The clinical benefits, clinical use, mechanism of action, bioanalysis, pharmacokinetics, pharmacogenetics, pharmacodynamics, drug resistance, toxicity, and patient instructions and recommendations for supportive care for pazopanib and axitinib are discussed.
Keywords: Pazopanib, Axitinib, Drug profile, Angiogenesis, VEGF
Learning Objectives
After completing this course, the reader will be able to:
Identify the current indications for pazopanib and axitinib.
Describe the mechanism of action and the pharmacokinetics of pazopanib and axitinib.
Enumerate the clinical benefits of pazopanib and axitinib, and describe the position of these drugs in the treatment paradigm of metastatic renal cell cancer.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Pazopanib and axitinib are both U.S. Food and Drug Administration approved ATP-competitive inhibitors of the vascular endothelial growth factor receptor. Pazopanib and axitinib have been shown to be effective and tolerable treatment options for patients with metastatic renal cell cancer and therefore have enlarged the armamentarium for this disease. This concise drug review discusses the clinical benefits, clinical use, mechanism of action, bioanalysis, pharmacokinetics, pharmacogenetics, pharmacodynamics, drug resistance, toxicity, and patient instructions and recommendations for supportive care for these two drugs.
Introduction
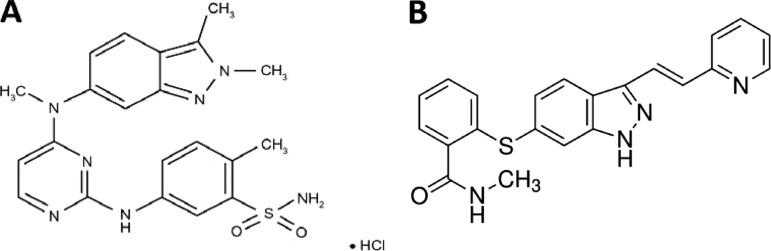
Pazopanib (Votrient®; GlaxoSmithKline, Brentford, U.K.) (Fig. 1A) is currently approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency for the treatment of patients with metastatic renal cell carcinoma (mRCC) [1]. In the pivotal phase III study in patients with mRCC, pazopanib led to a clinically relevant and statistically significant longer progression-free survival (PFS) time of 5 months versus placebo, and it has been shown to provide clinical benefit in patients with various other tumor types, including soft tissue sarcoma, non-small cell lung cancer, ovarian cancer, and thyroid cancer [2–6]. Axitinib (Inlyta®; Pfizer Inc., New York) (Fig. 1B) was recently approved by the FDA for the treatment of patients with mRCC after failure of one prior systemic therapy. This approval followed the FDA advisory committees, who described the favorable benefit–risk profile of axitinib in patients with previously treated advanced RCC based on a comparative phase III trial of axitinib versus sorafenib [7, 8]. Pazopanib and axitinib are both small molecule angiogenesis inhibitors with similar drug profiles (Table 1). Pazopanib is being studied for additional indications in adults, including adjuvant treatment of renal cell cancer, advanced soft tissue sarcoma and ovarian cancer, whereas axitinib is being studied as a single agent as well as in combination with chemotherapy across several tumor types, such as hepatocellular carcinoma, pancreatic cancer, thyroid cancer, and advanced refractory non-small cell lung cancer [9]. Furthermore, a phase I pediatric dose-finding study of pazopanib is currently ongoing, and phase II and III pediatric trials have been planned [10].
Figure 1.
Chemical structures. (A): Chemical structure of pazopanib. (B): Chemical structure of axitinib.
Table 1.
Summary table
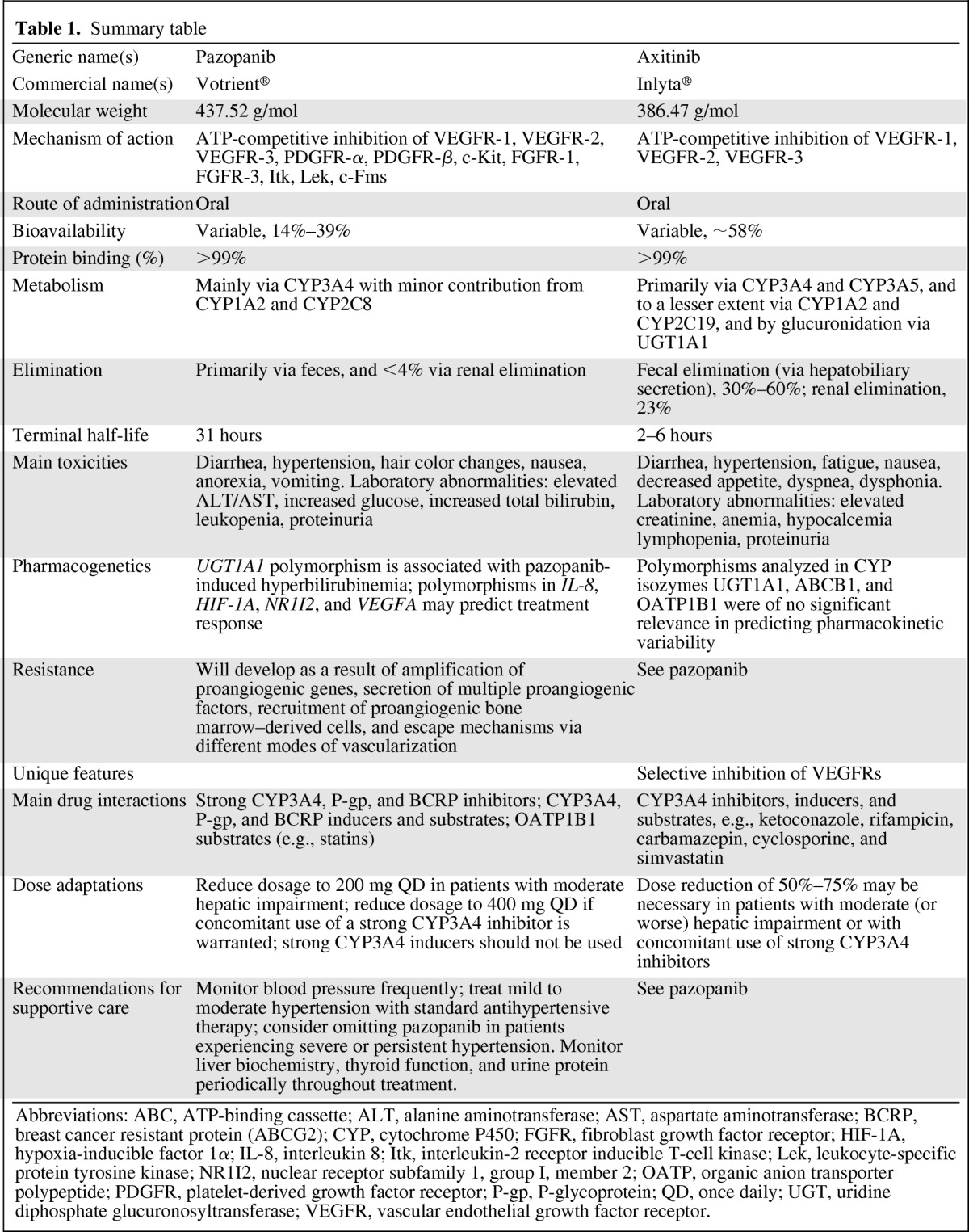
Abbreviations: ABC, ATP-binding cassette; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCRP, breast cancer resistant protein (ABCG2); CYP, cytochrome P450; FGFR, fibroblast growth factor receptor; HIF-1A, hypoxia-inducible factor 1α; IL-8, interleukin 8; Itk, interleukin-2 receptor inducible T-cell kinase; Lek, leukocyte-specific protein tyrosine kinase; NR1I2, nuclear receptor subfamily 1, group I, member 2; OATP, organic anion transporter polypeptide; PDGFR, platelet-derived growth factor receptor; P-gp, P-glycoprotein; QD, once daily; UGT, uridine diphosphate glucuronosyltransferase; VEGFR, vascular endothelial growth factor receptor.
Clinical Benefits
In a phase II study with pazopanib, 800 mg once daily (QD), in 225 patients with metastatic or locally recurrent RCC, an overall response rate of 34.7% (95% confidence interval [CI], 28%–41%) and a median PFS interval of 51.7 weeks were demonstrated. In a randomized placebo comparison (n = 55), the PFS interval was 11.9 months for pazopanib versus 6.2 months for placebo [11]. In the pivotal randomized phase III placebo-controlled clinical trial, pazopanib demonstrated a highly significant longer PFS time in both treatment-naive and cytokine pretreated patients with mRCC. The median PFS interval for patients receiving pazopanib was 9.2 months, versus 4.2 months for patients in the placebo group, with a more pronounced difference in the treatment-naive group (11.1 months versus 2.8 months) than in the cytokine-refractory group (7.4 months versus 4.2 months). These benefits, together with pazopanib's associated relatively low incidence of severe myelosuppression, hand–foot syndrome, stomatitis, and fatigue compared with the safety profile of other agents of this class, such as sunitinib, positions pazopanib as a therapeutic option for mRCC patients. However, studies directly comparing the activity and safety of pazopanib and other registered tyrosine kinase inhibitors for mRCC are lacking. Results from an ongoing clinical trial comparing the efficacy and safety profiles of pazopanib and sunitinib are expected to further determine the position of pazopanib in the treatment of patients with mRCC [2, 12].
In a randomized, phase III clinical trial, axitinib was shown to benefit patients with mRCC after failure of one previous systemic therapy. Compared with sorafenib, axitinib led to a statistically significant and clinically meaningful longer PFS time (6.7 months versus 4.7 months; hazard ratio [HR], 0.665; one-sided p < .0001) in this study group. The median PFS times were 12.1 months for axitinib versus 6.5 months for sorafenib (HR, 0.464; p < .0001) for patients previously treated with cytokines and 4.8 months versus 3.4 months (HR, 0.741; p = .0107), respectively, for patients who where previously treated with sunitinib. The objective response rates were 19% for axitinib and 9% for sorafenib (p = .001), with median durations of response of 11 months for axitinib versus 10.6 months for sorafenib. The overall survival (OS) time was not significantly different between the two groups [8]. Furthermore, in phase II studies, axitinib was demonstrated to have antitumor activity in patients with cytokine-refractory and sorafenib-refractory mRCC, with objective response rates of 44% and 22.6%, median PFS times of 15.7 months and 7.4 months, and median OS times of 29.9 months and 13.6 months, respectively [13, 14]. The 5-year OS data from the phase II study with axitinib in patients with cytokine-refractory mRCC were presented recently. The median follow-up for OS evaluation was 5.9 years and the 5-year survival rate was 20.6% (95% CI, 10.9%–32.4%). Axitinib treatment can result in OS times >5 years in a subgroup of patients with mRCC and could therefore be considered as a treatment option for second-line therapy of mRCC [15].
Clinical Use
Pazopanib is available as film-coated tablets of 200 mg and 400 mg. The recommended dose is 800 mg QD, administered at least 1 hour before or 2 hours after food intake. The dose of pazopanib should be reduced to 400 mg QD in patients who also use strong cytochrome P450 (CYP)3A4 inhibitors, and a dose reduction of 75% is advised for patients with moderate hepatic impairment. Individual dose adaptations based on tolerability should be taken in steps of 200 mg [2, 16]. In a phase I study in children (aged 4–21 years) with relapsed or refractory solid tumors, the maximum-tolerated dose of pazopanib was 450 mg/m2 [10].
Axitinib is available as 1 mg and 5 mg oral tablets, and the recommended starting dose is 5 mg twice daily (BID) on a continuous dosing schedule. The axitinib dose could be increased to 7 mg BID in patients who experience no adverse events grade >2, according to the Common Terminology Criteria for Adverse Events for ≥2 weeks, unless the patient's blood pressure is >150/90 mm Hg or the patient receives antihypertensive medication. Subsequently, the treatment dose could be further increased to 10 mg BID according to the same criteria. However, the clinical benefit of increasing the dose of axitinib in the treatment of patients with mRCC is yet to be defined in an ongoing phase II trial. If needed, the dose of axitinib could be decreased to 3 mg BID. If further dose reduction is necessary, the recommended dose is 2 mg BID [8, 17].
Mechanism of Action
Pazopanib and axitinib are second-generation potent inhibitors of multiple protein targets involved in tumor cell proliferation and angiogenesis. Angiogenesis plays a critical role in the progression of solid tumors as small as 1–2 mm in diameter. In this process, several proangiogenic factors are involved, with a central role for the vascular endothelial growth factor (VEGF) family. VEGF binds to the cell surface receptors VEGFR-1, VEGFR-2, and VEGFR-3, which subsequently leads to the recruitment of ATP. ATP in turn binds to the so-called ATP-binding pocket of VEGFR, causing activation of the VEGF signaling pathway, which ultimately results in cellular effects that are pivotal for angiogenesis. Inhibition of this pathway has demonstrated antitumor activity in several tumor types, including RCC, colorectal cancer, breast cancer, and non-small cell lung cancer [18]. Pazopanib inhibits this signaling pathway via ATP-competitive inhibition of VEGFR-1, VEGFR-2, and VEGFR-3, with in vitro 50% inhibitory concentrations of 10 nm, 30 nm, and 47 nm for VEGFR-1, VEGFR-2, and VEGFR-3, respectively. Similar activity was demonstrated against platelet-derived growth factor receptor (PDGFR)-α, PDGFR-β, fibroblast growth factor receptor (FGFR)-1, FGFR-3, and c-Kit. The transmembrane glycoprotein receptor tyrosine kinase (c-Fms) is inhibited as well, but with modest potency [19, 20]. Axitinib is a more selective tyrosine kinase inhibitor because it only shows activity against VEGFR-1, VEGFR-2, and VEGFR-3. Furthermore, its potency in vitro is higher than that of pazopanib and the first-generation VEGFR inhibitors such as sunitinib and sorafenib [8, 21, 22]. Additionally, in contrast to first-generation inhibitors, axitinib has no substantial inhibitory effect on PDGFRs, B-Raf, c-Kit, and Flt-3, which might contribute to less off-target adverse effects and a better therapeutic window [8].
Bioanalysis
Bioanalysis of pazopanib and axitinib can be exerted by high-performance liquid chromatography with tandem mass spectrometry [23, 24].
Pharmacokinetics
Absorption
At the recommended dose of 800 mg pazopanib QD, the maximum plasma concentration (Cmax) after the first dose of ∼19 ± 13 μg/mL is reached after 2–4 hours, and a geometric mean area under the plasma concentration–time curve (AUC) from time zero extrapolated to infinity (AUC0–∞) of ∼650 ± 500 μg·h/mL is obtained. Continuous dosing at 800 mg QD results in a mean AUC of 1,037 μg·h/mL and a Cmax of 58.1 μg/mL. The mean steady-state concentration (Ctrough) was ≥15 μg/mL, which appeared to correlate with clinical activity in patients with mRCC. Pazopanib doses >800 mg QD are not likely to produce higher plasma concentrations [16, 19, 24]. Systemic exposure to pazopanib did not increase in a dose-proportional manner in a phase I dose-escalation study in patients with hepatocellular carcinoma, and ranged from 151 μg·h/mL at 200 mg pazopanib QD to 214 μg·h/mL at 800 mg pazopanib QD. The mean Cmax values for the pazopanib doses used in this study also did not show linear pharmacokinetics and were 29.8 g/mL at 200 mg QD, 31.6 g/mL at 400 mg, 28.8 g/mL at 600 mg QD, and 39.5 g/mL at 800 mg QD. Ctrough levels were >15 μg/mL for all doses and were highest at the 800-mg pazopanib QD dose, that is, 28.1 μg/mL [25]. Administration of pazopanib with both low- and high-fat meals resulted in an approximately twofold higher systemic exposure, as indicated by twofold greater AUC and Cmax values. Pazopanib should therefore be administered to patients in the fasted state, at least 2 hours after or at least 1 hour before food intake [26]. Administration of a single 400-mg pazopanib tablet crushed resulted in an approximately twofold higher Cmax and a shorter tmax (by ∼2 hours) than with administration of the whole tablet [27].
After oral administration in the fed state, axitinib is rapidly absorbed from the gut, with peak plasma levels occurring within 2–6 hours after intake [28]. The rate and extent of drug absorption are higher in an overnight fasted state, with peak plasma concentrations occurring 0.5–4 hours post dosing [28–30]. At the recommended dose of 5 mg BID, the mean Cmax and AUC0–24 levels after the first dose are 37 ng/mL and 188 ng·h/mL, respectively, in the overnight fasted state versus 22 ng/mL and 194 ng·h/mL, respectively, in the fed state. These data indicate a food effect on the pharmacokinetics of axitinib. Further studies, however, have confirmed that overnight fasting is not required, and administration of axitinib with food is recommended [28, 31]. At doses of 2–10 mg BID, axitinib exhibits linear pharmacokinetics, and steady state is reached within 15 days, with no unexpected accumulation [28].
Protein Binding
Both pazopanib and axitinib are highly protein bound (>99%). In vitro studies demonstrated that axitinib binds mostly to albumin and, more restrictively, to α-1-acid glycoprotein [16, 32].
Metabolism
Results from in vitro studies demonstrated that pazopanib is metabolized by CYP3A4 and, to a lesser degree, by CYP1A2 and CYP2C8. Only 6% of the exposure in plasma represents the four principle pazopanib metabolites. One of these metabolites, GSK1268997, showed potency similar to that of pazopanib in vitro employing human umbilical vein endothelial cells, whereas the other metabolites are 10- to 20-fold less active [19, 33].
In vitro metabolism studies showed that axitinib metabolism consists primarily of oxidation via CYP3A4 and, to a lesser extent, via CYP2C19 and CYP1A2, with additional metabolism occurring by glucuronidation via diphosphate glucuronosyltransferase (UGT)1A1. The main metabolites of axitinib, a sulfoxide and an N-glucuronide, are inactive [28, 31].
Elimination
After administration of the recommended dose of 800 mg, elimination of pazopanib occurs slowly at a mean terminal plasma half-life (t1/2) of ∼31 hours [24]. Elimination of pazopanib and its metabolites occurs primarily via the feces and ≤4% of the orally administered dose is eliminated via renal excretion [19].
The t1/2 of axitinib is 2–5 hours, with hepatobiliary excretion being the major elimination pathway. Approximately 30%–60% of orally administered axitinib is eliminated in the feces, with renal elimination accounting for a further 23%. The predominant component of eliminated axitinib in feces is unchanged axitinib, whereas in urine <1% of the administered dose was found as unchanged drug [28, 32, 34].
Drug Interactions
Because pazopanib and axitinib are largely metabolized by CYP3A4, coadministration of agents known to be potent inducers or inhibitors of the CYP3A4 isozyme should be avoided. A study examining the concomitant administration of rifampicin, a potent inducer of CYP3A4, CYP1A2, and UGT1A1, and axitinib in healthy volunteers demonstrated 79% and 71% lower, respectively, geometric mean AUC0–∞ and Cmax values of axitinib [30]. Furthermore, data from a single patient demonstrated that, after concomitant use of phenytoin, a potent inducer of multiple CYP450 enzymes, the AUC0–24 and Cmax of axitinib were ∼10-fold lower. Despite earlier clinical evidence of response to axitinib, this lower plasma exposure and Cmax subsequently resulted in disease progression, which led to discontinuation from study treatment [28].
A phase I study examining the influence of ketoconazole, a potent CYP3A4 inhibitor, on the pharmacokinetics of axitinib demonstrated that concurrent administration of ketoconazole resulted in higher geometric mean AUC∞ and Cmax values of axitinib by twofold and 1.5-fold, respectively [29].
Concurrent administration of 1,500 mg lapatinib, a substrate for and weak inhibitor of CYP3A4 and P-glycoprotein (P-gp) and a potent inhibitor of breast cancer resistance protein (BCRP), with 800 mg pazopanib resulted in a greater systemic exposure of pazopanib, with 50%–60% higher AUC0–24 and Cmax values [35]. This greater pazopanib exposure is likely the result of P-gp and/or BCRP inhibition by lapatinib. Combination with strong P-gp and BCRP inhibitors and inducers should therefore be avoided [19].
Pazopanib itself is also able to inhibit several CYP enzymes, including CYP3A4, BCRP, P-gp, and organic anion transporter polypeptide (OATP)1B1. For example, coadministration of pazopanib (800 mg QD) and paclitaxel (80 mg/m2), a CYP3A4 and CYP2C8 substrate, once weekly resulted in higher AUC and Cmax values for paclitaxel, of 45% and 40%, respectively [36]. Care should therefore be taken when pazopanib is coadministered with other oral CYP3A4, BCRP, P-gp, and OATP1B1 substrates [19].
Alterations with Disease or Age
Although limited data are available on the use of pazopanib in patients aged ≥65 years, no clinically significant differences in the safety of pazopanib were observed in patients of different ages [19]. In patients with moderate hepatic impairment, as indicated by elevated bilirubin and aminotransferases, pazopanib clearance was 50% lower, resulting in twofold higher AUC and Cmax values than in individuals with normal hepatic function [37]. It is therefore recommended that patients with hepatic abnormalities be treated initially according to the pazopanib doses mentioned in Table 2. The starting dose of pazopanib should be reduced to 200 mg QD in patients with moderate hepatic impairment, and in patients with severe hepatic impairment pazopanib is contraindicated [19].
Table 2.
Pazopanib and axitinib starting dose in patients with hepatic impairment
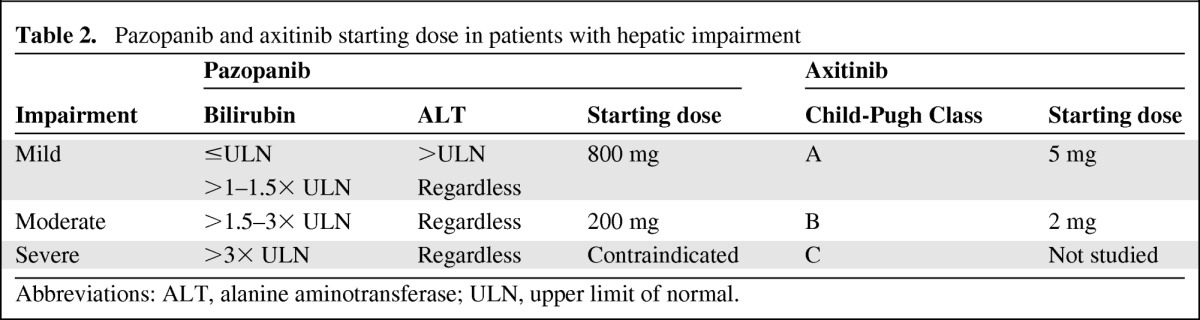
Abbreviations: ALT, alanine aminotransferase; ULN, upper limit of normal.
Currently, no age-related influences on the pharmacokinetics of axitinib have been reported, and information about the potential effects of diseases on its pharmacokinetics and pharmacodynamics is limited. In a phase I study evaluating the effects of hepatic impairment, as indicated by the Child-Pugh classification, on the pharmacokinetics and safety of a single oral dose of axitinib, individuals with mild hepatic dysfunction had pharmacokinetics similar to those of individuals with normal hepatic function. Patients with moderate hepatic impairment, however, had higher axitinib exposure than patients with normal hepatic function, as indicated by an approximately twofold higher AUC0–∞. The effect of severe hepatic impairment was not evaluated in that study. However, it can be reasonably expected to exceed or equal the effect of moderate hepatic dysfunction [32]. The recommended initial doses of axitinib are listed in Table 2. After the initial dose, subsequent doses can be increased or decreased based on individual safety and tolerability.
To date, no dedicated renal impairment trials for pazopanib and axitinib have been conducted. However, renal impairment is unlikely to significantly affect the pharmacokinetic properties of either drug because <4% of orally administered pazopanib is eliminated via renal excretion, and after oral administration of axitinib no renal elimination of unchanged drug could be found [17, 19]. Furthermore, based on the pharmacokinetic analysis, no significant difference in clearance of either compound was observed in patients with mild to severe renal impairment [16, 17].
Pharmacogenetics
Because metabolism of pazopanib and axitinib occurs principally via CYP isozymes, polymorphisms in genes encoding these enzymes might contribute to the pharmacokinetic variability of these compounds. Next to CYP isozymes, axitinib is also a substrate for UGT1A1 and the drug transporters P-gp, encoded by the ABCB1 gene, and OATP1B1, encoded by SLC01B1, which are also potential contributors to the interpatient variability in the pharmacokinetics of axitinib. However, in a meta-analysis using data pooled from 11 clinical pharmacological trials in healthy volunteers, none of the 15 polymorphisms analyzed were of significant relevance in explaining axitinib pharmacokinetic variability. Genotype-based dose adjustment is therefore not warranted after patients have been started on the initial dose of 5 mg BID [38].
Also for pazopanib, CYP3A4 polymorphisms have no known effect on the pharmacokinetics of this compound. However, in a study examining 27 single nucleotide polymorphisms in 13 genes in 397 mRCC patients receiving pazopanib, polymorphisms in the genes encoding interleukin 8 (IL-8), hypoxia-inducible factor 1α (HIF-1A), nuclear receptor subfamily 1, group I, member 2 (NR1I2), and VEGFA showed significant associations with PFS outcomes, when compared with wild-type genotypes [39]. Validation of these results is now ongoing to evaluate if these germline genetic markers can be used as predictive markers of the likelihood of response. In a preclinical in vivo study examining the effects of different B-Raf genotypes on the antitumor activity of pazopanib, significant differences were found among several xenograft tumor models. Only cell lines exhibiting either exon 11 mutations of B-Raf, human epidermal growth factor receptor 2 overexpression, or multiple pazopanib targets demonstrated a significant antiangiogenic response to pazopanib treatment. This result indicates a significant role for B-Raf in angiogenesis and suggests that B-Raf status might be a predictive marker for pazopanib efficacy [40].
Drug Resistance
Mechanisms of resistance to antiangiogenic therapy include amplification of proangiogenic genes, secretion of multiple proangiogenic factors (e.g., angiopoietin, VEGF(R), and ephrins), recruitment of proangiogenic bone marrow–derived cells, and escape mechanisms via different modes of vascularization [41]. The first three mechanisms ultimately result in greater levels of VEGF. Higher VEGF and VEGFR levels might lead to specific angiogenic inhibitor–related toxicities and faster regrowth of the tumor vasculature when the anti-VEGF drug is discontinued [42, 43].
Pharmacodynamics
The relationship between the dose of axitinib and response has not been extensively explored. In one study, the pharmacodynamic response to treatment with axitinib was measured by dynamic contrast-enhanced magnetic resonance imaging. Indicators of vascular response, such as the volume transfer constant (Ktrans), which correlates with tumor vascular permeability and perfusion, and initial AUC (IAUC), were calculated to examine the effect of axitinib treatment on tumor vascular function. By day 2 of therapy, 50% decreases in Ktrans and IAUC were demonstrated, which persisted through week 4 of treatment and corresponded to a plasma AUC0–24 >200 ng·h/mL. Furthermore, both the Ktrans and IAUC percentage changes correlated in a linear manner with the AUC0–24 and Cmax levels of axitinib in plasma [44]. Another study retrospectively examined the correlation between diastolic blood pressure (dBP) and antitumor response to axitinib. That study demonstrated that patients with at least one dBP measurement ≥90 mm Hg during therapy had a significantly lower relative risk for death than patients with dBP <90 mm Hg throughout therapy (HR, 0.55; p < .001). The relative risk for progression was lower in patients with a dBP measurement ≥90 mm Hg, and the objective response rate was significantly higher (43.9% versus 12%; p < .001). Furthermore, the median PFS interval (10.2 months versus 7.1 months) and median OS time (25.8 months versus 14.9 months) were greater for patients with a dBP measurement ≥90 mm Hg [45]. Additional studies to examine the pharmacodynamics of axitinib are currently ongoing.
The pharmacokinetic–pharmacodynamic relationships of pazopanib have not been extensively studied either. In a phase II trial, the relationship between plasma concentrations of pazopanib and drug exposure was investigated in 205 patients after 4 weeks and 12 weeks of treatment. Patients with a plasma concentration of pazopanib ≥20.6 μg/mL (n = 143) at week 4 had a median PFS time of 49.4 weeks and patients with a plasma concentration <20.6 μg/mL at week 4 had a median PFS time of 20.3 weeks (n = 62; p = .0041). The overall response rates in these patients were 45% versus 18% (p = .000017). Plasma levels of pazopanib ≥20.6 μg/mL may therefore be associated with better efficacy [46]. However, this needs to be proven in prospective studies. Furthermore, results of a phase I study of pazopanib in patients with advanced tumors suggested that hypertension may represent a general pharmacodynamic marker of the activity of pazopanib. Further research, however, is necessary to examine the clinical relevance of this finding [24]. In a phase II study with pazopanib in patients with RCC, no significant correlation was observed between the 12-week treatment response and the von Hippel-Lindau status or the status of other soluble markers including sVEGFR-1, VEGF, and circulating endothelial cells [47].
Toxicity
Chronic administration of axitinib and pazopanib is associated with manageable toxicities and a low rate of treatment discontinuation. The most common adverse events (all grades) reported in clinical trials with axitinib were diarrhea, hypertension, fatigue, nausea, decreased appetite, dyspnea, dysphonia, hand–foot syndrome, and decreased weight [8, 14, 28]. In the pivotal phase III trial of pazopanib, diarrhea, hypertension, hair color changes, nausea, anorexia, and vomiting were the most common adverse reactions (all grades). The most frequent adverse events of grade ≥3 were hypertension (16%), diarrhea (11%), and fatigue (11%), during treatment with axitinib, and hypertension (4%), diarrhea (3%), and asthenia (3%), during treatment with pazopanib. Creatinine elevation (55%), hypocalcemia (39%), anemia (33%), lymphocytopenia (33%), and lipase elevation (27%) were the most frequently reported laboratory abnormalities (all grades) in the pivotal phase III trial of axitinib, whereas alanine aminotransferase or aspartate aminotransferase increases (53%), hyperglycemia (41%), leukopenia (37%), total bilirubin increases (36%), and neutropenia (34%) accounted for the most common laboratory abnormalities during pazopanib treatment (Table 3) [2, 8].
Table 3.
Common treatment-emergent all-causality adverse events in the pivotal phase III trials
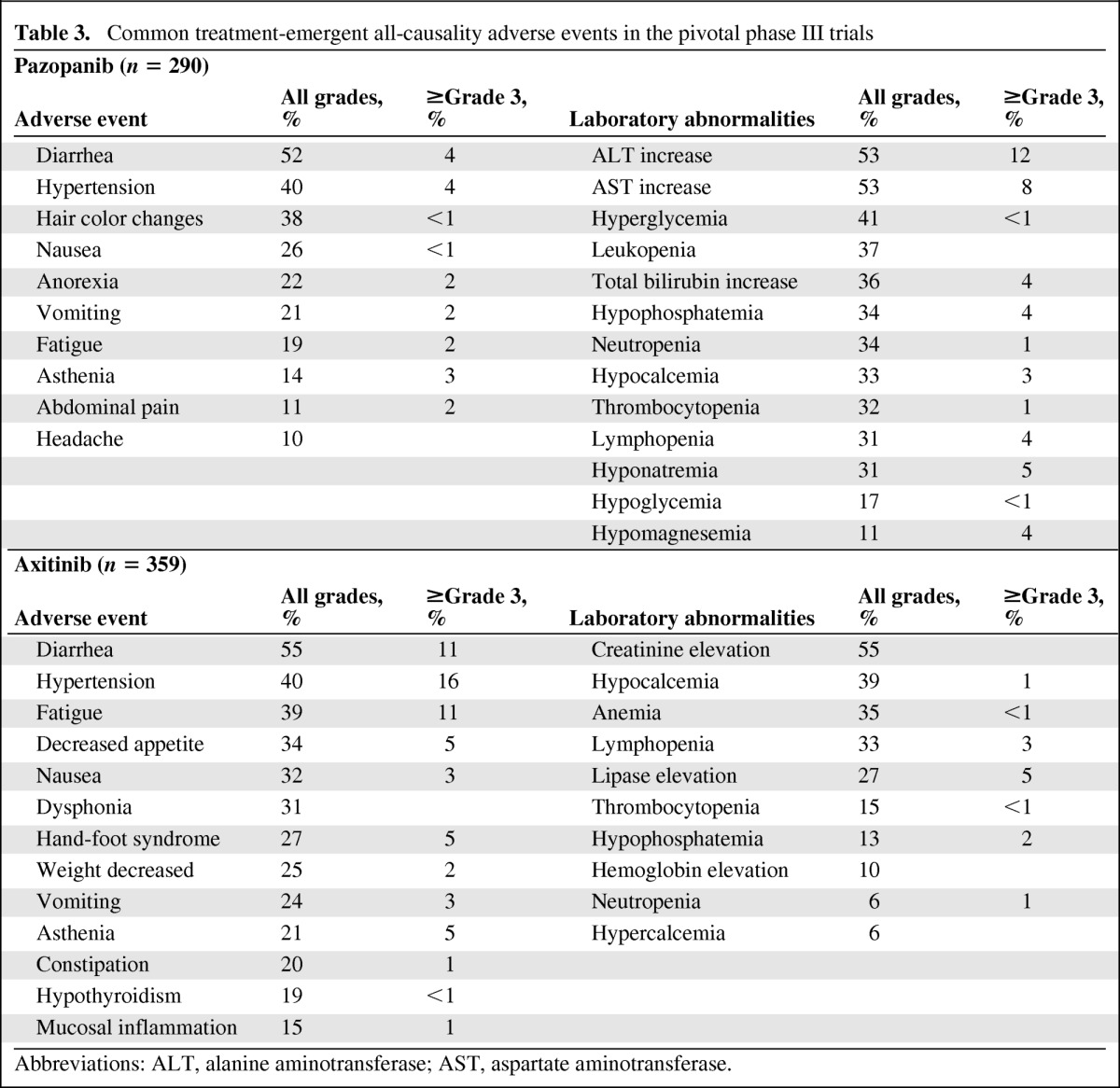
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Patient Instructions and Recommendations for Supportive Care
Pazopanib should be administered without food and the tablet must not be crushed. Axitinib may be taken with or without food. Because hypertension is a frequently occurring adverse reaction associated with both drugs, blood pressure should be monitored frequently. Mild to moderate hypertension should be treated with standard antihypertensive therapy and axitinib or pazopanib treatment may be continued. In patients who experience severe or persistent hypertension while using antihypertensive therapy, discontinuation of axitinib or pazopanib should be considered [13, 14, 16, 17]. Neither compound should be used during pregnancy because this may cause fetal harm, and men and women should use effective birth control during treatment with pazopanib or axitinib, because both drugs are teratogenic. Because pazopanib and axitinib may increase serum levels of transaminases and bilirubin, liver biochemistry should be measured before treatment initiation and regularly during treatment. Additionally, monitoring of thyroid function and urine protein is recommended during treatment with both pazopanib and axitinib [16, 17].
Conclusions
Pazopanib and axitinib are both FDA-approved ATP-competitive VEGFR inhibitors and represent treatment options for patients with mRCC. The efficacy of pazopanib appears to be comparable with that of sunitinib, one of the main treatment options for patients with mRCC, and it may have a better tolerability profile. Therefore, pazopanib appears to be a valuable addition to the treatment of mRCC patients. However, results from head-to-head comparative studies, such as the Pazopanib Versus Sunitinib in the Treatment of Subjects With Locally Advanced and/or Metastatic Renal Cell Carcinoma clinical trial, are to be awaited to make a definitive statement about the position of pazopanib in relation to other medications for mRCC.
Compared with sorafenib, axitinib resulted in a longer PFS time, higher objective response rate, and longer OS duration in previously treated patients with mRCC. This makes axitinib a treatment option for second-line therapy of mRCC, and ongoing clinical trials have to determine the suitability of axitinib in the first-line setting. To conclude, pazopanib and axitinib, together with other recently approved drugs, including sorafenib, sunitinib, temsirolimus, everolimus, and bevacizumab, altered the treatment paradigm of mRCC and offer patients multiple treatment options.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Jan H.M. Schellens
Collection and/or assembly of data: Robin M.J.M. van Geel
Data analysis and interpretation: Robin M.J.M. van Geel
Manuscript writing: Robin M.J.M. van Geel
Final approval of manuscript: Jan H.M. Schellens, Jos H. Beijnen
References
- 1.Bukowski RM, Yasothan U., Kirkpatrick P. Pazopanib. Nat Rev Drug Discov. 2010;9:17–18. doi: 10.1038/nrd3073. [DOI] [PubMed] [Google Scholar]
- 2.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 3.Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: A phase II study from the European Organisation for Research and Treatment of Cancer–Soft Tissue and Bone Sarcoma Group (EORTC study 62043) J Clin Oncol. 2009;27:3126–3132. doi: 10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]
- 4.Friedlander M, Hancock KC, Rischin D, et al. A phase II, open-label study evaluating pazopanib in patients with recurrent ovarian cancer. Gynecol Oncol. 2010;119:32–37. doi: 10.1016/j.ygyno.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 5.Altorki N, Lane ME, Bauer T, et al. Phase II proof-of-concept study of pazopanib monotherapy in treatment-naive patients with stage I/II resectable non-small-cell lung cancer. J Clin Oncol. 2010;28:3131–3137. doi: 10.1200/JCO.2009.23.9749. [DOI] [PubMed] [Google Scholar]
- 6.Bible KC, Suman VJ, Molina JR, et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: Results of a phase 2 consortium study. Lancet Oncol. 2010;11:962–972. doi: 10.1016/S1470-2045(10)70203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration (FDA) Silver Spring, MD: FDA; 2011. FDA Briefing Document, Oncologic Drugs Advisory Committee Meeting, NDA 202324 Axitinib (Inlyta®) [Google Scholar]
- 8.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 9.ClinicalTrials.gov. [accessed April 24, 2012]. Available at http://www.clinicaltrials.gov.
- 10.Glade Bender JL, Lee A, Adamson PC, et al. Phase I study of pazopanib in children with relapsed or refractory solid tumors (ADVL0815): A Children's Oncology Group Phase I Consortium. J Clin Oncol. 2011;29(suppl):9501. [Google Scholar]
- 11.Hutson TE, Davis ID, Machiels JP, et al. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2010;28:475–480. doi: 10.1200/JCO.2008.21.6994. [DOI] [PubMed] [Google Scholar]
- 12.Bukowski RM. Critical appraisal of pazopanib as treatment for patients with advanced metastatic renal cell carcinoma. Cancer Manag Res. 2011;3:273–285. doi: 10.2147/CMR.S15557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rini BI, Wilding G, Hudes G, et al. Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4462–4468. doi: 10.1200/JCO.2008.21.7034. [DOI] [PubMed] [Google Scholar]
- 14.Rixe O, Bukowski RM, Michaelson MD, et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: A phase II study. Lancet Oncol. 2007;8:975–984. doi: 10.1016/S1470-2045(07)70285-1. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, de La Motte Rouge T, Harzstark AL, et al. Axitinib second-line therapy for metastatic renal cell carcinoma (mRCC): Five-year (yr) overall survival (OS) data from a phase II trial. J Clin Oncol. 2011;29(suppl):4547. [Google Scholar]
- 16.Brentford, U.K.: GlaxoSmithKline; 2009. Votrient® (pazopanib) tablets [full prescribing information] [Google Scholar]
- 17.New York: Pfizer; 2012. Inlyta® (axitinib) tablets [full prescribing information] [Google Scholar]
- 18.Hamberg P, Verweij J, Sleijfer S. (Pre-)clinical pharmacology and activity of pazopanib, a novel multikinase angiogenesis inhibitor. The Oncologist. 2010;15:539–547. doi: 10.1634/theoncologist.2009-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Medicines Agency. Votrient® (Pazopanib): Summary of Product Characteristics. [accessed April 29, 2012]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001141/WC500094272.pdf.
- 20.Sonpavde G, Hutson TE. Pazopanib: A novel multitargeted tyrosine kinase inhibitor. Curr Oncol Rep. 2007;9:115–119. doi: 10.1007/s11912-007-0007-2. [DOI] [PubMed] [Google Scholar]
- 21.Scagliotti G, Govindan R. Targeting angiogenesis with multitargeted tyrosine kinase inhibitors in the treatment of non-small cell lung cancer. The Oncologist. 2010;15:436–446. doi: 10.1634/theoncologist.2009-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhargava P, Robinson MO. Development of second-generation VEGFR tyrosine kinase inhibitors: Current status. Curr Oncol Rep. 2011;13:103–111. doi: 10.1007/s11912-011-0154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sparidans RW, Iusuf D, Schinkel AH, et al. Liquid chromatography-tandem mass spectrometric assay for the light sensitive tyrosine kinase inhibitor axitinib in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:4090–4096. doi: 10.1016/j.jchromb.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 24.Hurwitz HI, Dowlati A, Saini S, et al. Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res. 2009;15:4220–4227. doi: 10.1158/1078-0432.CCR-08-2740. [DOI] [PubMed] [Google Scholar]
- 25.Yau T, Chen PJ, Chan P, et al. Phase I dose-finding study of pazopanib in hepatocellular carcinoma: Evaluation of early efficacy, pharmacokinetics, and pharmacodynamics. Clin Cancer Res. 2011;17:6914–6923. doi: 10.1158/1078-0432.CCR-11-0793. [DOI] [PubMed] [Google Scholar]
- 26.Heath EI, Chiorean EG, Sweeney CJ, et al. A phase I study of the pharmacokinetic and safety profiles of oral pazopanib with a high-fat or low-fat meal in patients with advanced solid tumors. Clin Pharmacol Ther. 2010;88:818–823. doi: 10.1038/clpt.2010.199. [DOI] [PubMed] [Google Scholar]
- 27.Heath EI, Forman K, Malburg L, et al. A phase I pharmacokinetic and safety evaluation of oral pazopanib dosing administered as crushed tablet or oral suspension in patients with advanced solid tumors. Invest New Drugs. 2011 Aug 3; doi: 10.1007/s10637-011-9725-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Rugo HS, Herbst RS, Liu G, et al. Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: Pharmacokinetic and clinical results. J Clin Oncol. 2005;23:5474–5483. doi: 10.1200/JCO.2005.04.192. [DOI] [PubMed] [Google Scholar]
- 29.Pithavala YK, Tong W, Mount J, et al. Effect of ketoconazole on the pharmacokinetics of axitinib in healthy volunteers. Invest New Drugs. 2012;30:273–281. doi: 10.1007/s10637-010-9511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pithavala YK, Tortorici M, Toh M, et al. Effect of rifampin on the pharmacokinetics of axitinib (AG-013736) in Japanese and Caucasian healthy volunteers. Cancer Chemother Pharmacol. 2010;65:563–570. doi: 10.1007/s00280-009-1065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly RJ, Rixe O. Axitinib (AG-013736) In: Martens UM, editor. Small Molecules in Oncology. Berlin, Germany: Springer; 2010. pp. 33–44. [Google Scholar]
- 32.Tortorici MA, Toh M, Rahavanedran SV, et al. Influence of mild and moderate hepatic impairment on axitinib pharmacokinetics. Invest New Drugs. 2011;29:1370–1380. doi: 10.1007/s10637-010-9477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Australian Government Department of Health and Ageing, Therapeutic Goods Administration. Australian Public Assessment Report for Pazopanib Hydrochloride. [accessed April 29, 2012]. Available at http://www.tga.gov.au/pdf/auspar/auspar-votrient.pdf.
- 34.Spano JP, Moore MJ, Pithavala YK, et al. Phase I study of axitinib (AG-013736) in combination with gemcitabine in patients with advanced pancreatic cancer. Invest New Drugs. 2011 Jun 14; doi: 10.1007/s10637-011-9697-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Monk BJ, Mas Lopez L, Zarba JJ, et al. Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. J Clin Oncol. 2010;28:3562–3569. doi: 10.1200/JCO.2009.26.9571. [DOI] [PubMed] [Google Scholar]
- 36.Tan AR, Dowlati A, Jones SF, et al. Phase I study of pazopanib in combination with weekly paclitaxel in patients with advanced solid tumors. The Oncologist. 2010;15:1253–1261. doi: 10.1634/theoncologist.2010-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibata S, Longmate J, Chung VM, et al. A phase I and pharmacokinetic single agent study of pazopanib (P) in patients (Pts) with advanced malignancies and varying degrees of liver dysfunction (LD) J Clin Oncol. 2010;28(15 suppl):2571. [Google Scholar]
- 38.Brennan M, Williams JA, Chen Y, et al. Meta-analysis of contribution of genetic polymorphisms in drug-metabolizing enzymes or transporters to axitinib pharmacokinetics. Eur J Clin Pharmacol. 2012;68:645–655. doi: 10.1007/s00228-011-1171-8. [DOI] [PubMed] [Google Scholar]
- 39.Xu CF, Bing NX, Ball HA, et al. Pazopanib efficacy in renal cell carcinoma: Evidence for predictive genetic markers in angiogenesis-related and exposure-related genes. J Clin Oncol. 2011;29:2557–2564. doi: 10.1200/JCO.2010.32.9110. [DOI] [PubMed] [Google Scholar]
- 40.Gril B, Palmieri D, Qian Y, et al. The B-Raf status of tumor cells may be a significant determinant of both antitumor and anti-angiogenic effects of pazopanib in xenograft tumor models. PLoS One. 2011;6:e25625. doi: 10.1371/journal.pone.0025625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loges S, Schmidt T, Carmeliet P. Mechanisms of resistance to anti-angiogenic therapy and development of third-generation anti-angiogenic drug candidates. Genes Cancer. 2010;1:12–25. doi: 10.1177/1947601909356574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran J, Master Z, Yu JL, et al. A role for survivin in chemoresistance of endothelial cells mediated by VEGF. Proc Natl Acad Sci U S A. 2002;99:4354. doi: 10.1073/pnas.072586399. 4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mancuso MR, Davis R, Norberg SM, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu G, Rugo HS, Wilding G, et al. Dynamic contrast-enhanced magnetic resonance imaging as a pharmacodynamic measure of response after acute dosing of AG-013736, an oral angiogenesis inhibitor, in patients with advanced solid tumors: Results from a phase I study. J Clin Oncol. 2005;23:5464–5473. doi: 10.1200/JCO.2005.04.143. [DOI] [PubMed] [Google Scholar]
- 45.Rini BI, Schiller JH, Fruehauf JP, et al. Diastolic blood pressure as a biomarker of axitinib efficacy in solid tumors. Clin Cancer Res. 2011;17:3841–3849. doi: 10.1158/1078-0432.CCR-10-2806. [DOI] [PubMed] [Google Scholar]
- 46.Suttle B, Ball HA, Molimard M, et al. Relationship between exposure to pazopanib (P) and efficacy in patients (pts) with advanced renal cell carcinoma (mRCC) J Clin Oncol. 2010;28(15 suppl):3048. [Google Scholar]
- 47.Hutson TE, Davis ID, Machiels JH, et al. Biomarker analysis and final efficacy and safety results of a phase II renal cell carcinoma trial with pazopanib (GW786034), a multi-kinase angiogenesis inhibitor. J Clin Oncol. 2008;26(suppl):5046. [Google Scholar]