Abstract
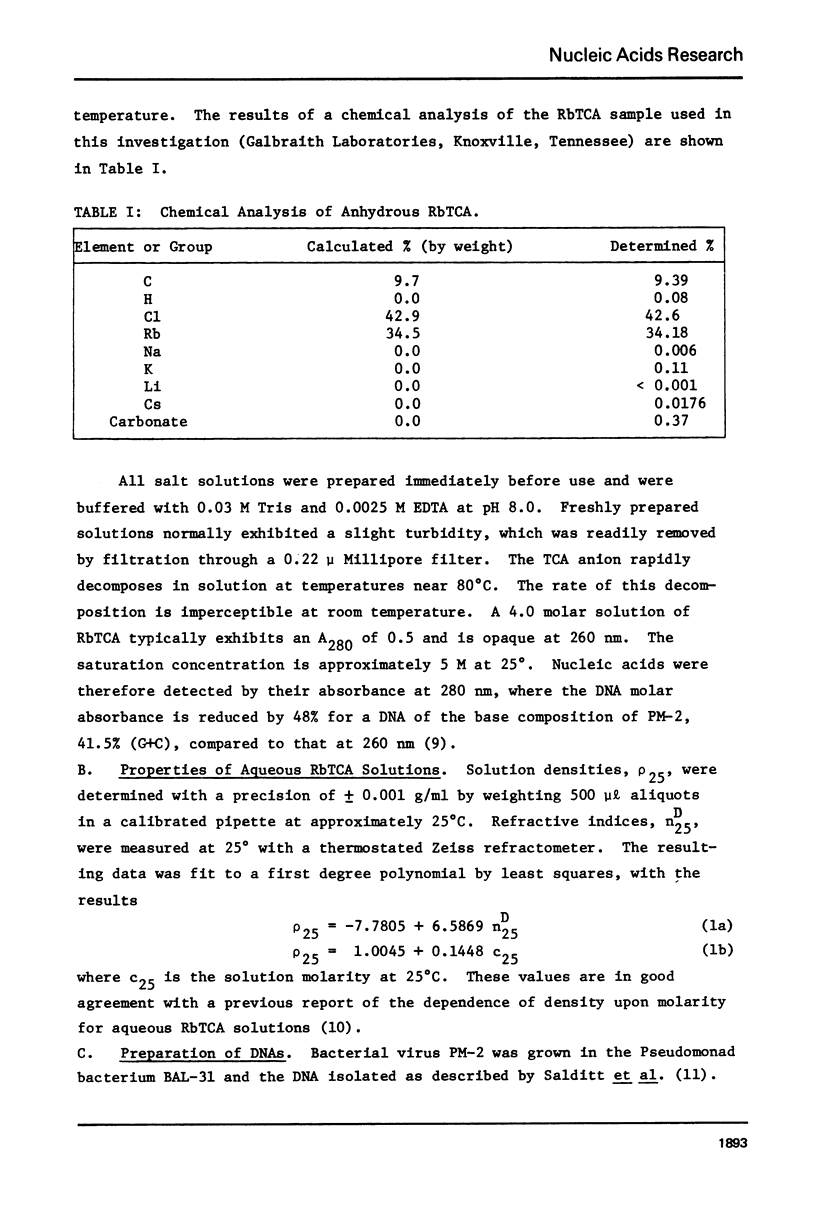
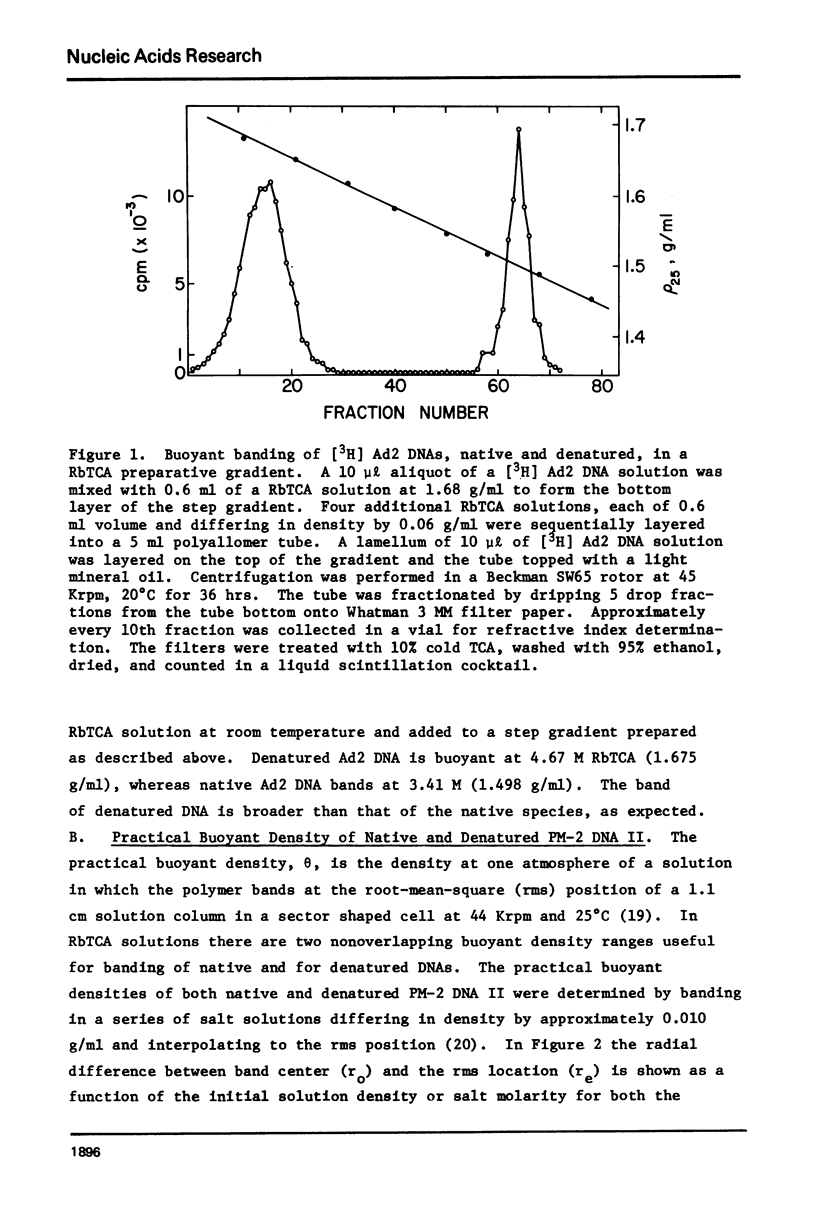
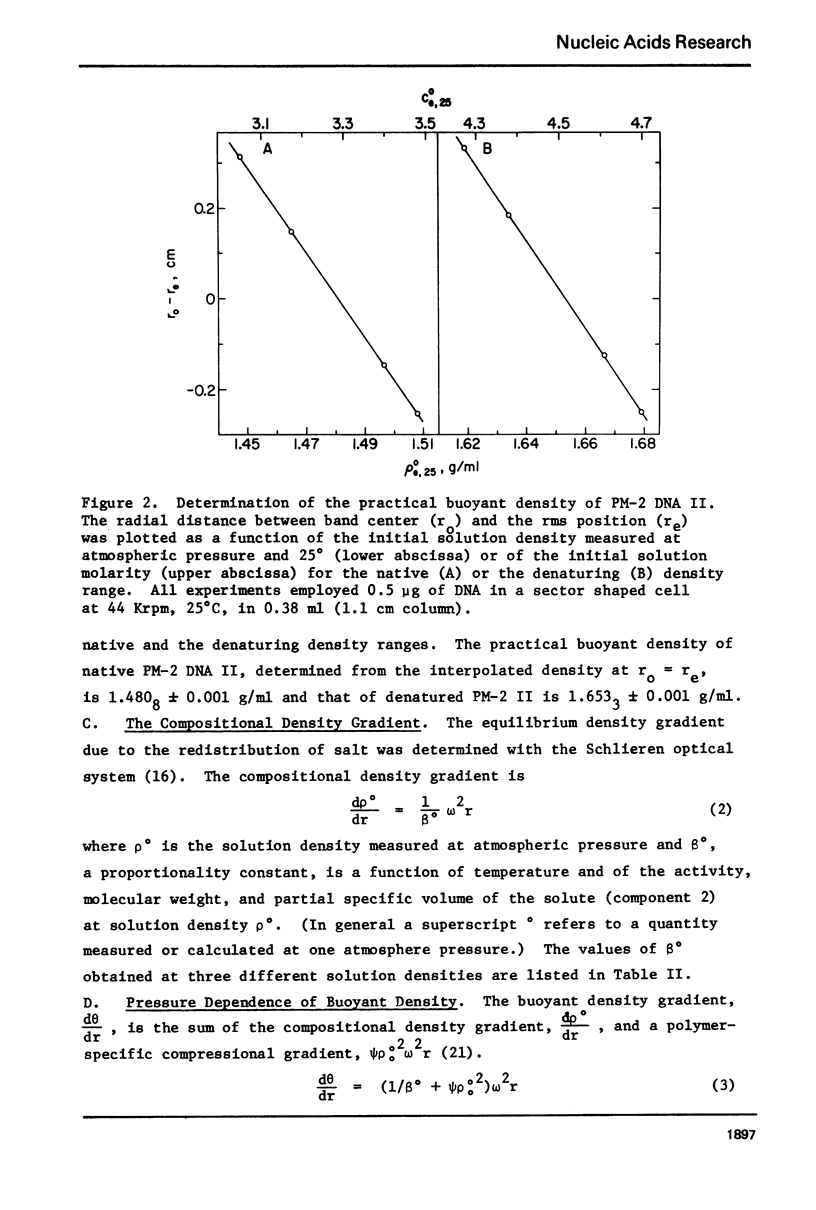
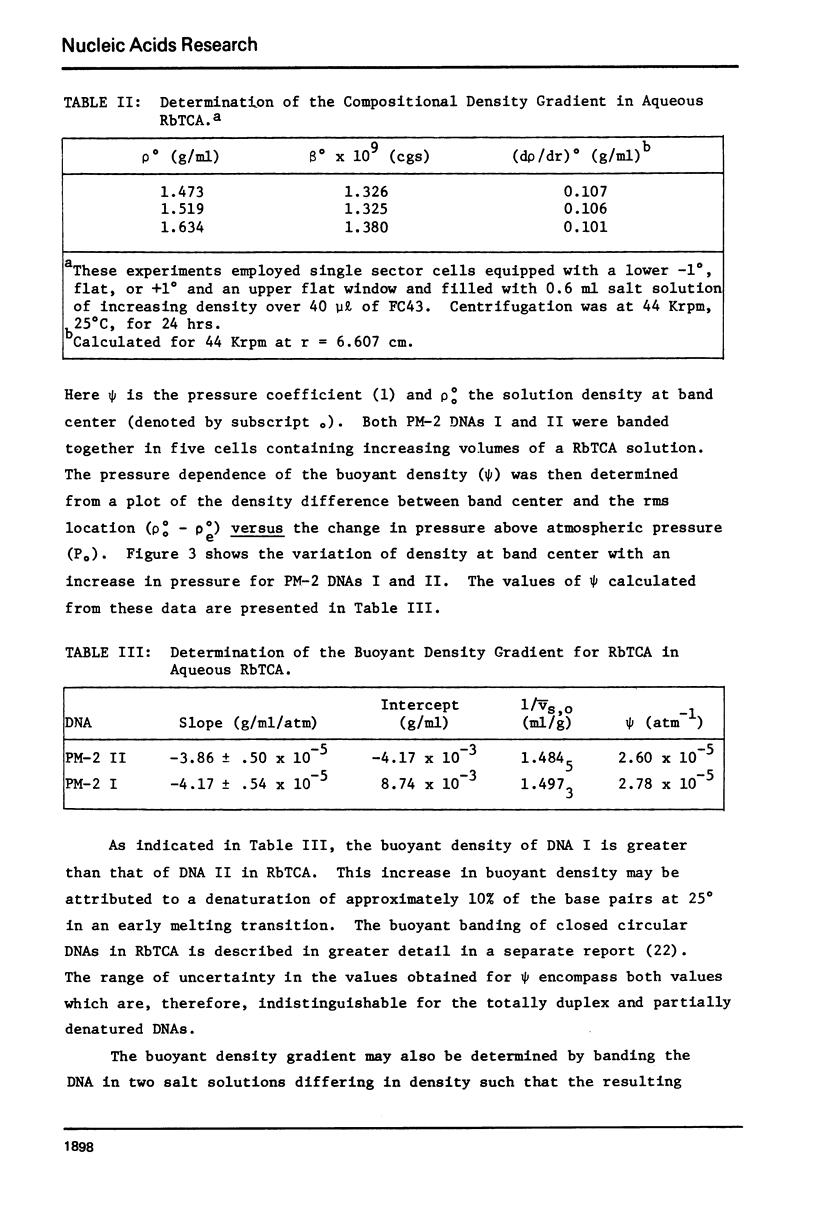
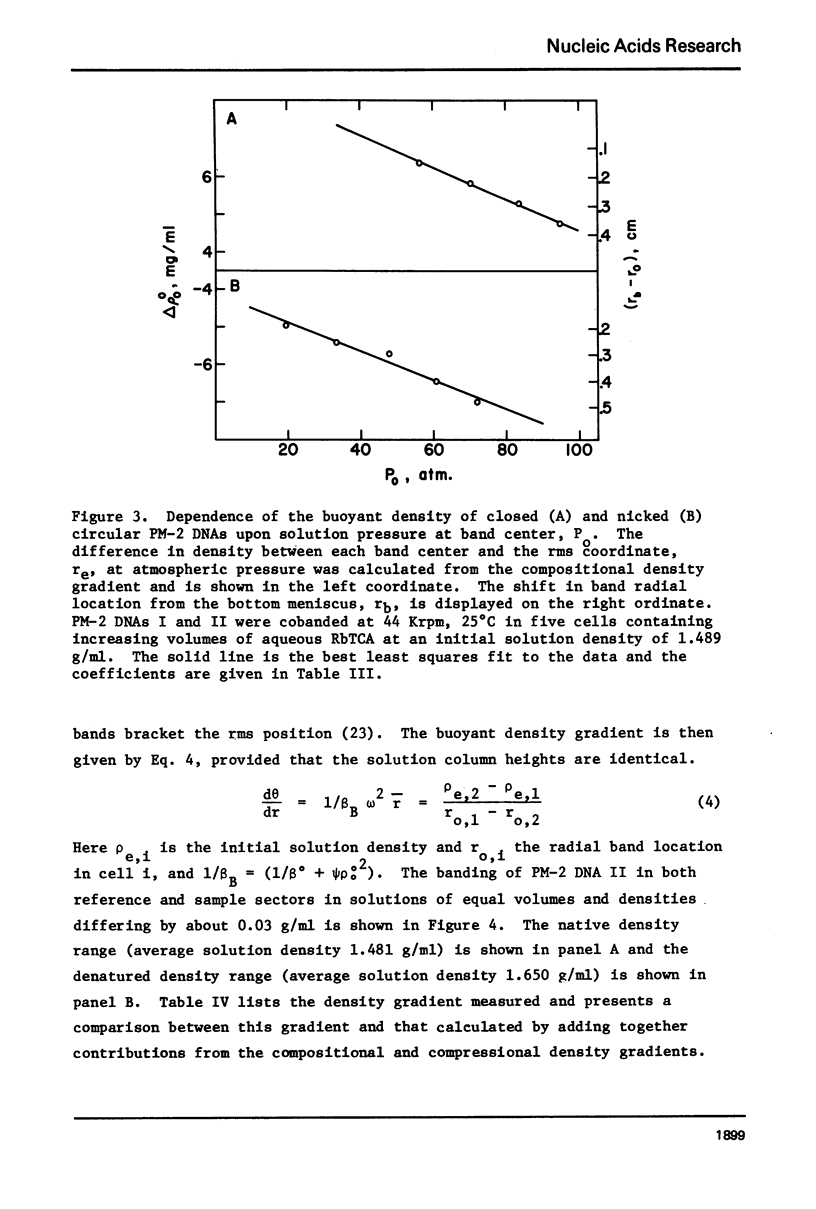
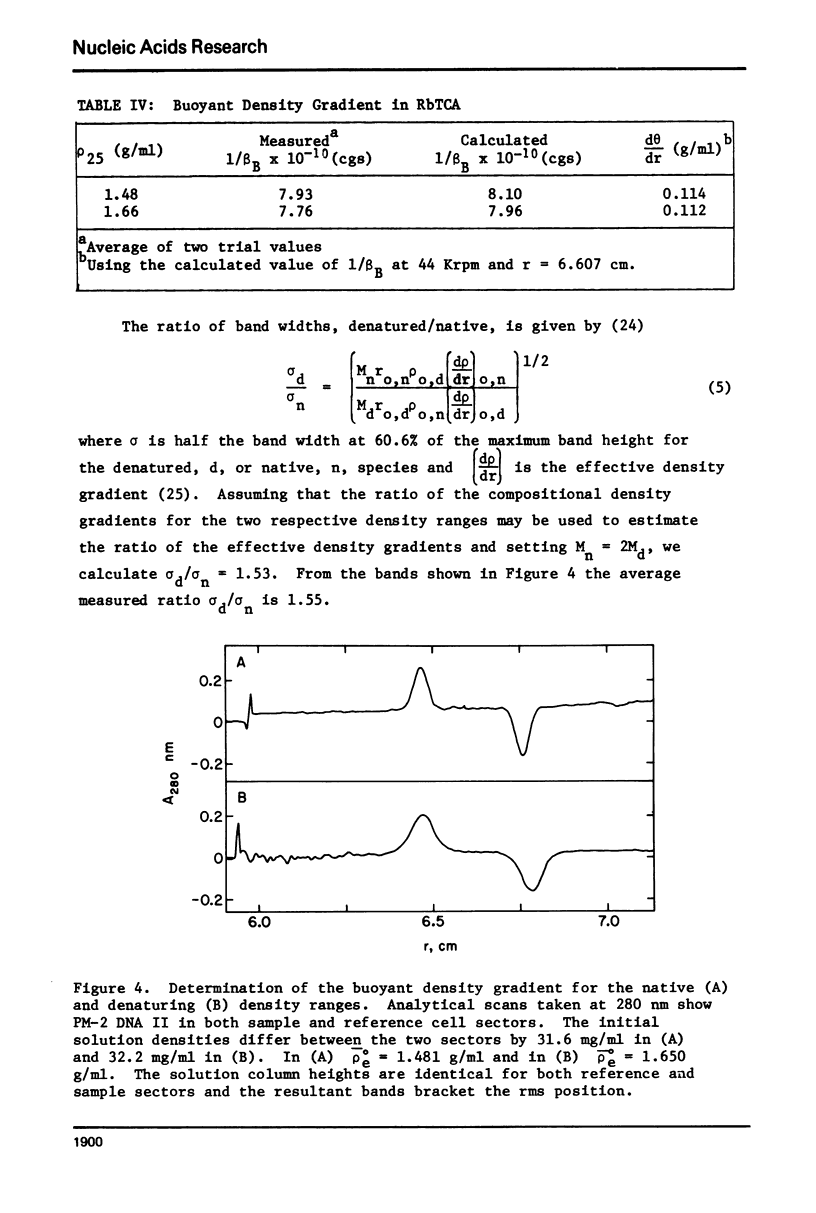
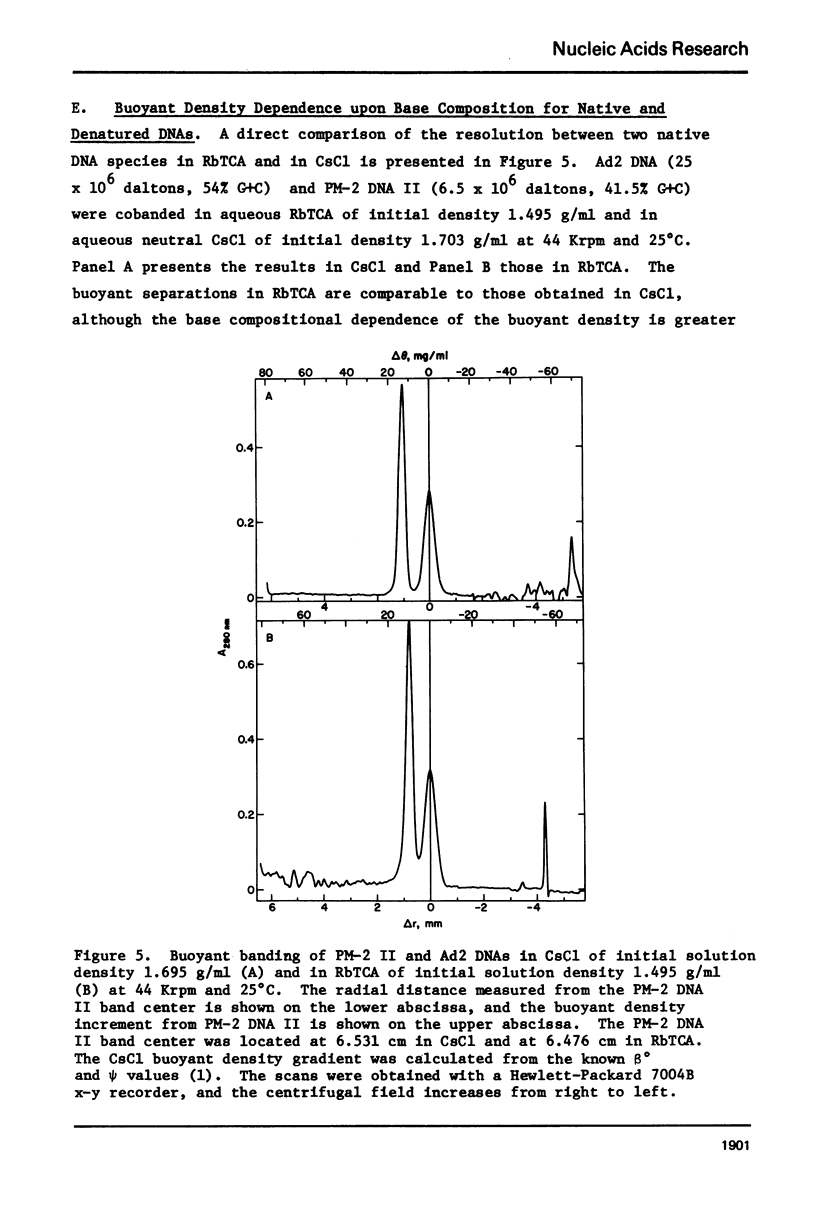
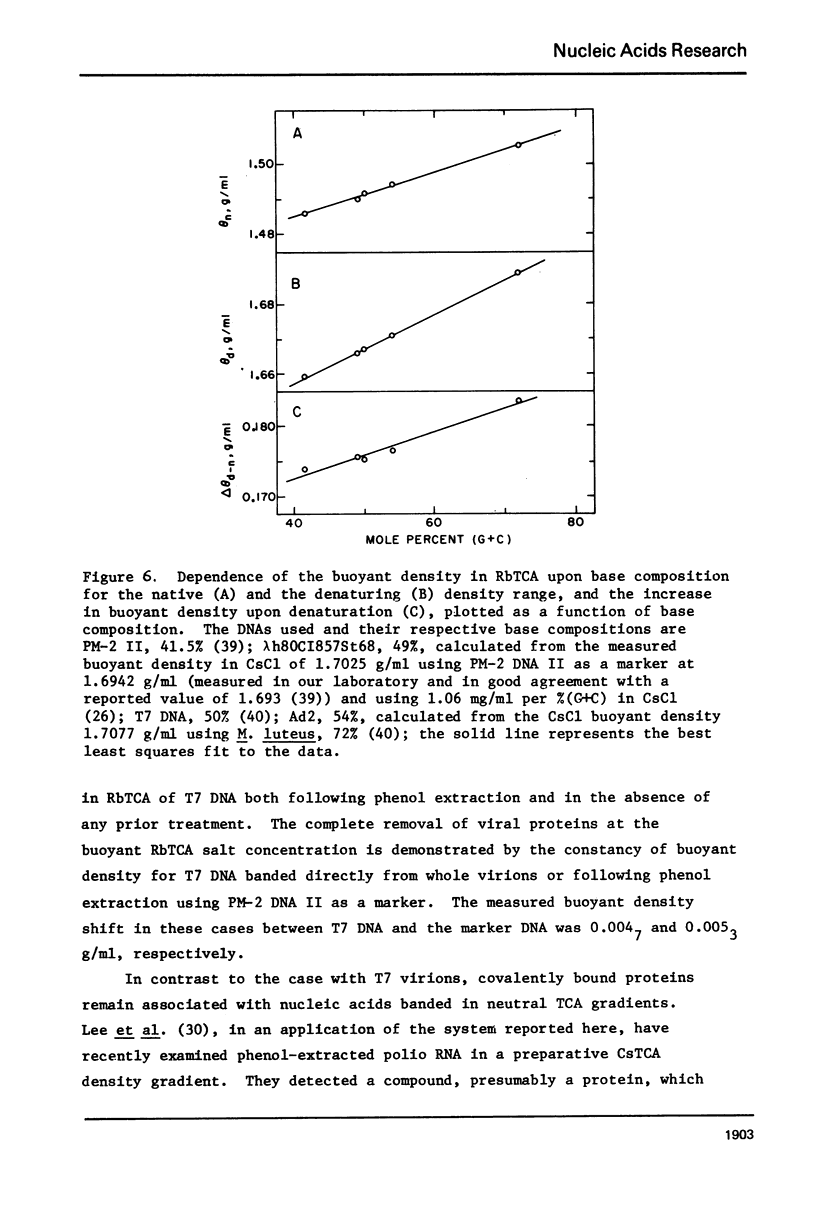
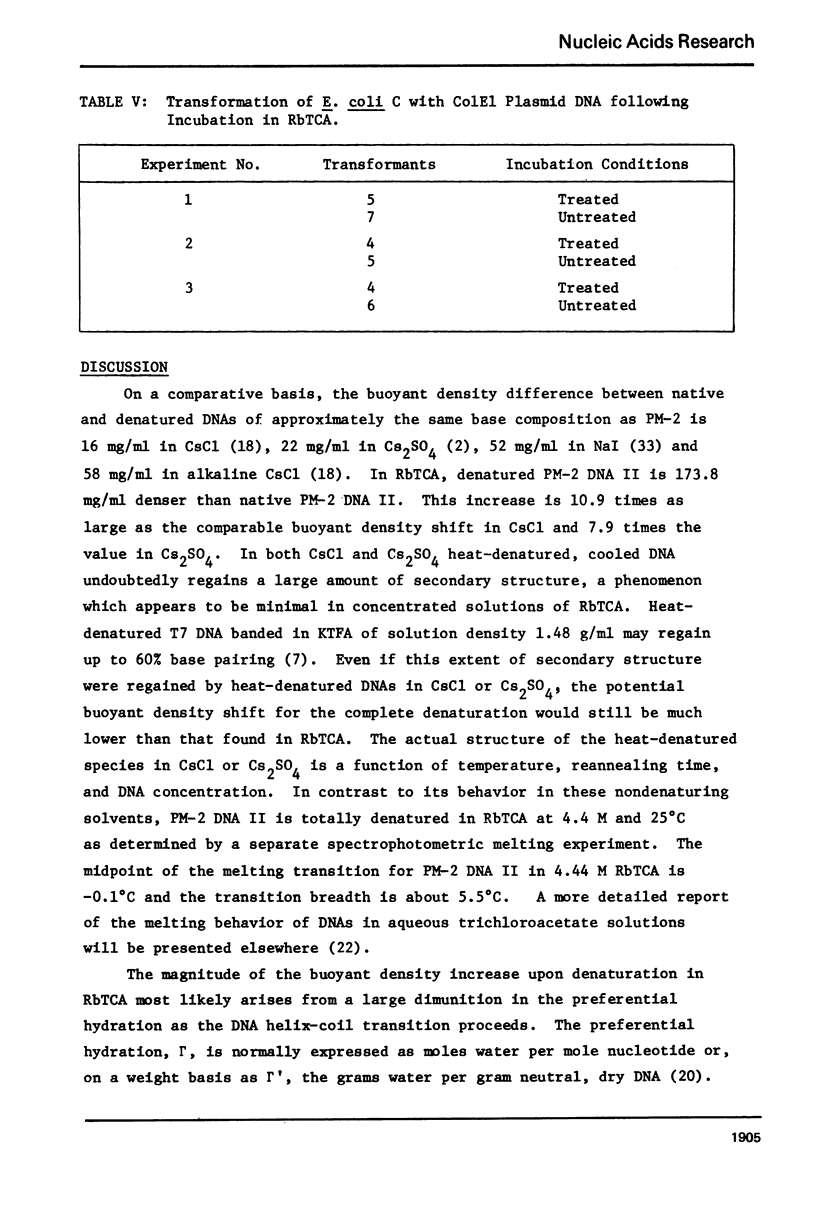
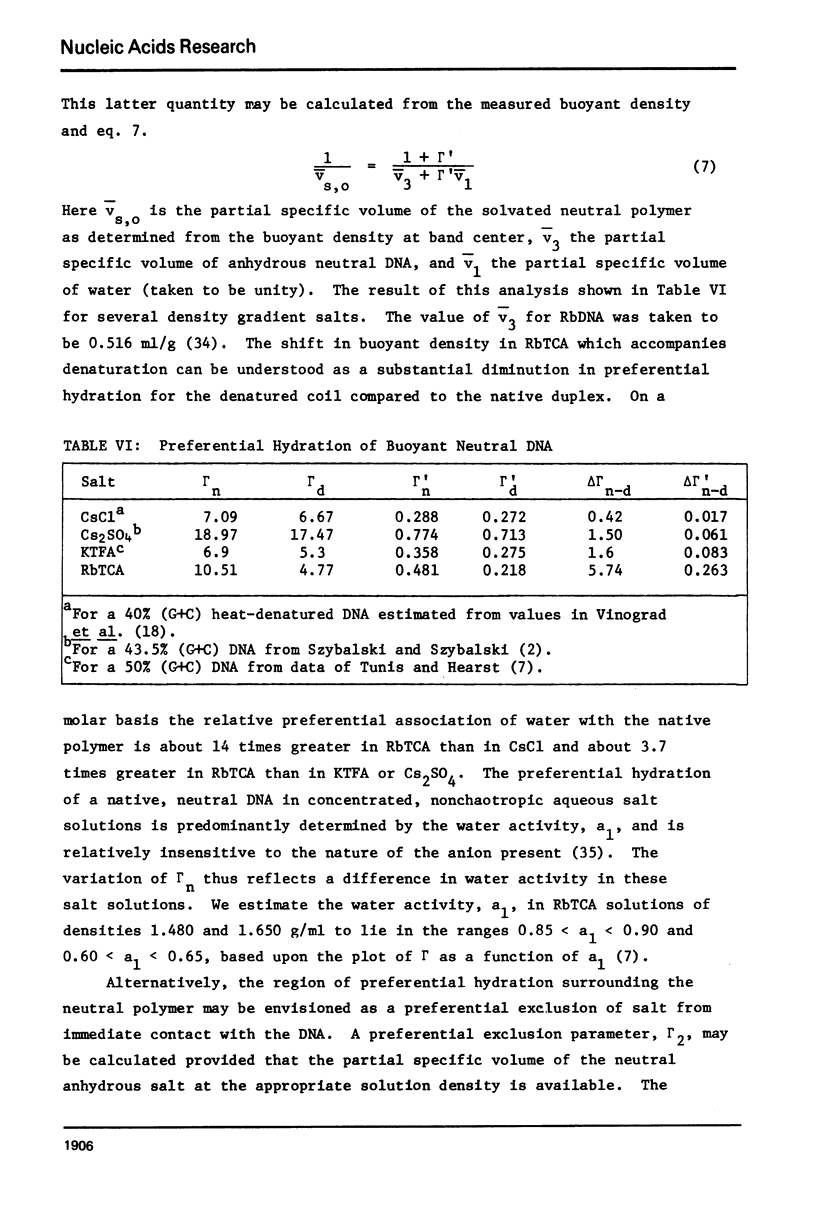
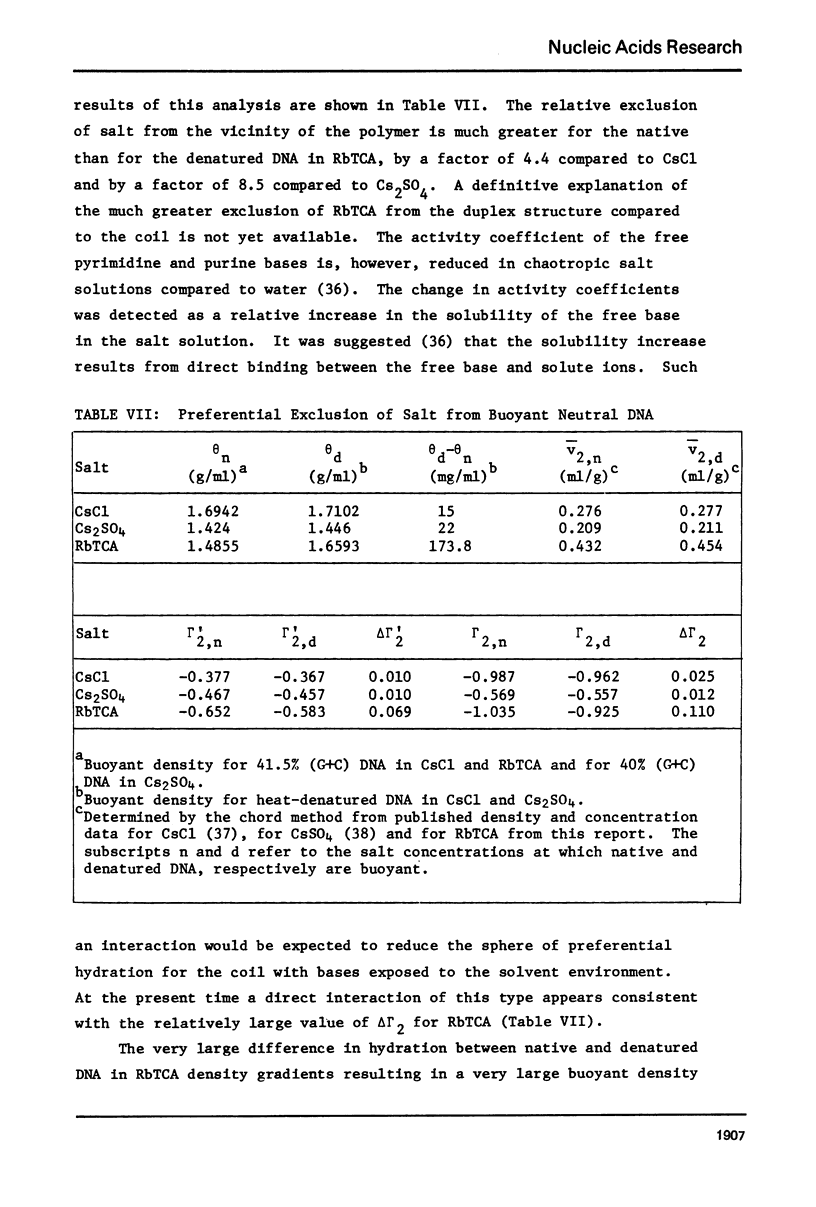
Aqueous RbTCA is generally suitable as a buoyant solvent for both native and denatured DNA at neutral pH and room temperature. Native PM-2 DNA II, for example, is buoyant at 3.29 M salt, 25°C; whereas the denatured strands band together at 4.52 M. Two properties of the solvent make this system uniquely useful for separations based upon the extent of secondary structure. First, the melting transition temperature for chemically unaltered DNA is depressed to room temperature or below. Second, the buoyant density increase accompanying denaturation is extraordinarily large, 174 mg/ml for PM-2 DNA II. This value is three times that found in aqueous NaI and ten times that for CsCl. The properties of the RbTCA buoyant solvent presented here include the compositional and buoyant density gradients and the buoyant density dependence upon base composition. The DNA remains chemically unaltered after exposure to RbTCA as shown by the absence of strand scissions for closed circular DNA and by the unimpaired biological activity in transformation assays. Intact virion DNA may be isolated by direct banding of whole virions in RbTCA gradients without prior phenol extraction. Strongly complexed or covalently bound proteins may be detected by their association with the buoyant polymer in the denaturing density gradient.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Bauer W., Vinogradj The interaction of closed circular DNA with intercalative dyes. 3. Dependence of the buoyant density upon superhelix density and base composition. J Mol Biol. 1970 Dec 14;54(2):281–298. doi: 10.1016/0022-2836(70)90430-4. [DOI] [PubMed] [Google Scholar]
- Birnie G. D. Separation of native and denatured DNA, RNA and hybrid on sodium iodide gradients. FEBS Lett. 1972 Oct 15;27(1):19–22. doi: 10.1016/0014-5793(72)80399-5. [DOI] [PubMed] [Google Scholar]
- Enea V., Zinder N. D. Guanidinium-CsCl density gradients for isopycnic analysis of nucleic acids. Science. 1975 Nov 7;190(4214):584–586. doi: 10.1126/science.1188358. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. DNA of bacteriophage PM2: a closed circular double-stranded molecule. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1164–1168. doi: 10.1073/pnas.63.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREIFELDER D. A NOVEL METHOD FOR THE RELEASE OF BACTERIOPHAGE DNA. Biochem Biophys Res Commun. 1965 Jan 4;18:141–144. doi: 10.1016/0006-291x(65)90897-1. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G., Hirschman S. Z. A neighbor-interaction analysis of the hypochromism and spectra of DNA. J Mol Biol. 1965 Sep;13(2):407–427. doi: 10.1016/s0022-2836(65)80106-1. [DOI] [PubMed] [Google Scholar]
- Franklin R. M., Salditt M., Silbert J. A. Structure and synthesis of a lipid-containing bacteriophage. I. Growth of bacteriophage PM2 and alterations in nucleic acid metabolism in the infected cell. Virology. 1969 Aug;38(4):627–640. doi: 10.1016/0042-6822(69)90182-2. [DOI] [PubMed] [Google Scholar]
- Goad W. B., Cann J. R. V. Chemically interacting systems. I. Theory of sedimentation of interacting systems. Ann N Y Acad Sci. 1969 Nov 7;164(1):192–225. doi: 10.1111/j.1749-6632.1969.tb14039.x. [DOI] [PubMed] [Google Scholar]
- Goebel W., Bonewald R. Class of small multicopy plasmids originating from the mutant antibiotic resistance factor R1 drd-19B2. J Bacteriol. 1975 Aug;123(2):658–665. doi: 10.1128/jb.123.2.658-665.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEARST J. E., IFFT J. B., VINOGRAD J. The effects of pressure on the buoyant behavior of deoxyribonucleic acid and tobacco mosaic virus in a density gradient at equilibrium in the ultracentrifuge. Proc Natl Acad Sci U S A. 1961 Jul 15;47:1015–1025. doi: 10.1073/pnas.47.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEARST J. E. The specific volume of various cationic forms of deoxyribonucleic acid. J Mol Biol. 1962 May;4:415–417. doi: 10.1016/s0022-2836(62)80024-2. [DOI] [PubMed] [Google Scholar]
- HEARST J. E., VINOGRAD J. The net hydration of T-4 bacteriophage deoxyribonuecleic acid and the effect of hydration on buoyant behavior in a density gradient at equilibrium in the ultracentrifuge. Proc Natl Acad Sci U S A. 1961 Jul 15;47:1005–1014. doi: 10.1073/pnas.47.7.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearst J. E., Vinograd J. A THREE-COMPONENT THEORY OF SEDIMENTATION EQUILIBRIUM IN A DENSITY GRADIENT. Proc Natl Acad Sci U S A. 1961 Jul;47(7):999–1004. doi: 10.1073/pnas.47.7.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. E., Fangman W. L. Scission of Escherichia coli deoxyribonucleic acid in alkali. Biochemistry. 1973 Apr 24;12(9):1772–1774. doi: 10.1021/bi00733a017. [DOI] [PubMed] [Google Scholar]
- Ifft J. B., Martin W. R., 3rd, Kinzie K. Density gradient proportionality constants for a number of aqueous binary solutions. Biopolymers. 1970;9(5):597–614. doi: 10.1002/bip.1970.360090505. [DOI] [PubMed] [Google Scholar]
- LUDLUM D. B., WARNER R. C. EQUILIBRIUM CENTRIFUGATION IN CESIUM SULFATE SOLUTIONS. J Biol Chem. 1965 Jul;240:2961–2965. [PubMed] [Google Scholar]
- Lee Y. F., Nomoto A., Detjen B. M., Wimmer E. A protein covalently linked to poliovirus genome RNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):59–63. doi: 10.1073/pnas.74.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozeron H. A., Szybalski W. Suppression of RNA precipitation during Cs2SO4 density gradient centrifugation. Biochem Biophys Res Commun. 1966 Jun 13;23(5):612–618. doi: 10.1016/0006-291x(66)90443-8. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Bellett J. D. A circular DNA-protein complex adenoviruses and its possible role in DNA replication. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):523–531. doi: 10.1101/sqb.1974.039.01.064. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Younghusband H. B., Bellett A. J. A circula DNA-protein complex from adenoviruses. Virology. 1973 Nov;56(1):54–69. doi: 10.1016/0042-6822(73)90287-0. [DOI] [PubMed] [Google Scholar]
- Robinson D. R., Grant M. E. The effects of aqueous salt solutions on the activity coefficients of purine and pyrimidine bases and their relation to the denaturation of deoxyribonucleic acid by salts. J Biol Chem. 1966 Sep 10;241(17):4030–4042. [PubMed] [Google Scholar]
- Salditt M., Braunstein S. N., Camerini-Otero R. D., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. X. Improved techniques for the purification of bacteriophage PM2. Virology. 1972 Apr;48(1):259–262. doi: 10.1016/0042-6822(72)90133-x. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Tunis M. J., Hearst J. E. On the hydration of DNA. I. Preferential hydration and stability of DNA in concentrated trifluoroacetate solution. Biopolymers. 1968;6(9):1325–1344. doi: 10.1002/bip.1968.360060908. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., HEARST J. E. Equilibrium sedimentation of macromolecules and viruses in a density gradient. Fortschr Chem Org Naturst. 1962;20:373–422. [PubMed] [Google Scholar]
- VINOGRAD J., MORRIS J., DAVIDSON N., DOVE W. F., Jr The bouyant behavior of viral and bacterial DNA in alkaline CsCl. Proc Natl Acad Sci U S A. 1963 Jan 15;49:12–17. doi: 10.1073/pnas.49.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. E., Vinograd J. The buoyant behavior of RNA and DNA in cesium sulfate solutions containing dimethylsulfoxide. Biochim Biophys Acta. 1971 Jan 28;228(2):423–439. doi: 10.1016/0005-2787(71)90048-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]


