with endopolygalacturonases as compared with homogalacturonan (Jensen et al., 2008). Endopolygalacturonases are synthesized and secreted by various plant pathogens, presumably to degrade the host cell walls and to facilitate progression throughout host tissues (Cervone et al., 1987a, 1987b, 1989). Therefore, xylogalacturonan could play a role in plant defense by reducing cell wall degradation and restraining pathogen penetration within the tissues. Consistent with such a role is the observation that XGD1 gene expression, which encodes a xylogalacturonan-specific xylosyltransferase, increases significantly in response to infection of Arabidopsis leaves by the fungus Botrytis cinerea or the oomycete Phytophthora infestans (Jensen et al., 2008). Whether xylogalacturonan produced by pea root border cells contributes specifically to the protection of root apex from pathogen infection remains to be clearly demonstrated.
In considering pea and B. napus, we were struck by the fact that pea is susceptible to infection by A. euteiches but the crucifer is not, and we wondered whether, given their differences, the arabinogalactan proteins produced by the two species were relevant. To this end, we studied to what extent key events in the establishment of infection could be influenced by arabinogalactan proteins. To infect their host, A. euteiches first have to find the plant (i.e. attraction), have to lose their apparatus for free motility (encystment), and have to grow hyphae, which carry out the infection (germination). In principle, cell wall polysaccharides could be involved in any of these events.
As shown previously (Donaldson and Deacon, 1993), gum arabic promotes the encystment of certain oomycetes, but arabinogalactan had no effects on zoospores of Pythium species. However, as shown in our chemotaxis assay, arabinogalactan proteins attract zoospores, with those obtained from pea root caps being more effective than those obtained from B. napus. In fact, the isolated arabinogalactan protein fraction from pea attracted zoospores nearly as effectively as did a complete root exudate. This is unusual given the complex nature of exudates and the typical finding that the mixture strongly outperforms any of its components (Hardham and Suzaki, 1986). The distinct attractiveness of the arabinogalactan protein fractions to the zoospores adds a bioassay to the compositional and electrophoretic analyses demonstrating qualitative differences between the fractions from the two species. To our knowledge, this is the first demonstration that arabinogalactan proteins can influence zoospore chemotaxis.
Given the complex nature of the interaction between pathogen and host, it is striking that, on their own, arabinogalactan proteins appear to be such strong attractants. Certainly, if A. euteiches zoospores rely on arabinogalactan proteins to find a target root, then their preference for those secreted by pea could partly explain why B. napus suffers mildly if at all from this pathogen. In vivo, the primary site of infection in pea is the root elongation zone, whereas root cap and border cells remain free of pathogen at early stages of root infection (Cannesan et al., 2011). Only a few encysted zoospores were present in the vicinity of the root tip (but not attached to root cap/border cells). Pea root border cells are known to produce antimicrobial compounds including proteins (Wen et al., 2007), extracellular DNA (Wen et al., 2009), and phytoalexin (Cannesan et al., 2011) that are secreted into the external environment. Arabinogalactan proteins are also secreted by root cap and border-like cells and are present in root mucilage that forms a halo surrounding the root tip (Durand et al., 2009; Driouich et al., 2010). One can expect that these arabinogalactan proteins, together with antimicrobial compounds, at the front line between root and soil, provide a chemical barrier to A. euteiches. Arabinogalactan proteins could contribute to avoid root cap infection by inducing zoospore encystment at the periphery of the root tip. We believe that arabinogalactan proteins are part of these complex molecular interactions between roots and zoospores of A. euteiches. Our hypothesis is that arabinogalactan proteins produced by the root cap and secreted into exudates have a “decoy” function to attract and immobilize the oomycete A. euteiches. Root border cells from pea have been reported to attract (stimulatory effect) and immobilize (inhibitory effect) soil-borne nematodes (Hawes et al., 2000). Root border cells also act as a barrier to the pathogenic fungus Nectria hematococa, preventing root cap infection (Gunawardena and Hawes, 2002). Arabinogalactan proteins are potential candidates to be involved in such a mechanism, thereby contributing to root cap protection via the attraction and immobilization/inhibition of pathogen progression.
The idea that arabinogalactan protein mediates interactions between a root and a microorganism is not without precedent (Seifert and Roberts, 2007). For example, treatment with β-GlcY reagent causes a significant reduction in the colonization of Arabidopsis root cap and border-like cells by rhizobacteria (Vicré et al., 2005). Likewise, an Arabidopsis mutant, rat1, deficient in a gene encoding for an arabinogalactan protein, is resistant to infection and transformation by Agrobacterium tumefaciens, a phenotype implying that the missing arabinogalactan protein is somehow required for a productive infection (Zhu et al., 2003). Xie et al. (2012) demonstrated that arabinogalactan proteins from pea exudates promote the polar attachment of the symbiotic bacterium Rhizobium leguminosarum to glass. Root exudates from the Arabidopsis mutant rat1 failed to induce such in vitro attachment with R. leguminosarum. These data underline the complexity of the molecular and cellular events involved in root-microbe interaction and are consistent with the fact that one or more arabinogalactan proteins in root exudates participate in bacteria attachment.
To conclude, this study highlights a novel function for arabinogalactan proteins from root caps in root-zoospore interaction (i.e. chemotaxis). Arabinogalactan proteins appear to be part of complex interactions between roots and zoospores of A. euteiches, and as a consequence, the nature of these proteoglycans in root caps is likely to impact the relations between roots and microbes. It is generally assumed that the biological functions of arabinogalactan proteins rely on the diversity and complexity of the carbohydrate moiety. However, recent data suggest that both glycomodules and the protein backbone are involved in the root-microorganism interaction (Xie et al., 2012). Understanding the molecular mechanisms involved in such communication is critical to control the behavior of plant pathogens, and it is of interest to further explore the motifs involved in zoospore interaction.
MATERIALS AND METHODS
Plant Material
Brassica napus (var. Expert) and pea (Pisum sativum var. Normand) seeds were surface sterilized and sown onto Murashige and Skoog medium containing 1.2% (w/v) Bacto Agar supplemented with 3% (w/v) Suc (Durand et al., 2009). Plants were grown in continuous light (120 μE m−2 s−1) at 24°C, as described by Vicré et al. (2005). To avoid the roots penetrating the agar and the subsequent loss of border and border-like cells, plants were grown in vertically orientated petri dishes. Root caps, including border and border-like cells, were harvested from 5-d-old seedlings. Root exudates were collected by suspending the roots in distilled water (four roots per 2 mL) for 15 min (adapted from Bacic et al., 1986). After being vortexed, the roots were removed, and the mucilage suspension was centrifuged (2,000g, 2 min) to remove cell debris.
Histochemical Staining and Light Microscopy
Roots were mounted on microscope slides in a drop of water for examination using bright-field microscopy. Ruthenium red dye (Sigma) was used at 0.05% (w/v) in deionized water for 15 min to detect pectins. Roots were carefully washed in deionized water and observed using a bright-field microscope (Durand et al., 2009). Staining of β-glucans including cellulose was performed using Calcofluor White M2R (Sigma; 1 mg L−1) for 30 min in the dark (Andème-Onzighi et al., 2002). After being carefully washed in deionized water, roots were observed using a microscope equipped with UV fluorescence (excitation filter, 359 nm; barrier filter, 461 nm). Staining with 5 µm calcein-AM (Sigma) was performed as described by Vicré et al. (2005). Briefly, roots were stained for 3 h, carefully washed in deionized water, and observed using a microscope equipped with epifluorescence optics and standard filter sets. Images were acquired with a Leica DFC 300 FX camera.
Immunofluorescence Localization of Arabinogalactan Protein Epitopes
The monoclonal antibodies specific for arabinogalactan proteins used in this study were as follows: JIM13 (Yates et al., 1996), JIM8 (Knox et al., 1991), and JIM14 (Knox et al., 1991; Supplemental Table S1). Roots from 5-d-old seedlings were fixed for 40 min in 1% glutaraldehyde, 4% paraformaldehyde in 50 mm PIPES, and 1 mm CaCl2, pH 7, and immunolabeled according to Willats et al. (2001). Roots were washed in phosphate-buffered saline (PBS) containing 1% (w/v) bovine serum albumin (BSA) and then incubated overnight in JIM13 (1:5), JIM8 (1:5), or JIM14 (1:5) diluted in PBS-1% BSA containing 1:30 normal goat serum, as described previously by Vicré et al. (2005). Roots were carefully washed and incubated with anti-rat IgG (dilution, 1:50) coupled to fluorescein isothiocyanate (Sigma). After washing in PBS-1% BSA, roots were mounted in antifade agent (Citifluor; Agar Scientific) and examined using epifluorescence with a Zeiss Axioscope microscope. Control experiments, in which the primary antiserum was omitted, were performed under identical illumination and photographic exposure conditions.
Isolation of Cell Wall Material
Root caps (100 mg) were frozen with liquid nitrogen and ground into a fine powder. The powder was treated sequentially with boiling 90% ethanol (2 × 30 min), chloroform and 95% methanol (overnight), 100% methanol (4 h), and acetone (overnight) according to Aboughe-Angone et al. (2008). The cell wall residue was freeze dried and kept until use. To extract pectin, root caps were ground to a fine powder in 70% ethanol using a pestle and mortar and suspended in boiling ethanol for 1 h. The powder was subjected to 0.8% boiling ammonium oxalate for 1 h, and the oxalate extracts were lyophilized.
Arabinogalactan proteins were isolated by precipitation with the β-GlcY reagent (Biosupplies Australia) as described previously by Ding and Zhu (1997). Briefly, root caps (including border or border-like cells) were ground to a fine powder in liquid nitrogen and incubated in extraction buffer (50 mm Tris-HCl, pH 8.0, 10 mm EDTA, 0.1% β-mercaptoethanol, and 1% [v/v] Triton X-100) for 16 h at 4°C. The supernatant was mixed by the addition of 5 volumes of 95% ethanol overnight at 4°C, and the precipitate was suspended in 10 mL of 50 mm Tris-HCl, pH 8.0. After being dialyzed against deionized water and freeze dried, the material was dissolved in 1% NaCl and arabinogalactan proteins were precipitated by the addition of an equal volume of a solution of 2 mg mL−1 β-GlcY reagent in 1% NaCl (adapted from Gane et al., 1995). The mixture was left to precipitate overnight at 4°C, and the arabinogalactan protein-β-GlcY complex was collected by centrifugation at 9,000 rpm for 1 h. The pellet was washed three times with 1% NaCl to remove excess β-GlcY reagent and three times with methanol. The pellet was dried and dissolved in 500 µL of dimethyl sulfoxide and 10% sodium dithionite. Fractions containing arabinogalactan proteins were pooled and dialyzed overnight against deionized water at 4°C and lyophilized.
Monosaccharide Analysis
The sugar composition of noncellulosic cell wall polysaccharides was determined by gas chromatography analysis of trimethylsilyl methylglycoside derivatives according to York et al. (1985) and using inositol as an internal standard. Briefly, samples were hydrolyzed in 2 m trifluoroacetic acid for 2 h at 110°C and then heated in dry 1 m HCl in methanol at 80°C for 24 h for methanolysis. After evaporation of the methanol, the methyl glycosides were then converted into their trimethylsilyl derivatives by heating the samples for 30 min at 80°C in hexamethyldisilizane:trimethylchlorosilane:pyridine (3:1:9). After evaporation of the reagent, the samples were suspended in cyclohexane before being injected on a DB-1 column (Supelco) as described previously by Nguema-Ona et al. (2006). Chromatographic data were integrated with GC Star Workstation software (Varian), each surface being corrected according to its response factor.
Sugar Linkage Analysis
Arabinogalactan proteins were methylated using potassium methyl-sulfinyl-methanide (200 μL, 2.5 m in dimethyl sulfoxide) as described by Ishii et al. (1999). Briefly, the permethylated polysaccharides were hydrolyzed with 2.0 m trifluoroacetic acid at 121°C for 1 h, reduced with 1 m NaBD4 in 1 m NH4OH for 1 h at room temperature, and acetylated using acetic anhydride at 121°C for 3 h. The partially methylated alditol acetates were analyzed by gas chromatography coupled to electron-impact mass spectrometry using SP-2330 columns.
Fractionation of Arabinogalactan Proteins by Anion-Exchange Chromatography
Anion-exchange chromatography was performed to separate arabinogalactan proteins from contaminating pectins in the β-GlcY reagent precipitate according to the protocol of Du et al. (1994) and Sims and Bacic (1995). β-GlcY reagent precipitate and pectin (extracted with 0.8% ammonium oxalate as described by Dardelle et al. [2010]) from pea root cap were dialyzed against 0.05 m Tris-HCl, pH 8, at 4°C. The arabinogalactan proteins or pectin extracts were loaded onto a Q Sepharose Fast Flow gel column (3 mL) equilibrated with 0.05 m Tris-HCl, pH 8. The column was washed to eliminate unbound material with 0.05 m Tris, pH 8. Bound material was eluted by applying a gradient of NaCl (from 0.05 to 0.5 m). Fractions were pooled, concentrated, dialyzed using centrifugal filter units (Amicon Ultra-10K-Millipore), and freeze dried (adapted from Sims and Bacic, 1995). Samples were transferred to a nitrocellulose membrane, and the presence of arabinogalactan proteins and pectins was monitored using β-GlcY reagent staining and dot blotting (Andème-Onzighi et al., 2000; Vicré et al., 2004). The primary antibodies used were as follows: JIM13 (1:50), JIM5 (1:100), LM16 (1:50), LM13 (1:50), and 2F4 (1:50).
Immunoblots and Electrophoresis
For SDS-PAGE analysis, arabinogalactan proteins were separated on 8% polyacrylamide minigels (Bio-Rad) and blotted as described previously (Willats and Knox, 1996). For dot-blot detection of arabinogalactan proteins, 25-µg samples were blotted on nitrocellulose membranes (Whatman; protan BA 83 nitrocellulose, 0.2 µm) and were blocked with 5% (w/v) low-fat dried milk in Tris-buffered saline (TBS; 20 mm Tris, 500 mm NaCl, pH 7.5). Samples were incubated with primary antibody (1:10) in TBS for 2 h. After four washes with TBS containing 1% Tween 20, blots were incubated for 2 h with anti-rat IgG peroxidase conjugate (Sigma) diluted 1:50 in TBS containing 5% low fat milk. After four washes with TBS containing 1% Tween 20 followed by one wash with TBS, enzyme activity was visualized with 30% hydrogen peroxide (diluted in TBS) and methanol 4-chloronaphthol horseradish peroxidase color reagent. The primary antibodies used were JIM5, JIM7, LM6, LM8, JIM13, JIM8, and JIM14 (obtained from PlantProbes).
For the radial diffusion assay, a 1% (w/v) agarose gel containing 0.15 m NaCl, 0.02% (w/v) NaNO3, and 10 µg mL−1 β-GlcY reagent was prepared according to van Holst and Clarke (1986). The wells were filled with arabinogalactan proteins (1 µg) and incubated overnight at room temperature. Gum arabic from acacia (Fisher Scientific) was used as a standard. For rocket and cross electrophoresis, gels were made in 1% agarose containing 90 mm Tris (pH 8.3), 90 mm boric acid, 2 mm EDTA, and 20 µg mL−1 β-GlcY reagent. Aliquots of 0.5 µg of arabinogalactan proteins were loaded in each well and run for 16 h at 10 mA, and the gels were rinsed with 2% (w/v) NaCl. The quantity of arabinogalactan proteins was estimated in relation to the peak area of gum arabic (three to four gels were used for quantification). Cross electrophoresis was performed according to van Holst and Clarke (1986). Samples were run in the first dimension as described above, and then the lanes were cut out and run in the second dimension on a gel with 30 µg mL−1 β-GlcY reagent for 16 h at 5 mA (Ding and Zhu, 1997; Girault et al., 2000).
Zoospore Production and Chemotaxis
Aphanomyces euteiches isolate RB84 was used. This isolate is the French reference strain for pea, previously described as being highly virulent, and was kindly provided by B. Tivoli (INRA Rennes). Zoospores were produced according to Moussart et al. (2001) and used at a concentration of about 105 zoospores mL−1 water. Zoospores could be observed microscopically moving freely on the surface of the glass slide. Chemotaxis assays were adapted from Zhao et al. (2000). Briefly, a droplet of zoospores (20 µL, approximately 500 spores) was placed on the surface of a glass slide, the test compound was added to the opposite side of the slide, and the percentage of attracted zoospores was determined over a period of 4 h. In order to check for the specificity of arabinogalactan proteins, control experiments were carried out using arabinogalactan protein fractions preincubated overnight with β-GlcY reagent. Gum arabic was used at 0.1% (w/v), citrus pectin at 1% (w/v), and β-GlcY at 5 µm. Three replicate slides were included for each treatment, and the whole test was repeated three times.
Assays for germination and encystment were based on those described previously by Deacon and Saxena (1998). Briefly, a 20-µL suspension of zoospores was added to a 20-µL droplet of a test substance on a slide and incubated in the dark for 1 to 4 h, observed with bright-field microscopy, and the percentage of germinated or encysted spores was counted manually. In some experiments, citrus pectin (Sigma) was used for comparison. Observations were made with a 40× objective, and images were acquired with a Leica DFC 300 FX camera. Data are means of three replicates based on at least 200 zoospores per replicate.
Statistical Analysis
Statistical significance was calculated by using one-way ANOVA and the Newman-Keuls test, and P < 0.05 was accepted as significant.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Bright-field micrographs of B. napus root apex grown through soil.
Supplemental Figure S2. Border-like cells from B. napus root stained with calcein-AM.
Supplemental Figure S3. Immunodot staining of arabinogalactan proteins using β-GlcY reagent.
Supplemental Figure S4. Immunofluorescence controls.
Supplemental Figure S5. Anion-exchange chromatography of β-GlcY precipitate or pectin extract from pea root caps.
Supplemental Figure S6. Western-blot analysis of selected fractions extracted from entire roots and root caps.
Supplemental Figure S7. Chemotactic response of A. euteiches zoospores to various concentrations of arabinogalactan proteins over a period of 4 h.
Supplemental Figure S8. Time course of encystment and germination responses of A. euteiches zoospores in the presence of water.
Supplemental Figure S9. Encystment of zoospores as a function of various concentrations of arabinogalactan proteins.
Supplemental Figure S10. Germination of encysted zoospores as a function of various concentrations of arabinogalactan proteins.
Supplemental Table S1. List of monoclonal antibodies.
Supplemental Table S2. Monosaccharide composition of β-GlcY precipitate obtained from pea roots and pea root caps including border cells.
Supplementary Material
Acknowledgments
We thank Christophe Rihouey (University of Rouen) and Sophie Aboughe-Angone (IPHAMETRA-CENAREST-Gabon) for excellent technical assistance during the preparation of samples for gas chromatography and Sophie Bernard (University of Rouen) for help and advice throughout this work. Special thanks are due to Prof. T. Baskin (University of Massachusetts) for critical reading and thoughtful comments on the manuscript. We also thank Rosemary Newton (Kew Gardens) for critical reading of the manuscript and Prof. J.P. Knox (University of Leeds) for providing some of the monoclonal antibodies used in this study.
Glossary
- β-GlcY
β-glucosyl Yariv
- PBS
phosphate-buffered saline
- BSA
bovine serum albumin
- TBS
Tris-buffered saline
References
- Aboughe-Angone S, Nguema-Ona E, Ghosh P, Lerouge P, Ishii T, Ray B, Driouich A. (2008) Cell wall carbohydrates from fruit pulp of Argania spinosa: structural analysis of pectin and xyloglucan polysaccharides. Carbohydr Res 343: 67–72 [DOI] [PubMed] [Google Scholar]
- Andème-Onzighi C, Lhuissier F, Vicré M, Yamada H, Driouich A. (2000) A (1→3,6)-β-d-galactosyl epitope containing uronic acids associated with bioactive pectins occurs in discrete cell wall domains in hypocotyl and root tissues of flax seedlings. Histochem Cell Biol 113: 61–70 [DOI] [PubMed] [Google Scholar]
- Andème-Onzighi C, Sivaguru M, Judy-March J, Baskin TI, Driouich A. (2002) The reb1-1 mutation of Arabidopsis alters the morphology of trichoblasts, the expression of arabinogalactan-proteins and the organization of cortical microtubules. Planta 215: 949–958 [DOI] [PubMed] [Google Scholar]
- Bacic A, Moody SF, Clarke AE. (1986) Structural analysis of secreted root slime from maize (Zea mays L.). Plant Physiol 80: 771–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow PW. (2003) The root cap: cell dynamics, cell differentiation and cap function. J Plant Growth Regul 21: 261–286 [Google Scholar]
- Borsics T, Webb D, Andeme-Ondzighi C, Staehelin LA, Christopher DA. (2007) The cyclic nucleotide-gated calmodulin-binding channel AtCNGC10 localizes to the plasma membrane and influences numerous growth responses and starch accumulation in Arabidopsis thaliana. Planta 225: 563–573 [DOI] [PubMed] [Google Scholar]
- Brigham LA, Woo HH, Wen F, Hawes MC. (1998) Meristem-specific suppression of mitosis and a global switch in gene expression in the root cap of pea by endogenous signals. Plant Physiol 118: 1223–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannesan MA, Gangneux C, Lanoue A, Giron D, Laval K, Hawes M, Driouich A, Vicré-Gibouin M. (2011) Association between border cell responses and localized root infection by pathogenic Aphanomyces euteiches. Ann Bot (Lond) 108: 459–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervone F, De Lorenzo G, Degrà L, Salvi G. (1987a) Elicitation of necrosis in Vigna unguiculata Walp. by homogeneous Aspergillus niger endo-polygalacturonase and by α-d-galacturonate oligomers. Plant Physiol 85: 626–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervone F, De Lorenzo G, Degrà L, Salvi G, Bergami M. (1987b) Purification and characterization of a polygalacturonase-inhibiting protein from Phaseolus vulgaris L. Plant Physiol 85: 631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervone F, Hahn MG, De Lorenzo G, Darvill A, Albersheim P. (1989) Host-pathogen interactions. XXXIII. A plant protein converts a fungal pathogenesis factor into an elicitor of plant defense responses. Plant Physiol 90: 542–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, May B, Kawata EE, Gu Q, Wu HM. (1993) Characterization of cDNAs for stylar transmitting tissue-specific proline-rich proteins in tobacco. Plant J 3: 151–160 [PubMed] [Google Scholar]
- Clausen MH, Willats WGT, Knox JP. (2003) Synthetic methyl hexagalacturonate hapten inhibitors of anti-homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydr Res 338: 1797–1800 [DOI] [PubMed] [Google Scholar]
- Dardelle F, Lehner A, Ramdani Y, Bardor M, Lerouge P, Driouich A, Mollet JC. (2010) Biochemical and immunocytological characterizations of Arabidopsis pollen tube cell wall. Plant Physiol 153: 1563–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon JW, Saxena G. (1998) Germination triggers of zoospore cysts of Aphanomyces euteiches and Phytophthora parasitica. Mycol Res 102: 33–41 [Google Scholar]
- Ding L, Zhu JK. (1997) A role for arabinogalactan-proteins in root epidermal cell expansion. Planta 203: 289–294 [DOI] [PubMed] [Google Scholar]
- Dolan L, Linstead P, Roberts K. (1995) An AGP epitope distinguishes a central metaxylem initial from other vascular initials in the Arabidopsis root. Protoplasma 189: 149–155 [Google Scholar]
- Donaldson SP, Deacon S. (1993) Effects of amino acids and sugars on zoospore taxis, encystment and cyst germination in Pythium aphanidermatum (Edson) Fitzp., P. catenulatum Matthews and P. dissotocum Drechs. New Phytol 123: 289–295 [Google Scholar]
- Driouich A, Baskin TI. (2008) Intercourse between cell wall and cytoplasm exemplified by arabinogalactan proteins and cortical microtubules. Am J Bot 95: 1491–1497 [DOI] [PubMed] [Google Scholar]
- Driouich A, Durand C, Cannesan MA, Percoco G, Vicré-Gibouin M. (2010) Border cells versus border-like cells: are they alike? J Exp Bot 61: 3827–3831 [DOI] [PubMed] [Google Scholar]
- Driouich A, Durand C, Vicré-Gibouin M. (2007) Formation and separation of root border cells. Trends Plant Sci 12: 14–19 [DOI] [PubMed] [Google Scholar]
- Du H, Simpson RJ, Moritz RL, Clarke AE, Bacic A. (1994) Isolation of the protein backbone of an arabinogalactan-protein from the styles of Nicotiana alata and characterization of a corresponding cDNA. Plant Cell 6: 1643–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand C, Vicré-Gibouin M, Follet-Gueye ML, Duponchel L, Moreau M, Lerouge P, Driouich A. (2009) The organization pattern of root border-like cells of Arabidopsis is dependent on cell wall homogalacturonan. Plant Physiol 150: 1411–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis M, Egelund J, Schultz CJ, Bacic A. (2010) Arabinogalactan-proteins: key regulators at the cell surface? Plant Physiol 153: 403–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gane AM, Craik D, Munro SLA, Howlett GJ, Clarke AE, Bacic A. (1995) Structural analysis of the carbohydrate moiety of arabinogalactan-proteins from stigmas and styles of Nicotiana alata. Carbohydr Res 277: 67–85 [DOI] [PubMed] [Google Scholar]
- Gaspar Y, Johnson KL, McKenna JA, Bacic A, Schultz CJ. (2001) The complex structures of arabinogalactan-proteins and the journey towards understanding function. Plant Mol Biol 47: 161–176 [PubMed] [Google Scholar]
- Girault R, His I, Andeme-Onzighi C, Driouich A, Morvan C. (2000) Identification and partial characterization of proteins and proteoglycans encrusting the secondary cell walls of flax fibres. Planta 211: 256–264 [DOI] [PubMed] [Google Scholar]
- Gunawardena U, Hawes MC. (2002) Role of border cells in localized root infection by pathogenic fungi. Mol Plant Microbe Interact 15: 1128–1136 [DOI] [PubMed] [Google Scholar]
- Hardham AR, Suzaki E. (1986) Encystment of zoospores of the fungus Phytophthora cinnamomi is induced by specific lectin and monoclonal antibody binding to the cell surface. Protoplasma 133: 165–173 [Google Scholar]
- Hawes MC, Bengough G, Cassab G, Ponce G. (2003) Root caps and rhizosphere. J Plant Growth Regul 21: 352–367 [Google Scholar]
- Hawes MC, Gunawardena U, Miyasaka S, Zhao X. (2000) The role of root border cells in plant defense. Trends Plant Sci 5: 128–133 [DOI] [PubMed] [Google Scholar]
- Hawes MC, Lin HJ. (1990) Correlation of pectolytic enzyme activity with the programmed release of cells from root caps of pea (Pisum sativum). Plant Physiol 94: 1855–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immerzeel P, Eppink MM, De Vries SC, Schols HA, Voragen AGJ. (2006) Carrot arabinogalactan proteins are interlinked with pectins. Physiol Plant 128: 18–28 [Google Scholar]
- Ishii T, Matsunaga T, Pellerin P, O’Neill MA, Darvill A, Albersheim P. (1999) The plant cell wall polysaccharide rhamnogalacturonan II self-assembles into a covalently cross-linked dimer. J Biol Chem 274: 13098–13104 [DOI] [PubMed] [Google Scholar]
- Jauh GH, Lord EM. (1996) Localization of pectins and arabinogalactan-proteins in lily (Lilium longiflorum L.) pollen tube and style, and their possible roles in pollination. Planta 199: 251–261 [Google Scholar]
- Jensen JK, Sørensen SO, Harholt J, Geshi N, Sakuragi Y, Møller I, Zandleven J, Bernal AJ, Jensen NB, Sørensen C, et al. (2008) Identification of a xylogalacturonan xylosyltransferase involved in pectin biosynthesis in Arabidopsis. Plant Cell 20: 1289–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knee EM, Gong FC, Gao M, Teplitski M, Jones AR, Foxworthy A, Mort AJ, Bauer WD. (2001) Root mucilage from pea and its utilization by rhizosphere bacteria as a sole carbon source. Mol Plant Microbe Interact 14: 775–784 [DOI] [PubMed] [Google Scholar]
- Knox JP, Linstead PJ, King J, Cooper C, Roberts K. (1990) Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181: 512–521 [DOI] [PubMed] [Google Scholar]
- Knox JP, Linstead PJ, Peart J, Cooper C, Roberts K. (1991) Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. Plant J 1: 317–326 [DOI] [PubMed] [Google Scholar]
- Knox OGG, Gupta VVSR, Nehl DB, Stiller WN. (2007) Constitutive expression of Cry proteins in roots and border cells of transgenic cotton. Euphytica 154: 83–90 [Google Scholar]
- Lu H, Chen M, Showalter AM. (2001) Developmental expression and perturbation of arabinogalactan-proteins during seed germination and seedling growth in tomato. Physiol Plant 112: 442–450 [DOI] [PubMed] [Google Scholar]
- Ma W, Muthreich N, Liao C, Franz-Wachtel M, Schütz W, Zhang F, Hochholdinger F, Li C. (2010) The mucilage proteome of maize (Zea mays L.) primary roots. J Proteome Res 9: 2968–2976 [DOI] [PubMed] [Google Scholar]
- Majewska-Sawka A, Nothnagel EA. (2000) The multiple roles of arabinogalactan proteins in plant development. Plant Physiol 122: 3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer JB, Bacic A, Pereira-Netto AB, Donatti L, Zawadzki-Baggio SF, Pettolino FA. (2010) Arabinogalactan-proteins from cell suspension cultures of Araucaria angustifolia. Phytochemistry 71: 1400–1409 [DOI] [PubMed] [Google Scholar]
- Moody SF, Clarke AE, Bacic A. (1988) Structural analysis of secreted slime from wheat and cowpea roots. Phytochemistry 27: 2857–2861 [Google Scholar]
- Moussart A, Wicker E, Duparque M, Rouxel F. (2001) Development of an efficient screening test for pea resistance to Aphanomyces euteiches. In Proceedings of the 4th European Conference on Grain Legumes, July 8–12, 2001, Krakow, Poland. European Association for Grain Legume Research, pp 272–273
- Nguema-Ona E, Andème-Onzighi C, Aboughe-Angone S, Bardor M, Ishii T, Lerouge P, Driouich A. (2006) The reb1-1 mutation of Arabidopsis: effect on the structure and localization of galactose-containing cell wall polysaccharides. Plant Physiol 140: 1406–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguema-Ona E, Bannigan A, Chevalier L, Baskin TI, Driouich A. (2007) Disruption of arabinogalactan proteins disorganizes cortical microtubules in the root of Arabidopsis thaliana. Plant J 52: 240–251 [DOI] [PubMed] [Google Scholar]
- Oosterveld A, Voragen AGP, Schols HA. (2002) Characterization of hop pectins show the presence of arabinogalactan-protein. Carbohydr Polym 49: 407–413 [Google Scholar]
- Pennell RI, Janniche L, Kjellbom P, Scofield GN, Peart JM, Roberts K. (1991) Developmental regulation of a plasma membrane arabinogalactan protein epitope in oilseed rape flowers. Plant Cell 3: 1317–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell RI, Knox JP, Scofield GN, Selvendran RR, Roberts K. (1989) A family of abundant plasma membrane-associated glycoproteins related to the arabinogalactan proteins is unique to flowering plants. J Cell Biol 108: 1967–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce G, Rasgado F, Cassab GI. (2008) How amyloplasts, water deficit and root tropisms interact? Plant Signal Behav 3: 460–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper ZA. (2011) Extraction and detection of arabinogalactan proteins. Methods Mol Biol 715: 245–254 [DOI] [PubMed] [Google Scholar]
- Roy S, Mittra B, Sharma S, Das TK, Babu CR. (2002) Detection of root mucilage using an anti-fucose antibody. Ann Bot (Lond) 89: 293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šamaj J, Šamajová O, Peters M, Baluška F, Lichtscheidl I, Knox JP, Volkmann D. (2000) Immunolocalization of LM2 arabinogalactan protein epitope associated with endomembranes of plant cells. Protoplasma 212: 186–196 [Google Scholar]
- Schildknecht PHPA, de M Castro M, Vidal BC. (2004) Histochemical analysis of the root epidermal mucilage in maize and wheat. Can J Bot 82: 1419–1428 [Google Scholar]
- Seifert GJ, Roberts K. (2007) The biology of arabinogalactan proteins. Annu Rev Plant Biol 58: 137–161 [DOI] [PubMed] [Google Scholar]
- Sims IM, Bacic A. (1995) Extracellular polysaccharides from suspension cultures of Nicotiana plumbaginifolia. Phytochemistry 38: 1397–1405 [Google Scholar]
- Thude S, Classen B. (2005) High molecular weight constituents from roots of Echinacea pallida: an arabinogalactan-protein and an arabinan. Phytochemistry 66: 1026–1032 [DOI] [PubMed] [Google Scholar]
- Tryfona T, Liang HC, Kotake T, Kaneko S, Marsh J, Ichinose H, Lovegrove A, Tsumuraya Y, Shewry PR, Stephens E, et al. (2010) Carbohydrate structural analysis of wheat flour arabinogalactan protein. Carbohydr Res 345: 2648–2656 [DOI] [PubMed] [Google Scholar]
- Tyler BM. (2002) Molecular basis of recognition between Phytophthora pathogens and their hosts. Annu Rev Phytopathol 40: 137–167 [DOI] [PubMed] [Google Scholar]
- van Hengel AJ, Roberts K. (2002) Fucosylated arabinogalactan-proteins are required for full root cell elongation in Arabidopsis. Plant J 32: 105–113 [DOI] [PubMed] [Google Scholar]
- van Holst GJ, Clarke AE. (1986) Organ-specific arabinogalactan-proteins of Lycopersicon peruvianum (Mill) demonstrated by crossed electrophoresis. Plant Physiol 80: 786–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicré M, Jauneau A, Knox JP, Driouich A. (1998) Immunolocalisation of β(1-6) and β(1-4) galactans in the cell wall and Golgi stacks of flax roots. Protoplasma 203: 26–34 [Google Scholar]
- Vicré M, Lerouxel O, Farrant JM, Lerouge P, Driouich A. (2004) Composition and desiccation-induced alterations of the cell wall in the resurrection plant Craterostigma wilmsii. Physiol Plant 120: 229–239 [DOI] [PubMed] [Google Scholar]
- Vicré M, Santaella C, Blanchet S, Gateau A, Driouich A. (2005) Root border-like cells of Arabidopsis: microscopical characterization and role in the interaction with rhizobacteria. Plant Physiol 138: 998–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen F, Celoy R, Price I, Ebolo JJ, Hawes MC. (2008) Identification and characterization of a rhizosphere β-galactosidase from Pisum sativum L. Plant Soil 304: 133–144 [Google Scholar]
- Wen F, VanEtten HD, Tsaprailis G, Hawes MC. (2007) Extracellular proteins in pea root tip and border cell exudates. Plant Physiol 143: 773–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen F, White GJ, VanEtten HD, Xiong Z, Hawes MC. (2009) Extracellular DNA is required for root tip resistance to fungal infection. Plant Physiol 151: 820–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats WGT, Knox JP. (1996) A role for arabinogalactan-proteins in plant cell expansion: evidence from studies on the interaction of β-glucosyl Yariv reagent with seedlings of Arabidopsis thaliana. Plant J 9: 919–925 [DOI] [PubMed] [Google Scholar]
- Willats WGT, Limberg G, Buchholt HC, van Alebeek GJ, Benen J, Christensen TM, Visser J, Voragen A, Mikkelsen JD, Knox JP. (2000) Analysis of pectic epitopes recognised by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides, and enzymatic degradation. Carbohydr Res 327: 309–320 [DOI] [PubMed] [Google Scholar]
- Willats WGT, Marcus SE, Knox JP. (1998) Generation of monoclonal antibody specific to (1→5)-α-L-arabinan. Carbohydr Res 308: 149–152 [DOI] [PubMed] [Google Scholar]
- Willats WGT, McCartney L, Knox JP. (2001) In-situ analysis of pectic polysaccharides in seed mucilage and at the root surface of Arabidopsis thaliana. Planta 213: 37–44 [DOI] [PubMed] [Google Scholar]
- Willats WGT, McCartney L, Steele-King CG, Marcus SE, Mort A, Huisman M, van Alebeek GJ, Schols HA, Voragen AGJ, Le Goff A, et al. (2004) A xylogalacturonan epitope is specifically associated with plant cell detachment. Planta 218: 673–681 [DOI] [PubMed] [Google Scholar]
- Xie F, Williams A, Edwards A, Downie JA. (2012) A plant arabinogalactan-like glycoprotein promotes a novel type of polar surface attachment by Rhizobium leguminosarum. Mol Plant Microbe Interact 25: 250–258 [DOI] [PubMed] [Google Scholar]
- Yariv J, Lis H, Katchalski E. (1967) Precipitation of arabic acid and some seed polysaccharides by glycosylphenylazo dyes. Biochem J 105: 1C–2C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates EA, Valdor JF, Haslam SM, Morris HR, Dell A, Mackie W, Knox JP. (1996) Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology 6: 131–139 [DOI] [PubMed] [Google Scholar]
- York WS, Darvill A, O’Neill M, Stevenson T, Albersheim P. (1985) Isolation and characterization of plant cell walls and cell wall components. Methods Enzymol 118: 3–40 [Google Scholar]
- Zhao X, Schmitt M, Hawes MC. (2000) Species-dependent effects of border cell and root tip exudates on nematode behavior. Phytopathology 90: 1239–1245 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Nam J, Humara JM, Mysore KS, Lee LY, Cao H, Valentine L, Li J, Kaiser AD, Kopecky AL, et al. (2003) Identification of Arabidopsis rat mutants. Plant Physiol 132: 494–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.