Abstract
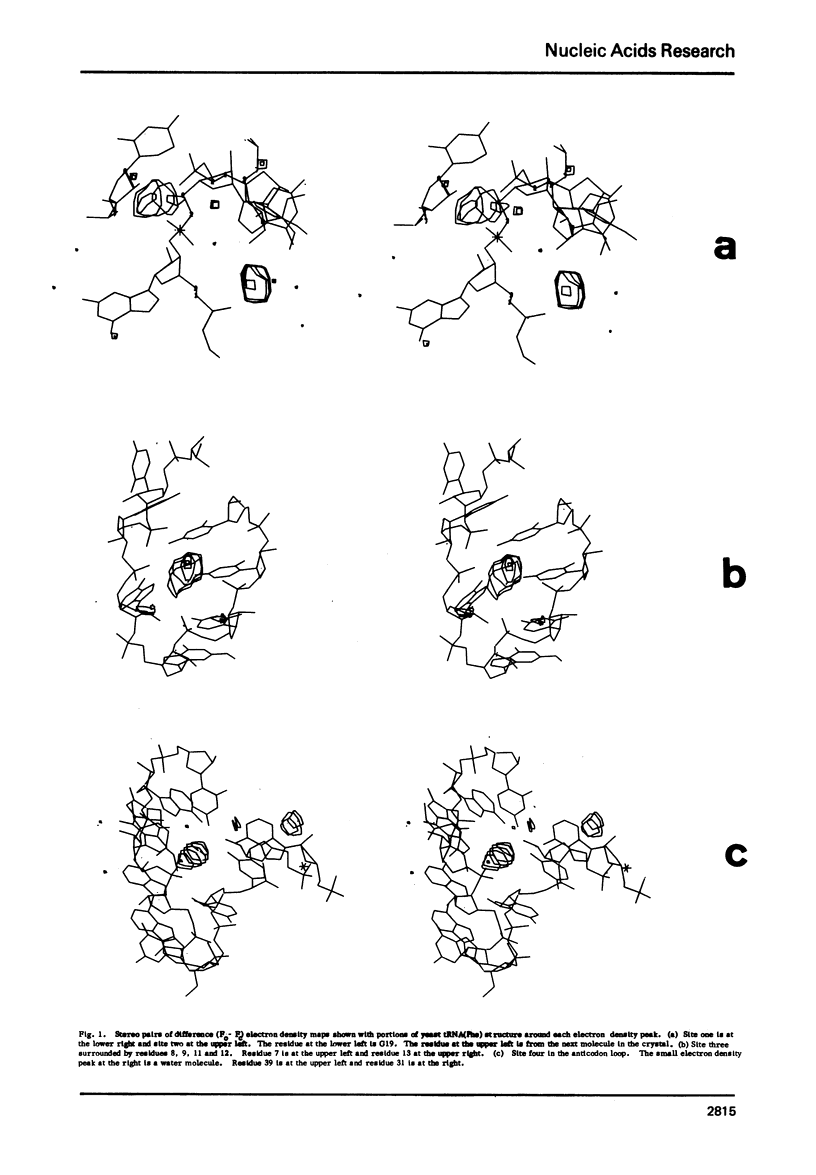
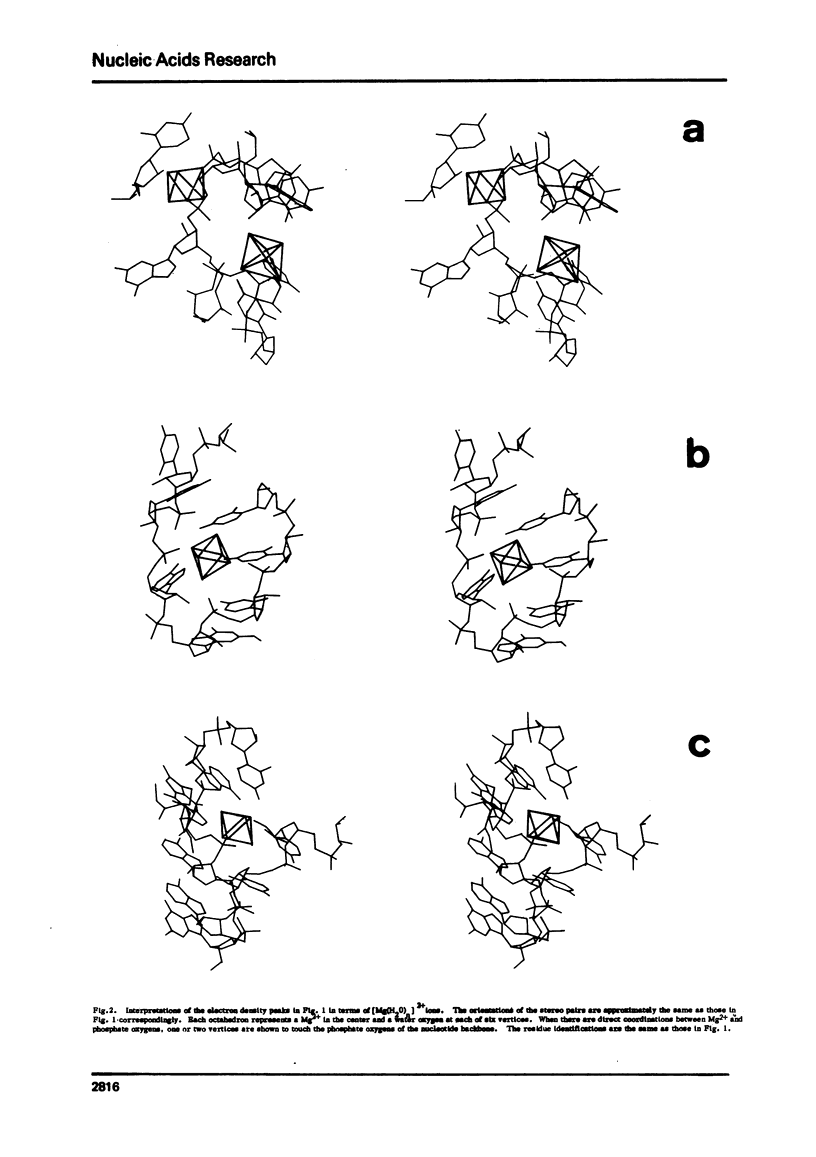
X-ray crystallagraphic studies studies indicate that there are at least four site-specifically bound hydrated Mg2+ ions, [Mg(H2O)n]2+, in yeast tRNAPhe. The size and the octahedral coordination geometry, rather than the charge, of [Mg(H2O)N]2+ appear to be the primary reasons for the specificity of magnesium ions in site-binding and in the stabilization of the tertiary structure of tRNA.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohn M., Danchin A., Grunberg-Manago M. Proton magnetic relaxation studies of marganous complexes of transfer RNA and related compounds. J Mol Biol. 1969 Jan 14;39(1):199–217. doi: 10.1016/0022-2836(69)90342-8. [DOI] [PubMed] [Google Scholar]
- Danchin A. A new method for specific labelling of tRNA: preliminary results on yeast tRNA Phe . Biochimie. 1972;54(3):333–337. doi: 10.1016/s0300-9084(72)80212-8. [DOI] [PubMed] [Google Scholar]
- Danchin A., Guéron M. Cooperative binding of manganese (II) to transfer RNA. Eur J Biochem. 1970 Nov;16(3):532–536. doi: 10.1111/j.1432-1033.1970.tb01113.x. [DOI] [PubMed] [Google Scholar]
- Danchin A. tRNA structure and binding sites for cations. Biopolymers. 1972;11(7):1317–1333. doi: 10.1002/bip.1972.360110702. [DOI] [PubMed] [Google Scholar]
- Fresco J. R., Adams A., Ascione R., Henley D., Lindahl T. Tertiary structure in transfer ribonucleic acids. Cold Spring Harb Symp Quant Biol. 1966;31:527–537. doi: 10.1101/sqb.1966.031.01.068. [DOI] [PubMed] [Google Scholar]
- Jack A., Ladner J. E., Rhodes D., Brown R. S., Klug A. A crystallographic study of metal-binding to yeast phenylalanine transfer RNA. J Mol Biol. 1977 Apr 15;111(3):315–328. doi: 10.1016/s0022-2836(77)80054-5. [DOI] [PubMed] [Google Scholar]
- Jones C. R., Kearns D. R. Investigation of the structure of yeast tRNAphe by nuclear magnetic resonance: paramagnetic rare earth ion probes of structure. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4237–4240. doi: 10.1073/pnas.71.10.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayne M. S., Cohn M. Cation requirements of isoleucyl-tRNA synthetase from Escherichia coli. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1285–1291. doi: 10.1016/s0006-291x(72)80114-1. [DOI] [PubMed] [Google Scholar]
- Kayne M. S., Cohn M. Enhancement of Tb(III) and Eu(III) fluorescence in complexes with Escherichia coli tRNA. Biochemistry. 1974 Sep 24;13(20):4159–4165. doi: 10.1021/bi00717a014. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Quigley G., Suddath F. L., Rich A. High-resolution x-ray diffraction patterns of crystalline transfer RNA that show helical regions. Proc Natl Acad Sci U S A. 1971 Apr;68(4):841–845. doi: 10.1073/pnas.68.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Suddath F. L., Quigley G. J., McPherson A., Sussman J. L., Wang A. H., Seeman N. C., Rich A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974 Aug 2;185(4149):435–440. doi: 10.1126/science.185.4149.435. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Adams A., Fresco J. R. Renaturation of transfer ribonucleic acids through site binding of magnesium. Proc Natl Acad Sci U S A. 1966 Apr;55(4):941–948. doi: 10.1073/pnas.55.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rialdi G., Levy J., Biltonen R. Thermodynamic studies of transfer ribonucleic acids. I. Magnesium binding to yeast phenylalanine transfer ribonucleic acid. Biochemistry. 1972 Jun 20;11(13):2472–2479. doi: 10.1021/bi00763a014. [DOI] [PubMed] [Google Scholar]
- Römer R., Hach R. tRNA conformation and magnesium binding. A study of a yeast phenylalanine-specific tRNA by a fluorescent indicator and differential melting curves. Eur J Biochem. 1975 Jun 16;55(1):271–284. doi: 10.1111/j.1432-1033.1975.tb02160.x. [DOI] [PubMed] [Google Scholar]
- Schreier A. A., Schimmel P. R. Interaction of manganese with fragments, complementary fragment recombinations, and whole molecules of yeast phenylalanine specific transfer RNA. J Mol Biol. 1974 Jul 5;86(3):601–620. doi: 10.1016/0022-2836(74)90183-1. [DOI] [PubMed] [Google Scholar]
- Stein A., Crothers D. M. Equilibrium binding of magnesium(II) by Escherichia coli tRNAfMet. Biochemistry. 1976 Jan 13;15(1):157–160. doi: 10.1021/bi00646a024. [DOI] [PubMed] [Google Scholar]
- Suddath F. L., Quigley G. J., McPherson A., Sneden D., Kim J. J., Kim S. H., Rich A. Three-dimensional structure of yeast phenylalanine transfer RNA at 3.0angstroms resolution. Nature. 1974 Mar 1;248(5443):20–24. doi: 10.1038/248020a0. [DOI] [PubMed] [Google Scholar]
- Sussman J. L., Kim S. H. Idealized atomic coordinates of yeast phenylalanine transfer RNA. Biochem Biophys Res Commun. 1976 Jan 12;68(1):89–96. doi: 10.1016/0006-291x(76)90014-0. [DOI] [PubMed] [Google Scholar]
- Wolfson J. M., Kearns D. R. Europium as a fluorescent probe of transfer RNA structure. Biochemistry. 1975 Apr 8;14(7):1436–1444. doi: 10.1021/bi00678a014. [DOI] [PubMed] [Google Scholar]


