Abstract
Carotenes and their oxygenated derivatives, the xanthophylls, are structural determinants in both photosystems (PS) I and II. They bind and stabilize photosynthetic complexes, increase the light-harvesting capacity of chlorophyll-binding proteins, and have a major role in chloroplast photoprotection. Localization of carotenoid species within each PS is highly conserved: Core complexes bind carotenes, whereas peripheral light-harvesting systems bind xanthophylls. The specific functional role of each xanthophyll species has been recently described by genetic dissection, however the in vivo role of carotenes has not been similarly defined. Here, we have analyzed the function of carotenes in photosynthesis and photoprotection, distinct from that of xanthophylls, by characterizing the suppressor of zeaxanthin-less (szl) mutant of Arabidopsis (Arabidopsis thaliana) which, due to the decreased activity of the lycopene-β-cyclase, shows a lower carotene content than wild-type plants. When grown at room temperature, mutant plants showed a lower content in PSI light-harvesting complex I complex than the wild type, and a reduced capacity for chlorophyll fluorescence quenching, the rapidly reversible component of nonphotochemical quenching. When exposed to high light at chilling temperature, szl1 plants showed stronger photoxidation than wild-type plants. Both PSI and PSII from szl1 were similarly depleted in carotenes and yet PSI activity was more sensitive to light stress than PSII as shown by the stronger photoinhibition of PSI and increased rate of singlet oxygen release from isolated PSI light-harvesting complex I complexes of szl1 compared with the wild type. We conclude that carotene depletion in the core complexes impairs photoprotection of both PS under high light at chilling temperature, with PSI being far more affected than PSII.
Carotenoids are polyisoprenoid pigments that are ubiquitously distributed among oxygenic photosynthetic organisms, from cyanobacteria to land plants (Britton et al., 2004). A molecular feature of these C40 molecules is a conjugated double-bond system, which is responsible for the strong absorption in the visible region of the spectrum and the antioxidant capacity of these pigments. In photosynthetic tissues of higher plants, carotenoids are mainly accumulated in the thylakoid membranes. Carotenoid composition is remarkably conserved among plant taxa, consisting of the hydrocarbons α- and β-carotene (accounting for one-fourth of total carotenoids) and their oxygenated derivatives called xanthophylls. The latter group include the β,ε-xanthophyll lutein, the most abundant plant carotenoid, and the β,β-xanthophylls violaxanthin, neoxanthin, antheraxanthin, and zeaxanthin (Demmig-Adams and Adams, 1992), whose biosynthesis is tightly controlled during plant acclimation to stressful conditions (Hirschberg, 2001; Alboresi et al., 2011). The carotenoid biosynthesis pathway from phytoene include a series of four desaturation reactions, leading to the formation of the C40 compound lycopene, which is then cyclized at both ends by β-cyclase (LCYB) to produce β-carotene. Alternatively, β-cyclization occurs at a single end, the other being processed by ε-cyclase (LUT2) to produce α-carotene. Thus, there exist two distinct branches in plant carotenoid biosynthesis, one leading to synthesis of β,β-hydroxylated xanthophylls from β-carotene, and the other to lutein from α-carotene. The hydroxylation of either α- or β-carotene is catalyzed by multiple enzymes with overlapping substrate specificity belonging to two different classes: CHY1 and CHY2 (ferredoxin-dependent diiron oxygenases) catalyze β-ring hydroxylation, while LUT1 and LUT5 (cytochromes P450) are involved in the hydroxylation of both ε-ring and β-ring of α-carotene (Tian et al., 2003, 2004; Fiore et al., 2006; Kim and DellaPenna, 2006; Kim et al., 2009). Complete lack of xanthophylls in the chy1chy2lut1lut5 mutant (Kim et al., 2009) confirms that these four genes encode the complete enzymatic complement catalyzing carotene hydroxylation in Arabidopsis (Arabidopsis thaliana).
The distribution of each carotenoid species between the different components of the photosynthetic machinery is the basis for their specific functions. Thus, a minor fraction is free in the lipid phase of thylakoids where it serves as antioxidant (Havaux et al., 2004) and modulates the fluidity of the lipid bilayer (Gruszecki and Strzałka, 2005). However, carotenoids are located mostly within specific binding sites of pigment-protein complexes, contributing to both light harvesting and photoprotection of these PS subunits. β-Carotene is bound to reaction center subunits of both PSI and PSII, whereas xanthophylls are bound to peripheral light-harvesting complexes (Lhc) subunits that comprise the antenna system. Core complexes of PSI and PSII bind, respectively, 15 and 11 β-carotenes (Amunts et al., 2010; Umena et al., 2011). Lhcb proteins, constituting the antenna system of PSII, bind lutein, violaxanthin, and neoxanthin at four distinct binding sites (Liu et al., 2004). Zeaxanthin, upon its synthesis from violaxanthin under excess light, can also bind to these antenna components in exchange for violaxanthin, in site V1 in the case of the major LHCII trimeric complex (Caffarri et al., 2001) or in site L2 in the case of the monomeric subunits CP26 (Lhcb5), CP29 (Lhcb4), and CP24 (Lhcb6; Croce et al., 2003; Betterle et al., 2010; Pan et al., 2011). PSI antenna proteins (Lhca1–4) and CP24 lack neoxanthin (Jensen et al., 2007; Passarini et al., 2009).
Besides their role as structural determinants, carotenoids are involved in photoprotective mechanisms. Indeed, they have coevolved with oxygenic photosynthesis to avoid photooxidative damage derived from photosensitizing action of porphyrins and reduction of oxygen by univalent photosynthetic electron transporters. This is particularly important under rapid fluctuations in light intensity, when photochemical quenching activity is exceeded, leading to photoinhibition (Külheim et al., 2002). Carotenoids protect chloroplasts from excess light by (1) modulating the nonradiative dissipation of excess excitation energy (Niyogi et al., 1998; Dall’Osto et al., 2005), and (2) by mediating direct quenching of chlorophyll (Chl) triplets (3Chl*) or (3) by scavenging the reactive oxygen species (ROS) generated during photosynthesis (Niyogi, 2000; Dall’Osto et al., 2005, 2007b; Havaux et al., 2007). The essential role of carotenoids in photoprotection was evidenced by the phenotype of carotenoid-less plants, unable to perform photoautotrophic growth (Herrin et al., 1992; Trebst and Depka, 1997; Kim et al., 2009).
The roles of xanthophylls have been subjected to dissection through genetic analysis. Several mutants with altered xanthophyll composition are impaired in photoprotection, implying that these pigments have a key role in plant fitness. Lutein depletion in lut2 plants resulted in higher photosensitivity in high light (HL) with respect to the wild type (Pogson et al., 1996), due to impaired 3Chl* quenching within LHCII (Dall’Osto et al., 2006). Lack of both lutein and zeaxanthin further enhances the photodamage in both HL-treated plants and green algae (Niyogi et al., 1997, 2001; Gilmore, 2001; Baroli et al., 2003; Dall’Osto et al., 2006). Mutation of three β-carotene hydroxylases CHY1, CHY2, and LUT5 in Arabidopsis, yielded a plant with lutein as the only xanthophyll, revealing unprecedented photosensitivity (Fiore et al., 2006; Dall’Osto et al., 2007b; Kim et al., 2009) and implying that singlet oxygen (1O2) scavenging is a constitutive component of photoprotection in antenna proteins together with Chl triplet quenching. Finally, a neoxanthin-less mutant showed enhanced sensitivity to superoxide anion (Dall’Osto et al., 2007a).
While xanthophyll biosynthesis mutants of Arabidopsis and Chlamydomonas have revealed distinct photoprotective roles in vivo for xanthophyll species, until recently no photoautotrophic mutant showing a selective β-carotene loss has been described, to our knowledge. Therefore, the role of β-carotene has been more difficult to identify. Early reports showed that isolated PSII reaction centers form 1O2 with high yield, thus causing photooxidation of P680 and other Chls (Barber et al., 1987; Telfer et al., 1994b). Indeed, the primary event upon PSII photoinhibition is the damage to the D1 subunit (Aro et al., 1993), and restoration of photosynthetic electron transport requires degradation and de novo synthesis of this subunit. These observations are consistent with the idea that the PSII reaction centers are a major source of 1O2 within the chloroplasts (Krieger-Liszkay, 2005; Telfer, 2005), and led to the conclusion, although indirect, that β-carotene ligands in the core complex have a special role in scavenging 1O2 (Telfer et al., 1994a; Telfer, 2005).
Recently, the Arabidopsis mutant szl1 was identified (Li et al., 2009) that carries a point mutation of LCYB gene, and thus exhibits a less-active lycopene β-cyclase with respect to the wild type. Due to the activity of the four carotene hydroxylase enzymes that catalyze the subsequent reactions leading to xanthophyll synthesis, a depletion in carotene with respect to wild-type plants is produced (Li et al., 2009), offering the opportunity of specifically probing carotene function in vivo in the presence of xanthophylls.
In this work, we have addressed the question of the function for the carotene ligands of both PS, and their importance in the photoprotection of the chloroplast. To this aim, we have compared a panel of Arabidopsis mutants affected in the biosynthesis of either xanthophylls or carotenes, and analyzed the effect of their depletion on the photodamage of chloroplast in vivo. We show that, although the szl1 mutation does not affect photosynthetic electron transport rate (ETR), mutant plants show a higher sensitivity to photoxidative stress with respect to the wild type when exposed to HL at low temperature (8°C). Interestingly, szl1 plants revealed stronger photoinhibition of PSI compared with PSII. These findings imply that β-carotene ligands of PSI have a crucial role in the photoprotection of the complex, especially in low-temperature conditions.
RESULTS
The szl1 Mutant of Arabidopsis Has Lower Carotene and Higher Xanthophyll Content Than Wild-Type Plants
The szl1 mutant (Li et al., 2009) showed, under our growth conditions (100 μmol photons m−2 s−1, 8 h light, 23°C/16 h dark, 20°C), similar leaf morphology and development rate with respect to wild-type plants. Pigment content of leaves from both genotypes was analyzed through diode array HPLC of leaf acetone extracts (Table I). We tested dark-adapted plants, a condition in which wild-type leaves accumulate violaxanthin, and plants transferred for 60 min under HL (550 μmol photons m−2 s−1), a condition in which violaxanthin is largely deepoxidated into zeaxanthin. Chl content, Chl a/b, and Chl/Car ratios were essentially the same in both genotypes (Table I), whereas β-carotene content was 60% lower in szl1 leaves (Table II), as reported previously (Li et al., 2009). The szl1 mutant showed a slight accumulation of α-carotene (a lutein precursor normally found in small amount in wild-type plants), a lower content in β,β-xanthophylls, and a higher content in lutein, thus a far lower β,β/ε,β-xanthophylls ratio than in the wild type. When plants were exposed to HL, deepoxidation was 30% lower in szl1 with respect to the wild type (Table II).
Table I. Pigment content and Chl fluorescence induction parameters.
Measurements were done on dark-adapted leaves of Arabidopsis wild type and mutants szl1, chy1chy2, and lut5. Data are expressed as mean ± sd (n ≥ 4). Chls, Total chlorophylls; Cars, total carotenoids; T2/3, time corresponding to two-thirds of the induction fluorescence rise in DCMU-treated leaves; a.u., arbitrary units. Values marked with the same letters are not significantly different from each other within a column (Student’s t test, P < 0.05).
| Genotypes | Chls/cm2 | Chl a/b | Chls/Cars | Xanthophyll/Cars | Carotene/Cars | Fo | Fm | Fv/Fm | T2/3−1 |
|---|---|---|---|---|---|---|---|---|---|
| μg | a.u. | 103, ms−1 | |||||||
| WT | 20.8 ± 1.1a | 3.0 ± 0.1a | 3.7 ± 0.2a,b | 0.7 ± 0.01a | 0.3 ± 0.01a | 388 ± 42a | 2136 ± 149a | 0.82 ± 0.01a | 4.3 ± 0.4a |
| szl1 | 17.5 ± 1.6a | 2.9 ± 0.1a | 3.5 ± 0.1a | 0.9 ± 0.01b | 0.1 ± 0.01b | 515 ± 39b | 1858 ± 165b | 0.72 ± 0.01b | 4.6 ± 0.4b |
| chy1chy2 | 20.2 ± 1.9a | 2.9 ± 0.1a | 4.0 ± 0.2b,c | 0.7 ± 0.01c | 0.3 ± 0.01c | 348 ± 35a,c | 1766 ± 173b | 0.80 ± 0.01a | 3.7 ± 0.4a |
| lut5 | 19.4 ± 3.5a | 3.0 ± 0.2a | 4.1 ± 0.2c | 0.6 ± 0.01d | 0.4 ± 0.02d | 388 ± 16c | 1883 ± 131b | 0.82 ± 0.01a | 3.1 ± 0.3c |
Table II. Photosynthetic pigment content of the wild type and mutants.
Pigment content was determined before and after leaves were illuminated for 60 min at 550 μmol photons m−2 s−1. Data are normalized to 100 Chl a + b molecules and are expressed as mean ± sd (n = 3). Values marked with the same letters are not significantly different from each other within a column and a light regime (Student’s t test, P < 0.05).
| Genotypes | Mol Pigment/100 Mol Chls |
β,β/ε,β Xanthophylls Ratio | ||||||
|---|---|---|---|---|---|---|---|---|
| Neoxanthin | Violaxanthin | Antheraxanthin | Lutein | Zeaxanthin | α-Carotene | β-Carotene | ||
| Dark adapted | ||||||||
| WT | 4.4 ± 0.4a | 2.8 ± 0.4a | 13.0 ± 0.1a | 0.2 ± 0.04a | 6.9 ± 0.4a | 0.6 ± 0.01a | ||
| szl1 | 1.3 ± 0.1b | 0.8 ± 0.2b | 22.3 ± 0.5b | 1.2 ± 0.2b | 2.7 ± 0.2b | 0.1 ± 0.01b | ||
| chy1chy2 | 0.7 ± 0.2b | 0.7 ± 0.1b | 15.6 ± 0.8c | 0.2 ± 0.1a | 8.0 ± 0.2c | 0.1 ± 0.03b | ||
| lut5 | 2.9 ± 0.4c | 2.0 ± 0.2c | 10.3 ± 0.5d | 6.3 ± 0.4c | 2.9 ± 0.2b | 0.5 ± 0.01c | ||
| HL | ||||||||
| WT | 4.2 ± 0.1a | 1.4 ± 0.1a | 0.3 ± 0.1a | 12.6 ± 0.3a | 1.1 ± 0.2a | 0.2 ± 0.1a | 6.1 ± 0.3a | 0.6 ± 0.01a |
| szl1 | 1.3 ± 0.1b | 0.6 ± 0.1b | 0.1 ± 0.01b | 20.8 ± 1.0b | 0.8 ± 0.1b | 1.1 ± 0.2b | 2.4 ± 0.5b | 0.1 ± 0.01b |
| chy1chy2 | 0.8 ± 0.2c | 0.6 ± 0.1b | 0.1 ± 0.01b | 15.2 ± 0.4c | 0.6 ± 0.1b,c | 0.2 ± 0.1a | 7.6 ± 0.4c | 0.1 ± 0.02b |
| lut5 | 3.1 ± 0.4d | 1.4 ± 0.1a | 0.1 ± 0.04b | 11.0 ± 0.7d | 0.6 ± 0.04c,d | 5.9 ± 0.4c | 3.0 ± 0.1b | 0.5 ± 0.04c |
The aim of this work was to address the function for carotene molecules bound to PS, and their relative importance in the photoprotection of the chloroplast. However, previous characterization of xanthophyll biosynthesis mutants (Dall’Osto et al., 2007b; Kim et al., 2009) clearly showed that xanthophyll composition of the LHCs also affects photoprotection. In particular, distinct roles were identified for β,β- and ε,β-xanthophylls. Relevant to this study, depletion of β,β-xanthophylls increased photosensitivity (Dall’Osto et al., 2007b). Since szl1 plants, besides a lower carotene content, also have a lower β,β/ε,β-xanthophylls ratio than the wild type, it is important to distinguish the effect of carotene depletion in core complexes from the increased lutein to β,β-xanthophyll ratio in the antenna moiety of the PS. Therefore, we included the chy1chy2 and lut5 genotypes in this characterization as controls (Tables I and II). The chy1chy2 double mutant has a reduced conversion of β-carotene into β,β-xanthophylls, yielding the same β,β/ε,β-xanthophylls ratio as the szl1 plants. In addition, we analyzed the lut5 genotype as a control with respect to the α-carotene accumulation. In fact, α-carotene competes with β-carotene binding sites on pigment-protein complexes (Dall’Osto et al., 2007b), and thus it might be responsible, in part, for changes in photoprotection activity. chy1chy2 and lut5 plants showed leaf Chl content and Chl a/b ratio identical to wild-type plants. Xanthophyll content per Chl was significantly lower than in the wild type (Table I), as expected from previous reports (Fiore et al., 2006; Kim et al., 2009).
Organization and Stoichiometry of Chl-Binding Proteins
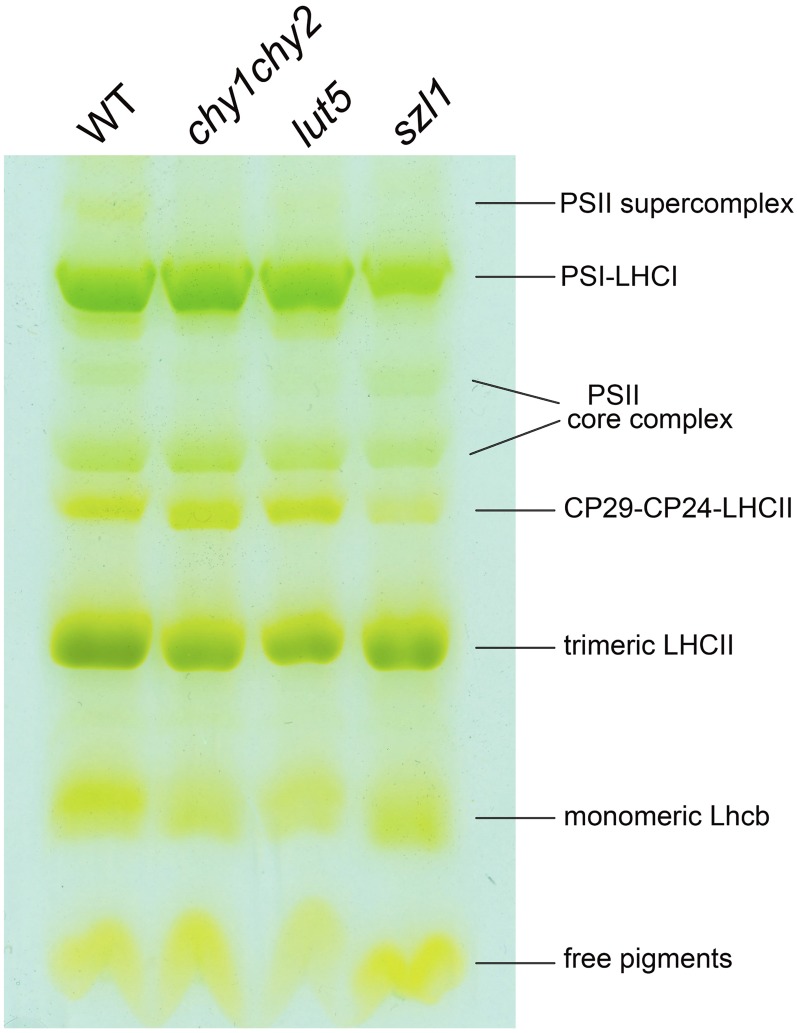
The organization of pigment-protein complexes in wild-type and mutant genotypes was analyzed by nondenaturing Deriphat-PAGE (Fig. 1). In agreement with a previous report (de Bianchi et al., 2008), seven major green bands were resolved upon solubilization of thylakoid membranes with 0.8% dodecyl-α-d-maltoside (α-DM). The uppermost band contained the PSII-LHCII supercomplex whose dissociation into components yielded the PSII core and the antenna moieties, namely CP29-CP24-(LHCII)3 supercomplex (Bassi and Dainese, 1992), trimeric LHCII, and monomeric Lhcb. The major green band just below the PSII supercomplex contained the PSI-LHCI supercomplex, which, different from PSII, is stable and does not yield dissociation products upon mild solubilization of wild-type thylakoids. Finally, the lowest band was composed of free pigments that dissociated during solubilization, mainly carotenoids. The distribution of Chl between PSI-LHCI, PSII core, and Lhcb components was determined from the densitometric analysis of the Deriphat-PAGE patterns. In szl1, the PSI-LHCI complex relative abundance was reduced versus wild-type thylakoids (−27%). Consistently, a higher PSII core/PSI-LHCI ratio was found in szl1 (0.61) with respect to the wild type (0.41). Minor differences were observed in the Lhcb/PSII core ratio, which was slightly lower (approximately 4.0) in chy1chy2 and lut5 with respect to the wild type (5.5), accompanied by a higher PSII core/PSI-LHCI ratio (approximately 0.5 versus 0.4).
Figure 1.
Analysis of pigment-protein complexes of the wild type and mutants. Thylakoid pigment-protein complexes were separated by nondenaturing Deriphat-PAGE upon solubilization with 0.8% α-DM. Thylakoids corresponding to 25 μg of Chls were loaded in each lane. Composition of each band is indicated. [See online article for color version of this figure.]
We first proceeded to determine whether the light harvesting and energy transfer to reaction center activity was affected by the mutations. The functional antenna size of PSII was measured on leaves by estimating the rise time of Chl florescence in the presence of 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU). chy1chy2 and lut5 leaves showed a significant reduction in the PSII antenna size with respect to the wild type (Table I), thus suggesting that carotenoid depletion did impair the overall light-harvesting capacity, as suggested by densitometric analysis of the Deriphat-PAGE. However, the PSII functional antenna size was not significantly affected by the szl1 mutation.
Green bands were eluted from the gel, and their pigment composition was determined by HPLC (Table III). Monomeric and trimeric Lhcb complexes from mutant genotypes showed a lower content in carotenoids per unit Chl versus the corresponding fractions from the wild type (around −20%). Furthermore, antenna proteins isolated from szl1 and chy1chy2 thylakoids had a lower content in β,β-xanthophylls (−90% in monomeric Lhcb, −75% in trimeric LHCII) and a compensatory increase in lutein (+25% in monomeric Lhcb, +10% in trimeric LHCII), while the relative abundance of ε,β- and β,β-xanthophylls were similar in antenna proteins from the wild type and lut5. The PSII core complexes purified from wild-type and mutant thylakoids only bound Chl α,β-carotene and α-carotene. However, while the Chl/Car ratios were essentially the same in the wild type, chy1chy2, and lut5, the PSII core complex purified from szl1 showed a far lower content in carotenes (−40%). Moreover, 33% of bound carotenes in the PSII core complex from szl1, are made by α-carotene, versus 72% in lut5; PSII core complexes bound almost exclusively β-carotene in both the wild type and chy1chy2.
Table III. Pigment composition of Chl proteins purified from thylakoids of the wild type, szl1, chy1chy2, and lut5.
Data are normalized to 100 Chl a + b molecules and are expressed as mean ± sd (n = 3). See “Materials and Methods” for details of purification. Abbreviations: Chls, Total Chl; Xanths, total xanthophylls; Cars, total carotenoids; ε-β,ε,β-xanthophylls, β-β,β,β-xanthophylls. Values marked with the same letters are not significantly different from each other within a column and a complex (Student’s t test, P < 0.05).
| Complexes | Mol Pigment/100 Mol Chls |
Chls/Cars | ε,β/Xanths | β,β/Xanths | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chl a | Chl b | Neoxanthin | Violaxanthin | Lutein | α-Carotene | β-Carotene | ||||
| Monomeric Lhcb | ||||||||||
| WT | 64.6 ± 0.3a | 35.5 ± 0.2a | 5.8 ± 0.4a | 4.1 ± 0.2a | 16.1 ± 0.1a | 3.8 ± 0.01a | 0.6 ± 0.01a | 0.4 ± 0.01a | ||
| szl1 | 65.7 ± 0.1b | 34.3 ± 0.1b | 0.1 ± 0.01b | 0.9 ± 0.02b | 20.4 ± 0.6b | 4.7 ± 0.1b | 1.0 ± 0.01b | 0.04 ± 0.01b | ||
| chy1chy2 | 60.3 ± 0.5c | 39.7 ± 0.5c | 0.2 ± 0.01b | 1.1 ± 0.1b | 20.0 ± 0.7b | 4.7 ± 0.2b | 1.0 ± 0.01b | 0.06 ± 0.01b | ||
| lut5 | 62.4 ± 0.1d | 37.6 ± 0.1d | 3.0 ± 0.1c | 4.4 ± 0.2a | 15.5 ± 0.4a | 4.4 ± 0.1c | 0.7 ± 0.01c | 0.3 ± 0.01c | ||
| Trimeric LHCII | ||||||||||
| WT | 58.0 ± 0.3a | 42.0 ± 0.3a | 7.3 ± 0.6a | 1.7 ± 0.1a | 21.1 ± 0.3a | 3.3 ± 0.1a | 0.7 ± 0.01a | 0.3 ± 0.01a | ||
| szl1 | 59.7 ± 0.4b | 40.3 ± 0.4b | 1.7 ± 0.1b | 0.8 ± 0.02b | 22.5 ± 0.1b | 4.0 ± 0.01b | 0.9 ± 0.01b | 0.1 ± 0.01b | ||
| chy1chy2 | 56.2 ± 0.2c | 43.8 ± 0.2c | 1.2 ± 0.1b | 0.8 ± 0.04b | 22.3 ± 0.1b | 4.1 ± 0.01c | 0.9 ± 0.01b | 0.1 ± 0.01b | ||
| lut5 | 56.0 ± 0.8a,c | 44.0 ± 0.8a,c | 5.0 ± 0.4c | 1.6 ± 0.1a | 19.7 ± 0.3c | 3.8 ± 0.01d | 0.8 ± 0.01c | 0.3 ± 0.01c | ||
| PSII core complex | ||||||||||
| WT | 100.0 ± 0.0 | 0.8 ± 0.1a | 17.6 ± 1.6a | 5.5 ± 0.5a | ||||||
| szl1 | 100.0 ± 0.0 | 3.6 ± 0.1b | 6.8 ± 0.4b | 9.7 ± 0.3b | ||||||
| chy1chy2 | 100.0 ± 0.0 | 0.7 ± 0.1a | 16.4 ± 0.1a | 5.9 ± 0.01a | ||||||
| lut5 | 100.0 ± 0.0 | 12.5 ± 0.2c | 5.0 ± 0.2c | 5.7 ± 0.03a | ||||||
| PSI-LHCI | ||||||||||
| WT | 88.8 ± 0.1a | 11.3 ± 0.1a | 3.2 ± 0.2a | 6.2 ± 0.4a,d | 0.5 ± 0.1a | 14.1 ± 1.3a | 4.2 ± 0.2a | 0.7 ± 0.03a | 0.3 ± 0.03a | |
| szl1 | 88.4 ± 0.1a | 11.6 ± 0.1a | 0.7 ± 0.1b | 7.4 ± 0.3a,c | 2.7 ± 0.1b | 7.2 ± 0.4b | 5.6 ± 0.1b | 0.9 ± 0.01b | 0.1 ± 0.01b | |
| chy1chy2 | 86.1 ± 0.3b | 13.9 ± 0.3b | 0.8 ± 0.1b | 7.9 ± 0.1c | 0.4 ± 0.02a | 15.0 ± 0.5a | 4.2 ± 0.1a | 0.9 ± 0.01b | 0.1 ± 0.01b | |
| lut5 | 87.0 ± 0.2c | 13.0 ± 0.2c | 2.0 ± 0.1c | 5.6 ± 0.4d | 10.3 ± 0.4c | 6.3 ± 0.3c | 4.1 ± 0.1a | 0.7 ± 0.03c | 0.3 ± 0.03c | |
A similar effect was observed on the pigment composition of the PSI-LHCI complexes purified from wild-type and mutant thylakoids: Carotenoid content per unit Chl was essentially the same in the wild type, chy1chy2, and lut5, while it was reduced in szl1 due to a lower carotene content (−30%). The relative abundance of ε,β- and β,β-xanthophylls was similar in PSI-LHCI from the wild type and lut5, while complexes isolated from szl1 and chy1chy2 had a far lower content in β,β-xanthophylls and a compensatory increase in lutein. While β-carotene is the main carotene found in PSI-LHCI from the wild type and chy1chy2, α-carotene accounts for 28% and 62% of total carotenes of the complexes in szl1 and lut5, respectively.
Photosynthesis-Related Functions: ETR and Excess Energy Dissipation
Analysis of the fluorescence yield in dark-adapted leaves (Butler, 1978) revealed a significant decrease of the PSII maximum quantum efficiency (Fv/Fm) in szl1 with respect to the other genotypes (Table I). In all mutants, the absolute values of Fm is slightly reduced with respect to the wild type, while only in szl1 the F0 value is 35% higher than the corresponding wild type. Thus, the decline in Fv/Fm in slz1 is mainly due to F0 rise (Table I), meaning that a larger fraction of absorbed energy is lost as fluorescence in this mutant; it suggests either that the connection between the major LHC and PSII reaction center is less efficient in carotene-depleted plants, or that PSII reaction center trapping efficiency is reduced.
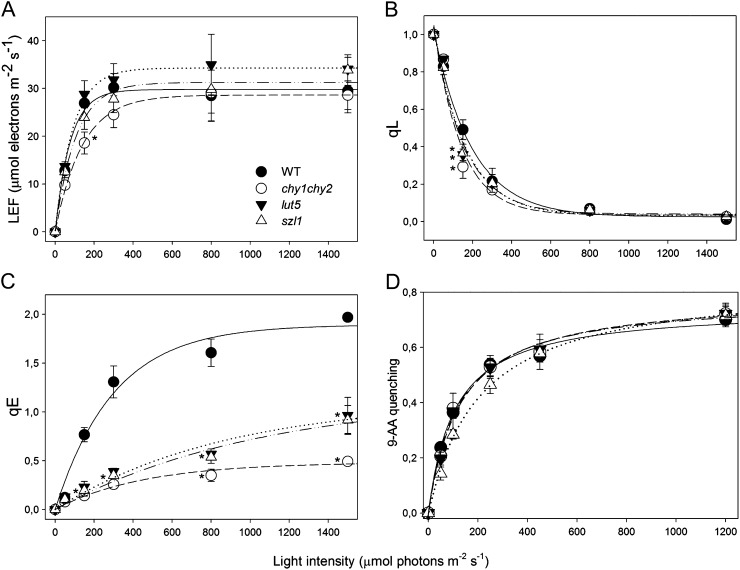
To test the hypothesis that carotene content might affect photosynthetic electron transport, PSII function during photosynthesis was analyzed by Chl fluorometry. szl1, chy1chy2, and lut5 showed no major differences with respect to wild-type plants either in the linear ETR or in the QA redox state (qL), as measured on leaves at different light intensities in the presence of saturating CO2 (Fig. 2, A and B).
Figure 2.
Analysis of room temperature Chl fluorescence during photosynthesis in wild-type and mutant plants. A, Dependence of the linear electron flow (LEF) on light intensity was measured in wild-type and mutant leaves. ETR was calculated as ΦPSII·PAR·Aleaf·fractionPSII (see “Materials and Methods” for details). B, Amplitude of qL measured at different light intensities. qL reflects the redox state of the primary electron acceptor QA, thus the fraction of open PSII centers. C, Dependence of qE, the rapidly reversible component of NPQ, on light intensity. Data are expressed as means ± sd (n = 4). D, Amplitude of light-dependent quenching of 9-AA fluorescence, measured at different light intensities on intact chloroplasts. 9-AA fluorescence quenching is induced by actinic illumination of chloroplasts and reflects the amplitude of transthylakoid ΔpH buildup. Values that are significantly different (P < 0.05) from the wild type are marked with an asterisk (*).
Capacity for Chl fluorescence quenching (qE), the rapidly reversible component of nonphotochemical quenching (NPQ), was plotted as a function of light intensity (Fig. 2C), and a reduction in qE activity was measured in chy1chy2 and lut5 plants. These results are consistent with previous reports (Niyogi et al., 1998; Dall’Osto et al., 2007b; Kim et al., 2009) showing a correlation between xanthophyll content, accumulation of zeaxanthin, and amplitude of qE. szl1 leaves were also analyzed for their fluorescence quenching capacity, to investigate if carotene depletion affected qE amplitude. szl1 showed a maximum value of qE lower than the wild type but similar to that of the other mutants. Since PsbS content was the same in wild-type and szl1 plants (Li et al., 2009), we determined the capacity of intact chloroplasts to produce changes in lumenal pH by following the light-induced quenching of 9-aminoacridine (AA; Johnson et al., 1994). All mutants performed similar to the wild type at all light intensities (Fig. 2D). This suggests that the reduction of qE in szl1 can be attributed to its lower xanthophyll cycle pool size, similar to that of chy1chy2 mutant, rather than to the lower content of carotenes in the core complex of both PS.
Photosensitivity under HL at Chilling Temperature
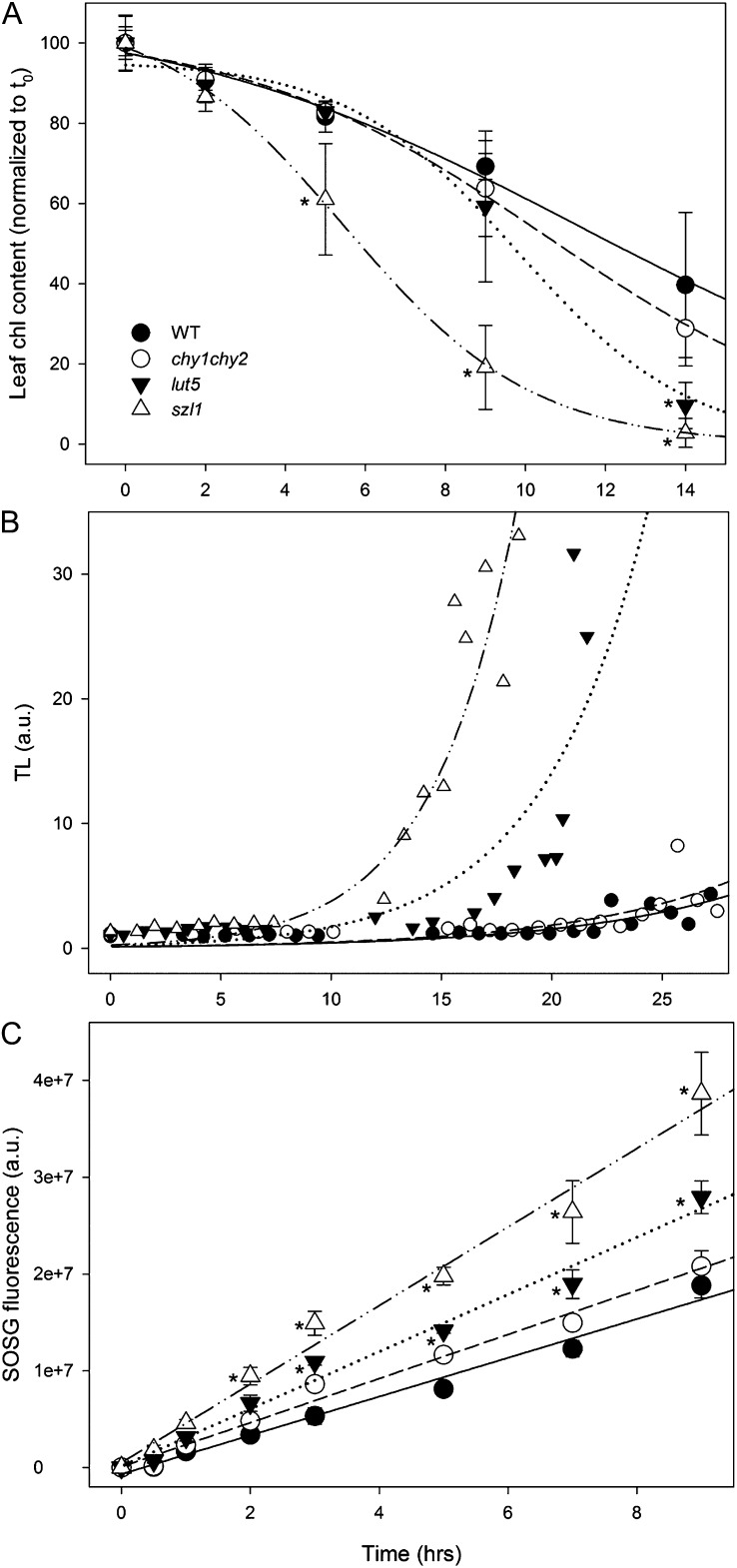
Treatment of plants with strong light produced photoxidative stress, whose severity was enhanced by low temperature. Under these conditions, enhanced release of 1O2 caused bleaching of pigments, lipid peroxidation, and PSII photoinhibition (Zhang and Scheller, 2004). To analyze the effect of missing carotenoids on the sensitivity to photoxidative stress, leaf discs from the wild type and mutants were subjected to HL + cold stress (2,400 μmol photons m−2 s−1, 8°C), then the time course of pigment photobleaching was measured (Fig. 3A). Results indicate that the Chl bleaching rate was higher in szl1 leaves, lower in the wild type and chy1chy2, while lut5 leaves showed an intermediate behavior.
Figure 3.
Photooxidation of Arabidopsis wild-type and mutant genotypes under photoxidative stress. A, Detached leaves floating on water were treated at 2,400 μmol photons m−2 s−1 at 8°C, and kinetics of Chl bleaching were recorded. Data are expressed as means ± sd (n = 6). B, Wild-type and mutant leaves floating on water were exposed to 800 μmol photons m−2 s−1 at 8°C, and photooxidation was estimated from the extent of lipid peroxidation measured by high-temperature TL. Each experimental point corresponds to a different sample. a.u., Arbitrary units. See “Materials and Methods” for details. C, Wild-type and mutant detached leaves were vacuum infiltrated with 5 μm SOSG, a 1O2-specific fluorogenic probe. SOSG increases its fluorescence emission upon reaction with 1O2. The increase in the probe emission was followed during illumination with red actinic light (550 μmol photons m−2 s−1) at 8°C. a.u., Arbitrary units. Values that are significantly different (P < 0.05) from the wild type are marked with an asterisk (*).
The level of stress caused by HL + cold treatment in the wild type and mutants was further investigated by measuring the extent of lipid peroxidation as detected by thermoluminescence (TL; Ducruet and Vavilin, 1999). Figure 3B shows plots of TL amplitudes at different time points during exposure of leaf discs to HL + cold stress (800 μmol photons m−2 s−1, 8°C). The highest levels of lipid peroxidation upon HL treatment was observed in szl1, followed by lut5 while chy1chy2 was only slightly more photosensitive than the wild type. Measurement of the 1O2 production in leaves (Fig. 3C) was consistent with pigment bleaching and lipid peroxidation measurements: At the end of the treatment, the wild type showed the lowest level of singlet oxygen sensor green (SOSG) fluorescence, while szl1 the highest; chy1chy2 and lut5 had intermediate behavior. Instead, after illumination with HL, szl1 leaves showed significantly lower yield in reduced forms of ROS (namely, hydrogen peroxide, O2−, and OH·) with respect to all the other genotypes at each time point (Supplemental Fig. S1).
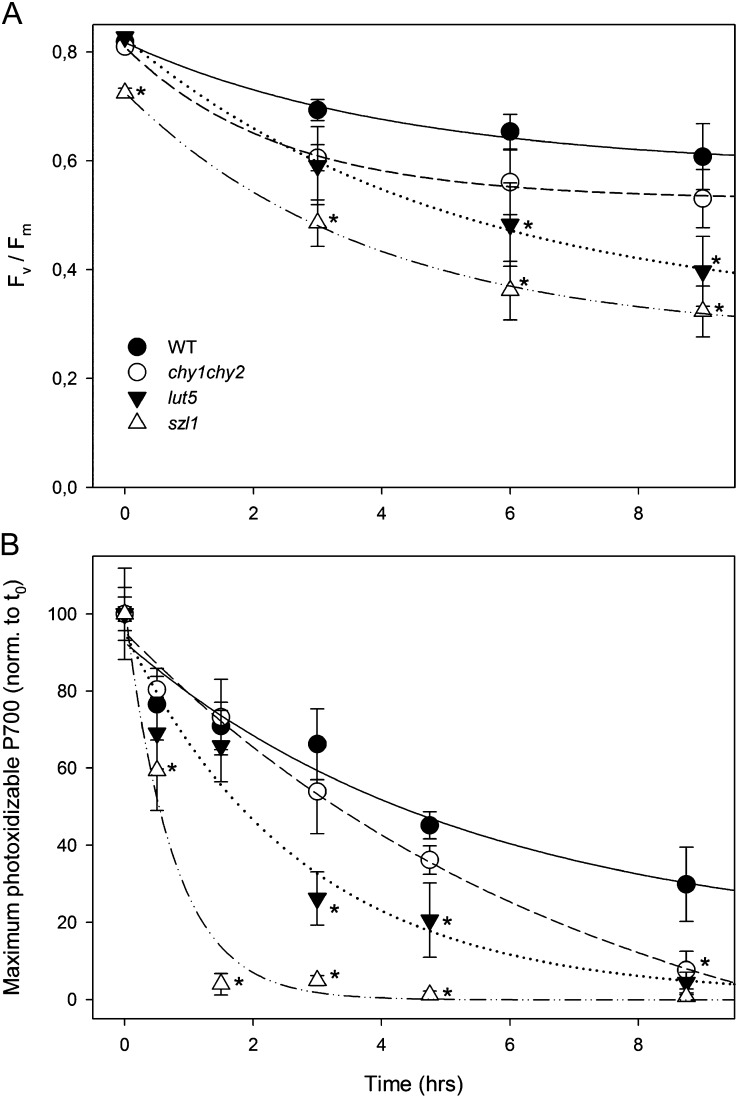
The enhanced photosensitivity of szl1 plants could be caused by impaired photoprotection at either PSII, PSI, or both. To determine the primary target of photooxidation in szl1 leaves upon exposure at HL and low temperature, kinetics of PSII and PSI photoinhibition were determined. The sensitivity to photoxidative stress in wild-type and mutant plants was assessed upon their transfer from control conditions to HL + cold stress (550 μmol photons m−2 s−1, 8°C), upon which the levels of Fv/Fm, the maximal photochemical yield of PSII, were monitored for 9 h; results are reported on Figure 4A. In wild-type plants Fv/Fm gradually decreased from 0.8 to 0.6 during the treatment (halftime of PSII photoinhibition of approximately 20 h), similar to the behavior of chy1chy2. In lut5, however, Fv/Fm was more affected by the treatment, down to a value of 0.4 at the end of the treatment. The szl1 plants were as photosensitive as lut5, since their Fv/Fm decreased to 0.35 at the end of the treatment, corresponding to a halftime for PSII photoinhibition of approximately 5.5 h. Measurements of Fv/Fm recovery after photoinhibitory treatment clearly showed the same rate of PSII quantum efficiency recovery in all genotypes (Supplemental Fig. S2), implying that the higher photosensitivity is due to a less-effective photoprotection rather than to impaired PSII repair mechanism (Aro et al., 1994).
Figure 4.
Photoinhibition of the wild type and mutants exposed to HL and low temperature. Kinetics of Fv/Fm decay (PSII photoinhibition) and maximum photoxidizable P700 decay (PSI photoinhibition) were measured on the wild type and mutants. Whole plants were exposed to 550 μmol photons m−2 s−1 at 8°C. Data are expressed as means ± sd (n ≥ 5). Values that are significantly different (P < 0.05) from the wild type are marked with an asterisk (*). t0, Time zero.
Upon exposure to photoxidative stress, F0 and Fm changes showed different kinetics in wild-type and mutant leaves (Supplemental Fig. S3). Stress treatment resulted in a decrease of Fm in all genotypes, likely due to photoinactivation of PSII reaction centers, which then dissipate excitation energy as heat rather than as photochemistry (Baker, 2008). Instead, while HL + cold stress was associated with an increase in F0 in chy1chy2, lut5, and wild-type plants, as expected upon oxidative damage of PSII RC, szl1 plants showed a slight reduction of F0 value with time of treatment (Supplemental Fig. S3). The szl1 F0 changes could be traced back to the massive Chl bleaching of this genotype upon photoxidative stress (Fig. 3A) that are likely to affect the fluorescence emission per leaf surface area.
The kinetic of PSI photoinhibition was assessed by measuring the maximum content of photoxidizable P700 upon transfer of plants from control conditions to HL + cold stress. These stress conditions had a much more dramatic effect on photoinhibition of PSI with respect to that of PSII in Arabidopsis, in agreement with previous results (Zhang and Scheller, 2004). Indeed, the photoxidizable P700 gradually decreased to 50% of its initial value in 4.5 h and to 30% at the end of the 9-h treatment in wild-type plants (Fig. 4B). The halftime of photoinhibition was shorter for chy1chy2 and lut5 genotypes (being 50% inhibited in 2.5 and 4 h, respectively). Surprisingly, the halftime of PSI photoinhibition was far shorter for szl1 plants (approximately 0.6 h) than in the other genotypes.
To further quantify the PSI damage, the maximum level of P700+ was measured upon a saturating flash under a far-red-light background, before and after HL treatment (Munekage et al., 2002). The decrease in the P700+ level might be caused not only by PSI photoinhibition, but also by an overreduced state of acceptors and activation of cyclic electron flow (Sonoike, 2011; Rutherford et al., 2012). To evaluate the extent of PSI photoinhibition, leaves were vacuum infiltrated with methyl viologen upon the HL treatment; electron acceptance from PSI by methyl viologen restored the maximum oxidation of P700. Results (Supplemental Table S1) show that in the wild type less than 25% of PSI was damaged, whereas in szl1 up to 80% of PSI was inactivated.
1O2 Production from Purified Pigment-Protein Complexes
The above results (Figs. 3 and 4) suggest a role for carotenes in photoprotection of both PSII and PSI reaction centers, possibly in limiting the 1O2 release into the lipid phase. Although this result is consistent with carotene location in PSI-LHCI and PSII core complexes, it is relevant to experimentally assess whether photodamage is due to the properties of the pigment-binding proteins or is caused by pleiotropic factors. To this aim, we purified PSII core, Lhcb antenna proteins, and PSI-LHCI supercomplex and determined their 1O2 production when illuminated with strong light (see “Materials and Methods” for details). When isolated Chl-binding complexes are exposed to strong light, 1O2 is produced as the main ROS involved in the photoinhibition of both PS (Triantaphylidès et al., 2008) deriving from the reaction of excited Chl (3Chl*) with molecular oxygen. The level of 1O2 production by isolated complexes strongly depends on carotenoid composition and is inversely correlated with the capacity of 3Chl* quenching and ROS scavenging by bound xanthophylls (Mozzo et al., 2008; Dall’Osto et al., 2010; de Bianchi et al., 2011).
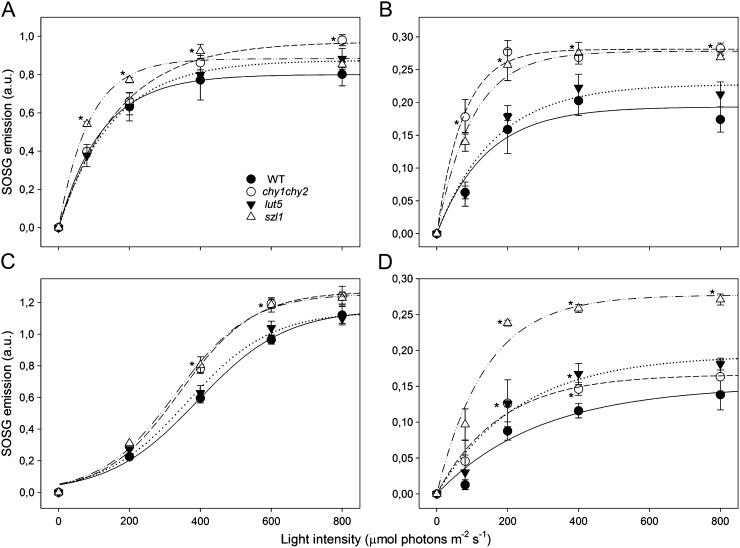
Results of 1O2 production at 20°C by the different complexes isolated from the wild type and szl1 are reported in Figure 5. PSII core complexes from the wild type, chy1chy2, and lut5 showed a similar yield of 1O2 at each light intensity tested, up to 800 μmol photons m−2 s−1. The complexes from szl1 showed an approximately 30% increase in 1O2 production at low and moderate light intensities while the differences were less evident at higher light when the signal from all complexes tended to saturation (Fig. 5A). Measurements of Lhcb fractions (Fig. 5B) from the different genotypes did not show the same pattern. In fact, the complexes from chy1chy2 and szl1 showed an approximately 70% increase in 1O2 production with respect to the corresponding fraction from the wild type and lut5, consistent with the lower β,β-xanthophyll content (Dall’Osto et al., 2007b). Similar results were obtained by measuring PSII-LHCII: Supercomplexes from szl1 and chy1chy2 showed an approximately 25% increase in 1O2 production at moderate light intensities, with respect to both the wild type and lut5. In the case of PSI, the core complex and the antenna moieties form a stable complex that cannot be dissociated without some level of damage. We therefore measured 1O2 production on PSI-LHCI supercomplexes. Clearly, the 1O2 yield was 2-fold higher in the preparation from szl1 with respect to that of the wild type (Fig. 5D). In contrast, 1O2 yield from the PSI-LHCI complex of chy1chy2 and lut5 was only slightly higher than the wild type. Pigment-protein photoprotection was further evaluated from the ability to prevent Chl photobleaching via thermal deactivation of 3Chl* by carotenoids under strong white light illumination in atmosphere. The PSII core complex from szl1 did not show any reduction of its rate of bleaching (t50% bleaching) with respect to the wild type. On the contrary, PSI-LHCI from szl1 plants was less photoprotected, as shown by the 28% reduction in t50% bleaching (Supplemental Fig. S4).
Figure 5.
1O2 production from carotenoid-binding complexes. Dependence of 1O2 release on light intensity was measured on purified PSII core (A), Lhcb (B), PSII-LHCII (C), and PSI-LHCI (D) supercomplexes. SOSG was used as 1O2-specific fluorogenic probe, since it increases its fluorescence emission upon reaction with 1O2 in solution. Symbols and error bars show means ± sd (n = 3). Values that are significantly different (P < 0.05) from the wild type are marked with an asterisk (*).
DISCUSSION
In this work, we have investigated the role of carotene versus xanthophyll ligands in the photosynthetic apparatus, focusing on their photoprotective capacity for both PS. A panel of Arabidopsis mutants affected in the carotenoid biosynthesis pathways was compared: In fact, due to the intermediate position of carotenes in the carotenoids biosynthesis pathway, it is impossible to affect their abundance without inducing changes in the xanthophylls, which are downstream in the metabolic pathway. The major target of our analysis was the mutant szl1, depleted in carotenes due to a lower β-cyclase activity (Li et al., 2009). When exposed to HL at low temperature, szl1 plants showed the highest levels of pigment bleaching and lipid peroxidation among the genotypes considered in this study. Interestingly, carotene depletion in szl1 plants preferentially affects PSI activity, since mutant plants revealed far stronger photoinhibition of PSI with respect to PSII. Thus, it appears that photoprotection efficiency strongly depends on carotene content of PS. It should be noted, however, that the szl1 mutation, besides decreasing carotene content, also favors ε-branch versus β-branch xanthophylls, thus modifying the chromophore composition of the xanthophyll-binding Lhc subunits of the antenna system and increasing the α-carotene, usually a minor component, versus the normally found β-carotene. Comparison with the photoprotection phenotype of the chy1chy2 and lut5 is thus important to assess if the phenotype can be attributed in part to changes in xanthophyll composition, which is very similar in szl1 and chy1chy2, or to the enrichment in α-carotene, a feature observed in both szl1 and lut5.
Altered Xanthophyll Composition Affects Photoprotection Capacity
Photoprotection by carotenoids is performed by multiple mechanisms including quenching of Chl triplet states (Peterman et al., 1995; Mozzo et al., 2008), scavenging of both superoxide and hydroxyl radicals (Trevithick-Sutton et al., 2006), and quenching of 1O2, thus preventing lipid peroxidation (Havaux and Niyogi, 1999). In addition, ROS production can be prevented by quenching of singlet Chl excited states, a function that is enhanced by lutein and zeaxanthin (Niyogi et al., 2001; Dall’Osto et al., 2005). The strong phenotypes of Arabidopsis mutants with altered xanthophyll composition (Niyogi et al., 2001; Dall’Osto et al., 2007b; Kim et al., 2009) showed that the presence and relative amounts of these pigments is relevant for plant fitness. We observed that chy1chy2 plants are more sensitive to photoxidative stress than the wild type (Fig. 3). The total xanthophyll content is only slightly reduced in this mutant (Table II), and yet the β,β/ε,β-xanthophyll ratio is 8-fold decreased in the mutant, and in its LHC proteins (Table III). Previous work showed that optimal photoprotection of Lhc proteins depends on the balanced activity in ROS scavenging by β,β-xanthophylls and Chl triplet quenching by lutein (Dall’Osto et al., 2007b). Indeed, we observed an increased rate of 1O2 release from antenna proteins purified from chy1chy2, with β,β-xanthophylls partially replaced by lutein, with respect to the corresponding preparation from the wild type (Fig. 5B). In particular, it appears that depletion in neoxanthin is a major factor, consistent with recent reports (Mozzo et al., 2008). Besides neoxanthin, violaxanthin and zeaxanthin are also effective in preventing photodamage since the effect of their depletion is observed in PSI-LHCI complex, which does not bind neoxanthin, isolated from chy1chy2 plants (Table III). It should be noted that the assembly of PSI-LHCI supercomplexes is not impaired in chy1chy2, as shown by the migration rate of the supercomplex in the native PAGE identical to the wild type (Fig. 1).
Since Lhca and Lhcb proteins from chy1chy2 and szl1 have essentially the same β,β/ε,β-xanthophyll ratio and the same total xanthophyll content, the extreme sensitivity of szl1 to excess light cannot be explained based on differences in the xanthophyll composition and/or organization of the antenna proteins, implying that additional sensitivity factors are present in szl1 plants.
Altered Xanthophyll/Carotene Ratio Leads to Enhanced Photoxidation
Kinetics of pigment bleaching and lipid peroxidation clearly showed that lut5 and szl1 are much more sensitive to photoxidative stress with respect to both the wild type and chy1chy2 (Fig. 3) while the discussion above led to the conclusion that this is not due to their xanthophyll composition. A striking difference between lut5 and szl1 plants is their carotenoid composition: a low xanthophyll content per Chl in the former (−25% with respect to the wild type, see Table II), and a high xanthophyll/carotene ratio in the latter (+50% compared with the wild type), and yet both conditions lead to a strong light sensitivity with respect to wild-type plants. We have shown above that the β,β/ε,β-xanthophyll ratio cannot be the cause for differential photosensitivity, since this is the same in chy1chy2 and szl1, which exhibit very different levels of lipid peroxidation (Fig. 3). Overreduction of the plastoquinone pool is commonly associated with photoinhibition in wild-type plants, and yet the plastoquinone redox state was not significantly different in lut5 or szl1 with respect to chy1chy2 (Fig. 2). Zeaxanthin accumulation in HL, which provides photoprotection by both modulating qE and scavenging 1O2 in the lipid phase (Niyogi et al., 1998; Havaux and Niyogi, 1999), cannot account for the higher sensitivity of szl1 plants. Indeed, szl1 showed a marked increase in both the accumulation of zeaxanthin (+40% compared with lut5, Table II) and rate of 1O2 release in HL (Fig. 3C), while release of reduced ROS is far lower than in other genotypes (Supplemental Fig. S1).
Important differences between szl1 and lut5 are the strong depletion in Lhcb proteins (Fig. 1) and reduction in PSII functional antenna size (Table I) in lut5, while the PSII antenna size of szl1 plants is the same as the wild type (Table I). It should be noted that only the abundance of Lhcb proteins is affected by the lut5 mutation, whereas the Lhca assembly into PSI-LHCI supercomplexes is not disturbed, as shown by the identical migration of PSI-LHCI supercomplexes in native PAGE (Fig. 1; Dall’Osto et al., 2007b, 2010). We conclude that carotenes, although present in abundance, cannot replace xanthophylls in stabilizing Lhc proteins, and Lhcb proteins contribute to PSII photoprotection. Despite the observation that lut5 and szl1 are the most contrasting genotypes with respect to their Lhcb content (Fig. 1), lut5 plants are still less photosensitive than szl1 (Fig. 4), implying that the reason(s) for higher photosensitivity of szl1 cannot be attributed to differences in composition or size of the antenna moiety of the PS.
Differences in the xanthophyll composition of Lhcb proteins from the wild type and mutants account for differential photosensitivity (Fig. 5), with proteins from szl1 producing more 1O2 at all light intensities with respect to both the wild type and lut5, and similar to the same fraction from chy1chy2 (Tables II and III). Although caution should be applied when considering assays on isolated complexes as the reflection of an in vivo situation, it is worth noting that szl1 is clearly more sensitive than chy1chy2, thus an additional source of sensitivity is present in szl1, deriving from a different component of the photosynthetic apparatus, specifically affected by the mutation. We observed that the 1O2 production from purified PSII core complexes is similar in the complexes from all genotypes but szl1 (Fig. 5A), which evolves more 1O2, particularly at low-light intensity, a feature that correlates with the depletion in β-carotene content of PSII cores and strongly suggests this is a factor for increased photosensitivity of szl1. Yet, the increase in 1O2 yield from the carotenoid-depleted PSII core is rather small (Fig. 5A) when compared with the effect of altered β,β/ε,β-xanthophyll ratio in the Lhc proteins (Fig. 5B).
β-Carotene depletion in szl1 is evident not only in the PSII core but in PSI as well. Figure 5D shows that PSI-LHCI complex from szl1 showed a marked increase in the rate of 1O2 release (2-fold) in HL with respect to the wild-type complex, while PSI-LHCI from chy1chy2 and lut5 were less affected (Fig. 5C). Taken together, the above results show that szl1 plants are specifically affected in PSI complex, leading to a dramatic photosensitivity of PSI activity at any light intensity. It is particularly remarkable that PSI, which in the literature has been considered to be the more resistant of the two PS, was preferentially affected by the deficiency of carotenes.
Besides carotene depletion, a further feature of szl1 mutants is the presence of α-carotene in both PSI-LHCI and PSII core complexes, partially replacing β-carotene and potentially being a cause for photosensitivity (Table III). However, the lut5 genotype has a far higher α/β-carotene ratio than szl1 in both thylakoid (Table II) and isolated PSII core complexes (Table III), and yet isolated PSII core complexes and PSI-LHCI from this genotype did not show major differences in 1O2 production with respect to the complexes from the wild type (Fig. 5A), implying that α-carotene is not the major cause of photosensitivity.
What Is the Origin of the Extreme PSI Photosensitivity in szl1 Plants?
In the PSII core complex, most of the β-carotene molecules are in close contact with Chls, as required for effective quenching of 3Chl* (Ferreira et al., 2004). The only exception is represented by the two β-carotene ligands in the PSII reaction center, whose distance from the special pair P680 is higher, implying that they cannot quench 3P680* by triplet-triplet transfer and rather, they likely act as scavengers for 1O2 produced during charge recombination (Telfer et al., 1991, 1994b). PSII has been indicated as the primary target of photoinhibition (Andersson et al., 1992; Aro et al., 1993), since the D1 subunit is easily damaged in HL and rapidly turned over. Instead, P700+ was reported to be protective for PSI, since it can quench excitation energy and oxidize the reduced electron acceptor of PSI and remove excess reducing power (Sonoike, 2011). However, 3P680 and 3P700 lie close in energy level, thus both are prone to react with oxygen and yield 1O2. 3P700 results from charge recombination, therefore its yield is increased by acceptor side limiting conditions (Rutherford et al., 2012). Furthermore, exposure of PSI-LHCI to HL generates 3Car* mainly associated with LHCI (Santabarbara and Carbonera, 2005), while a selective bleaching of lutein molecules located in the outer antenna was observed (Andreeva et al., 2007), thus raising the question of what the role for carotene ligands is.
When comparing photoinhibition of szl1 and lut5 plants at HL + cold stress, the halftime of Fv/Fm decay (PSII photoinhibition, see Fig. 4A), as well as PSII repair efficiency (Supplemental Fig. S2), were very similar for both genotypes, while the szl1 genotype was far more sensitive to PSI photoinhibition with respect to the other genotypes, as shown by a 6-fold-faster PSI photoinhibition rate (Fig. 4B). This is consistent with the quantification of 1O2 released in HL by purified pigment-protein complexes: The 1O2 yield was 2-fold higher in the PSI-LHCI from szl1 with respect to that from the wild type (Fig. 5C), while PSII core complexes from all genotypes showed a similar yield of 1O2 at each light intensity tested (Fig. 5A). What is the origin of the extreme PSI photosensitivity in szl1 plants?
While PSII has an efficient repair machinery (Aro et al., 1993), a similar mechanism is not known for PSI: After light-induced damage, recovery of PSI from photoinhibition takes several days (Sonoike and Terashima, 1994; Sonoike, 2011); indeed, the damage to PSI is considered to be essentially irreversible and involves degradation and resynthesis of the whole complex.
Because of its irreversibility, PSI photoinhibition must be specially avoided. This is accomplished by a number of protective mechanisms, namely cyclic electron transport (Munekage et al., 2002) and the stromal scavenging enzyme systems superoxide dismutase and ascorbate peroxidase, which scavenge reduced ROS released by PSI (Asada, 1999). In several plant species including Arabidopsis, PSI becomes more sensitive to photoinhibition under specific environmental conditions such as chilling temperature, likely because the protective mechanisms are less efficient at low temperature (Sonoike, 2011); furthermore, the sink of reductants is decreased at low temperature, thus overreduction of the electron chain occurs and the yield of 3P700 and 1O2 increases. Indeed, the primary target for PSI photoinhibition upon illumination was located in the iron-sulfur centers FX, FB, and FA, and was caused by ROS (Inoue et al., 1986; Tjus and Andersson, 1993). Here, we show that HL + cold stress is effective in damaging PSI even in wild-type plants (Fig. 4B), and that this effect is greatly enhanced in carotene-depleted szl1 plants, implying that carotene ligands in PSI are crucial in ensuring the maintenance of PSI activity under this condition.
When searching for the molecular mechanism(s) behind the preferential damage of PSI in szl1, it can be hypothesized that carotene composition might affect the susceptibility to photoinhibition of the mutant. Indeed, a clear difference between the wild type and szl1 is the presence of α-carotene in PSI-LHCI of the mutant, partially replacing β-carotene. It is worth noting that PSI-LHCI from lut5 plants has a higher α/β-carotene ratio than szl1, and roughly the same xanthophyll content (Table III). However, a faster PSI photoinhibition (Fig. 4B) and a higher release of 1O2 from PSI-LHCI (Fig. 5C) were observed with respect to the wild type. On this basis, we cannot completely exclude the possibility that part of the PSI photosensitivity of szl1 plants was related to the α-carotene content of either core complex or LHCI.
Moreover, PSI stability might be limited by the amount of carotene molecules available. Indeed, a recent improved model of plant PSI (Amunts et al., 2010) suggested that most of β-carotene molecules are coordinated by either different subunits or distant region of the same subunit; therefore, these pigments might have a key role for PSI structural integrity, and it is consistent with the high degree of conservation of their positions and coordination between plants and cyanobacteria (Amunts et al., 2010). In szl1 plants, a general weakening of the PSI-LHCI structure would make the complex more susceptible to ROS attack thus causing degradation, as shown by native-PAGE analysis (Fig. 1). However, the photobleaching kinetics of PSI-LHCI complexes, challenged with strong light (Supplemental Fig. S4), show that this pigment-protein complex from szl1 is rather stable.
Alternatively, rather than the PSI core complex, the peripheral light-harvesting system might be more affected by carotene depletion. Indeed, it should be stressed that β-carotene is a ligand not only of the wild-type PSI core complex, but also of LHCI antenna moiety (Wehner et al., 2004). A recent report (Alboresi et al., 2009) showed that preferential degradation of LHCI upon illumination of isolated PSI-LHCI is effective in protecting the catalytic activity of the complex. Recovery from PSI photoinhibition is an energetically demanding process, since it necessarily requires degradation and resynthesis of the whole complex; thus, sacrificing the antennae would be a photoprotective strategy evolved to limit photoxidative damage into LHCI moiety and preserve the integrity of iron-sulfur clusters. The role of LHCI proteins as safety valves for PSI is related to the red absorption forms (Carbonera et al., 2005; Alboresi et al., 2009), Chls of the outer antenna with low energy level that concentrate the excitation energy before transfer to the reaction center (Croce et al., 1996); 3Chl* eventually formed by the red Chls are quenched by nearby carotenoids (Carbonera et al., 2005). This model implies that, as shown for Lhcb, Lhca proteins constitutively undergo formation of 3Chl* and production of 1O2. Carotenoid species bound to the LHCI, namely lutein, violaxanthin, and β-carotene, could be directly involved in triplet quenching and/or ROS scavenging, and likely occupy selective binding sites and serve distinct roles. Impairing one of these functions by changing the occupancy of specific carotenoid binding sites through mutations in the biosynthetic pathway leads to photosensitivity, similar to what observed previously within PSII antenna system (Dall’Osto et al., 2007b).
CONCLUSION
Here we show that the szl1 plants, which carry a point mutation in the LCYB gene and thus a less-active β-cyclase than the wild type (Li et al., 2009), have a lower carotene content in both PS with respect to wild-type plants and altered xanthophyll composition of the light-harvesting component of the antenna systems. Physiological characterization of the szl1 mutant offered the possibility of probing carotene function in vivo differentially from the effect on xanthophyll complement. When challenged with HL + cold stress, szl1 mutant plants undergo more photodamage than the wild type, particularly within PSI-LHCI, despite the fact that PSI-LHCI was less depleted in carotenes than PSII. Comparison with the chy1chy2 and lut5 mutants, which respectively share with szl1 alterations in xanthophyll composition and α-carotene accumulation, showed that these features were not the major factors causing enhanced susceptibility to photoinhibition and pointed to carotene depletion in photosynthetic core complexes as the major source of photodamage. It is evident that regulation of PSI Chl excited states under HL + cold stress is crucial for protection of the photosynthetic apparatus.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Wild-type plants of Arabidopsis (Arabidopsis thaliana) ecotype Columbia and mutants chy1chy2, lut5, and szl1 were obtained as previously reported (Fiore et al., 2006; Li et al., 2009). T-DNA knockout lines used are: chy1 (SAIL line 49A07), chy2 (SAIL line 1242B12), and lut5 (SALK line 116660). Plants were grown for 4 weeks on Sundermisch potting mix (Gramoflor) in controlled conditions of 8 h light, 23°C/16 h dark, 20°C, with a light intensity of 100 μmol photons m−2 s−1.
Stress Conditions
For HL treatments, light was provided by 150-W halogen lamps (Focus 3, Prisma). Short-term HL treatment was performed for 1 h at 550 μmol photons m−2 s−1 at 8°C to measure maximum zeaxanthin accumulation on detached leaves floating on water. Samples for HPLC analysis were rapidly frozen in liquid nitrogen prior to pigment extraction. Photooxidative stress was induced in either whole plants or detached leaves by a strong light treatment. Whole Arabidopsis plants were exposed to HL (550 μmol photons m−2 s−1 with a photoperiod day/night of 16/8 h) at 8°C for 2 d; detached leaves were exposed to either 800 or 2,400 μmol photons m−2 s−1 at 8°C for 14 h.
Chloroplasts and Thylakoids Isolation
Chloroplasts and stacked thylakoid membranes were isolated from wild-type and mutant leaves as previously described (Casazza et al., 2001).
Pigment Analyses
Pigments were extracted from leaves with 80% acetone, then separated and quantified by HPLC (Gilmore and Yamamoto, 1991). Chl content was determined by fitting the spectrum of the sample’s acetone extract with the spectra of individual pigments, as described previously (Croce et al., 2000).
Gel Electrophoresis
Nondenaturing Deriphat-PAGE was performed following the method described previously (Peter et al., 1991) but using 3.5% (w/v) acrylamide (38:1 acrylamide/bisacrylamide) in the stacking gel and in the resolving gel an acrylamide concentration gradient from 4.5% to 11.5% (w/v) stabilized by a glycerol gradient from 8% to 16%. Thylakoids concentrated at 1 mg/mL Chl were solubilized with either 0.8% α-DM or 1% β-DM, and 20 μg of Chls were loaded in each lane. Signal amplitude was quantified using the GelPro 3.2 software (BIORAD). Purified pigment-protein complexes were excised from gel and eluted with a pestle in a buffer containing 10 mm HEPES pH 7.5, 0.05% α-DM.
Analysis of Chl Fluorescence and P700 Redox Kinetics
PSII function during photosynthesis was measured through Chl fluorescence on whole leaves at room temperature with a PAM 101 fluorimeter (Heinz-Walz; Andersson et al., 2001), using a saturating light pulse of 4,500 μmol photons m−2 s−1, 0.6 s, and white actinic light ranging from 50 to 1,100 μmol photons m−2 s−1, supplied by a KL1500 halogen lamp (Schott). NPQ, ϕPSII, photochemical quenching (qP), qL, and ETR were calculated according to the following equations (Van Kooten and Snel, 1990; Baker, 2008): NPQ = (Fm − Fm′)/Fm′, ϕPSII = (Fm′ − Fs)/Fm′, qP = (Fm′ − Fs)/(Fm′ − F0′), qL = qP·F0′/Fs, ETR = ϕPSII·PAR·Aleaf·fractionPSII, where F0/F0′ is the minimal fluorescence from dark/light-adapted leaf, Fm/Fm′ is the maximal fluorescence from dark/light-adapted leaves measured after the application of a saturating flash, Fs the stationary fluorescence during illumination, and PAR the photosynthetic active radiation; Aleaf (leaf absorptivity) was 0.67 ± 0.04 for the wild type, 0.59 ± 0.04 for chy1chy2, 0.61 ± 0.03 for lut5, 0.58 ± 0.05 for szl1; fractionPSII was measured by densitometric analysis of Deriphat-PAGE. Calculation of ΔpH-dependent component of qE was performed as described previously (Walters and Horton, 1995). Fluorescence kinetics were measured with a home-built setup, in which leaves were vacuum infiltrated with 3.0 × 10−5 m DCMU, 150 mm sorbitol, and were excited with green light at 520 nm (Luxeon, Lumileds), and emission was measured in the near far red (Rappaport et al., 2007). The halftime of the fluorescence rise was taken as a measure of the functional antenna size of PSII (Malkin et al., 1981).
P700 redox state measurements were performed using a LED spectrophotometer (JTS10, Biologic Science Instruments) in which absorption changes were sampled by weak monochromatic flashes (10-nm bandwidth).
Spectroscopy
Steady-state spectra were obtained using samples in 10 mm HEPES pH 7.5, 0.05% α(β)-DM, 0.2 m Suc. Absorption measurements were performed using a SLM-Aminco DW-2000 spectrophotometer at room temperature. Fluorescence emission spectra were measured at room temperature using a Jobin-Yvon Fluoromax-3 spectrofluorimeter, equipped with a fiberoptic to measure emission of fluorogenic probes on vacuum-infiltrated leaves. Measure of ΔpH—the kinetics of ΔpH formation across the thylakoid membrane was measured using the method of 9-AA fluorescence quenching (Johnson et al., 1994) with modifications described in de Bianchi et al. (2008).
Measurements of ROS Production
Measurements of 1O2 production from purified pigment-protein complexes were performed with a specific fluorogenic probes, SOSG (Invitrogen; Dall’Osto et al., 2010). SOSG is a 1O2 highly selective fluorescent probe, that increases its 530-nm emission band in presence of this ROS species (Flors et al., 2006). Pigment-protein complexes were diluted in a reaction buffer (10 mm HEPES pH 7.5, 0.05% α-DM, 2 μm SOSG) to the same absorption area in the wavelength range 600 to 750 nm (about 2 μg Chls/mL), to measure 1O2 yield for complexes having the same level of light-harvesting capacity. Isolated complexes were illuminated with red light (λ > 600 nm, 20°C, 5 min) and fluorescence yield of SOSG were determined before and after HL treatment (Dall’Osto et al., 2007a). Measurements of 1O2 production from leaves were performed with leaf discs vacuum infiltrated with 200 μm SOSG in 50 mm phosphate buffer (pH 7.5), then illuminated with red light (λ > 600 nm, 550 μmol photons m−2 s−1, 8°C) as previously described (Dall’Osto et al., 2010). At different times, leaf discs were harvested and SOSG fluorescence was measured. Measurements of reduced ROS (hydrogen peroxide, OH·, and O2−) production from leaves were performed with dichlorofluorescein diacetate, a specific fluorogenic ROS probe, and nitroblue tetrazolium. Leaf discs, after the different times of HL treatment, were infiltrated under vacuum with a solution of 200 μm dichlorofluorescein diacetate in 50 mm phosphate buffer (pH 7.5) and maintained in the dark for 30 min. Following excitation at 490 nm, the fluorescence emission at 530 nm was then detected. The NBT staining method was used for in situ detection and quantification of superoxide radical, as previously described (Ramel et al., 2009).
Determination of the Sensitivity to Photoxidative Stress
HL treatment was performed for 2 d at 550 μmol photons m−2 s−1, 8°C on whole plants. Decay kinetics of maximal quantum yield of PSII photochemistry (Fv/Fm; Havaux et al., 2004) and maximum content of photooxidizable P700 (ΔAmax at 705 nm; Yang et al., 2010) were recorded on detached leaves during illumination to assess inhibition of PSII and PSI, respectively. Content of oxidizable P700 (ΔAmax) was recorded during far-red-light illumination (2,500 μmol photons m−2 s−1, λmax = 720 nm); to have a precise estimation of the PSI photoinhibition, ΔAmax has been determined in detached leaves, vacuum infiltrated with 50 μm dibromothymoquinone and 1 mm methyl viologen (Sonoike, 2011). The maximum contents of P700+ was also determined on methylviologen-treated leaves using a saturating flash (3,000 μmol photons m−2 s−1) under a 520 μE far-red-light background (Munekage et al., 2002). Photooxidative stress was measured on whole plants by thermoluminometry, with a custom-made apparatus that has been described (Ducruet, 2003). The amplitude of the TL peak at 135°C was used as an index of lipid peroxidation (Havaux, 2003). Chl bleaching was followed on 1-cm-diameter leaf discs, harvested from mature leaves. Leaf discs were kept floating on water and then exposed to white light (2,400 μmol m−2 s−1, 8°C). Discs were rapidly frozen in liquid nitrogen prior to pigment extraction and quantification.
Statistics
Significance analyses were performed using an ANOVA with a pairwise multiple comparison procedure in Origin. Error bars represent the sd.
Sequence data from this article can be found in the EMBL/GenBank data libraries under accession numbers At4g25700 (chy1), At5g52570 (chy2), At1g31800 (lut5), and At2g32640 (szl1).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Production of reduced ROS in wild-type and mutant leaves.
Supplemental Figure S2. PSII repair efficiency under photoxidative stress.
Supplemental Figure S3. F0 and Fm changes upon PSII photoinhibition.
Supplemental Figure S4. Photobleaching of pigment-protein complexes purified from wild-type and szl1 plants.
Supplemental Table S1. Photosensitivity of PSI.
Supplementary Material
Glossary
- ROS
reactive oxygen species
- HL
high light
- 1O2
singlet oxygen
- ETR
electron transport rate
- Chl
chlorophyll
- α-DM
dodecyl-α-d-maltoside
- DCMU
3-(3,4-dichlorophenyl)-1,1-dimethylurea
- Ql
QA redox state
- NPQ
nonphotochemical quenching
- TL
thermoluminescence
- SOSG
singlet oxygen sensor green
- qE
chlorophyll fluorescence quenching
- AA
aminoacridine
- DCF
dichlorofluorescein diacetate
References
- Alboresi A, Ballottari M, Hienerwadel R, Giacometti GM, Morosinotto T. (2009) Antenna complexes protect photosystem I from photoinhibition. BMC Plant Biol 9: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alboresi A, Dall’osto L, Aprile A, Carillo P, Roncaglia E, Cattivelli L, Bassi R. (2011) Reactive oxygen species and transcript analysis upon excess light treatment in wild-type Arabidopsis thaliana vs a photosensitive mutant lacking zeaxanthin and lutein. BMC Plant Biol 11: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts A, Toporik H, Borovikova A, Nelson N. (2010) Structure determination and improved model of plant photosystem I. J Biol Chem 285: 3478–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson B, Salter AH, Virgin I, Vass I, Styring S. (1992) Photodamage to photosystem-II—primary and secondary events. J Photochem Photobiol B 15: 15–31 [Google Scholar]
- Andersson J, Walters RG, Horton P, Jansson S. (2001) Antisense inhibition of the photosynthetic antenna proteins CP29 and CP26: implications for the mechanism of protective energy dissipation. Plant Cell 13: 1193–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreeva A, Abarova S, Stoitchkova K, Picorel R, Velitchkova M. (2007) Selective photobleaching of chlorophylls and carotenoids in photosystem I particles under high-light treatment. Photochem Photobiol 83: 1301–1307 [DOI] [PubMed] [Google Scholar]
- Aro E-M, Virgin I, Andersson B. (1993) Photoinhibition of photosystem II: inactivation, protein damage and turnover. Biochim Biophys Acta 1143: 113–134 [DOI] [PubMed] [Google Scholar]
- Aro EM, McCaffery S, Anderson JM. (1994) Recovery from photoinhibition in peas (Pisum sativum L.) acclimated to varying growth irradiances (role of D1 protein turnover). Plant Physiol 104: 1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K. (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601–639 [DOI] [PubMed] [Google Scholar]
- Baker NR. (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59: 89–113 [DOI] [PubMed] [Google Scholar]
- Barber J, Chapman DJ, Telfer A. (1987) Characterisation of a PS II reaction centre isolated from the chloroplasts of Pisum sativum. FEBS Lett 220: 67–73 [Google Scholar]
- Baroli I, Do AD, Yamane T, Niyogi KK. (2003) Zeaxanthin accumulation in the absence of a functional xanthophyll cycle protects Chlamydomonas reinhardtii from photooxidative stress. Plant Cell 15: 992–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi R, Dainese P. (1992) A supramolecular light-harvesting complex from chloroplast photosystem-II membranes. Eur J Biochem 204: 317–326 [DOI] [PubMed] [Google Scholar]
- Betterle N, Ballottari M, Hienerwadel R, Dall’Osto L, Bassi R. (2010) Dynamics of zeaxanthin binding to the photosystem II monomeric antenna protein Lhcb6 (CP24) and modulation of its photoprotection properties. Arch Biochem Biophys 504: 67–77 [DOI] [PubMed] [Google Scholar]
- Britton G, Liaaen-Jensen S, Pfander H. (2004) Carotenoids Hand Book. Birkhauser, Basel, Switzerland
- Butler WL. (1978) Energy distribution in the photochemical apparatus of photosynthesis. Annu Rev Plant Physiol 29: 345–378 [Google Scholar]
- Caffarri S, Croce R, Breton J, Bassi R. (2001) The major antenna complex of photosystem II has a xanthophyll binding site not involved in light harvesting. J Biol Chem 276: 35924–35933 [DOI] [PubMed] [Google Scholar]
- Carbonera D, Agostini G, Morosinotto T, Bassi R. (2005) Quenching of chlorophyll triplet states by carotenoids in reconstituted Lhca4 subunit of peripheral light-harvesting complex of photosystem I. Biochemistry 44: 8337–8346 [DOI] [PubMed] [Google Scholar]
- Casazza AP, Tarantino D, Soave C. (2001) Preparation and functional characterization of thylakoids from Arabidopsis thaliana. Photosynth Res 68: 175–180 [DOI] [PubMed] [Google Scholar]
- Croce R, Cinque G, Holzwarth AR, Bassi R. (2000) The Soret absorption properties of carotenoids and chlorophylls in antenna complexes of higher plants. Photosynth Res 64: 221–231 [DOI] [PubMed] [Google Scholar]
- Croce R, Müller MG, Caffarri S, Bassi R, Holzwarth AR. (2003) Energy transfer pathways in the minor antenna complex CP29 of photosystem II: a femtosecond study of carotenoid to chlorophyll transfer on mutant and WT complexes. Biophys J 84: 2517–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce R, Zucchelli G, Garlaschi FM, Bassi R, Jennings RC. (1996) Excited state equilibration in the photosystem I-light-harvesting I complex: P700 is almost isoenergetic with its antenna. Biochemistry 35: 8572–8579 [DOI] [PubMed] [Google Scholar]
- Dall’Osto L, Caffarri S, Bassi R. (2005) A mechanism of nonphotochemical energy dissipation, independent from PsbS, revealed by a conformational change in the antenna protein CP26. Plant Cell 17: 1217–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Osto L, Cazzaniga S, Havaux M, Bassi R. (2010) Enhanced photoprotection by protein-bound vs free xanthophyll pools: a comparative analysis of chlorophyll b and xanthophyll biosynthesis mutants. Mol Plant 3: 576–593 [DOI] [PubMed] [Google Scholar]
- Dall’Osto L, Cazzaniga S, North H, Marion-Poll A, Bassi R. (2007a) The Arabidopsis aba4-1 mutant reveals a specific function for neoxanthin in protection against photooxidative stress. Plant Cell 19: 1048–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Osto L, Fiore A, Cazzaniga S, Giuliano G, Bassi R. (2007b) Different roles of α- and β-branch xanthophylls in photosystem assembly and photoprotection. J Biol Chem 282: 35056–35068 [DOI] [PubMed] [Google Scholar]
- Dall’Osto L, Lico C, Alric J, Giuliano G, Havaux M, Bassi R. (2006) Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol 6: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bianchi S, Betterle N, Kouril R, Cazzaniga S, Boekema E, Bassi R, Dall’Osto L. (2011) Arabidopsis mutants deleted in the light-harvesting protein Lhcb4 have a disrupted photosystem II macrostructure and are defective in photoprotection. Plant Cell 23: 2659–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bianchi S, Dall’Osto L, Tognon G, Morosinotto T, Bassi R. (2008) Minor antenna proteins CP24 and CP26 affect the interactions between photosystem II subunits and the electron transport rate in grana membranes of Arabidopsis. Plant Cell 20: 1012–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW. (1992) Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol 43: 599–626 [Google Scholar]
- Ducruet JM. (2003) Chlorophyll thermoluminescence of leaf discs: simple instruments and progress in signal interpretation open the way to new ecophysiological indicators. J Exp Bot 54: 2419–2430 [DOI] [PubMed] [Google Scholar]
- Ducruet JM, Vavilin D. (1999) Chlorophyll high-temperature thermoluminescence emission as an indicator of oxidative stress: perturbating effects of oxygen and leaf water content. Free Radic Res (Suppl) 31: S187–S192 [DOI] [PubMed] [Google Scholar]
- Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S. (2004) Architecture of the photosynthetic oxygen-evolving center. Science 303: 1831–1838 [DOI] [PubMed] [Google Scholar]
- Fiore A, Dall’osto L, Fraser PD, Bassi R, Giuliano G. (2006) Elucidation of the beta-carotene hydroxylation pathway in Arabidopsis thaliana. FEBS Lett 580: 4718–4722 [DOI] [PubMed] [Google Scholar]
- Flors C, Fryer MJ, Waring J, Reeder B, Bechtold U, Mullineaux PM, Nonell S, Wilson MT, Baker NR. (2006) Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, singlet oxygen sensor green. J Exp Bot 57: 1725–1734 [DOI] [PubMed] [Google Scholar]
- Gilmore AM. (2001) Xanthophyll cycle-dependent nonphotochemical quenching in photosystem II: mechanistic insights gained from Arabidopsis thaliana L. mutants that lack violaxanthin deepoxidase activity and/or lutein. Photosynth Res 67: 89–101 [DOI] [PubMed] [Google Scholar]
- Gilmore AM, Yamamoto HY. (1991) Zeaxanthin formation and energy-dependent fluorescence quenching in pea chloroplasts under artificially mediated linear and cyclic electron transport. Plant Physiol 96: 635–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruszecki WI, Strzałka K. (2005) Carotenoids as modulators of lipid membrane physical properties. Biochim Biophys Acta 1740: 108–115 [DOI] [PubMed] [Google Scholar]
- Havaux M. (2003) Spontaneous and thermoinduced photon emission: new methods to detect and quantify oxidative stress in plants. Trends Plant Sci 8: 409–413 [DOI] [PubMed] [Google Scholar]
- Havaux M, Dall’osto L, Bassi R. (2007) Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol 145: 1506–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Dall’Osto L, Cuiné S, Giuliano G, Bassi R. (2004) The effect of zeaxanthin as the only xanthophyll on the structure and function of the photosynthetic apparatus in Arabidopsis thaliana. J Biol Chem 279: 13878–13888 [DOI] [PubMed] [Google Scholar]
- Havaux M, Niyogi KK. (1999) The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA 96: 8762–8767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrin DL, Battey JF, Greer K, Schmidt GW. (1992) Regulation of chlorophyll apoprotein expression and accumulation: requirements for carotenoids and chlorophyll. J Biol Chem 267: 8260–8269 [PubMed] [Google Scholar]
- Hirschberg J. (2001) Carotenoid biosynthesis in flowering plants. Curr Opin Plant Biol 4: 210–218 [DOI] [PubMed] [Google Scholar]
- Inoue K, Sakurai M, Hiyama T. (1986) Photoinactivation sites of photosystem I in isolated chloroplasts. Plant Cell Physiol 27: 961–968 [Google Scholar]
- Jensen PE, Bassi R, Boekema EJ, Dekker JP, Jansson S, Leister D, Robinson C, Scheller HV. (2007) Structure, function and regulation of plant photosystem I. Biochim Biophys Acta 1767: 335–352 [DOI] [PubMed] [Google Scholar]
- Johnson GN, Young AJ, Horton P. (1994) Activation of non-photochemical quenching in thylakoids and leaves. Planta 194: 550–556 [Google Scholar]
- Kim J, DellaPenna D. (2006) Defining the primary route for lutein synthesis in plants: the role of Arabidopsis carotenoid beta-ring hydroxylase CYP97A3. Proc Natl Acad Sci USA 103: 3474–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Smith JJ, Tian L, Dellapenna D. (2009) The evolution and function of carotenoid hydroxylases in Arabidopsis. Plant Cell Physiol 50: 463–479 [DOI] [PubMed] [Google Scholar]
- Krieger-Liszkay A. (2005) Singlet oxygen production in photosynthesis. J Exp Bot 56: 337–346 [DOI] [PubMed] [Google Scholar]
- Külheim C, Agren J, Jansson S. (2002) Rapid regulation of light harvesting and plant fitness in the field. Science 297: 91–93 [DOI] [PubMed] [Google Scholar]
- Li Z, Ahn TK, Avenson TJ, Ballottari M, Cruz JA, Kramer DM, Bassi R, Fleming GR, Keasling JD, Niyogi KK. (2009) Lutein accumulation in the absence of zeaxanthin restores nonphotochemical quenching in the Arabidopsis thaliana npq1 mutant. Plant Cell 21: 1798–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Yan H, Wang K, Kuang T, Zhang J, Gui L, An X, Chang W. (2004) Crystal structure of spinach major light-harvesting complex at 2.72 A resolution. Nature 428: 287–292 [DOI] [PubMed] [Google Scholar]
- Malkin S, Armond PA, Mooney HA, Fork DC. (1981) Photosystem II photosynthetic unit sizes from fluorescence induction in leaves: correlation to photosynthetic capacity. Plant Physiol 67: 570–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozzo M, Dall’Osto L, Hienerwadel R, Bassi R, Croce R. (2008) Photoprotection in the antenna complexes of photosystem II: role of individual xanthophylls in chlorophyll triplet quenching. J Biol Chem 283: 6184–6192 [DOI] [PubMed] [Google Scholar]
- Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T. (2002) PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110: 361–371 [DOI] [PubMed] [Google Scholar]
- Niyogi KK. (2000) Safety valves for photosynthesis. Curr Opin Plant Biol 3: 455–460 [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Björkman O, Grossman AR. (1997) The roles of specific xanthophylls in photoprotection. Proc Natl Acad Sci USA 94: 14162–14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Grossman AR, Björkman O. (1998) Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10: 1121–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Shih C, Soon Chow W, Pogson BJ, Dellapenna D, Björkman O. (2001) Photoprotection in a zeaxanthin- and lutein-deficient double mutant of Arabidopsis. Photosynth Res 67: 139–145 [DOI] [PubMed] [Google Scholar]
- Pan X, Li M, Wan T, Wang L, Jia C, Hou Z, Zhao X, Zhang J, Chang W. (2011) Structural insights into energy regulation of light-harvesting complex CP29 from spinach. Nat Struct Mol Biol 18: 309–315 [DOI] [PubMed] [Google Scholar]
- Passarini F, Wientjes E, Hienerwadel R, Croce R. (2009) Molecular basis of light harvesting and photoprotection in CP24: unique features of the most recent antenna complex. J Biol Chem 284: 29536–29546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter GF, Takeuchi T, Thornber JP. (1991) Solubilization and two-dimensional electrophoretic procedures for studying the organization and composition of photosynthetic membrane polypeptides. Methods 3: 115–124 [Google Scholar]
- Peterman EJ, Dukker FM, van Grondelle R, van Amerongen H. (1995) Chlorophyll a and carotenoid triplet states in light-harvesting complex II of higher plants. Biophys J 69: 2670–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson B, McDonald KA, Truong M, Britton G, DellaPenna D. (1996) Arabidopsis carotenoid mutants demonstrate that lutein is not essential for photosynthesis in higher plants. Plant Cell 8: 1627–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel F, Sulmon C, Bogard M, Couée I, Gouesbet G. (2009) Differential patterns of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets. BMC Plant Biol 9: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport F, Béal D, Joliot A, Joliot P. (2007) On the advantages of using green light to study fluorescence yield changes in leaves. Biochim Biophys Acta 1767: 56–65 [DOI] [PubMed] [Google Scholar]
- Rutherford AW, Osyczka A, Rappaport F. (2012) Back-reactions, short-circuits, leaks and other energy wasteful reactions in biological electron transfer: redox tuning to survive life in O(2). FEBS Lett 586: 603–616 [DOI] [PubMed] [Google Scholar]
- Santabarbara S, Carbonera D. (2005) Carotenoid triplet states associated with the long-wavelength-emitting chlorophyll forms of photosystem I in isolated thylakoid membranes. J Phys Chem B 109: 986–991 [DOI] [PubMed] [Google Scholar]
- Sonoike K. (2011) Photoinhibition of photosystem I. Physiol Plant 142: 56–64 [DOI] [PubMed] [Google Scholar]
- Sonoike K, Terashima I. (1994) Mechanism of photosystem-I photoinhibition in leaves of Cucumis-Sativus l. Planta 194: 287–293 [Google Scholar]
- Telfer A. (2005) Too much light? How β-carotene protects the photosystem II reaction centre. Photochem Photobiol Sci 4: 950–956 [DOI] [PubMed] [Google Scholar]
- Telfer A, Bishop SM, Phillips D, Barber J. (1994a) Isolated photosynthetic reaction center of photosystem II as a sensitizer for the formation of singlet oxygen: detection and quantum yield determination using a chemical trapping technique. J Biol Chem 269: 13244–13253 [PubMed] [Google Scholar]
- Telfer A, De Las Rivas J, Barber J. (1991) Beta-carotene within the isolated photosystem II reaction centre: photooxidation and irreversible bleaching of this chromophore by oxidised P680. Biochim Biophys Acta 1060: 106–114 [Google Scholar]
- Telfer A, Dhami S, Bishop SM, Phillips D, Barber J. (1994b) β-Carotene quenches singlet oxygen formed by isolated photosystem II reaction centers. Biochemistry 33: 14469–14474 [DOI] [PubMed] [Google Scholar]
- Tian L, Magallanes-Lundback M, Musetti V, DellaPenna D. (2003) Functional analysis of beta- and epsilon-ring carotenoid hydroxylases in Arabidopsis. Plant Cell 15: 1320–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Musetti V, Kim J, Magallanes-Lundback M, DellaPenna D. (2004) The Arabidopsis LUT1 locus encodes a member of the cytochrome p450 family that is required for carotenoid epsilon-ring hydroxylation activity. Proc Natl Acad Sci USA 101: 402–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjus SE, Andersson B. (1993) Loss of the trans-thylakoid proton gradient is an early event during photoinhibitory illumination of chloroplast preparations. Biochim Biophys Acta 1183: 315–322 [Google Scholar]
- Trebst A, Depka B. (1997) Role of carotene in the rapid turnover and assembly of photosystem II in Chlamydomonas reinhardtii. FEBS Lett 400: 359–362 [DOI] [PubMed] [Google Scholar]
- Trevithick-Sutton CC, Foote CS, Collins M, Trevithick JR. (2006) The retinal carotenoids zeaxanthin and lutein scavenge superoxide and hydroxyl radicals: a chemiluminescence and ESR study. Mol Vis 12: 1127–1135 [PubMed] [Google Scholar]
- Triantaphylidès C, Krischke M, Hoeberichts FA, Ksas B, Gresser G, Havaux M, Van Breusegem F, Mueller MJ. (2008) Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol 148: 960–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umena Y, Kawakami K, Shen JR, Kamiya N. (2011) Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473: 55–60 [DOI] [PubMed] [Google Scholar]
- Van Kooten O, Snel JFH. (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25: 147–150 [DOI] [PubMed] [Google Scholar]
- Walters RG, Horton P. (1995) Acclimation of Arabidopsis thaliana to the light environment: changes in photosynthetic function. Planta 197: 306–312 [DOI] [PubMed] [Google Scholar]
- Wehner A, Storf S, Jahns P, Schmid VH. (2004) De-epoxidation of violaxanthin in light-harvesting complex I proteins. J Biol Chem 279: 26823–26829 [DOI] [PubMed] [Google Scholar]
- Yang J-S, Wang R, Meng J-J, Bi Y-P, Xu P-L, Guo F, Wan S-B, He Q-W, Li XG. (2010) Overexpression of Arabidopsis CBF1 gene in transgenic tobacco alleviates photoinhibition of PSII and PSI during chilling stress under low irradiance. J Plant Physiol 167: 534–539 [DOI] [PubMed] [Google Scholar]
- Zhang S, Scheller HV. (2004) Photoinhibition of photosystem I at chilling temperature and subsequent recovery in Arabidopsis thaliana. Plant Cell Physiol 45: 1595–1602 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.