Abstract
Insulin-like growth factors (IGFs) are critical for development and growth of skeletal muscles, but because several tissues produce IGFs, it is not clear which source is necessary or sufficient for muscle growth. Because it is critical for production of both IGF-I and IGF-II, we ablated glucose-regulated protein 94 (GRP94) in murine striated muscle to test the necessity of local IGFs for normal muscle growth. These mice exhibited smaller skeletal muscles with diminished IGF contents but with normal contractile function and no apparent endoplasmic reticulum stress response. This result shows that muscles rely on GRP94 primarily to support local production of IGFs, a pool that is necessary for normal muscle growth. In addition, body weights were ∼30% smaller than those of littermate controls, and circulating IGF-I also decreased significantly, yet glucose homeostasis was maintained with little disruption to the growth hormone pathway. The growth defect was complemented on administration of recombinant IGF-I. Thus, unlike liver production of IGF-I, muscle IGF-I is necessary not only locally but also globally for whole-body growth.—Barton, E. R., Park, S., James, J. K., Makarewich, C. A., Philippou, A., Eletto, D., Lei, H., Brisson, B., Ostrovsky, O., Li, Z., Argon, Y. Deletion of muscle GRP94 impairs both muscle and body growth by inhibiting local IGF production.
Keywords: chaperones, endocrine growth control, glucose homeostasis, glucose regulated protein 94, IGFs
Muscle physiology depends on the action of insulin-like growth factors (IGFs) in multiple ways. Initial myoblast proliferation, differentiation, and fiber formation are orchestrated by IGF-I and IGF-II in concert with additional growth factors. Both the fusion of activated satellite cells to form muscle fibers as well as protein synthesis in the fibers is governed by IGF. In addition, regeneration after injury uses these same pathways to resolve muscle damage and regain functional capacity. Thus, IGF-I, in particular, has been a central therapeutic target for enhancing muscle function in aging and disease. The beneficial effects of IGF-I for muscle have been demonstrated in several animal models, including tissue-specific transgenic expression (1, 2), viral-mediated gene transfer (3), and directed recombinant IGF-I delivery (4). All of these strategies result in functional hypertrophy in young adult animals, in maintenance of mass and regenerative capacity in senescent animals, and in a boost of muscle recovery to counter acute and chronic damage.
Because there are many sources for IGF-I in the body, an important unresolved question is the identity of the most relevant IGF-I sources for muscle physiology. Circulating IGF-I is produced primarily by the liver, yet ablation of this source does not have a dramatic effect on muscle size nor on general postnatal body growth (5–7), indicating that skeletal muscles can rely on other pools of IGF-I. Because muscles themselves produce both IGF-I and IGF-II (8), the most likely source is local autocrine/paracrine IGFs.
At the cellular level, myoblasts and other cell types initiate production of IGF-I and IGF-II in response to several stress conditions, such as injury or mechanical stretch (9, 10). The production of IGF by these cells depends on the activity of the stress protein glucose-regulated protein 94 (GRP94; gene name HSP90B1) (11, 12). GRP94 is a member of the HSP90 family of stress proteins, whose expression is regulated by a variety of metabolic conditions, such as glucose tension, redox state, and changes to calcium homeostasis (13). It is ubiquitously expressed in multicellular organisms and is highly conserved in evolution, yet it has a small number of clients and interacting proteins (14). Despite the limited clientele, GRP94 is essential at the organismal level: germ line deletion in plants, invertebrates, and mammals leads to developmental arrests and lethality. Without active GRP94, cells cannot respond to stress by producing IGF and other GRP94 clients (11).
GRP94 had been related to several aspects of muscle physiology. First, GRP94 is completely contained within the sarcoplasmic reticulum (SR) lumen (15) in skeletal and cardiac muscle (16). Second, GRP94 functions as a stress protein in muscle: levels increase transiently in fibrillating atrial myocytes, and overexpression of GRP94 in stressed cardiomyocytes protected them from cell death (17). The stress-protecting role of GRP94 is not shared by other endoplasmic reticulum (ER) chaperones such as BiP or calreticulin (17). Finally, GRP94 is essential for muscle differentiation; grp94−/− embryonic stem cells cannot differentiate into any of the muscle sublineages (18), and reduction in GRP94 levels in skeletal myoblasts leads to loss of myocyte fusion competence (12). Because all of these effects on muscle have not yet been related to the molecular modes of action of GRP94, we investigated whether the GRP94-IGF axis is relevant for muscle in vivo and the extent to which it underlies the effects of GRP94 on development and maintenance of skeletal muscle.
MATERIALS AND METHODS
Mouse growth and tissue measurements
All experiments were approved by the Children's Hospital of Philadelphia and the University of Pennsylvania animal care committees. Mice containing a floxed allele of GRP94 (grp94flox; ref. 19) were crossed with muscle creatine kinase (MCK)-Cre transgenic mice (on a C57BL/6 background, stock 006475; The Jackson Laboratory, Bar Harbor, MI, USA). Double heterozygous progeny were then bred to grp94flox/flox mice to deplete GRP94 within skeletal and cardiac muscle. After weaning, mice were weighed twice a week, and their lengths were measured from the tip of the nose to the base of the tail. Animals were sacrificed at 4 or 8 wk, and blood and tissue samples were harvested for morphological, biochemical, and functional measurements.
Blood samples were centrifuged at 2000 g after clotting for 20 min and stored at −80°C for subsequent use. Tissues were blotted, weighed, and rapidly frozen in liquid nitrogen for biochemical measurements or mounted, surrounded by optimal cutting temperature (OCT) compound (Sakura, Torrance, CA, USA), and rapidly frozen in melting isopentane for morphology.
Complementation of growth deficits was performed by daily subcutaneous injections of mecasermin rinfabate (IPLEX; a generous gift from Insmed Inc., Monmouth Junction, NJ, USA), a complex of recombinant IGF-I and IGF binding protein (IGFBP) 3. It was injected at 50 mg/kg and compared with saline injections.
Immunohistochemistry (IHC)
Frozen cross-sections (10 μm) from the midbelly of each muscle were subjected to IHC for laminin (rabbit Ab-1; Neomarkers, Fremont, CA, USA) to outline the muscle fibers. Fibers were typed with antibodies recognizing myosin heavy chain (MHC) 2a (SC-71), MHC 2b (BF-F3), and MHC 1/β (BAF-8) (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA). Nuclei were counterstained with DAPI. Integrin complex localization was determined by IHC for β1D integrin (Abcam, Cambridge, UK). Sarcoglycan complex localization was determined by IHC for γ-sarcoglycan (rabbit polyclonal antibody, gift from Dr. E. M. McNally, University of Chicago, Chicago, IL, USA). Stained sections were visualized on a Leica DMR microscope (Leica Microsystems, Wetzlar, Germany), and digital images were analyzed using OpenLab software (Improvision, Coventry, UK). Cross-sectional areas were measured in >500 fibers from 4 nonoverlapping fields at ×100 view from 4 muscle pairs.
Immunoblotting
GRP94 deletion was assessed by immunoblotting with the 9G10 mAb (Enzo Life Sciences, Farmingdale, NY, USA) as described in ref. 11. Other primary antibodies included calreticulin and integrin β1D (Abcam), calsequestrin, phospho-Akt and total Akt, phospho-ERK1/2 and total ERK1/2, phospho-S6RP (Cell Signaling Technology, Beverly, MA, USA), IGF-I, IGFBP1, IGFBP3, IGF-acid-labile subunit (ALS; R&D Systems, Minneapolis, MN, USA), talin and tubulin (Sigma-Aldrich, St. Louis, MO, USA), KDEL (Assay Designs, Ann Arbor, MI, USA), and 14-3-3 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Secondary antibodies included either horseradish peroxidase (HRP)-conjugated antibodies recognizing mouse, rat, and goat (Cell Signaling and R&D Systems, respectively) or fluorescently conjugated antibodies recognizing mouse, rat, or rabbit (LI-COR Biosciences, Lincoln, NE, USA). Immunoblots were imaged on film with the ImageQuant 4000 (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) or the Odyssey Imaging System (LI-COR).
Analysis of muscle contraction
Eight-week-old mice were anesthetized with ketamine/xylazine and exsanguinated. The soleus and extensor digitorum longus (EDL) muscles were removed and placed in a bath of Ringer's solution gas-equilibrated with 95% O2/5% CO2. The muscles were subjected to isolated muscle mechanical measurements using a previously described apparatus (Aurora Scientific, Aurora, ON, Canada; ref. 3). After determining optimum length using single supramaximal twitch stimulation, maximum isometric tetanus was measured in the muscles from both limbs. The muscles were then frozen for morphological measurements.
Quantitative reverse transcription–polymerase chain reaction (qRT-PCR)
Total RNA was isolated from gastrocnemius muscles and livers. Equal amounts of total RNA from each sample were subjected to single-strand reverse transcription (Applied Biosystems, Foster City, CA, USA). The resultant cDNA was used for qRT-PCR with oligonucleotides specific for genes of interest (Supplemental Table S3) using the Applied Biosystems 7300 system and reagents (LightCycler FastStart DNA MasterPLUS SYBR Green I; Roche Applied Science, Indianapolis, IN, USA) as described previously (3).
Primary myoblast cultures
Primary myoblasts were generated as described in refs. 20 and 21. In brief, single myofibers with the associated satellite cells were dissociated from flexor digitorum brevis muscles of 8-wk-old mice by collagenase digestion and passages through a wide-bore glass pipette, in Tyrode's solution/10% FBS. Fibers were cultured in 12-well plates coated with Matrigel (BD Biosciences, Bedford, MA, USA) with ∼12 fibers/well in growth medium consisting of 20% (v/v) FBS, 0.5% penicillin-streptomycin (Mediatech, Herndon, VA, USA), 0.4% amphotericin B (Fungizone; Gibco, Grand Island, NY, USA), 0.2% gentamicin (Gibco) and 0.01% basic fibroblast growth factor (MP Biomedicals, Irvine, CA, USA) in Ham's F-10 medium (Gibco). Satellite cells dissociated from their fibers 18–24 h after plating and started to migrate and proliferate within 24 h. When the cultures reached 70–80% confluence, they were switched to differentiation medium, consisting of 10% (v/v) horse serum, 0.5% chicken embryo extract, 0.5% penicillin-streptomycin, 0.4% amphotericin B, and 0.2% gentamicin in DMEM (Gibco). Both the Matrigel and the horse serum contained IGF-I that allowed satellite cell differentiation. Primary myoblasts were allowed to differentiate for 3 d, and medium samples were collected every day. On d 3, medium and cells were collected for protein isolation and quantification.
IGF, insulin, and growth hormone (GH) measurements
IGF-I content in tissues, serum, and medium was determined with a commercially available ELISA kit specific for rodent IGF-I (R&D Systems). Equal volumes of serum (15 μl) were also subjected to immunoblotting for IGF-I protein, using goat anti-IGF-I (R&D Systems) and procedures described previously (3, 22). IGF-II levels in the medium were determined by ELISA for mouse IGF-II (R&D Systems). The level of insulin in serum was measured using ELISA for mouse/rat insulin (EZRMI-13K; Millipore, Billerica, MA). The level of GH in the circulation was measured using a Rat/Mouse Growth Hormone ELISA (EZRMGH-45K; Millipore). Because there is inherent variability in murine serum GH, expression measurements of liver responsive genes was also performed by qRT-PCR.
Serum glucose measurements
Mice were unfed for 16 h before testing. Fasting blood glucose was measured on a tail-blood sample using a glucometer (Bayer Glucometer Elite; Bayer USA, Tarrytown, NY, USA). Glucose clearance was determined after an intraperitoneal injection of glucose at 2 g/kg body weight (20% glucose w/v in sterile saline). Blood glucose levels were monitored for 120 min after injection.
Statistics
Data are presented as means ± se. Unpaired t tests were used for comparisons between mGRP94−/− samples and those from age- and sex-matched control samples. Fiber size distributions were compared using the Mann-Whitney test, assuming unequal variances. Statistical significance was accepted for P < 0.05. Correlations between serum IGF-I and body weight used age- and sex-matched mice from both mGRP94−/− and wild-type (WT) groups.
RESULTS
Conditional ablation of GRP94 in muscles
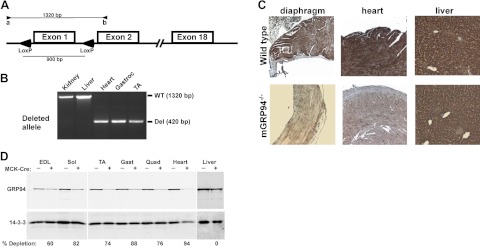
Tissue-specific ablation of GRP94 was achieved by breeding mice with a “floxed” GRP94 gene (ref. 19 and Fig. 1A) with mice expressing Cre recombinase driven by the MCK promoter (23), generating MCK-Cre/+;GRP94Fl/Fl progeny (hereafter termed mGRP94−/− mice). Use of the MCK promoter afforded late embryonic onset of Cre expression so that muscle specification during development could occur, unlike in the nonconditional knockout of GRP94, which leads to early embryonic lethality (18). Mendelian segregation of all expected genotypes among the newborn mice (Supplemental Table S1) showed that no significant prenatal lethality had occurred. Deletion of the GRP94 gene was observed in all striated muscles examined (by PCR), including the limb muscles and heart (Fig. 1A, B) and confirmed by IHC for GRP94 protein expression (Fig. 1C). Livers displayed no deletion of GRP94, consistent with the specificity of the MCK-Cre transgene. Quantification of the extent of GRP94 deletion by tissue immunoblots showed that in muscles of 20 of 24 mice, the GRP94 level was depleted by >67%, and the level of depletion was consistent in all the skeletal muscles of a given mouse (Fig. 1D).
Figure 1.
Muscle-specific deletion of GRP94. A) Schematic representation of the GRP94 gene showing the loxP sites flanking exon 1, whose deletion removes 900 bp of DNA. B) Example of PCR assays used to genotype mouse tissues. When the WT allele is amplified with primers a (5′ to the gene) and b (within exon 2), it yields a 1320-bp product from nondeleted tissues (liver and kidney), but only a 420-bp product if the floxed gene segment is deleted. C) IHC of tissue sections for GRP94 expression. The detecting antibody is mAb 9G10, developed with an HRP reaction. D) Quantitative assessment of GRP94 depletion by Western blot analysis using extracts from the muscles of WT (MCK-Cre−) or mutant (MCK-Cre+). GRP94 is detected with mAb 9G10. Percentage depletion was calculated as the ratio of GRP94 in the Cre+ and Cre− muscle in each pair, normalized to 14-3-3 (loading control). MCK-Cre is not active in liver, only in skeletal and cardiac muscles. EDL, extensor digitorum longus; sol, soleus, TA, tibialis anterior; gast, gastrocnemius; quad, quadriceps.
IGF secretion is inhibited in mGRP94−/− muscle
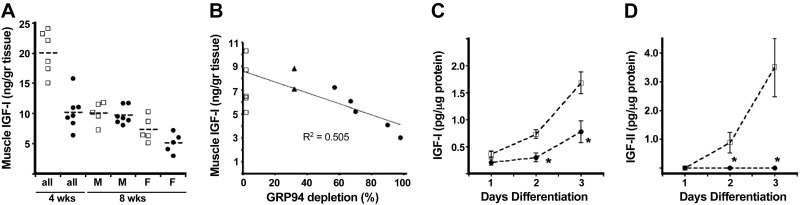
Pharmacological inhibition of GRP94 activity or RNA interference knockdown reduces IGF-I/II secretion in all cell types studied, including the myoblast cell lines C2C12 and 10T1/2 (12). To determine whether this relationship applies in vivo, we measured the IGF-I content in muscle extracts (3, 24), because IGF-I is the predominant IGF for murine postnatal growth. Four-week-old mGRP94−/− mice of both genders exhibited a significant reduction in muscle IGF-I, as did 8-wk-old mGRP94−/− female mice, compared with age- and sex-matched controls (Fig. 2A and Supplemental Table S2). Production of IGF-I was inversely correlated with the extent of GRP94 gene deletion (Fig. 2B). For unknown reasons, mGRP94−/− male mice, which at 4 wk had reduced muscle IGF-I, had almost normal content of muscle IGF-I at 8 wk of age, but this did not reflect IGF-I production, as shown in Fig. 2C. Eight-week-old female muscles did exhibit a deficit in IGF-I (Fig. 2A, B).
Figure 2.
Effect of GRP94 deletion on production of muscle IGF. A) IGF-I contents of extracts of tibialis anterior and gastrocnemius muscles from the indicated ages, and genders were determined by ELISA. □, WT mice; ●, mGRP94−/− mice. Each point represents an individual mouse. The 4-wk-old cohort consisted of 8 males and 5 females. B) Skeletal muscle IGF-I level correlates with the level of GRP94 protein. The values of tissue IGF-I from individual 8-wk mGRP94−/− females (●) and mGRP94+/− females (▲) were plotted as a function of the amount of GRP94 protein from the same samples, estimated by Western blots of the harvested TA or gastrocnemius muscles. The nondepleted samples were from WT littermates (□). C) Quantitation of IGF-I secreted by primary myoblasts. Cultures were set up in triplicate as described in Materials and Methods, supernatants were collected at the indicated time points, and their IGF-I contents were measured by ELISA. Shown is a representative experiment out of two independent repetitions, with cultures from 3 mice per genotype. *P < 0.05 vs. corresponding WT; unpaired t test. D) Quantitation of IGF-II secreted by primary myoblasts (as in C).
Because the ELISA assay cannot distinguish between local and circulating sources of IGF-I, primary myoblasts were isolated from mGRP94−/− and WT littermate muscles (including 8-wk-old males) and differentiated in permissive medium. IGF-I in horse serum allowed differentiation but was undetectable by the rodent IGF-I ELISA assay. There was dramatic reduction in the ability of mGRP94−/− cultures to secrete IGF-I compared with WT cultures (Fig. 2C). Further, IGF-II secretion from differentiating myoblasts was also prevented (Fig. 2D), consistent with our previous results with GRP94-silenced cells (11). Thus, both in vivo and in vitro measurements showed that targeted deletion of GRP94 in striated muscles inhibited local IGF production.
Consequences of GRP94 depletion on muscle growth
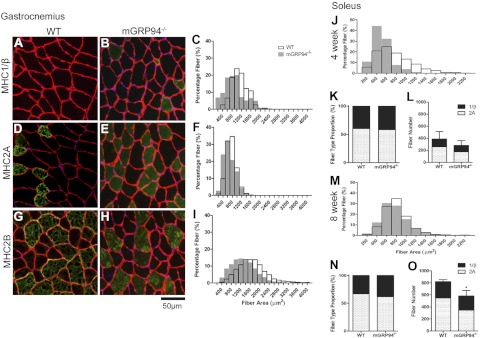
The mGRP94−/− mice were viable, but striated muscles were significantly smaller than those in their WT littermates. At 4 wk of age, male mice displayed a 20% reduction in muscle mass, and females had a more dramatic loss (∼40%) in all muscles examined (Supplemental Table S2). By 8 wk, muscles of both male and female mice remained ∼20% smaller than those of age- and sex-matched littermates. Both skeletal muscles and the heart were similarly affected, consistent with the specificity of the MCK promoter (Table 1). Because loss of mass can occur either from fewer muscle fibers or from smaller fibers, fiber number and cross-sectional area distributions were compiled for several limb muscles. A predominant factor contributing to the reduction in muscle mass was a shift in the fiber size distribution toward smaller fibers in all muscles examined (Fig. 3 and Supplemental Fig. S1). In addition, mGRP94−/− muscles had fewer fibers (Fig. 3L, O and Supplemental Fig. S1). Fiber size analysis after IHC for MHC isoforms showed that all fiber types had smaller fiber areas (Fig. 3A–I). Finally, we observed a fast-to-slow shift in all muscles studied including the soleus and gastrocnemius as well as the appearance of more MHC 1/β fibers in the EDL and tibialis anterior from 4- and 8-wk-old animals (Fig. 3 and Supplemental Fig. S1), but this shift could not by itself account for the reduction in muscle mass. We concluded that mGRP94−/− muscles were smaller because of reduced size of all major fiber types as well as lower numbers of muscle fibers.
TABLE 1.
Organ sizes and muscle functional parameters in 8-wk-old mice
| Parameter | 8-wk-old male |
8-wk-old female |
||
|---|---|---|---|---|
| WT | mGRP94−/− | WT | mGRP94−/− | |
| Body weight (g) | 24.46 ± 1.62 (11) | 20.91 ± 2.13 (11)* | 19.24 ± 1.07 (9) | 16.42 ± 1.66 (9)* |
| EDL (mg) | 10.33 ± 1.30 (11) | 8.48 ± 1.32 (11)* | 8.19 ± 0.74 (9) | 6.77 ± 0.87 (9)* |
| Tibialis anterior (mg) | 45.92 ± 3.11 (11) | 39.61 ± 4.01 (11)* | 36.28 ± 2.70 (9) | 30.54 ± 3.50 (9)* |
| Soleus (mg) | 8.00 ± 2.08 (11) | 6.85 ± 1.33 (11)* | 7.07 ± 0.96 (9) | 5.52 ± 1.06 (9)* |
| Gastrocnemius (mg) | 127.48 ± 15.05 (11) | 106.45 ± 10.43 (8)* | 96.61 ± 6.38 (9) | 81.91 ± 8.95 (7)* |
| Quadriceps (mg) | 188.15 ± 16.28 (11) | 157.56 ± 19.69 (11)* | 148.10 ± 9.87 (8) | 124.84 ± 12.16 (8)* |
| Heart (mg) | 119.13 ± 9.56 (11) | 105.57 ± 12.13 (11)* | 101.48 ± 8.26 (8) | 85.66 ± 10.37 (7) |
| Liver (mg) | 1270.9 ± 214.0 (5) | 893.4 ± 150.69 (4) | 856.5 ± 93.49 (5) | 765.4 ± 125.52 (3)* |
| Kidney (mg) | 143.23 ± 17.23 (6) | 128.13 ± 20.40 (4)* | 118.38 ± 18.28 (5) | 98.52 ± 9.95 (4) |
| Body length (mm) | 94.00 ± 2.00 (6) | 84.4 ± 6.27 (5)* | 91.29 ± 6.97 (7) | 82.14 ± 2.67 (7)* |
| Femur length (mm) | 14.88 ± 0.27 (6) | 14.35 ± 0.46 (4)* | 14.48 ± 0.40 (4) | 13.51 ± 0.09 (2)* |
| EDL (n = 7) |
Soleus (n = 4) |
|||
|---|---|---|---|---|
| WT | mGRP94−/− | WT | mGRP94−/− | |
| Cross-sectional area (mm2) | 1.7 ± 0.25 | 1.42 ± 0.20* | 1.03 ± 0.17 | 0.86 ± 0.15 |
| Twitch (mN) | 74.76 ± 20.16 | 63.02 ± 18.68 | 25.97 ± 4.90 | 19.23 ± 6.95 |
| One-half relaxation time (ms) | 41.14 ± 6.34 | 45.00 ± 9.12 | 65.67 ± 10.88 | 75.75 ± 18.90 |
| Maximum tetanus (mN) | 396.57 ± 27.32 | 327.35 ± 34.85* | 195.26 ± 10.89 | 155.18 ± 26.67* |
| Specific force (N/cm2) | 23.21 ± 2.73 | 23.41 ± 4.18 | 18.63 ± 1.37 | 18.00 ± 2.53 |
Data are means ± se; values in parentheses indicate number of animals for each experiment.
P < 0.05 vs. age- and sex-matched WT mice.
Figure 3.
Size distribution of fiber types. A, B) Transverse sections of gastrocnemius muscles from 8-wk-old WT (A) and mGRP94−/− (B) animals were double-stained with anti-laminin (red), to delineate the fiber circumference, and anti-MHC 1/β isoform (green). C) Histogram of size distributions of MHC1/β-positive fibers from WT (□) or mutant (■) muscles, determined from images as in A and B. Plots represent percentage of fibers in each size bin. Fibers are significantly smaller in mGRP94−/− muscles compared with those in WT fibers (Mann-Whitney test). n = 4 muscles/genotype, with >500 muscle fibers sampled to generate the size distributions. D–F) Sections and histograms (as in A–C) for MHC2A-positive fibers. G–I) Sections and histograms (as in A–C) for MHC2B-positive fibers. J, M) Histograms of fiber distributions of soleus muscles from 4-wk-old (J) and 8-wk-old (M) animals show that mGRP94−/− muscles have smaller muscle fibers. n = 4-5 for each age and genotype. K, N) Fiber type proportions for soleus muscles from 4-wk-old (K) and 8-wk-old (N) animals. L, O) Fiber numbers in 4-wk-old (L) and 8-wk-old (O) animals. Numbers of fibers are lower in soleus muscles from mGRP94−/− mice, losing predominantly MHC 2A fibers.
To address the possibility that smaller muscles are due to diminished IGF-I receptor-mediated signaling (25), we examined IGF-I receptor expression and signaling. Receptors were seen in concentrated patches and puncta on the sarcolemma in muscles from both WT and mGRP94−/− muscles (Supplemental Fig. S3A). In addition, we measured phosphorylation levels of Akt and ERK1/2, members of the IGF-I signaling pathway, as well as that of S6 ribosomal protein, the downstream target of p70 S6 kinase. In both 4- and 8-wk-old animals, these signaling mediators are phosphorylated, affirming that the main deficiency is in production of IGF-1 rather than in sensing it (Supplemental Fig. S3B). There is no significant difference between WT and mGRP94−/− muscles and, if anything, there is a tendency toward hyperphosphorylation, the molecular cause of which is currently unknown. Thus, although there was less IGF-I in the mGRP94−/− muscles, receptors were still available for activation, and signaling levels were maintained, if not increased, but muscle growth is retarded.
Effects of GRP94 depletion on muscle function
Targeted ablation of GRP94 in muscles could impair force production, either through inhibition of IGF-I secretion or through alternate mechanisms, and thus force generation was measured in isolated EDL and soleus muscles. Twitch and tetanic force in mGRP94−/− muscles was lower in proportion to the reduction in muscle mass (Table 1). However, specific force (force normalized to cross-sectional area) did not differ between mGRP94−/− muscles and littermate controls (Table 1).
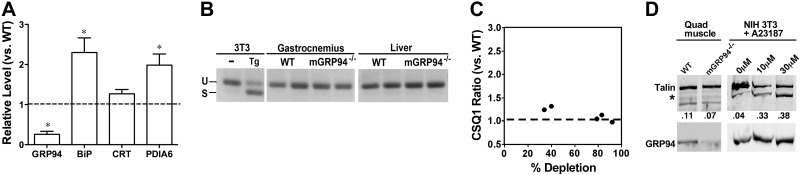
Because GRP94 is a major stress protein involved in the ER stress response (26) and because IGF-I signaling is related to this response (27), we examined whether mGRP94−/− muscles were under chronic ER stress. The ablation of muscle GRP94 caused a corresponding increase in the steady-state expression of the major ER chaperone, BiP/GRP78, and of the oxidoreductase PDIA6 (Fig. 4A). Previous cell culture experiments indicated that the response of these two genes is inversely proportional to the level of GRP94 (ref. 12 and unpublished results). However, the response in vivo was selective, because the expression of calreticulin was not affected by deletion of muscle GRP94 (Fig. 4A). Furthermore, none of the mGRP94−/− muscles displayed splicing of XBP-1 (Fig. 4B), a sensitive assay for activation of the IRE-1 branch of ER stress signaling. Thus, taken together, results of the immunoblots and the splicing assay show that GRP94-deficient muscles are not under chronic or global ER stress.
Figure 4.
Changes in the ER of GRP94-depleted muscles. A) Immunoblotting of muscle for luminal proteins shows no evidence of global ER stress. GRP94 levels are significantly reduced in muscles of mGRP94−/− mice, causing a compensatory increase BiP/GRP78 and PDIA6, but no change in calreticulin. Data are shown as means ± se for n = 6 samples/ genotype, *P < 0.05 vs. WT; unpaired t test. B) XBP-1 splicing assay to monitor ER stress. Positive and negative controls were generated from cDNAs of 3T3 cells in the presence or absence of thapsigargin (Tg), respectively, which cause splicing (S) of XBP-1. Neither muscle nor liver samples from WT and mGRP94−/− mice show significant XBP-1 splicing, supporting the lack of ER stress. U, unspliced. C) Calsequestrin 1 (CSQ1) levels do not differ between muscles from n = 5 matched pairs of WT and mGRP94−/− mice. Ratios are plotted as a function of the GRP94 deletion in the same mice, determined by blotting with anti-GRP94 antibody. D) GRP94 deficiency in the muscle does not lead to Ca2+-dependent cleavage of talin. In neither WT nor mutant muscles does talin display appreciable cleavage by calpain. As a positive control, NIH 3T3 cells were treated with the indicated concentrations of the Ca2+ ionophore A23187, to increase artificially free Ca2+ and activate calpain cleavage of talin (band marked with *). Blots of total protein extracts were probed with anti-talin monoclonal 8D4. Ratio of the cleavage product to full-length talin is provided below each lane.
Because GRP94 serves as an important calcium buffer within the ER/SR (15, 28), loss of GRP94 could modulate functional kinetics that are most evident in the rates of force relaxation (29, 30). However, there was only a modest increase in the relaxation time after twitch stimulation (Table 1). The lack of calcium phenotype was also substantiated by unchanging calsequestrin 1 (Fig. 4C), which binds twice as much Ca2+ as GRP94 on a molar basis and is also more abundant than GRP94 in the SR (31). A third indication that calcium buffering was normal in mGRP94−/− muscles was lack of calpain activity. Calpains are proteases that are sensitive to increased cytoplasmic Ca2+ (32), and, as an indirect measure of calpain activation, we monitored cleavage of one of its substrates, talin. However, there was no change in the extent of talin cleavage in the mGRP94−/− quadriceps muscles, compared with that in littermate controls (Fig. 4D). Thus, the loss of calcium buffering capacity by GRP94 was not sufficient to impair force generation or muscle stability.
Another possible mechanism was an effect on muscle integrins, which are also GRP94 clients (19, 33). Because one of the major integrins in muscle fibers is the α7/β1D integrin dimer, we tested β1D levels by immunoblotting and IHC. We observed no significant difference in the amount of this integrin and, more importantly, in its membrane localization in mGRP94−/− muscles (Supplemental Fig. S2). Thus, although there are likely to be additional clients of GRP94 affected in this animal model, they do not appear to have large effects on the normal resting function or growth of skeletal muscle. Last, a generalized defect in protein folding and processing could also reduce muscle mass. To address this possibility, we measured γ-sarcoglycan content and localization, which is not a known client of GRP94. Not only were normal levels of this protein determined by immunoblotting; it was also properly localized in the muscle membrane (Supplemental Fig. S2). Thus, we propose that the major cause of the smaller striated muscles is the inhibition of production of local IGF-I.
mGRP94−/− mice display impaired organismal growth
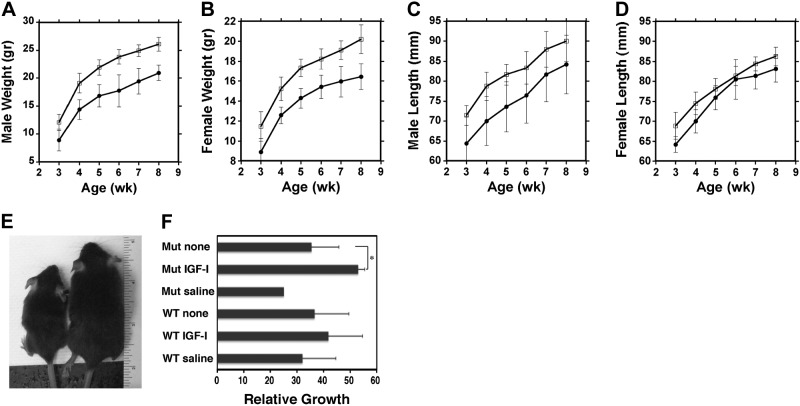
As shown above, muscle-targeted ablation of GRP94 reduced muscle mass as well as IGF-I secretion from striated muscle. As a consequence, mGRP94−/− animals were noticeably smaller (Fig. 5E). Body weights and lengths were lower for both mGRP94−/− males and females starting at weaning (3 wk old) and remained lower throughout (Fig. 5A–D). Consistent with smaller size, the organ weights were proportionally reduced, and femur bone length was shorter in mGRP94−/− mice (Table 1 and Supplemental Table S2). The reduction in growth was proportional, because skeletal muscle weight/body weight ratios showed no differences between WT and mGRP94−/− animals (Supplemental Fig. S3C).
Figure 5.
Growth of WT and mGRP94−/− mice. A, B) Male (A) and female (B) weight growth curves based on 16 mGRP94−/− and 19 WT males and 14 mGRP94−/− and 12 WT females. Error bars are sd. The differences between mutant and WT mice in the weight growth curves are statistically significant at P < 0.05 (unpaired t test). C, D) Male (C) and female (D) body length growth curve. Graphs are based on 9 mGRP94−/− and 8 WT males and 14 mGRP94−/− and 12 WT females. □, WT mice; ●, mGRP94−/− mice. E) Example of an mGRP94−/− mouse (left) and a WT littermate (right) at 8 wk. F) Restoration of growth by exogenous IGF-I. Histogram depicts the growth rate of mGRP94−/− (Mut) and WT females between 4 and 8 wk, when either injected daily with recombinant IGF-I (IPLEX) or saline or not injected (none). Note that mGRP94−/− mice respond even better than WT mice to administration of IGF-I. n = 5 females/condition, except Mut + saline, for which only 1 animal was available (4 mutant mice did not survive the full course of injections) *P < 0.05; unpaired t test.
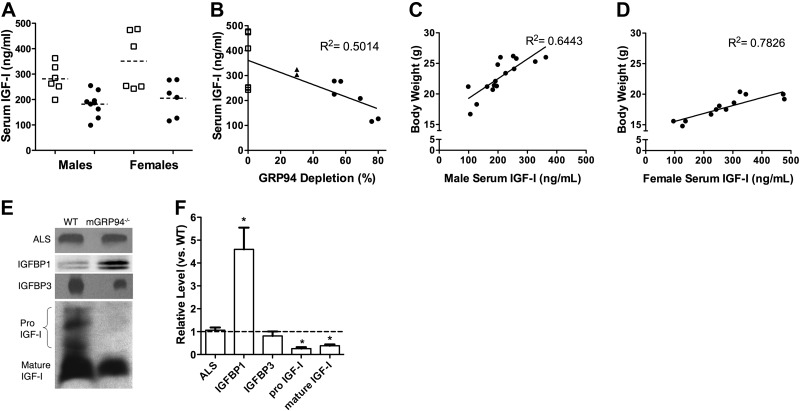
Given the effect on the entire body plan, we measured IGF-I levels in the circulation by immunoblotting and ELISA. Unexpectedly, a significant reduction was found in total serum IGF-I of mGRP94−/− mice compared with that in littermate controls, regardless of age or sex (Fig. 6A and Supplemental Table S2). Furthermore, when the levels of circulating IGF-I in 8-wk-old mGRP94−/− mice were compared with the extent of GRP94 deletion, there was a strong correlation between these variables: extensive deletion of GRP94 led to low serum IGF-I (Fig. 6B). We examined the effect of circulating IGF-I on body size by plotting body weights as a function of serum IGF-I for young adult mice (8-wk-old animals; Fig. 6C, D). Both male and female mice exhibited significantly high correlations between body weight and serum IGF-I (R2=0.6443 for males; R2=0.7826 for females). Thus, blocking IGF-I production from skeletal muscles by targeting GRP94 impaired not only muscle mass but also circulating IGF-I and body size.
Figure 6.
Effect of GRP94 deletion on serum IGF-I levels. A) Serum IGF-I levels in 8-wk-old male or female mice. ■, WT mice; ●, mGRP94−/− mice. Each point represents an individual mouse, whose IGF-I was measured by ELISA. Horizontal dashed lines are the means of each group of values. Mean serum values for mGRP94−/− mice are significantly lower than serum IGF-I values for sex-matched WT mice by unpaired t test. B) Serum IGF-I levels as a function of GRP94 deletion in the muscles. Each point is the IGF-I value determined by ELISA from an individual mouse and the corresponding GRP94 deletion determined by immunoblotting. ▲, mice heterozygous for both Cre and loxP. C, D) Body weight of 8-wk-old mGRP94−/− mice as a function of their serum IGF-I levels. Each point is an individual animal and shows a high correlation between body weight and circulating IGF-I for both males (C) and females (D). E, F) Comparison of ALS, IGFBP1, IGFBP3, and IGF-I levels in sera from WT and mGRP94−/− mice. E) Immunoblot. F) Quantification of data. IGFBP1 is elevated in the mGRP94−/− serum, whereas there is no significant change in the levels of IGFALS or IGFBP3. IGF-I levels are diminished in the mGRP94−/− serum, with a marked decrease in both mature and pro-IGF-I.
To demonstrate that the growth phenotype is due to the IGF-I deficiency, we administered recombinant IGF-I to WT and mutant mice and monitored the change in growth from 4 to 8 wk. Both cohorts responded to the exogenous IGF, but the growth rate of the mutant mice with IGF was significantly greater than that of the WT mice (>50%; Fig. 5F). Therefore, the reduction in circulating IGF-I can be complemented, leading to more vigorous growth.
The availability of circulating IGF-I is tightly controlled by IGFBPs. The major effector of this regulation is the ternary complex, comprisingIGFBP3 and the ALS (IGF-ALS). Both the pro and mature forms of IGF-I were found in the circulation, and both were reduced in the sera of mGRP94−/− mice (Fig. 6E, F). Even though circulating IGF-I was reduced, there was no apparent change in the amount of the ternary complex members in the serum (Fig. 6E, F). Another regulator of free IGF-I is IGFBP1. Unlike the lack of change in IGFBP3 or ALS, serum IGFBP1 protein levels were markedly elevated in serum of mGRP94−/− mice (Fig. 6E, F), despite reduced levels of total circulating IGF-I. Thus, higher levels of IGFBP1 may compound the loss of available IGF-I for growth.
Systemic consequences of inhibiting muscle IGF-I secretion
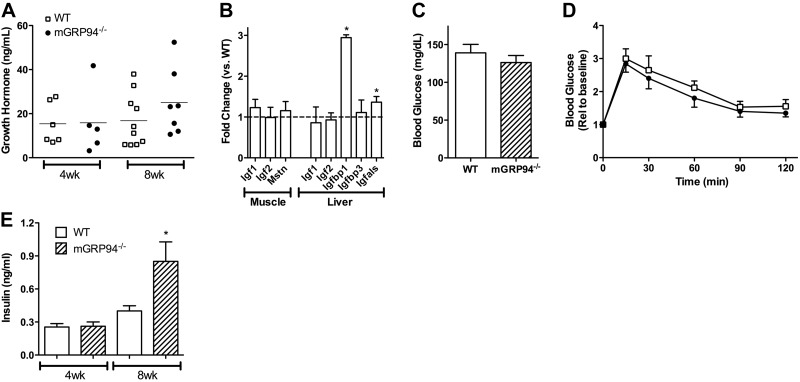
Work with several animal models helped establish the existence of feedback regulation relating IGF-I expression to GH (34). GH is released from the pituitary gland when circulating IGF-I levels are low (35), and when liver IGF-I production is inhibited GH levels rise (6, 36). Because the circulating pool of total IGF-I decreased, we anticipated that GH would rise to drive more expression of IGF-I and its binding partners. The mean levels of GH in 8-wk-old animals were somewhat elevated (Fig. 7A), but because of the intrinsic variability of circulating mouse GH due to its pulsatile release (37), we did not have sufficient statistical power to evaluate this change. However, GH effects on liver expression of IGF-I, IGFBP3, and IGF-ALS served as surrogate markers for GH levels, as shown in Fig. 7B. Igf1 transcription in the liver and muscle did not differ between WT and mGRP94−/− animals, and although IGF-I content was reduced in the muscle, there was no significant change in IGF-I levels in the liver. IGF-ALS expression by the liver increased ∼30%, which was consistent with the modest increase in GH levels, but although increased transcription of IGF-ALS was evident, there was no significant change in the circulating levels of this protein (Fig. 6E–F). Igfbp3 showed no difference in transcription in liver. Additional GH-independent changes could also affect muscle growth. These include IGFBP1, which is controlled by glucose homeostasis, ER stress, and insulin (38–40). Consistent with the rise in serum levels of IGFBP1, Igfbp1 transcription was significantly up-regulated in the livers of mGRP94−/− mice (Fig. 7B). Finally, there was no compensatory change in expression of myostatin, a negative regulator of muscle growth (ref. 41 and Fig. 7B). Thus, the depletion of circulating IGF-I through the inhibition of muscle production of this growth factor did not trigger a major response of the GH-IGF-I axis to correct IGF-I levels or other regulators of muscle mass but did result in elevated IGFBP1 levels through increased transcription.
Figure 7.
Metabolic consequences of GRP94 depletion. A) Levels of growth hormone in the blood of 4- and 8-wk-old mice. □, WT mice. ●, mGRP94−/− mice. There was no statistical difference between GH levels in mGRP94−/− mice and in WT mice. B) Relative expression of muscle Igf1, Igf2, and myostatin and liver Igf1, Igf2, Igfbp1, Igfbp3, and Igfals compared with that of WT controls by qRT-PCR. n = 4–6 samples/genotype from 8-wk-old mice were used for analysis. There were no differences in expression in muscle samples, and only Igfbp1 and Igfals expression was increased in the livers of mGRP94−/− mice. Data are presented as means ± se of the fold change in expression. *P < 0.05; unpaired t test. C) Fasting blood glucose levels measured from 8-wk-old WT and mGRP94−/− mice (n=6). D) Glucose tolerance curves performed on the same mice as in C. Differences are not statistically significant. E) Circulating insulin levels in 4- and 8-wk-old WT and mGRP94−/− mice. There is a 2-fold increase in insulin at 8 wk of age in the mGRP94−/− mice. Data represent means ± se for n = 10–12 samples/age and genotype. *P < 0.05 vs. age-matched WT; unpaired t test.
Glucose homeostasis is predominantly regulated by insulin and its receptors, although IGF-I can also contribute to glucose clearance through binding to hybrid IGF-I/insulin receptors (42). Further, skeletal muscle is a primary site for glucose uptake via GLUT4 (43), and thus a loss of muscle mass could interfere with normal glucose clearance. To determine whether the reduction in circulating IGF-I or the diminished muscle mass impaired glucose clearance, fasting glucose levels and glucose clearance were measured in 8-wk-old animals. Glucose levels did not differ significantly in the two mouse lines after 16 h of food withdrawal (Fig. 7C). Further, on a glucose challenge test, the mGRP94−/− mice cleared glucose at a normal, if not enhanced, rate (Fig. 7D). To clarify how efficient glucose clearance was maintained, we measured circulating insulin levels. At 4 wk of age, there was no difference in insulin between WT and mGRP94−/− mice. However, at 8 wk of age, insulin levels increased almost 2-fold (Fig. 7E), suggesting that IGF-I depletion led to an up-regulation of insulin but without insulin resistance. Thus, the ablation of GRP94 in striated muscle does not impair glucose homeostasis significantly, because insulin may compensate for the loss of circulating IGF and muscle size reduction.
DISCUSSION
This work shows that muscle-specific ablation of GRP94 caused smaller muscles primarily by limiting local production of muscle IGF, providing an in vivo model for the requirement of GRP94 for the biosynthesis of both IGFs. Remarkably, inhibition of muscle IGF-I secretion also caused reduced circulating IGF-I and led to smaller animal size, an effect that was proportional to the extent of muscle GRP94 deletion and could be complemented with exogenous IGF-I. The global growth defect implies that muscle IGF-I is not only important for muscle growth but also contributes to the endocrine pool of this potent growth factor.
The conditional deletion used in this work obviates the fetal lethality observed previously (18) and allows development of muscles that, although functional, are small because of both smaller fiber size and lower fiber number. In culture, GRP94 inhibition affects myoblast fusion and myotube formation (12), and this could translate into a reduction in the number of fibers within a given muscle. Fiber number in the soleus muscles shows a significant and age-dependent reduction, and, in addition, fiber size measurements showed a clear reduction irrespective of the fiber type. Therefore, deficits in both fiber size and number contribute to decreased mass in mGRP94−/− muscle.
We were surprised that of the 4 groups of mice measured, 8-wk-old males did not show a reduction in IGF-I content, despite a dramatic growth phenotype. Whether there was a greater contribution of circulating IGF-I pool to the local muscle pool in these animals or a sex-dependent ability to accumulate the limited IGF-I produced was not addressed. However, it is clear from the primary myoblast cultures of cells from 8-wk-old male and female mGRP94−/− muscles that both are equally deficient in IGF secretion.
Several observations strongly suggest that the primary effect of GRP94 deletion on muscle is mediated through IGF, although other proteins also require GRP94 activity. Because previous studies showed that GRP94 activity is required for proper surface expression of a subset of integrins and Toll-like receptors (TLRs; refs. 19, 33), we examined the level of β1D integrin, a major component of skeletal muscle sarcolemma, but found no evidence of integrin loss. Other muscle integrins, whose loss leads to a dystrophic phenotype (44), may also be dependent on GRP94, but muscles from the mGRP94−/− mice functioned normally with no signs of degeneration/regeneration. We did not pursue the expression of TLR4 and TLR9 in the muscle (45), because TLR4-deficient mice do not seem to have either a muscle or organismal growth defect (46).
Another possible cause of the fiber type changes is the GRP94 effect on calcium levels in the SR. Because GRP94 is one of the major Ca2+-buffering proteins in the SR (28), its loss could affect the level of stored Ca2+ (47). However, there was no evidence for impaired Ca2+ handling in the mGRP94−/− mice, based on a lack of extended relaxation times after contraction, the absence of calsequestrin compensation, and a lack of increased calpain activity (48). Thus, we conclude that the effect on IGF production is the primary in vivo mechanism through which GRP94 affects muscle biology.
GRP94 is one of the major stress proteins in the ER that help cope with various metabolic stresses, so when it is depleted, there is the potential for chronic ER stress. However, GRP94-depleted muscles exhibited no global up-regulation of ER stress target proteins; rather, BiP and PDIA6 are up-regulated, but other abundant luminal proteins such as calreticulin are not induced. Second, the muscles do not exhibit any XBP-1 splicing, a sensitive and proximal measure of ER stress. Thus, the phenotype of the GRP94-deleted muscle is not due to ER stress.
An important finding in this study is that deletion of GRP94 in muscles significantly lowers systemic IGF-I. This striking effect implies that IGF-I locally produced in the muscle is a notable contributor to the circulating (endocrine) pool of IGF-I. To our knowledge, this is the first indication for an endocrine role for muscle-derived IGF-I, for not only are the muscles affected but also the whole animal displays impaired growth (Table 1), including femur length. Unlike ablation of liver IGF-I production, there was a high correlation between total circulating IGF-I and body weight (Fig. 6). A component of the IGF-I produced by the muscle is pro-IGF-I, which may have different interactions within the ternary complex that normally stabilizes IGF-I in the bloodstream. We expected that the reduction in circulating IGF-I would cause an increase in GH secretion and up-regulation of GH responsive genes in the liver. However, no dramatic change in GH was detected, and expression of genes involved in the GH-IGF axis was only modestly increased. Thus, a central question that arises is whether the pools of IGF-I produced in any given tissue are equivalent. What makes the IGF-I supplied by the muscle distinct from that produced by the liver is unknown. Because the igf1 gene contains sites for alternative splicing and for differential glycosylation, distinct post-translational processing might alter the actions of the resultant protein. Further, pro-IGF-I in the serum (Fig. 6E, F) may be derived from muscle, and this pool may escape detection by the regulatory feedback for maintaining IGF-I homeostasis.
A rich history examining the importance of endocrine IGF-I for growth precedes the current work and, in particular, focuses on liver IGF-I production. The general consensus is that limiting liver IGF-I or the proteins in the circulating ternary complex (IGFBP3 and ALS) does not control the growth of many of the tissues that make their own IGF-I, such as skeletal muscle, bone, or brain, supporting the fact that local IGF-I is sufficient to sustain normal growth of these tissues. One important exception is that reduced circulating IGF-I limits bone density (49), suggesting that bone growth not only relies on paracrine IGF-I (50) but also is dependent on endocrine IGF-I. Alternatively, boosting circulating levels of IGF-I by increasing liver production (51) or through daily injections of recombinant IGF-I increases body (and muscle) weight, supporting the fact that circulating IGF-I can enter the local tissue environment and have enhanced anabolic effects. Thus, the capacity of muscle utilization surpasses its normal production. Studies in which muscle IGF-I production is increased through transgenic overexpression (1, 2) or by viral-mediated gene transfer (3) take advantage of the mismatch between capacity for IGF-I and its production to promote functional hypertrophy. In the absence of any IGF-I, growth is severely impaired, demonstrated initially by gene targeting of the igf1 locus (52), and is also evident in patients with short stature with mutations in members of the IGF-I pathway (53). This finding supports the necessity of IGF-I for all growth but does not address the necessity of local IGF-I. Using GRP94 deletion as a tool to limit production of IGF-I, we also conclude that muscle IGF-I contributes to both local tissue growth and whole animal growth.
The interplay among the proteins regulating IGF levels and bioavailability may help to distinguish the pools of IGF-I. Unlike previous studies, in which depletion of liver IGF-I led to a decrease in IGFBP1 and IGFBP3 levels and an increase in GH and insulin (6), we observed quite different responses to depletion of muscle IGF-I production. There was no dramatic change in serum GH or GH-responsive genes in the liver (IGF-I, IGFALS, and IGFBP3). However, there were increases in both insulin and IGFBP1, which is counter to the expected reduction in IGFBP1 to boost the free IGF-I pool and to the known inhibition of IGFBP1 production by insulin (40). Thus, this animal model sets the stage for exploring potentially novel and distinct regulatory mechanisms of the endocrine system associated with different IGF-I sources.
The impaired organismal growth found in the mGRP94−/− mice could be due to two nonexclusive causes. First, the inhibition of IGF-I secretion from muscle directly affects muscle growth and leads to smaller muscles. In turn, small muscles indirectly affect bone growth through reduced force applied to bone growth plates. Alternatively, inhibition of IGF-I secretion from muscle not only may reduce muscle size through autocrine/paracrine actions but also may reduce the endocrine pool of IGF-I by a GH-independent mechanism. Unlike other models in which circulating IGF was diminished, this reduction may directly affect organismal growth. The current study cannot distinguish between these two mechanisms, but compelling evidence from this and previous publications support the possibility that both may operate.
The action of GRP94 on IGFs establishes a new post-translational regulatory point for production of IGFs. We have shown that limiting GRP94 levels directly affects IGF-i production and ultimately inhibits normal growth. Furthermore, increasing GRP94 activity enhances cell survival via increased IGF-I production (54). Processing of IGFs in the ER may involve components other than GRP94, because the glycosidase NET37 also interacts with pro-IGF and is needed for its secretion (55). Thus, modulating the activity of such ER components may provide a new strategy to tune IGF levels. Increased activity of interacting proteins such as GRP94 and, by extension, heightened IGF-I levels, would be beneficial in many clinical applications, such as prevention of sarcopenia, boosting muscle growth after injury, or countering muscle degeneration associated with genetic disease. Harnessing the potential of GRP94-mediated modulation of IGF production will be an important step in developing therapeutic strategies for muscle health.
Supplementary Material
Acknowledgments
The authors are grateful to the technical expertise provided by Jalpa Modi in mouse husbandry, Magdalena Sikora and Zuozhen Tian in muscle functional measurements and glucose clearance tests, and Monjir Bakshi in muscle primary cultures. The authors also thank Dr. Yina Dong for help with the quantitation of GRP94 deletion, and Dr. Beth McNally (University of Chicago, Chicago, IL, USA) for the gift of antibodies. IPLEX was provided by David Green (Insmed Inc., Monmouth Junction, NJ, USA).
This work was supported by U.S. National Institutes of Health grants AG18001 and GM04788 (Y.A.), AR057363 and AR052646 (E.R.B), and AI070603 and AI077283 (Z.L.). O.O. was a fellow of the Arthritis Foundation.
The authors declare no conflicts of interest. Author contributions: Y.A., E.R.B., S.P., and Z.L. conceived or designed the experiments and wrote the manuscript; S.P., J.K.J., C.A.M., A.P., D.E., H.L., B.B., and O.O. performed the experiments; E.R.B., S.P., J.K.J., and Y.A. analyzed the data.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- ALS
- acid-labile subunit
- EDL
- extensor digitorum longus
- ER
- endoplasmic reticulum
- GH
- growth hormone
- GRP94
- glucose-regulated protein 94
- HRP
- horseradish peroxidase
- IGF
- insulin-like growth factor
- IGFBP
- insulin-like growth factor binding protein
- IHC
- immunohistochemistry
- MHC
- myosin heavy chain
- MCK
- muscle creatine kinase
- qRT-PCR
- quantitative reverse transcription–polymerase chain reaction
- SR
- sarcoplasmic reticulum
- TLR
- Toll-like receptor
- WT
- wild type
REFERENCES
- 1. Coleman M. E., DeMayo F., Yin K. C., Lee H. M., Geske R., Montgomery C., Schwartz R. J. (1995) Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J. Biol. Chem. 270, 12109–12116 [DOI] [PubMed] [Google Scholar]
- 2. Musaro A., McCullagh K., Paul A., Houghton L., Dobrowolny G., Molinaro M., Barton E. R., Sweeney H. L., Rosenthal N. (2001) Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat. Genet. 27, 195–200 [DOI] [PubMed] [Google Scholar]
- 3. Barton E. R. (2006) Viral expression of insulin-like growth factor-I isoforms promotes different responses in skeletal muscle. J. Appl. Physiol. 100, 1778–1784 [DOI] [PubMed] [Google Scholar]
- 4. Adams G. R., McCue S. A. (1998) Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J. Appl. Physiol. 84, 1716–1722 [DOI] [PubMed] [Google Scholar]
- 5. Sjogren K., Liu J. L., Blad K., Skrtic S., Vidal O., Wallenius V., LeRoith D., Tornell J., Isaksson O. G., Jansson J. O., Ohlsson C. (1999) Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc. Natl. Acad. Sci. U. S. A. 96, 7088–7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yakar S., Liu J. L., Fernandez A. M., Wu Y., Schally A. V., Frystyk J., Chernausek S. D., Mejia W., Le Roith D. (2001) Liver-specific igf-1 gene deletion leads to muscle insulin insensitivity. Diabetes 50, 1110–1118 [DOI] [PubMed] [Google Scholar]
- 7. Yakar S., Liu J. L., Stannard B., Butler A., Accili D., Sauer B., LeRoith D. (1999) Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. U. S. A. 96, 7324–7329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Turner J. D., Rotwein P., Novakofski J., Bechtel P. J. (1988) Induction of mRNA for IGF-I and -II during growth hormone-stimulated muscle hypertrophy. Am. J. Physiol. 255, E513–E517 [DOI] [PubMed] [Google Scholar]
- 9. Pelosi L., Giacinti C., Nardis C., Borsellino G., Rizzuto E., Nicoletti C., Wannenes F., Battistini L., Rosenthal N., Molinaro M., Musaro A. (2007) Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. FASEB J. 21, 1393–1402 [DOI] [PubMed] [Google Scholar]
- 10. Yang C. Q., Zhan X., Hu X., Kondepudi A., Perdue J. F. (1996) The expression and characterization of human recombinant proinsulin-like growth factor II and a mutant that is defective in the O-glycosylation of its E domain. Endocrinology 137, 2766–2773 [DOI] [PubMed] [Google Scholar]
- 11. Ostrovsky O., Ahmed N. T., Argon Y. (2009) The chaperone activity of GRP94 toward insulin-like growth factor II is necessary for the stress response to serum deprivation. Mol. Biol. Cell 20, 1855–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ostrovsky O., Eletto D., Makarewich C., Barton E. R., Argon Y. (2010) Glucose regulated protein 94 is required for muscle differentiation through its control of the autocrine production of insulin-like growth factors. Biochim. Biophys. Acta 1803, 333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Little E., Ramakrishnan M., Roy B., Gazit G., Lee A. S. (1994) The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation, and applications. Crit. Rev. Eukaryot. Gene Expr. 4, 1–18 [DOI] [PubMed] [Google Scholar]
- 14. Yang Y., Li Z. (2005) Roles of heat shock protein gp96 in the ER quality control: redundant or unique function? Mol. Cells 20, 173–182 [PubMed] [Google Scholar]
- 15. Cala S. E., Jones L. R. (1994) GRP94 resides within cardiac sarcoplasmic reticulum vesicles and is phosphorylated by casein kinase II. J. Biol. Chem. 269, 5926–5931 [PubMed] [Google Scholar]
- 16. Vitadello M., Colpo P., Gorza L. (1998) Rabbit cardiac and skeletal myocytes differ in constitutive and inducible expression of the glucose-regulated protein GRP94. Biochem. J. 332, 351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vitadello M., Penzo D., Petronilli V., Michieli G., Gomirato S., Menabo R., Di Lisa F., Gorza L. (2003) Overexpression of the stress protein Grp94 reduces cardiomyocyte necrosis due to calcium overload and simulated ischemia. FASEB J. 17, 923–925 [DOI] [PubMed] [Google Scholar]
- 18. Wanderling S., Simen B. B., Ostrovsky O., Ahmed N. T., Vogen S., Gidalevitz T., Argon Y. (2007) GRP94 is essential for mesoderm induction and muscle development because it regulates IGF secretion. Mol. Biol. Cell 18, 3764–3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang Y., Liu B., Dai J., Srivastava P. K., Zammit D. J., Lefrancois L., Li Z. (2007) Heat shock protein gp96 is a master chaperone for Toll-like receptors and is important in the innate function of macrophages. Immunity 26, 215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bischoff R. (1986) Proliferation of muscle satellite cells on intact myofibers in culture. Dev. Biol. 115, 129–139 [DOI] [PubMed] [Google Scholar]
- 21. Shefer G., Yablonka-Reuveni Z. (2005) Isolation and culture of skeletal muscle myofibers as a means to analyze satellite cells. Methods Mol. Biol. 290, 281–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pfeffer L. A., Brisson B. K., Lei H., Barton E. R. (2009) The insulin-like growth factor (IGF)-I E-peptides modulate cell entry of the mature IGF-I protein. Mol. Biol. Cell 20, 3810–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jaynes J. B., Johnson J. E., Buskin J. N., Gartside C. L., Hauschka S. D. (1988) The muscle creatine kinase gene is regulated by multiple upstream elements, including a muscle-specific enhancer. Mol. Cell. Biol. 8, 62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barton E. R., DeMeo J., Lei H. (2010) The insulin-like growth factor (IGF)-I E-peptides are required for isoform-specific gene expression and muscle hypertrophy after local IGF-I production. J. Appl. Physiol. 108, 1069–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fernandez A. M., Kim J. K., Yakar S., Dupont J., Hernandez-Sanchez C., Castle A. L., Filmore J., Shulman G. I., Le Roith D. (2001) Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev. 15, 1926–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eletto D., Dersh D., Argon Y. (2010) GRP94 in ER quality control and stress responses. Semin. Cell Dev. Biol. 21, 479–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Novosyadlyy R., Kurshan N., Lann D., Vijayakumar A., Yakar S., LeRoith D. (2008) Insulin-like growth factor-I protects cells from ER stress-induced apoptosis via enhancement of the adaptive capacity of endoplasmic reticulum. Cell Death Differ. 15, 1304–1317 [DOI] [PubMed] [Google Scholar]
- 28. Van P. N., Peter F., Soling H. D. (1989) Four intracisternal calcium-binding glycoproteins from rat liver microsomes with high affinity for calcium. No indication for calsequestrin-like proteins in inositol 1,4,5-trisphosphate-sensitive calcium sequestering rat liver vesicles. J. Biol. Chem. 264, 17494–17501 [PubMed] [Google Scholar]
- 29. Schwaller B., Dick J., Dhoot G., Carroll S., Vrbova G., Nicotera P., Pette D., Wyss A., Bluethmann H., Hunziker W., Celio M. R. (1999) Prolonged contraction-relaxation cycle of fast-twitch muscles in parvalbumin knockout mice. Am. J. Physiol. 276, C395–C403 [DOI] [PubMed] [Google Scholar]
- 30. Chin E. R., Grange R. W., Viau F., Simard A. R., Humphries C., Shelton J., Bassel-Duby R., Williams R. S., Michel R. N. (2003) Alterations in slow-twitch muscle phenotype in transgenic mice overexpressing the Ca2+ buffering protein parvalbumin. J. Physiol. 547, 649–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murphy R. M., Larkins N. T., Mollica J. P., Beard N. A., Lamb G. D. (2009) Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat. J. Physiol. 587, 443–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beckerle M. C., Burridge K., DeMartino G. N., Croall D. E. (1987) Colocalization of calcium-dependent protease II and one of its substrates at sites of cell adhesion. Cell 51, 569–577 [DOI] [PubMed] [Google Scholar]
- 33. Randow F., Seed B. (2001) Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat. Cell Biol. 3, 891–896 [DOI] [PubMed] [Google Scholar]
- 34. Ohlsson C., Mohan S., Sjogren K., Tivesten A., Isgaard J., Isaksson O., Jansson J. O., Svensson J. (2009) The role of liver-derived IGF-I. Endocr. Rev. 30, 494–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rotwein P., Chia D. J. (2010) Gene regulation by growth hormone. Pediatr. Nephrol. 25, 651–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wallenius K., Sjogren K., Peng X. D., Park S., Wallenius V., Liu J. L., Umaerus M., Wennbo H., Isaksson O., Frohman L., Kineman R., Ohlsson C., Jansson J. O. (2001) Liver-derived IGF-I regulates GH secretion at the pituitary level in mice. Endocrinology 142, 4762–4770 [DOI] [PubMed] [Google Scholar]
- 37. Borges J. L., Blizzard R. M., Evans W. S., Furlanetto R., Rogol A. D., Kaiser D. L., Rivier J., Vale W., Thorner M. O. (1984) Stimulation of growth hormone (GH) and somatomedin C in idiopathic GH-deficient subjects by intermittent pulsatile administration of synthetic human pancreatic tumor GH-releasing factor. J. Clin. Endocrinol. Metab. 59, 1–6 [DOI] [PubMed] [Google Scholar]
- 38. Kelley K. M., Oh Y., Gargosky S. E., Gucev Z., Matsumoto T., Hwa V., Ng L., Simpson D. M., Rosenfeld R. G. (1996) Insulin-like growth factor-binding proteins (IGFBPs) and their regulatory dynamics. Int. J. Biochem. Cell Biol. 28, 619–637 [DOI] [PubMed] [Google Scholar]
- 39. Marchand A., Tomkiewicz C., Magne L., Barouki R., Garlatti M. (2006) Endoplasmic reticulum stress induction of insulin-like growth factor-binding protein-1 involves ATF4. J. Biol. Chem. 281, 19124–19133 [DOI] [PubMed] [Google Scholar]
- 40. Powell D. R., Suwanichkul A., Cubbage M. L., DePaolis L. A., Snuggs M. B., Lee P. D. (1991) Insulin inhibits transcription of the human gene for insulin-like growth factor-binding protein-1. J. Biol. Chem. 266, 18868–18876 [PubMed] [Google Scholar]
- 41. Lee S. J. (2004) Regulation of muscle mass by myostatin. Annu. Rev. Cell Dev. Biol. 20, 61–86 [DOI] [PubMed] [Google Scholar]
- 42. Fernandez A. M., Dupont J., Farrar R. P., Lee S., Stannard B., Le Roith D. (2002) Muscle-specific inactivation of the IGF-I receptor induces compensatory hyperplasia in skeletal muscle. J. Clin. Invest. 109, 347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mueckler M. (1994) Facilitative glucose transporters. Eur. J. Biochem. 219, 713–725 [DOI] [PubMed] [Google Scholar]
- 44. Burkin D. J., Kaufman S. J. (1999) The α7β1 integrin in muscle development and disease. Cell Tissue Res. 296, 183–190 [DOI] [PubMed] [Google Scholar]
- 45. Nishimura M., Naito S. (2005) Tissue-specific mRNA expression profiles of human Toll-like receptors and related genes. Biol. Pharm. Bull. 28, 886–892 [DOI] [PubMed] [Google Scholar]
- 46. Hoshino K., Takeuchi O., Kawai T., Sanjo H., Ogawa T., Takeda Y., Takeda K., Akira S. (1999) Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162, 3749–3752 [PubMed] [Google Scholar]
- 47. Biswas C., Ostrovsky O., Makarewich C. A., Wanderling S., Gidalevitz T., Argon Y. (2007) The peptide binding activity of GRP94 is regulated by calcium. Biochem. J. 405, 233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang J., Forsberg N. E. (1998) Role of calpain in skeletal-muscle protein degradation. Proc. Natl. Acad. Sci. U. S. A. 95, 12100–12105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yakar S., Rosen C. J., Beamer W. G., Ackert-Bicknell C. L., Wu Y., Liu J. L., Ooi G. T., Setser J., Frystyk J., Boisclair Y. R., LeRoith D. (2002) Circulating levels of IGF-1 directly regulate bone growth and density. J. Clin. Invest. 110, 771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Govoni K. E., Lee S. K., Chung Y. S., Behringer R. R., Wergedal J. E., Baylink D. J., Mohan S. (2007) Disruption of insulin-like growth factor-I expression in type IIαI collagen-expressing cells reduces bone length and width in mice. Physiol. Genomics 30, 354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Elis S., Wu Y., Courtland H. W., Sun H., Rosen C. J., Adamo M. L., Yakar S. (2011) Increased serum IGF-1 levels protect the musculoskeletal system but are associated with elevated oxidative stress markers and increased mortality independent of tissue igf1 gene expression. Aging Cell 10, 547–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Baker J., Liu J. P., Robertson E. J., Efstratiadis A. (1993) Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75, 73–82 [PubMed] [Google Scholar]
- 53. Rosenfeld R. G. (1996) Biochemical diagnostic strategies in the evaluation of short stature: the diagnosis of insulin-like growth factor deficiency. Horm. Res. 46, 170–173 [DOI] [PubMed] [Google Scholar]
- 54. Ostrovsky O., Makarewich C., Snapp E. L., Argon Y. (2009) An essential role for ATP binding and hydrolysis in the chaperone activity of GRP94 in cells. Proc. Natl. Acad. Sci. U. S. A. 106, 11600–11605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Datta K., Guan T., Gerace L. (2009) NET37, a nuclear envelope transmembrane protein with glycosidase homology, is involved in myoblast differentiation. J. Biol. Chem. 284, 29666–29676 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.