Abstract
There is a clear need for efficient methods to produce protein therapeutics requiring mannose-termination for therapeutic efficacy. Here we report on a unique system for production of active human lysosomal acid β-glucosidase (glucocerebrosidase, GCase, EC 3.2.1.45) using seeds of the Arabidopsis thaliana complex-glycan-deficient (cgl) mutant, which are deficient in the activity of N-acetylglucosaminyl transferase I (EC 2.4.1.101). Gaucher disease is a prevalent lysosomal storage disease in which affected individuals inherit mutations in the gene (GBA1) encoding GCase. A gene cassette optimized for seed expression was used to generate the human enzyme in seeds of the cgl (C5) mutant, and the recombinant GCase was mainly accumulated in the apoplast. Importantly, the enzymatic properties including kinetic parameters, half-maximal inhibitory concentration of isofagomine and thermal stability of the cgl-derived GCase were comparable with those of imiglucerase, a commercially available recombinant human GCase used for enzyme replacement therapy in Gaucher patients. N-glycan structural analyses of recombinant cgl-GCase showed that the majority of the N-glycans (97%) were mannose terminated. Additional purification was required to remove ~15% of the plant-derived recombinant GCase that possessed potentially immunogenic (xylose-and/or fucose-containing) N-glycans. Uptake of cgl-derived GCase by mouse macrophages was similar to that of imiglucerase. The cgl seed system requires no addition of foreign (non-native) amino acids to the mature recombinant GCase protein, and the dry transgenic seeds represent a stable repository of the therapeutic protein. Other strategies that may completely prevent plant-like complex N-glycans are discussed, including the use of a null cgl mutant.
Keywords: Arabidopsis cgl mutant, Gaucher disease, human glucocerebrosidase, mannose-terminated N-glycans, N-glycosylation
Introduction
Transgenic plants, seeds and cultured plant cells are potentially one of the most economical systems for large-scale production of recombinant proteins for industrial and pharmaceutical uses (Kermode 2006; Lau and Sun 2009; Kermode 2012). Seeds are particularly attractive as production hosts due to their high rates of protein synthesis during seed maturation, and their ability to remain viable in the mature dry (quiescent) and stored state (Twyman et al. 2003; Stoger and Ma 2005). The stability of proteins in dry seeds allows for the additional advantage of a “decoupling” of the processing of the materials to obtain the purified recombinant protein from the generation and harvesting of the transgenic seeds (Boothe et al. 2010).
Over one-third of approved pharmaceutical proteins are glycoproteins (Saint-Jore-Dupas et al. 2007; Gomord et al. 2010), and even minor differences in N-glycan structures can change the distribution, activity or longevity of recombinant proteins when compared with their native counterparts, altering their efficacy as therapeutics. Thus, one of the major challenges of using plants as systems for pharmaceutical glycoprotein production is to produce these pharmaceuticals with “humanized” N-glycans. Notably, certain processes of N-glycosylation that occur in post-endoplasmic reticulum (ER) compartments are markedly different in plant cells versus mammalian cells. Although the early steps and components of the N-glycosylation process in the ER (including the involvement of the dolichol lipid intermediate and ER oligosaccharide transferase), and the Golgi-localized N-acetylglucosaminyl transferase I (GnT I), are the same in plant and mammalian cells, differences occur during later stages as proteins transit through the Golgi complex (Lerouge et al. 1998). For example, in the plant Golgi complex, enzymes convert the original high-mannose N-glycans of proteins to plant-specific hybrid and complex N-glycans by a series of sequential reactions that rely on the accessibility of the glycan chain(s) to the Golgi processing machinery (Kermode 1996; Gomord and Faye 2004). Plant-specific sugars that are associated with these “matured” N-glycans, such as β-1,2-xylose and α-1,3-fucose, may induce immune responses in humans, particularly when plant-made pharmaceutical glycoproteins are parenterally administrated (Gomord et al. 2010).
Several strategies have been developed to reduce or eliminate plant-specific N-glycan maturation, including ER retention, targeting the protein of interest to protein storage vacuoles via a pathway that bypasses the Golgi complex, or by knocking out the genes that specify xylosyltransferase and fucosyltransferase activities (Kermode 2012). Another approach is to use the Arabidopsis thaliana cgl (complex-glycan-deficient) mutant, which lacks GnT I activity due to a mutation in the gene encoding GnT I (von Schaewen et al. 1993; Strasser et al. 2005). GnT I is the first enzyme in the pathway of hybrid and complex N-glycan biosynthesis. Without the addition of N-acetylglucosamine to the trimmed glycan, xylosyl-and fucosyltransferases are unable to add β-1,2-xylose and α-1,3-fucose, respectively. Thus, N-glycans on endogenous proteins synthesized in this mutant are in the “high-mannose” or oligomannosidic form, predominantly Man5-GlcNAc2, with minor amounts of Man6, Man7 and Man8.
Gaucher disease is a prevalent human lysosomal storage disease and it is caused by a hereditary deficiency of the lysosomal enzyme acid β-glucosidase (glucocerebrosidase, GCase, EC 3.2.1.45). GCase catalyzes the hydrolysis of the glycosphingolipid, glucocerebroside (glucosylceramide) to generate glucose and ceramide (Beutler and Grabowski 2001). The disease has been broadly defined as three major clinical subtypes (1, 2 and 3), with type 1 representing non-neuronopathic disease and types 2 and 3 represent neuronopathic disease (Grabowski 2008). Progressive accumulation of glucocerebroside in the lysosomes of macrophages in various tissues of the reticuloendothelial system leads to visceral organ manifestations, which are common to all Gaucher disease subtypes (Grabowski 1997). The visceral manifestations of Gaucher disease can be treated by enzyme replacement therapy (ERT). The current Federal Drug Agency (FDA)-approved ERT is imiglucerase, a recombinant human GCase produced in Chinese hamster ovary (CHO) cells (Cerezyme®; Genzyme Corp., MA, USA). After its expression and purification, imiglucerase is modified by treatment with three glycosidases (α-neuraminidase, β-galactosidase and β-N-acetylglucosaminidase) (Furbish et al. 1981; Bijsterbosch et al. 1996; Friedman et al. 1999) to expose the terminal mannose residues on the N-glycans of the recombinant GCase. These terminal mannose sugars are recognized by the mannose receptor located on the macrophage plasma membrane; this downstream processing of GCase improves its targeting to and internalization by macrophages. The annual average cost for each patient ranges from 125,000 to more than 500,000 USD (Schmitz et al. 2007).
Here, active human GCase was produced in seeds of an Arabidopsis thaliana cgl mutant. The enzymatic properties including kinetic parameters, half-maximal inhibitory concentration (IC50) of isofagomine (IFG) and thermal stability of cgl-derived GCase are similar to those of imiglucerase. The major N-glycan components of the recombinant GCase were of the oligomannosidic type (85%), with the remainder being complex and hybrid types (15%). Mannose-terminated N-glycans represented 97% of the N-glycans on the cgl-GCase. The addition of a purification step (an anti-horseradish peroxidase affinity column) effectively removed the recombinant GCase-containing xylose and/or fucose.
The present strategy demonstrates the potential for producing appropriate recombinant therapeutics if a null cgl mutant is used (e.g. the cgl1 C6 mutant; see Discussion), or after extensive purification of the proteins to remove antigenic (complex/hybrid N-glycan) forms of the enzyme.
The uptake of cgl-derived GCase into mouse macrophages was similar to that of imiglucerase. Two advantages that may render the present system, a viable alternative for GCase production for ERT, include: (i) a three-step (in vitro) enzymatic processing of cgl-GCase is not needed to generate mannose-terminated glycans, which contrasts with CHO-cell-derived GCase and (ii) there is no need for additional amino acids on the mature recombinant GCase protein, as is the case for the carrot cell-derived GCase for targeting of the enzyme to a protein-storage-vacuole destination (Shaaltiel et al. 2007).
Results
Recombinant human GCase of cgl seeds is active
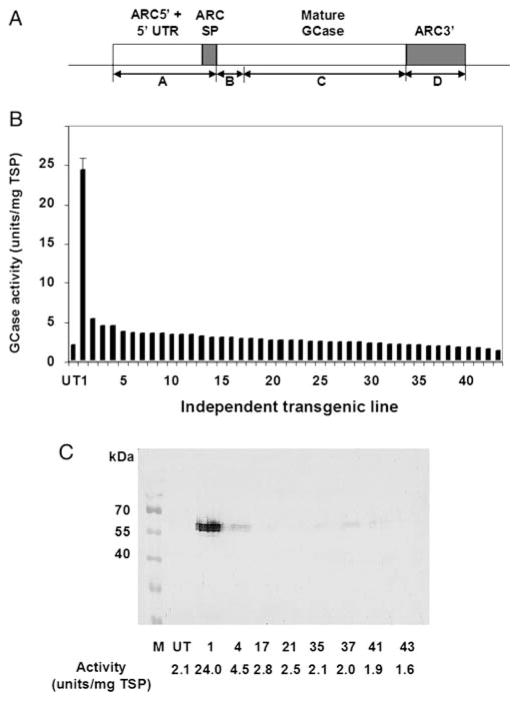
The regulatory sequences of the seed protein gene arcelin 5-I (ARC5-I; accession no. Z50202) of the common bean (Phaseolus vulgaris) are very effective in enhancing heterologous gene expression in plants, including Arabidopsis and tobacco (Goossens et al. 1999; De Jaeger et al. 2002; Kermode et al. 2007). In addition, the sequence encoding the arcelin signal peptide combined with the arcelin promoter, 5′ UTR, and 3′ flanking regions of the arcelin gene synergistically enhance recombinant human α-L-iduronidase expression in Arabidopsis thaliana cgl seeds (Downing et al. 2006, 2007; reviewed in Kermode 2006). Here, these sequences were employed to achieve high-level production of the human recombinant GCase. The construct (Figure 1A) was transformed into plants using the floral dip method of Agrobacterium-mediated gene transfer (Clough and Bent 1998) and T2 generation seeds were collected from 43 plants. Proteins were extracted from these seeds and screened for GCase activity. Some background activity was present in the untransformed cgl seeds (Figure 1B), presumably due to endogenous enzymes that can hydrolyze the artificial substrate. This was confirmed by treatment of the transgenic seed extracts from line 1 with the inhibitor conduritol B epoxide, in which the fraction of activity that was sensitive to this GCase-specific inhibitor was 92%. The transgenic lines showed variable GCase activity above or near this background level. One transgenic line had high GCase activity (24.0 ± 1.8 units/mg total soluble protein [TSP]; Figure 1B). Western blot analysis (Figure 1C) of seed extracts from a subset of the transgenic lines shown in Figure 1B indicated a ~60 kDa protein which was detectable at variable levels. The line with the highest level of GCase activity showed the highest accumulation of GCase protein. There was no cross reactivity of the GCase polyclonal antibody with any endogenous proteins in untransformed cgl seeds (Figure 1C, UT).
Fig. 1.
Production of GCase in Arabidopsis cgl seeds. (A) Schematic representation of construct used to produce GCase in cgl seeds. ARC 5′ + 5′ UTR, ARC3′ and ARC SP represent sequences from the ARC5-I gene, the promoter and 5′-UTR, the 3′ end, and the signal peptide (SP)-encoding sequences, respectively. Mature GCase refers to the human GBA cDNA minus the signal-peptide-encoding sequences. A, B, C and D indicate parts of the expression construct referred to in the experimental procedures section. (B) GCase activity of independent transgenic lines. Activities associated with lines 2–43 represent the average value of duplicate assays on the same extract. Activity associated with line 1 was the average value ± S.D. derived from three independent experiments on this transgenic line in which assays were done in duplicate or triplicate. (C) Western blot analysis of GCase protein in cgl seeds of selected independent transgenic lines. Total soluble protein (100 μg) was loaded in each lane. GCase-specific activities associated with the independent lines (units/mg TSP) are noted. UT, untransformed cgl seeds (negative control); M, pre-stained protein marker.
GCase of cgl storage parenchyma cells is secreted
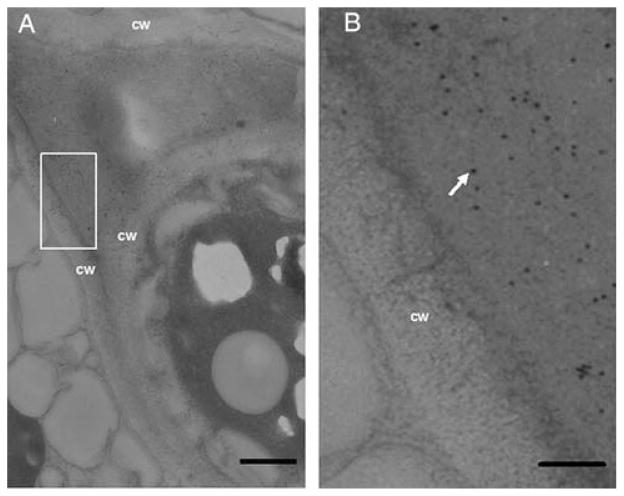
To determine the subcellular localization of GCase, immuno-labeling was carried out on ultra-thin sections of cgl seeds prepared from high-pressure freezing/frozen substitution of the tissues. The sections were sequentially incubated with anti-GCase antibody, and then with gold-conjugated secondary antibody. Sections of the untransformed cgl seeds were used as negative controls. Electron micrograph images of the transgenic seed sections indicate that the gold particles were predominantly present within the extracellular spaces (Figure 2), indicating that most of the GCase were secreted into the apoplast, as expected.
Fig. 2.
Immunogold EM localization of GCase in transgenic cgl seeds. (A) Lower magnification of gold-labeled ultrathin section of transgenic cgl seed. (B) Higher magnification of the inset in (A). The arrow indicates a gold particle. CW, cell wall.
Properties of purified cgl-derived GCase are comparable with those of imiglucerase
To further characterize the cgl-derived GCase, the enzyme was purified by a three-step procedure that used concanavalin A (Con A) chromatography, Butyl FF chromatography and Bio-Gel P 100 chromatography (Table I). The purified GCase was resolved by SDS–PAGE and stained with Coomassie Brilliant Blue. After destaining, a single band on the gel indicated that GCase was purified to homogeneity (Figure 3). The diffuse nature of the band was suggestive of variation in the pattern of N-glycosylation/N-glycan maturation of GCase. There are five consensus sites for N-glycosylation on GCase (Asn-X-Ser/Thr); generally four sites are utilized in mammalian cells (Jonsson et al. 1987; Bergmann and Grabowski 1989; Berg-Fussman et al. 1993), and most of the N-glycans are in complex forms (see Discussion).
Table I.
Purification of GCase from transgenic cgl seeds
| Purification step | Total activity (units) | Specific activity (units/mg TSP) | Purification fold | Yield |
|---|---|---|---|---|
| Crude extract | 57,360 | 22.3 | 1 | 100 |
| Con A-Sepharose chromatography | 36,882 | 3345 | 150 | 64.3 |
| Butyl FF chromatography | 13,931 | 43,537 | 2034 | 24.3 |
| Bio-Gel P 100 | 7170 | 48,940 | 2195 | 12.5 |
Fig. 3.
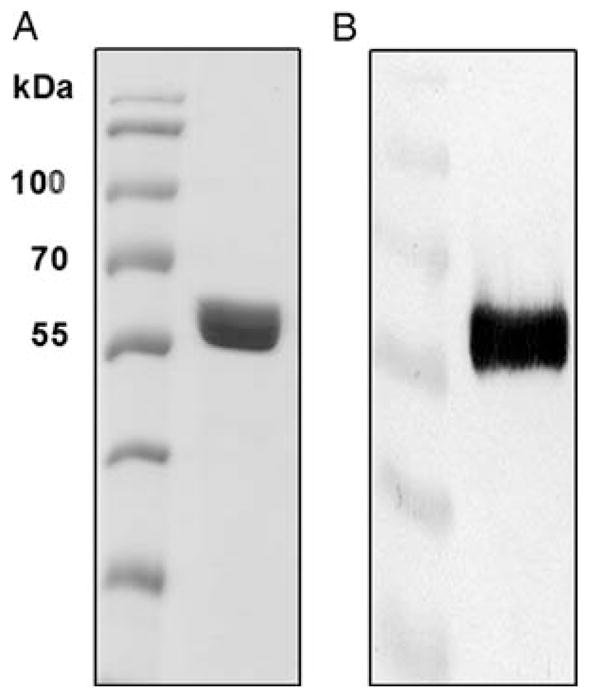
Purified GCase from transgenic cgl seeds. (A) Purified GCase (4 μg) fractionated by SDS–PAGE. (B) Western blot analysis of purified GCase (200 ng of purified GCase was loaded).
Kinetic analyses were carried out to compare cgl-GCase and imiglucerase using the fluorogenic substrate 4-methylumbelliferyl β-D-glucopyranoside (4-MUGP). cgl-GCase and imiglucerase exhibited similar Vmax and Km values (Figure 4 and Table II). IFG is a potent inhibitor of GCase (Lieberman et al. 2007). The IC50 values of IFG for the two enzymes were also similar (Table II). The IC50 value of IFG for imiglucerase at pH 5.2 using the same substrate was reported as 30 nM (Steet et al. 2006). A fluorescence denaturation assay was used to determine the thermal stability of both enzymes, as characterized by the melting temperature (Tm) in the presence and in the absence of IFG. Significantly, the Tm’s of cgl-GCase and imiglucerase in the absence or in the presence of 300 μM IFG were similar (Table II). These results demonstrate that cgl-GCase and imiglucerase have similar enzymatic properties.
Fig. 4.
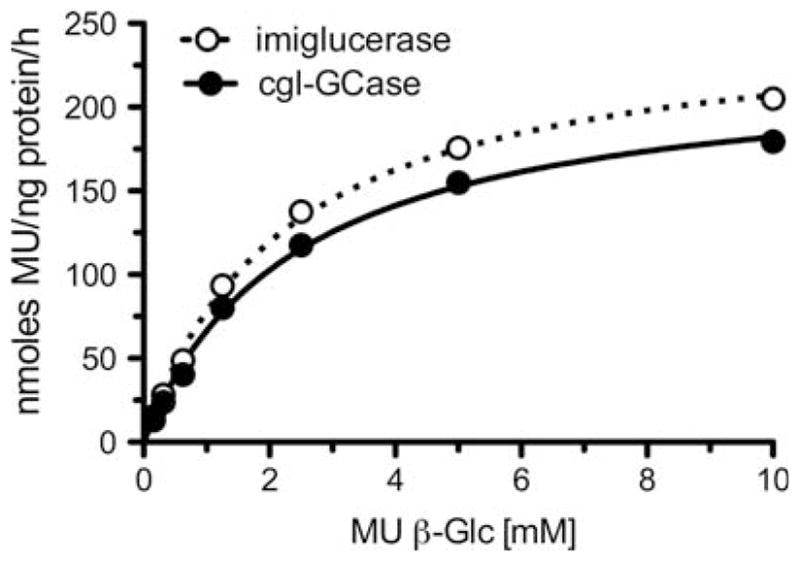
Michaelis–Menten plots for cgl-GCase and imiglucerase. Data are the means of three replicate experiments.
Table II.
Comparison of enzymatic properties of imiglucerase and cgl-GCase
| Imiglucerase | cgl-GCase | |
|---|---|---|
| Vmax (nmol MU/ng protein/h) | 254 ± 4 | 226 ± 4 |
| Km (mM) | 2.34 ± 0.1 | 2.4 ± 0.1 |
| IFG IC50a | 18 ± 1 | 19 ± 1 |
| Tm (°C) | 48.2 ± 0.6 | 48.4 ± 0.3 |
| Tm-IFG (°C) | 64.0 ± 0.3 | 63.8 ± 0.8 |
Determined in the presence of 5 mM substrate 4-MUGP.
N-glycans of cgl-derived GCase are mannose-terminated and predominantly oligomannosidic
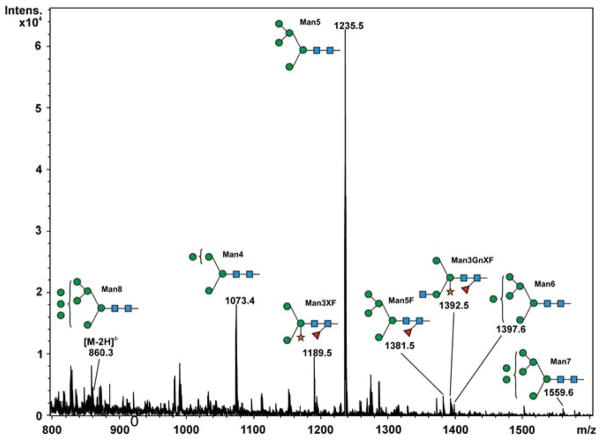
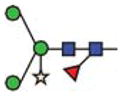
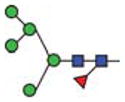
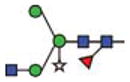
Purified GCase from cgl seeds was analyzed for its constituent N-glycans by carbon LC ESI MS/MS. The results show that ~85% of the N-glycan components detected were of the oligomannosidic type (i.e. those containing 1–7 hexose residues in addition to the pentasaccharide N-glycan core). The remaining 15% were paucimannose and hybrid N-glycans containing xylose and/or fucose (Figure 5 and Table III). Man5-type glycans accounted for ~60%. Structures like Man5F are indicative of exoglycosidase activities on the N-glycans during expression and/or purification of the protein. N-glycans possessing terminal mannose residues accounted for ~97%. Since 15% of the cgl-GCase possessed potentially immunogenic hybrid/complex N-glycans, we investigated a method to remove the recombinant proteins possessing xylose- and/or fucose-containing N-glycans. A purification step implementing an anti-horseradish peroxidase column was conducted; this step was effective in removing the recombinant proteins containing matured N-glycans (Figure 6).
Fig. 5.
N-glycan profile of cgl-GCase. Peaks identified as N-glycan signals are labeled. Signals were detected as singly negatively charged [M–H]− unless stated otherwise and were confirmed by MS/MS.
Table III.
N-glycans of cgl-derived GCase
| Abbrev. | Proposed structure | Rel. amount (%) | Hex | HexNAc | Fuc | Xyl | |
|---|---|---|---|---|---|---|---|
| Paucimannose and hybrid type structures | Man3XF |
|
9.2 | 3 | 2 | 1 | 1 |
| Man5F |
|
3.1 | 5 | 2 | 1 | ||
| Man3GnXF |
|
2.7 | 3 | 2 | 1 | 1 | |
| Oligomannosidic structures | Man4 |
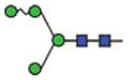
|
17.4 | 4 | 2 | ||
| Man5 |
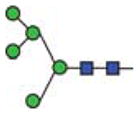
|
60.2 | 5 | 2 | |||
| Man6 |
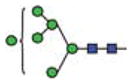
|
1.8 | 6 | 2 | |||
| Man7 |
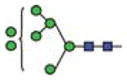
|
1.2 | 7 | 2 | |||
| Man8 |
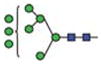
|
4.5 | 8 | 2 |
Fig. 6.
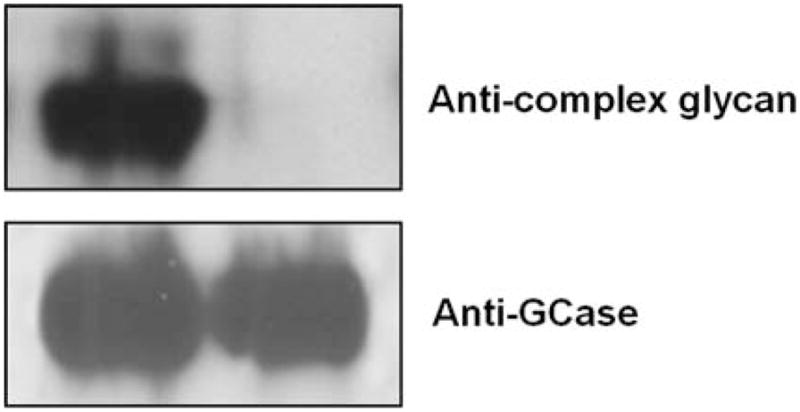
Anti-horseradish-peroxidase affinity chromatography to remove GCase containing N-glycan xylose and/or fucose. Western blot analysis used an antibody specific for plant complex N-glycans (Lauriere et al. 1989). Lane 1, 50 ng purified GCase; lane 2, 50 ng GCase after passing through an anti-horseradish-peroxidase affinity column.
The efficiency of mouse macrophages to internalize cgl-GCase and imiglucerase via their cell-surface mannose receptors is comparable
Mouse macrophage cells (RAW 264.7) were used to determine the efficiency of GCase uptake. Equal amounts of cgl-GCase and imiglucerase (3000 ± 300 nmol MU/h, ~ 1 μg) were applied to the RAW cells (Supplementary data, Fig. S1A). Following the 3-h incubation period, total intracellular GCase activity was determined and found to be significantly increased (~20%) in cells treated with cgl-GCase and imiglucerase relative to untreated cells (Supplementary data, Fig. S1B). To reduce the high endogenous murine GCase activity in RAW cells, an antibody that specifically recognizes human GCase was used to “pull down” and directly measure the increase in enzyme activity produced by the internalized imiglucerase or cgl-GCase. This procedure resulted in a 300-fold reduction in background levels of murine GCase activity relative to cells that were treated with either of the recombinant GCase proteins (Figure 7). Importantly, similar amounts of imiglucerase and cgl-GCase were internalized by the cultured macrophages. Furthermore, the internalization of either enzyme was similarly reduced by ~7-fold when yeast mannan was included in the culture media. These data indicate that the major cellular mechanism of endocytosis is the same for either form of GCase, i.e. via mannose receptors expressed on the plasma membrane of macrophages. As has been demonstrated by the efficacy of imiglucerase when used for ERT, following receptor-mediated endocytosis the receptor–ligand complex is transported to the lysosome where GCase can hydrolyze its stored substrate.
Fig. 7.
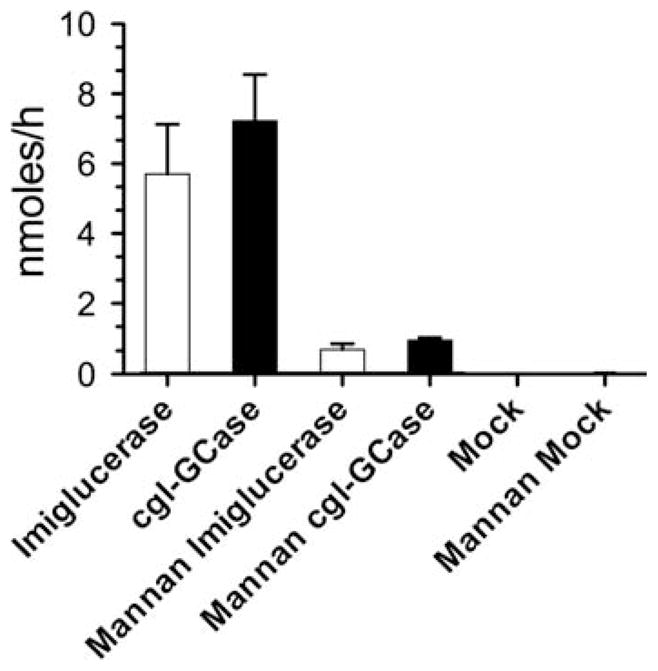
Comparison of uptake of imiglucerase and cgl-GCase by RAW mouse macrophages. GCase activity immunoprecipitated by a rabbit polyclonal IgG antibody specific for human GCase from lysates of treated cells.
GCase is moderately stable during seed storage
To determine the post-harvest stability of GCase, seeds were harvested when siliques had dried out (3 weeks after pollination). These seeds were maintained at room temperature for 25 d for further drying (seeds lost fresh weight during the first 2 weeks), and were then transferred to 4°C, during which time they were maintained over silica gel as a desiccant (Table IV). The GCase activities declined during the initial storage period when seeds were kept at room temperature but the activities recovered following the transfer of seeds to 4°C within 2 months. At the end of the storage period (at d 115 of storage), the GCase activities were ~75% of the original. Interestingly, the pattern showing a transient increase in GCase activities following the transfer of seeds to 4°C storage conditions is also observed for another lysosomal hydrolase (α-L-iduronidase) produced as a recombinant protein in cgl seeds (Downing et al. 2007). These data suggest that cgl-derived GCase is moderately stable during seed storage.
Table IV.
Stability of GCase during storage of mature cgl seeds
| Storage treatment | Total storage days | Activity (units/mg TSP) and % retention of original activity |
|---|---|---|
| Freshly harvested | 0 | 24.5 |
| 7 days, RT | 7 | 21.1 (86.1%) |
| 14 days, RT | 14 | 20.7 (84.5%) |
| 25 days, RT | 25 | 16.7 (68.2%) |
| 25 days, RT; 7 days, 4°C | 32 | 18.1 (73.9%) |
| 25 days, RT; 30 days, 4°C | 55 | 22.3 (91.0%) |
| 25 days, RT; 90 days, 4°C | 115 | 18.8 (74.5%) |
RT, room temperature.
Discussion
GCase produced in cgl seeds is highly active and possesses similar characteristics to those of imiglucerase
Arabidopsis seeds constitute an attractive host for the expression of complex therapeutic glycoproteins: (i) The seeds are not a food crop; thus regulatory issues are far less contentious (e.g. issues pertaining to a need to separate edible food crops destined for consumption, from those destined for biopharmaceutical use); (ii) The stability of the recombinant protein in the mature dry cool-stored seeds (including GCase in the present study) is a significant advantage, and allows one to generate many seeds that can conveniently stored until the protein is required. (iii) Automated sowing/harvesting of pilled seeds for bulk production of Arabidopsis biomass has been developed or is in the process of being developed (Loos et al. 2011).
In the present study, the highest expressing cgl line of Arabidopsis accumulated GCase at a protein amount of ~0.1% TSP (T3 seeds). Using this line and our present purification protocol, 1 mg of purified GCase can be generated from ~25–30 g of mature dry seed. There is likely the potential to obtain lines with even higher GCase activity and protein levels with more screening; expression of human α-L-iduronidase in cgl seeds using the same (arcelin gene) regulatory sequences, yielded an exceptional line in which the α-L-iduronidase protein was estimated to be 18 μg/mg TSP or 1.8% TSP (Downing et al. 2006; Downing et al. 2007).
Most importantly, the enzymatic properties of purified cgl-derived GCase, including kinetic parameters (Vmax and Km), thermal stability and IC50 of IFG, were similar to those of imiglucerase.
Cgl seeds generate GCase with mannose-terminated N-glycans and the GCase protein is efficiently sequestered by mammalian macrophages
Immunogold electron microscopy (EM) localization of GCase in transgenic Arabidopsis cgl seeds demonstrated that the majority of recombinant GCase was secreted into the apoplast associated with embryo storage parenchyma cells. In human tissues, GCase appears to be loosely associated with the lysosomal membrane (van Weely et al. 1990). In mammalian cells, GCase is targeted to the lysosome independently of the mannose-6-phosphate receptor (Aerts et al. 1988); the lysosomal integral membrane protein LIMP-2 plays a specific role in this targeting process, and in its absence, the majority of GCase is secreted (Reczek et al. 2007).
Since the transport pathway of GCase to the plant cell surface most likely occurs via transit through the Golgi complex, it was important to determine the maturation status of the N-linked glycans of the recombinant GCase. The recombinant GCase predominantly possessed oligomannose-type N-glycans, with 60.2% Man5 and 17.4% Man4. The core fucosylated mannosidic Man5F and the Man3GnXF accounted for 3.1 and 2.7% of the N-glycans, respectively. Some of the paucimannosidic N-glycan structures (Man3) containing xylose and/or fucose accounted for ~9% and may be generated by the action of vacuolar or plasma membrane β-N-acetylhexosaminidases (HEXO1 and HEXO 2/3, respectively) (Strasser et al. 2007). The immunogold EM analyses detected a minor fraction of the GCase in protein storage vacuoles of the cgl seeds (Supplemenatry data, Fig. S2); most of the recombinant enzyme was secreted (Figure 2). Cgl-GCase containing these N-glycan species probably initially contained a terminal GlcNAc residue (allowing for the addition of xylose and/or fucose in the plant Golgi); subsequently, the terminal GlcNAc may have been removed by one or more of the β-N-acetylhexosaminidases.
This study represents the first detailed analysis of the glycan profile of a human therapeutic glycoprotein generated within an Arabidopsis seed cgl background. The mutant is characterized by one amino acid change in GnT I (Asp144 to Asn). This creates an additional N-glycosylation site that leads to a misfolded and unstable protein with no detectable enzyme activity (Strasser et al. 2005). Earlier reports of the cgl mutant showed an absence of complex glycans on glycoproteins of plant leaf extracts or callus extracts, with the predominant type of glycan being Man5GlcNAc2 (von Schaewen et al. 1993; Strasser et al. 2005). More recently, Frank et al. (2008) in their analysis of three cgl1 alleles detected a low level of GnT I activity in this particular cgl mutant under conditions that promote “underglycosylation” of proteins. For example, tunicamycin treatment relieves the “folding block” for the mutant GnT I protein, permitting its transit to the Golgi complex. Thus, the conditional nature of this particular cgl mutant (cgl1 C5) has been demonstrated. Partial restoration of GnT I activity during seed development is a distinct possibility in the cgl1 C5 mutant. Our glycan data on cgl-derived GCase, showing ~15% of N-glycans containing xylose and/or fucose, suggest that this may indeed be the case. Glycan trimming appears to play a critical role during seed development (Boisson et al. 2001), and it is possible that complex N-glycans are likewise important. Conditional lethality of animal cells occurs when there is GnT I deficiency. For example, deficiency of GnT I is an embryonic lethal in mice (Ioffe and Stanley 1994), whereas CHO cell mutants that lack GnT I activity are healthy (Kumar et al. 1990). The present strategy demonstrates the potential for producing appropriate recombinant therapeutics, including GCase, if the cgl1 C6 mutant is used, as this line is a true loss-of-function mutant. Alternatively, extensive purification of the cgl1 C5-derived proteins to remove antigenic forms of the enzyme could be implemented. For example, we show that a chromatography step in the purification scheme is an effective means to remove the ~15% complex/hybrid-glycan-containing CGase.
N-glycosylation (e.g. at Asn-19) is critical for the acquisition of GCase activity (Berg-Fussman et al. 1993). There are five predicted N-glycosylation sites in GCase; the first four of them are generally utilized (Berg-Fussman et al. 1993). Sequential in vitro enzymatic remodeling of the three complex N-glycans of placental GCase to “uncover” terminal mannose residues generates a modified GCase that is more efficiently internalized by macrophages because of its improved ability to interact with mannose receptors on macrophages (Furbish et al. 1981). This strategy was used to modify the N-glycans on CHO-cell-derived recombinant human GCase, imiglucerase (in which the N-glycans are predominantly complex types at all four sites), and the remodeled imiglucerase showed an increased clinical efficacy for Gaucher disease (Grabowski et al. 1995). To eliminate the need for carbohydrate remodeling, alternative expression systems that generate GCase with mannose-terminated N-glycans in vivo have attracted attention, such insect cells (Sinclair and Choy 2002), Lec1 mutant CHO cells (Van Patten et al. 2007) and kifunensine (mannosidase I inhibitor)-treatment of a cultured human cell line induced to produce gene-activated human GCase (“velaglucerase alfa”) (Brumshtein et al. 2010).
Plant-based systems are viewed as one of the more promising alternative expression systems for cost-effective production of recombinant proteins, possessing distinct advantages, even over the above-mentioned systems. However, the sugars that are added during N-glycan maturation (i.e. formation of complex glycans), such as β-1,2-xylose and α-1,3-fucose, may pose problems in part because of their potential immunogenicity, and this has limited the use of plant systems for production of human therapeutic proteins. GCase has been expressed in tobacco leaves, tobacco seeds and carrot suspension cells (Reggi et al. 2005; Shaaltiel et al. 2007). GCase derived from tobacco seeds is problematic since terminal mannose residues account for only a small proportion of the N-glycans on tobacco-expressed recombinant GCase; uptake of this protein by human R120W fibroblasts was 5-fold lower when compared with imiglucerase. Shaaltiel et al. (2007) used carrot suspension cells to express a modified human GBA1 gene encoding glucocerebrosidase (GBA) cDNA, whereby the recombinant GCase was targeted to storage vacuoles by appending a vacuolar targeting sequence. Because of the glycosidases that are resident in the protein storage vacuole, recombinant human GCase with mannose-terminated N-glycans was produced. The recombinant GCase expressed in carrot cells was shown to contain over 90% mannose-terminated N-glycans, and to exhibit the same rate of uptake by thioglycolate-elicited peritoneal macrophages as imiglucerase. It is noteworthy that the recombinant enzyme produced in this expression system has two modifications that may be of concern relative to cgl-GCase and imiglucerase. First, the mature recombinant GCase protein has two extra amino acids on the N-terminus (derived from the linker used for the fusion of a plant-specific signal peptide; glutamic acid and phenyl-alanine), and seven extra amino acids on the C-terminus (DLLVDTM, constituting the vacuolar sorting determinant of tobacco chitinase A) (Neuhaus et al. 1991). Secondly, the dominant N-glycans contain xylose and/or fucose residues, which are potentially antigenic structures. Although a single-dose test did not lead to any toxic responses in mice (Shaaltiel et al. 2007), and a subsequent primate study uncovered no adverse effects (Aviezer et al. 2009), the US FDA, nonetheless, has requested further testing of this carrot cell-derived therapeutic. As a potential plant-based ERT for Gaucher disease, cgl-GCase does not share these concerns.
We have shown that GCase produced in the cgl1 C5 background of Arabidopsis naturally contains mannose-terminated N-glycans (97%) that are predominantly oligomannosidic (85%). This obviates the need for further in vitro enzymatic remodeling. However, the xylose- and/or fucose-containing recombinant cgl-GCase molecules (comprising ~15%) need to be removed. One strategy, as demonstrated here, is to remove this fraction using an anti-horseradish peroxidase affinity column as one of the purification steps. It may be preferable to perform this step prior to a final Bio-gel filtration step; this would avoid any IgG leaching from the affinity column (and therefore potentially introducing immunogenicity). Another strategy is to host production of the recombinant protein in the null cgl (C6) mutant.
Thus the cgl system provides proof-of-principle of a viable plant-based expression platform to produce recombinant GCase with mannose-terminated N-glycans. Macrophage uptake studies confirmed that cgl-GCase was taken up to a similar extent as imiglucerase, and primarily via mannose receptors. Both cgl-GCase and imiglucerase have similar enzyme kinetic and thermostability properties. These results provide the foundation for future experiments to evaluate the in vivo stability and therapeutic efficacy of Arabidopsis-derived GCase to reduce the substrate storage in our murine model of Gaucher disease (Sinclair et al. 2007).
Materials and methods
Generation of the expression construct for production of recombinant human GCase, and generation of Arabidopsis transformants
Procedures for DNA manipulation were conducted according to standard protocols (Sambrook et al. 1989) using enzymes from Fermentas Canada Inc. (Burlington, ON, Canada) and Invitrogen Life Technologies (Burlington, ON, Canada). Oligonucleotide primers for PCR amplification reactions were synthesized by Sigma-Genosys (www.sigma-genosys.com); PCR-generated sequences were confirmed by sequence analysis (University Core DNA and Protein Services, University of Calgary, Calgary, AB, Canada).
The planned sequence of ligations required, in one instance, the elimination of a single EcoRI site and, in another, elimination of HindIII and EcoRI sites from the plasmid used in the majority of the subcloning, pT7T3 18u. This was accomplished by cutting with the respective restriction enzymes and filling in with Klenow fragment. The resulting plasmids were designated pTE and pTHE, respectively.
A PCR was performed to take advantage of the unique MfeI site near the 5′ end of the GBA open-reading frame and accomplish the dual goal of adding an EcoRI site and excising a HindIII site. The DNA sequence encoding the 5′ end of the human GBA cDNA was PCR synthesized using pBS-GC1.6/E5 as template, with the forward primer p(9) containing the new EcoRI site (5′-CCATCGATGAATT CCTGGCGATGCCACAGGTA-3′) and the reverse primer p (10) comprised of 5′-AGGACCCAATTGGGTGCGT-3′. After restriction of the PCR-amplified product with MfeI and ClaI, the 323-bp fragment encoding the C-terminus of the mature GCase protein was isolated for cloning. The original plasmid containing the GBA cDNA (pBS-GC1.6/E5) was digested with MfeI and ClaI, treated with calf intestinal alkaline phosphatase and purified. Re-integration with the digested and phosphorylated PCR fragment led to the generation of the new plasmid pBS-GC Cla/Mfe.
Two PCRs were performed to generate two fragments, which are denoted by A and B (Figure 1A). For A, a fragment containing DNA regulatory sequences of the arcelin gene (the ARC 5-I gene promoter, and 5′-UTR and signal peptide-encoding sequences) was PCR synthesized using pWD 66 (Downing et al. 2006) as a template, forward primer p(1) (5′-ACGCCCGGGG TTATTTCCTCATCACCAGAC-3′) and reverse primer p(2) (5′-TGAGTTTGC GTGTGTGAGAA-3′). For B, the DNA sequence encoding the 5′ end of the GBA open-reading frame (minus its signal peptide-encoding sequences) was PCR synthesized using pBS-GC1.6/E5 as template, forward primer p(3) (5′-GCCCGCCCCTGCATCC-3′) and reverse primer p(4)(5′CGCGTCTAGAGGGTACCC GGATGATGTTAT-3′). After restriction of the PCR-amplified products (A with XmaI, and B with HindIII), the fragments were isolated, phosphorylated and purified for cloning. The two fragments were ligated into pTHE (which had been digested with XmaI and HindIII, treated with calf intestinal alkaline phosphatase and purified on an agarose gel), giving rise to pTHE AB for later cloning.
The DNA sequence encoding the C-terminus of the GCase protein was PCR synthesized using pBS-GC Cla/Mfe as template, forward primer p(14) (5′-CCCTAAAAGCTTCGG CTACA-3′) and the reverse primer p(15) (5′-TCACTGG CGATGCCACAGGTA-3′). The DNA sequence comprising the 3′ end of the arcelin gene was released from pWD66 (Downing et al. 2006) by restriction digestion with XbaI. Both fragments were gel-purified and ligated to generate pTECD.
To make the final construct for production of GCase in cgl seeds (Figure 1A), pTECD was digested with HindIII and XbaI, and the fragment gel-purified and ligated into pTHE AB generating pTHE ABCD. The XmaI/XbaI fragment from the pTHE ABCD was recovered from the gel and ligated into the binary vector pRD 400 XmaI/XbaI site. Plasmid pRD400 carries the NPTII gene for kanamycin resistance (Datla et al. 1991).
The recombinant binary plasmid was introduced into competent Agrobacterium tumefaciens GV3101/pMP90 cells (Koncz and Schell 1986) according to Katavic et al. (1994). Transformed cells were selected on media containing 50 μg/mL kanamycin, 25 μg/mL rifampicin and 25 μg/mL gentamycin.
Arabidopsis thaliana cgl seeds were obtained from the Arabidopsis Biological Resource Center (stock number CS6192). This particular mutant is referred to as cglI C5 in Frank et al. (2008). Arabidopsis plants were transformed according to the fioral dip method described by Clough and Bent (1998). T1 generation transformants were selected on medium containing 50 μg/mL kanamycin according to Katavic et al. (1994). Plants were grown at 20°C in long days (16 h light, 8 h dark; 90–120 μE m−2 s−1 photosynthetically active radiation [PAR]) on prepared soil mixture (Terra-Lite Redi Earth; W.R. Grace and Company of Canada Ltd., Ajax, ON, Canada). Seeds of the T2 generation were used for screening (western blot analyses and GCase activity assays). Seeds of the T3 generation of the highest expressing line were used for GCase purification.
GCase activity assays
GCase activities in the crude extracts or purified fractions were assayed using the fiuorogenic substrate 4-MUGP (Sigma) according to Sawkar et al. (2002) and Dale and Beutler (1976). Protein was extracted from cgl seeds by grinding seeds with a plastic pestle in buffer [50 mM sodium phosphate, pH 6.0, 0.1% sodium taurocholic acid, 1 mM EDTA, 0.5 mM phenylmethanesulfonylfluoride (PMSF)]. The homogenate was centrifuged at 13,000 rpm for 10 min and the resulting supernatant was assessed for GCase activity and TSP (quantitative) assays. The activity assay was carried out by adding 5 μL of protein extract to 50 μL of the assay buffer containing 0.1 M potassium phosphate (pH 5.0), 0.15% Triton, 0.125% sodium taurocholate and 1.5 mM 4-MUGP. The mixture was incubated at 37°C for 0.5–1 h and terminated by adding 1.4 mL glycine buffer (0.2 M glycine, 0.125 M sodium carbonate, pH 10.7). Fluorescence of the reaction product, 4 MU, was determined (λex = 365 nm, λem = 460 nm). One unit of activity is defined as the amount of enzyme required to release 1 nmol 4 MU/min. Protein concentrations were determined by the Bio-Rad DC assay (Bio-Rad Laboratories Inc.).
Western blot analysis
The alkaline phosphatase detection system was used for western blots to identify the transgenic cgl lines producing recombinant GCase using methods described previously (Downing et al. 2006). The primary polyclonal anti-GCase antibody (Grabowski et al. 1985) was diluted 1:1000. Purified cgl-GCase was subjected to western blot analysis, before and after passage through an anti-horseradish-peroxidase affinity column, to detect the GCase proteins that contained plant-specific complex N-glycans (see below). For these analyses, the Lumigen™ TMA-6 detection kit was used as per manufacturer’s instructions (GE Healthcare UK Limited, Little Chalfont Buckinghamshire, UK). The anti-complex glycan antibody (Lauriere et al. 1989) was diluted 1:2000.
Immunogold EM localization of GCase
The general procedures for immunogold EM studies to localize GCase in transgenic cgl seeds were performed essentially as described previously (Tse et al. 2004). Dry seeds of untransformed cgl and transgenic cgl plants were imbibed in water at 4°C for 12 h before high-pressure freezing, and subsequent substitution of frozen sections with hydrophobic Lowicryl resin HM20. Immunolabeling was carried out using GCase-specific polyclonal antibodies at a 1:300 dilution and gold-coupled secondary antibodies at a 1:50 dilution. Aqueous uranyl acetate/lead citrate poststained sections were examined in a JOEL JEM-1200EX II transmission electron microscope (JOEL, Tokyo, Japan) operating at 80 kV.
Purification of GCase from cgl seeds
The purification protocol was developed based on Furbish et al. (1977), Dale and Beutler (1976), Choy (1989), and Lee et al. (1994). Seeds of the T3 generation (line 1) were ground in liquid N2 and proteins were extracted using the following buffer: 20 mM Tris, pH 7.0, 150 mM NaCl, 0.5% taurocholic acid, 1 mM PMSF. The conditions for Con A column binding and elution were optimized to facilitate maximum binding and elution of GCase (based on activity assays). After centrifugation at 15,000 rpm for 45 min, the supernatant was collected and loaded onto a Con A-sepharose column using a peristaltic pump (Pump-P1, Amersham) recycling for 24 h at 4°C. After washing, glycoproteins were eluted from the column using 15% methyl-α-mannoside in the above buffer recycling for 16–24 h with four exchanges of elution buffer. The eluant was concentrated using Microcon 30 kDa (Amicon) (Millipore, Billerica, MA, USA) and an exchange buffer with 100 mM sodium acetate, pH 5.2, 14% (w/v) ammonium sulfate (buffer A). The concentrated eluant was filtered through a 0.45-μm filter and loaded onto a Butyl FF column (1 mL; GE Healthcare Life Science, Quebec, Canada) using ÄKTA™ FPLC™ (GE Healthcare Life Science) at a flow rate of 0.5 mL/min. The column was then washed with 15 mL buffer A and 15 mL 50 mM ammonium sulfate followed by 15 mL of 24% ethanol (v/v) in 100 mM acetate buffer, pH 6.0. The GCase was then eluted with an elution buffer (150 mM acetate buffer, pH 6.0 containing 36% ethanol v/v; final pH 6.5). The eluted fractions were collected and assayed for GCase activity, and active fractions were pooled and concentrated with Microcon 30 kDa. The concentrated eluant was diluted 1:1 with 150 mM acetate buffer (pH 6.0) and loaded onto a Bio-Gel P 100 (BioRad) column (2.5 × 15 cm). GCase was eluted with 150 mM acetate buffer, pH 6.0, containing 18% ethanol at a flow rate of 0.5 mL/min. Fractions with GCase activity were concentrated with Microcon 10 kDa, then fractionated by SDS–PAGE to examine the purity of GCase. For long-term storage, the purified GCase fractions were combined and then dialyzed in 0.1 M citrate buffer, pH 6.0, containing 50% ethylene glycol.
Thermal denaturation experiments
The thermal stabilities of imiglucerase and cgl-GCase in the presence or in the absence of 300 μM IFG (isofagamine tartrate; Toronto Research Chemicals, North York, Canada) were evaluated using the previously described fluorescence denaturation assay based on the environmentally sensitive fluorophore NanoOrange (Kornhaber et al. 2008). All assays were performed in 100 mM phosphate–50 mM citric acid buffer at pH 7 in the presence of 1 μg cgl-GCase/imiglucerase and a 1/100 dilution of NanoOrange stock (Invitrogen). Thermal denaturation was carried out using a Mini-Opticon RT PCR (BioRad) instrument. Tm’s of enzymes in the presence or in the absence IFG were determined using the software provided by supplier.
Kinetic evaluation of GCase derivatives
Activities of enzymes were evaluated at pH 5.5 in 200 mM sodium phosphate–100 mM sodium citrate buffer containing 0.2% taurodeoxycholate at 37°C as described previously (Tropak et al. 2008). The IC50 of IFG (IFG tartrate) for imiglucerase or cgl-GCase was determined in the presence of 5 mM 4-MUGP substrate. Km and Vmax values for cgl-GCase and imiglucerase were determined in the presence of a 2-fold dilution series of decreasing concentrations of substrate (10–0.3 mM). Values were determined following nonlinear fitting of the data using the Michaelis–Menten equation within Prism 5.0.
Glycan composition analyses
The graphitized carbon liquid chromatography tandem mass spectrometry (carbon LC MS/MS) system was used to characterize the N-glycan status of recombinant GCase (Wilson et al. 2002). Purified GCase (4 μg) was resolved by 10% SDS–PAGE and the GCase protein band was recovered from the gel. N-glycans were released using PNGase A from tryptic peptides obtained after in-gel digestion as described by Kolarich and Altmann (2000). The released N-glycans were reduced and desalted and were analyzed by carbon LC ESI MS/MS using a Thermo Hypercarb column (180 μm ID × 100 mm) with an Agilent 1100 capillary LC and an Agilent ion trap for detection using negative ion mode according to Wilson et al. (2002). Oligosaccharide structures were assigned based on mass, MS/MS fragmentation and on the knowledge about plant N-glycosylation synthetic pathways (Leonard et al. 2004). The relative N-glycan distribution was calculated from the signal intensities of the monoisotopic m/z signals in the combined MS spectrum, which was summed over the entire range in which the N-glycan elute. If the singly and doubly charged signals were detected, both intensities were taken into account.
Anti-horseradish-peroxidase affinity column for removal of GCase containing plant complex N-glycans
Approximately 15% of the GCase derived from cgl seeds contained matured N-glycans, i.e. N-glycans with xylose and/or fucose. This fraction of the GCase was removed from the sample by passing the purified GCase (a single band on SDS–PAGE) through an anti-horseradish-peroxidase affinity column (recycling overnight at 4°C). The antibody specifically binds to xylose and/or fucose residues (Wilson et al. 1998), allowing for the removal of any GCase containing these sugars from the sample. The polyclonal anti-horseradish-peroxidase antibodies (Sigma-Aldrich Canada, Oakville, Ontario, Canada) were cross-linked to Affi-Gel 10 (Bio-Rad Laboratories Inc., Mississauga, ON, Canada) according to the manufacturer’s instructions.
Uptake of GCase by mouse macrophages
GCase uptake experiments were conducted according to a protocol of Brumshtein et al. (2010) with modifications. RAW 264.7 mouse macrophage cells were distributed into 24-well plates (0.5 × 105 cells/well) and grown in RPMI (Roswell Park Memorial Institute) medium supplemented with 10% fetal calf serum overnight at 37°C in a CO2 humidified incubator. The following day, the growth medium was removed, cells were washed twice with phosphate-buffered saline (containing calcium and magnesium) and replaced with RPMI growth media supplemented with 0.025% human serum albumin (RPMIH) or RPMIH containing 20 mg/mL yeast mannan (RPMIHM). Following a 1-h incubation at 37°C, the corresponding buffers were substituted with fresh buffer containing equivalent activity units of GCase (~ 1 μg, 3000 nmol MU/h units of imiglucerase [Genzyme, USA] or cgl-GCase), or lacking enzyme. Prior to the incubation period, an aliquot was removed to confirm the amount of units of enzyme added. Uptake was allowed to proceed for 3 h at 37°C in a CO2 humidified incubator. Following uptake, the incubation buffer was removed and the cells were extensively washed with three changes of phosphate-buffered saline containing calcium and magnesium (2 mL). Cells were lysed at 4°C for 30 min using 200 μL citrate phosphate (McIlvaine) buffer, pH 5.5, containing 0.4% Triton X 100 and 0.2% taurodeoxycholate. One-fifth of the lysate was used to measure the total intracellular GCase and β-N-acetyl hexo-saminidase activity as described previously (Tropak et al. 2008). The remainder of the lysate was used to immunoprecipitate endocytosed recombinant human GCase (imiglucerase or cgl-GCase). To each lysate sample was added 1 μL of rabbit anti-human GCase (Tropak et al. 2008) together with 20 μL of a 50% slurry of GammaBind™ Plus Sepharose™ beads (GE Healthcare, USA). Binding was allowed to proceed overnight with end-over-end mixing. Beads were then spun down by brief centrifugation, followed by three washes with 200 μL lysis buffer. The majority of wash buffer was removed and replaced with 25 μL lysis buffer followed by an equal volume of 4-MUGP (to give a final concentration of 5 mM) in McIVaine buffer, pH 5.5. Reactions were carried out for 1 h at 37°C and monitored as described previously (Tropak et al. 2008).
Supplementary Material
Acknowledgments
Funding
This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Strategic grant (STPGP 350770-07) and a Michael Smith Foundation for Health Research (MSFHR) Senior Scholar Investigatorship awarded to A.R.K.
Abbreviations
- 4-MUGP
4-methylumbelliferyl β-D-glucopyranoside
- cgl
complex-glycan-deficient
- Con A
concanavalin A
- EM
electron microscopy
- FDA
Federal Drug Agency
- GBA gene
GBA1 gene encoding glucocerebrosidase
- GCase
glucocerebrosidase
- GnT I
N-acetylglucosaminyl transferase I
- IC50
half-maximal inhibitory concentration
- IFG
isofagomine tartrate
- TSP
total soluble protein
Footnotes
Supplementary data for this article is available online at http://glycob.oxfordjournals.org/.
References
- Aerts JM, Schram AW, Strijland A, van Weely S, Jonsson LM, Tager JM, Sorrell SH, Ginns EI, Barranger JA, Murray GJ. Glucocerebrosidase, a lysosomal enzyme that does not undergo oligosaccharide phosphorylation. Biochim Biophys Acta. 1988;964:303–308. doi: 10.1016/0304-4165(88)90030-x. [DOI] [PubMed] [Google Scholar]
- Aviezer D, Brill-Almon E, Shaaltiel Y, Hashmueli S, Bartfeld D, Mizrachi S, Liberman Y, Freeman A, Zimran A, Galun E. A plant-derived recombinant human glucocerebrosidase enzyme—A preclinical and phase I investigation. PLoS ONE. 2009;4:e4792. doi: 10.1371/journal.pone.0004792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg-Fussman A, Grace ME, Ioannou Y, Grabowski GA. Human acid beta-glucosidase. N-glycosylation site occupancy and the effect of glycosylation on enzymatic activity. J Biol Chem. 1993;268:14861–14866. [PubMed] [Google Scholar]
- Bergmann JE, Grabowski GA. Posttranslational processing of human lysosomal acid beta-glucosidase: a continuum of defects in Gaucher disease type 1 and type 2 fibroblasts. Am J Hum Genet. 1989;44:741–750. [PMC free article] [PubMed] [Google Scholar]
- Beutler E, Grabowski GA. Gaucher disease. In: Scriver CR, et al., editors. The metabolic and molecular bases of inherited disease. 8. III. New York: McGraw-Hill; 2001. pp. 3635–3668. [Google Scholar]
- Bijsterbosch MK, Donker W, van de Bilt H, van Weely S, van Berkel TJ, Aerts JM. Quantitative analysis of the targeting of mannose-terminal glucocerebrosidase. Predominant uptake by liver endothelial cells. Eur J Biochem. 1996;237:344–349. doi: 10.1111/j.1432-1033.1996.00344.x. [DOI] [PubMed] [Google Scholar]
- Boisson M, Gomord V, Audran C, Berger N, Dubreucq B, Granier F, Lerouge P, Faye L, Caboche M, Lepiniec L. Arabidopsis glucosidase I mutants reveal a critical role of N-glycan trimming in seed development. EMBO J. 2001;20:1010–1019. doi: 10.1093/emboj/20.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothe J, Nykiforuk C, Shen Y, Zaplachinski S, Szarka S, Kuhlman P, Murray E, Morck D, Moloney MM. Seed-based expression system for plant molecular farming. Plant Biotechnol J. 2010;8:588–606. doi: 10.1111/j.1467-7652.2010.00511.x. [DOI] [PubMed] [Google Scholar]
- Brumshtein B, Salinas P, Peterson B, Chan V, Silman I, Sussman JL, Savickas PJ, Robinson GS, Futerman AH. Characterization of gene-activated human acid-beta-glucosidase: crystal structure, glycan composition, and internalization into macrophages. Glycobiology. 2010;20:24–32. doi: 10.1093/glycob/cwp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy FYM. Purification of lysosomal membrane-bound glucocerebrosidase from human cultured fibroblasts using high-performance liquid chromatography. Anal Biochem. 1989;179:312–318. doi: 10.1016/0003-2697(89)90135-8. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Dale GL, Beutler E. Enzyme replacement therapy in Gaucher’s disease: a rapid, high-yield method for purification of glucocerebrosidase. Proc Natl Acad Sci USA. 1976;73:4672–4674. doi: 10.1073/pnas.73.12.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datla RSS, Hammerlindl JK, Panchuk B, Pelcher LE, Keller W. A bifunctional fusion between β-glucuronidase and neomycin phosphotransferase: a broad-spectrum marker enzyme for plants. Gene. 1991;122:383–384. doi: 10.1016/0378-1119(91)90417-a. [DOI] [PubMed] [Google Scholar]
- De Jaeger G, Scheffer S, Jacobs A, Zambre M, Zobell O, Goossens A, Depicker A, Angenon G. Boosting heterologous protein production in transgenic dicotyledonous seeds using Phaseolus vulgaris regulatory sequences. Nat Biotechnol. 2002;20:1265–1268. doi: 10.1038/nbt755. [DOI] [PubMed] [Google Scholar]
- Downing WL, Galpin JD, Clemens S, Lauzon SM, Samuels AL, Pidkowich MS, Clarke LA, Kermode AR. Synthesis of enzymatically active human α-L-iduronidase in Arabidopsis cgl (complex glycan-deficient) seeds. Plant Biotechnol J. 2006;4:169–181. doi: 10.1111/j.1467-7652.2005.00166.x. [DOI] [PubMed] [Google Scholar]
- Downing WL, Hu X, Kermode AR. Post-transcriptional factors are important for high-level expression of the human α-L-iduronidase gene in Arabidopsis cgl (complex glycan-deficient) seeds. Plant Sci. 2007;172:327–334. [Google Scholar]
- Frank J, Kaulfürst-Soboll H, Rips S, Koiwa H, von Schaewen A. Comparative analyses of Arabidopsis complex glycan 1 mutants and genetic interaction with staurosporin and temperature sensitive3a. Plant Physiol. 2008;148:1354–1367. doi: 10.1104/pp.108.127027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman B, Vaddi K, Preston C, Mahon E, Cataldo JR, McPherson JM. A comparison of the pharmacological properties of carbohydrate remodeled recombinant and placental-derived beta-glucocerebrosidase: implications for clinical efficacy in treatment of Gaucher disease. Blood. 1999;93:2807–2816. [PubMed] [Google Scholar]
- Furbish FS, Blair HE, Shiloach J, Pentchev PG, Brady RO. Enzyme replacement therapy in Gaucher’s disease: large-scale purification of glucocerebrosidase suitable for human administration. Proc Natl Acad Sci USA. 1977;74:3560–3563. doi: 10.1073/pnas.74.8.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbish FS, Steer CJ, Krett NL, Barranger JA. Uptake and distribution of placental glucocerebrosidase in rat hepatic cells and effects of sequential deglycosylation. Biochim Biophys Acta. 1981;673:425–434. doi: 10.1016/0304-4165(81)90474-8. [DOI] [PubMed] [Google Scholar]
- Gomord V, Faye L. Posttranslational modification of therapeutic proteins in plants. Curr Opin Plant Biol. 2004;7:171–181. doi: 10.1016/j.pbi.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Gomord V, Fischette AC, Menu-Bouaouiche L, Saint-Jore-Dupas C, Plasson C, Michaud D, Faye L. Plant-specific glycosylation patterns in the context of therapeutic protein production. Plant Biotechnol J. 2010;8:564–587. doi: 10.1111/j.1467-7652.2009.00497.x. [DOI] [PubMed] [Google Scholar]
- Goossens A, Dillen W, De Clercq J, Van Montagu M, Angenon G. The arcelin-5 gene of Phaseolus vulgaris directs high seed-specific expression in transgenic Phaseolus acutifolius and Arabidopsis plants. Plant Physiol. 1999;120:1095–1104. doi: 10.1104/pp.120.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski GA. Gaucher disease: Gene frequencies and genotype/phenotype correlations. Genet Test. 1997;1:5–12. doi: 10.1089/gte.1997.1.5. [DOI] [PubMed] [Google Scholar]
- Grabowski GA. Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet. 2008;372:1263–1271. doi: 10.1016/S0140-6736(08)61522-6. [DOI] [PubMed] [Google Scholar]
- Grabowski GA, Barton NW, Pastores G, Dambrosia JM, Banerjee TK, McKee MA, Parker C, Schiffmann R, Hill SC, Brady RO. Enzyme therapy in type 1 Gaucher disease: comparative efficacy of mannose-terminated glucocerebrosidase from natural and recombinant sources. Ann Intern Med. 1995;122:33–39. doi: 10.7326/0003-4819-122-1-199501010-00005. [DOI] [PubMed] [Google Scholar]
- Grabowski GA, Goldblatt J, Dinur T, Kruse J, Svennerholm L, Gatt S, Desnick RJ. Genetic heterogeneity in Gaucher disease: physicokinetic and immunologic studies of the residual enzyme in cultured fibroblasts from non-neuronopathic and neuronpathic patients. Am J Med Genet. 1985;21:529–549. doi: 10.1002/ajmg.1320210316. [DOI] [PubMed] [Google Scholar]
- Ioffe E, Stanley P. Mice lacking N-acetylglucosaminyltransferase I activity die at mid-gestation, revealing an essential role for complex or hybrid N-linked carbohydrates. Proc Natl Acad Sci USA. 1994;91:728–732. doi: 10.1073/pnas.91.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson LM, Murray GJ, Sorrell SH, Strijland A, Aerts JF, Ginns EI, Barranger JA, Tager JM, Schram AW. Biosynthesis and maturation of glucocerebrosidase in Gaucher fibroblasts. Eur J Biochem. 1987;164:171–179. doi: 10.1111/j.1432-1033.1987.tb11008.x. [DOI] [PubMed] [Google Scholar]
- Katavic V, Haugh GW, Reed D, Martin M, Kunst L. In planta transformation of Arabidopsis thaliana. Mol Gen Genet. 1994;245:363–370. doi: 10.1007/BF00290117. [DOI] [PubMed] [Google Scholar]
- Kermode AR. Mechanisms of intracellular protein transport and targeting. Crit Rev Plant Sci. 1996;15:285–423. [Google Scholar]
- Kermode AR. Plants as factories for production of biopharmaceutical and bioindustrial proteins: lessons from cell biology. Can J Bot. 2006;84:679–694. [Google Scholar]
- Kermode AR. Seed Expression Systems for Molecular Farming. In: Wang A, Ma S, editors. Molecular farming in plants: recent advances and future prospects. New York: Springer; 2012. pp. 89–123. [Google Scholar]
- Kermode AR, Zeng Y, Hu X, Lauson S, Abrams SR, He X. Ectopic expression of a conifer Abscisic Acid Insensitive3 transcription factor induces high-level synthesis of recombinant human α-L-iduronidase in transgenic tobacco leaves. Plant Mol Biol. 2007;63:763–776. doi: 10.1007/s11103-006-9122-y. [DOI] [PubMed] [Google Scholar]
- Kolarich D, Altmann F. N-Glycan analysis by matrix-assisted laser desorption/ionization mass spectrometry of electrophoretically separated non-mammalian proteins: application to peanut allergen Ara h 1 and olive pollen allergen Ole e 1. Anal Biochem. 2000;285:64–75. doi: 10.1006/abio.2000.4737. [DOI] [PubMed] [Google Scholar]
- Koncz C, Schell J. The promoter of TL-DNA gene 5 controls the tissue- specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet. 1986;204:383–396. [Google Scholar]
- Kornhaber GJ, Tropak MB, Maegawa GH, Tuske SJ, Coales SJ, Mahuran DJ, Hamuro Y. Isofagomine induced stabilization of glucocerebrosidase. Chembiochem. 2008;9:2643–2649. doi: 10.1002/cbic.200800249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Yang J, Larsen RD, Stanley P. Cloning and expression of N-acetylglucosaminaltransferase I, the medial Golgi transferase that initiates complex N-linked carbohydrate formation. Proc Natl Acad Sci USA. 1990;87:9948–9952. doi: 10.1073/pnas.87.24.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau OS, Sun SSM. Plant seeds as bioreactors for recombinant protein production. Biotechnol Adv. 2009;27:1015–1022. doi: 10.1016/j.biotechadv.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Lauriere M, Lauriere C, Chrispeels MJ, Johnson KD, Sturm A. Characterization of a xylose-specific antiserum that reacts with the complex asparagine-linked glycans of extracellular and vacuolar glycoproteins. Plant Physiol. 1989;90:1182–1188. doi: 10.1104/pp.90.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LS, Muthukumar G, Czuba B, Shorr RGL. WO/1994/014837 Purification of protein-aceous material. 1994
- Leonard R, Kolarich D, Paschinger K, Altmann F, Wilson IB. A genetic and structural analysis of the N-glycosylation capabilities of rice and other monocotyledons. Plant Mol Biol. 2004;55:631–644. doi: 10.1007/s11103-004-1558-3. [DOI] [PubMed] [Google Scholar]
- Lerouge P, Cabanes-Macheteau M, Rayon C, Fischette-Lainé AC, Gomord V, Faye L. N-glycosylation of recombinant pharmaceutical glycoproteins produced in transgenic plants: towards an humanisation of plant N-glycans. Plant Mol Biol. 1998;38:31–48. [Google Scholar]
- Lieberman RL, Wustman BA, Huertas P, Powe AC, Pine CW, Khanna R, Schlossmacher MG, Ringe D, Petsko GA. Structure of acid beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease. Nat Chem Biol. 2007;3:101–107. doi: 10.1038/nchembio850. [DOI] [PubMed] [Google Scholar]
- Loos A, Droogenbroeck BV, Hillmer S, Grass J, Kunert R, Cao J, Robinson DG, Depicker A, Steinkellner H. Production of monoclonal antibodies with a controlled N-glycosylation pattern in seeds of Arabidopsis thaliana. Plant Biotechnol J. 2011;9:179–192. doi: 10.1111/j.1467-7652.2010.00540.x. [DOI] [PubMed] [Google Scholar]
- Neuhaus JM, Sticher L, Meins FJ, Boller T. A short C-terminal sequence is necessary and sufficient for the targeting of chitinases to the plant vacuole. Proc Natl Acad Sci USA. 1991;88:362–366. doi: 10.1073/pnas.88.22.10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reczek D, Schwake M, Schröder J, Hughes H, Blanz J, Jin X, Brondyk W, Van Patten S, Edmunds T, Saftig P. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell. 2007;131:770–783. doi: 10.1016/j.cell.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Reggi S, Marchetti S, Patti T, De Amicis F, Cariati R, Bembi B, Fogher C. Recombinant human acid β-glucosidase stored in tobacco seed is stable, active and taken up by human fibroblasts. Plant Mol Biol. 2005;57:101–113. doi: 10.1007/s11103-004-6832-x. [DOI] [PubMed] [Google Scholar]
- Saint-Jore-Dupas C, Faye L, Gomord V. From planta to pharma with glycosylation in the toolbox. Trends Biotechnol. 2007;25:317–323. doi: 10.1016/j.tibtech.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sawkar AR, Cheng WC, Beutler E, Wong CH, Balch WE, Kelly JW. Chemical chaperones increase the cellular activity of N370S beta-glucosidase: a therapeutic strategy for Gaucher disease. Proc Natl Acad Sci USA. 2002;99:15428–15433. doi: 10.1073/pnas.192582899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Poll LW, vom Dahl S. Therapy of adult Gaucher disease. Hematol J. 2007;92:148–152. doi: 10.3324/haematol.11193. [DOI] [PubMed] [Google Scholar]
- Shaaltiel Y, Bartfeld Y, Hashmueli S, Baum G, Brill-Almon E, Galili G, Dym O, Boldin-Adamsky SA, Silman I, Sussman JL, et al. Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of Gaucher’s disease using a plant cell system. Plant Biotechnol J. 2007;5:579–590. doi: 10.1111/j.1467-7652.2007.00263.x. [DOI] [PubMed] [Google Scholar]
- Sinclair G, Choy FY. Synonymous codon usage bias and the expression of human glucocerebrosidase in the methylotrophic yeast. Protein Exp Purif. 2002;26:96–105. doi: 10.1016/s1046-5928(02)00526-0. [DOI] [PubMed] [Google Scholar]
- Sinclair GB, Jevon G, Colobong KE, Randall DR, Choy FYM, Clarke LA. Generation of a conditional knockout of murine glucocerebrosidase: Utility for the study of Gaucher disease. Mol Genet Metab. 2007;90:148–156. doi: 10.1016/j.ymgme.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Steet RA, Chung S, Wustman BA, Powe AC, Do H, Kornfeld SA. The iminosugar isofagomine increases the activity of N370S mutant acid beta-glucosidase in Gaucher fibroblasts by several mechanisms. Proc Natl Acad Sci USA. 2006;103:13813–13818. doi: 10.1073/pnas.0605928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoger E, Ma JK. Sowing the seeds of success: Pharmaceutical proteins from plants. Curr Opin Biotechnol. 2005;16:167–173. doi: 10.1016/j.copbio.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Strasser R, Bondili JS, Schoberer J, Svoboda B, Liebminger E, Glössl J, Altmann F, Steinkellner H, Mach L. Enzymatic properties and sub-cellular localization of Arabidopsis beta-N-acetylhexosaminidases. Plant Physiol. 2007;145:5–16. doi: 10.1104/pp.107.101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R, Stadlmann J, Svoboda B, Altmann F, Glössl J, Mach L. Molecular basis of N-acetylglucosaminyltransferase I deficiency in Arabidopsis thaliana plants lacking complex N-glycans. Biochem J. 2005;387:385–391. doi: 10.1042/BJ20041686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropak MB, Kornhaber GJ, Rigat BA, Maegawa GH, Buttner JD, Blanchard JE, Murphy C, Tuske SJ, Coales SJ, Hamuro Y, et al. Identification of pharmacological chaperones for Gaucher disease and characterization of their effects on beta-glucocerebrosidase by hydrogen/deuterium exchange mass spectrometry. Chembiochem. 2008;9:2650–2662. doi: 10.1002/cbic.200800304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG, Jiang L. Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell. 2004;16:672–693. doi: 10.1105/tpc.019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman RM, Stoger E, Schillberg S, Christou P, Fischer R. Molecular farming in plants: Host systems and expression technology. Trends Biotechnol. 2003;21:570–578. doi: 10.1016/j.tibtech.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Van Patten SM, Hughes H, Huff MR, Piepenhagen PA, Waire J, Qiu H, Ganesa DR, Kutako JP, Edmunds T. Effect of mannose chain length on targeting of glucocerebrosidase for enzyme replacement therapy of Gaucher disease. Glycobiology. 2007;17:467–478. doi: 10.1093/glycob/cwm008. [DOI] [PubMed] [Google Scholar]
- van Weely S, Aerts JM, Van Leeuwen MB, Heikoop JC, Donker-Koopman WE, Barranger JA, Tager JM, Schram AW. Function of oligosaccharide modification in glucocerebrosidase, a membrane-associated lysosomal hydrolase. Eur J Biochem. 1990;191:667–677. doi: 10.1111/j.1432-1033.1990.tb19173.x. [DOI] [PubMed] [Google Scholar]
- von Schaewen A, Sturm A, O’Neill J, Chrispeels MJ. Isolation of a mutant Arabidopsis plant that lacks N-acetyl glucosaminyl transferase I and is unable to synthesize Golgi-mediated complex N-linked glycans. Plant Physiol. 1993;102:1109–1118. doi: 10.1104/pp.102.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IBH, Harthill JE, Mullin N, Ashford D, Altmann F. Core α1,3-fucose is a key part of the epitope recognized by antibodies reacting against plant N-linked oligosaccharides and is present in a wide variety of plant extracts. Glycobiology. 1998;8:651–661. doi: 10.1093/glycob/8.7.651. [DOI] [PubMed] [Google Scholar]
- Wilson NL, Schulz BL, Karlsson NG, Packer NH. Sequential analysis of N- and O-linked glycosylation of 2D-PAGE separated glycoproteins. J Proteome Res. 2002;1:521–529. doi: 10.1021/pr025538d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.