This study analyzes a variety of chimeric phytochromes to identify distinct structural modules within the phytochrome A photoreceptor that confer specific properties.
Abstract
Phytochrome is a red (R)/far-red (FR) light–sensing photoreceptor that regulates various aspects of plant development. Among the members of the phytochrome family, phytochrome A (phyA) exclusively mediates atypical phytochrome responses, such as the FR high irradiance response (FR-HIR), which is elicited under prolonged FR. A proteasome-based degradation pathway rapidly eliminates active Pfr (the FR-absorbing form of phyA) under R. To elucidate the structural basis for the phyA-specific properties, we systematically constructed 16 chimeric phytochromes in which each of four parts of the phytochrome molecule, namely, the N-terminal extension plus the Per/Arnt/Sim domain (N-PAS), the cGMP phosphodiesterase/adenyl cyclase/FhlA domain (GAF), the phytochrome domain (PHY), and the entire C-terminal half, was occupied by either the phyA or phytochrome B sequence. These phytochromes were expressed in transgenic Arabidopsis thaliana to examine their physiological activities. Consequently, the phyA N-PAS sequence was shown to be necessary and sufficient to promote nuclear accumulation under FR, whereas the phyA sequence in PHY was additionally required to exhibit FR-HIR. Furthermore, the phyA sequence in PHY alone substantially increased the light sensitivity to R. In addition, the GAF phyA sequence was important for rapid Pfr degradation. In summary, distinct structural modules, each of which confers different properties to phyA, are assembled on the phyA molecule.
INTRODUCTION
Because of their sessile nature, plants must modulate their growth and development in response to the surrounding environment. Because plants use light as an energy source, they have a special need to monitor and adapt to changes in light conditions. Therefore, plants have evolved divergent photoreceptors, including three classes of blue light–sensing photoreceptors, cryptochrome, phototropin, and ZEITLUPE/FLAVIN BINDING KELCH REPEAT F-BOX/LOV DOMAIN KELCH PROTEIN2 (Cashmore et al., 1999; Briggs et al., 2001; Kami et al., 2010), as well as the red (R)/far-red (FR) light–sensing phytochrome (Neff et al., 2000; Smith, 2000).
Phytochromes are unique pigments capable of photoreversible conformational changes between two spectrally distinct forms, specifically, an R-absorbing form (Pr) and an FR-absorbing form (Pfr). Upon absorption of R, the Pr form is converted to the biologically active Pfr form, whereas FR inactivates phytochrome by converting Pfr back to Pr. To be exact, light exposure establishes an equilibrium between the Pr and Pfr forms, even under monochromatic light, because the absorption spectra of these two forms partially overlap. Consequently, R and FR establish 80 and 1% Pfr ratios at photoequilibrium states, respectively (Mancinelli, 1994). Depending on this photoequilibrium state, major developmental steps are regulated throughout the plant life cycle.
Phytochromes constitute a small gene family in all plant species. In Arabidopsis thaliana, the phytochrome family consists of five members, phytochromes A to E (phyA to phyE) (Abe et al., 1989; Clack et al., 1994; Sharrock and Quail, 1989). These phytochromes share a common domain structure consisting of the chromophore-bearing N-terminal moiety, which exhibits a photoreversible conformational change, and the C-terminal dimerization moiety (Montgomery and Lagarias, 2002). Among the five family members, phyA and phyB are the most important (Reed et al., 1994; Quail et al., 1995). Accordingly, these two species are conserved among all of the angiosperms tested to date (Mathews, 2010). Of these two phytochromes, phyB is the major photoreceptor mediating the R high-irradiance response and the classical R/FR reversible low fluence response (Mancinelli, 1994; Shinomura et al., 1996). The other phytochromes, with the exception of phyA, mainly act as secondary photoreceptors to phyB.
In contrast with the other phytochromes, phyA exclusively mediates the very low fluence response (VLFR), which is elicited with a notably small amount of light (Shinomura et al., 1996), and the FR high irradiance response (FR-HIR), which is observed under continuous FR (Nagatani et al., 1993; Parks and Quail, 1993; Whitelam et al., 1993). Characteristically, VLFR cannot be reversed by a subsequent FR pulse (Mancinelli, 1994). As opposed to phyB-mediated responses, a low ratio of the active Pfr form is sufficient to induce a full phyA response (<0.1% Pfr for VLFR and 1% Pfr at the photoequilibrium for FR-HIR), implying that phyA has evolved as a highly sensitive photoreceptor. In early angiosperm history, the sensitization of phyA may have been an important step because the first angiosperms are thought to have emerged in dense shade (Mathews, 2006). Indeed, the Arabidopsis phyA mutant does not survive in deeply shaded conditions (Yanovsky et al., 1995).
Phytochrome molecules undergo dynamic changes in their subcellular localization. Phytochromes are synthesized in the Pr form and are mainly localized in the cytoplasm in the dark. Once converted to the Pfr form, phytochromes accumulate in the nucleus (Kircher et al., 1999, 2002; Yamaguchi et al., 1999; Hisada et al., 2000; Chen et al., 2005), where they interact with signaling partners, such as the basic helix-loop-helix transcription factors PHYTOCHROME INTERACTING FACTORs (PIFs), in a Pfr-dependent manner (Ni et al., 1998, 1999; Huq and Quail, 2002; Huq et al., 2004; Khanna et al., 2004; Leivar et al., 2008a). This interaction induces PIF degradation (Park et al., 2004; Bauer et al., 2004; Al-Sady et al., 2006; Shen et al., 2007, 2008; Lorrain et al., 2008), which, in turn, leads to the altered expression of target genes (Tepperman et al., 2001, 2004, 2006; Oh et al., 2006, 2007, 2009; Leivar et al., 2008b, 2009; Shin et al., 2007, 2009). Hence, nuclear accumulation is a key process for the signal transduction mechanism of phytochromes.
Nuclear translocation is required for both phyA- and phyB-mediated seedling deetiolation (Huq et al., 2003; Matsushita et al., 2003; Genoud et al., 2008; Toledo-Ortiz et al., 2010). Accordingly, phyA accumulates in the nucleus during VLFR and FR-HIR (Kircher et al., 1999; Kim et al. 2000). Recently, FAR-RED ELONGATED HYPOCOTYL1 (FHY1) and its homolog FHY1-LIKE (FHL) have been shown to play key roles in phyA nuclear accumulation under continuous FR (Hiltbrunner et al., 2005, 2006; Rösler et al., 2007; Genoud et al., 2008; Pfeiffer et al., 2009; Rausenberger et al., 2011). The widespread distribution of functional homologs of FHY1 and FHL among angiosperms implies the importance of these molecules in the sensitization process of phyA responses (Genoud et al., 2008).
To balance the increased sensitivity of phyA, plants have evolved a desensitization mechanism to remove phyA Pfr rapidly. Indeed, the phyA Pr protein that is accumulated at high levels in the dark is rapidly degraded by a proteasome-mediated mechanism upon photoconversion from Pr to Pfr (Jabben et al., 1989a, 1989b; Vierstra, 1994). This light-dependent degradation of phyA occurs both in the cytoplasm and the nucleus (Toledo-Ortiz et al., 2010; Debrieux and Fankhauser, 2010). In striking contrast, the levels of phyB remain constant regardless of light conditions. More recently, it has been shown that CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1) is involved in the ubiquitination of phyA (Seo et al., 2004) and phyB (Jang et al., 2010) in the light.
Although the increased sensitivity of the phyA-mediated responses can be partly explained by the higher abundance of phyA in darkness (8.5 times higher than phyB, according to Sharrock and Clack, 2002), this increased abundance is not sufficient to explain the observed sensitivity difference. For instance, phyB never exhibits FR-HIR activity, even when artificially overaccumulated (Wagner et al., 1996b). Hence, the phyA molecule is intrinsically sensitized to the light more than the phyB molecule. Furthermore, chimeric phytochrome analyses have narrowed the structure required for the specific functions of phyA to its N-terminal moiety (Wagner et al., 1996a; Clough et al., 1999). Namely, a chimeric phytochrome with phyA N- and phyB C-terminal moieties exhibited phyA features, whereas phyB-type responses were observed in the reverse combination (Wagner et al., 1996a; Clough et al., 1999). Hence, the N-terminal moiety primarily determines the properties evolved in phyA.
Recently, crystal structures of the chromophore-bearing regions have been determined in a few bacterial phytochromes (Wagner et al., 2005; Yang et al., 2007, 2008; Essen et al., 2008). Consequently, four consecutive domains, specifically, the N-terminal extension, the N-terminal Per/Arnt/Sim domain (PAS), the cGMP phosphodiesterase/adenyl cyclase/FhlA domain (GAF), and the phytochrome domain (PHY), were recognized within the N-terminal photosensory moiety, as has been proposed previously (Montgomery and Lagarias, 2002, Nagatani 2010). The GAF domain constitutes the chromophore binding pocket in the center of the N-terminal moiety (Wagner et al., 2005), whereas the PHY domain next to it stabilizes phytochrome in the Pfr form (Oka et al., 2004). Interestingly, an unusual three-dimensional structure, designated as the light-sensing knot, is found between the PAS and GAF domains (Wagner et al., 2005). In addition, a tongue-like protuberance from PHY makes contact with the chromophore pocket in the GAF (Essen et al., 2008; Yang et al., 2008).
In this study, we examined each of the above-mentioned domains with respect to phyA-specific properties. The phyA/phyB chimeric phytochromes, in which these domains were systematically swapped, were expressed in the Arabidopsis phyA phyB double mutant background. The resulting lines were tested for phyA-specific responses, including FR-induced nuclear accumulation and the inhibition of hypocotyl elongation under continuous FR and R-induced degradation of Pfr. In addition, the hypocotyl response under continuous R, which is a typical response of phyB, was also tested. Our results indicate that each of the phyA-specific properties is based on the local structure in different parts of the phyA molecule.
RESULTS
Preparation of Transgenic Lines Expressing phyA/phyB Chimeric Proteins
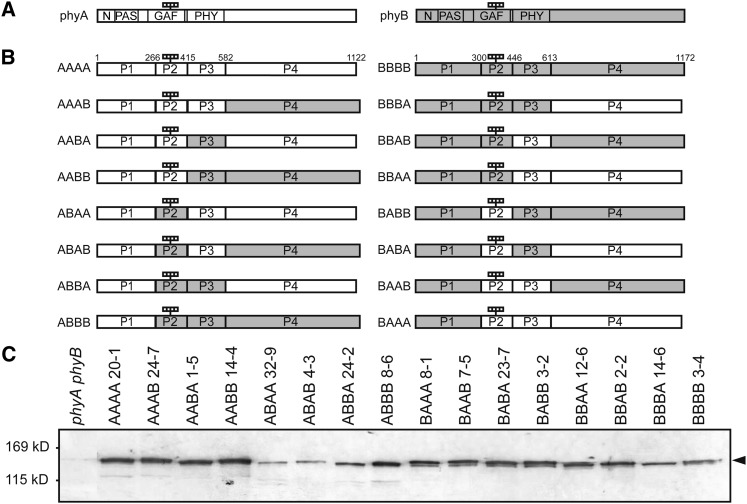
In previous works, chimeric phytochromes between oat (Avena sativa) phyA and rice (Oryza sativa) phyB (Wagner et al., 1996a) or between potato (Solanum tuberosum) phyA and tobacco (Nicotiana tabacum) phyB (Clough et al., 1999) were expressed in wild-type Arabidopsis. To extend those works, we divided the phytochrome molecule into four parts based on its domain structure (Montgomery and Lagarias, 2002; Figure 1A). Specifically, the N-terminal moiety was divided into three parts. Part 1 (N-PAS) encompassed the N-terminal extension, the PAS domain, and a small N-terminal part of the GAF domain, which was included for technical reasons. Parts 2 (GAF) and 3 (PHY) encompassed the remainder of the GAF domain and the entire PHY domain, respectively. Finally, the entire C-terminal half constituted part 4 (C-terminal). Accordingly, 16 possible phyA/phyB chimeric phytochromes in which each of the four parts was occupied by either the phyA or phyB sequence were constructed (Figure 1B).
Figure 1.
Preparation of Transgenic Arabidopsis Lines Expressing phyA/phyB Chimeric Proteins.
(A) Diagram of phyA and phyB. White and gray boxes indicate the phyA and phyB sequences, respectively. N, N-terminal extension (1 to 78 in phyA; 1 to 102 in phyB); PAS, PAS domain (79 to 185 in phyA; 103 to 219 in phyB); GAF, GAF domain (218 to 402 in phyA; 252 to 433 in phyB); PHY, PHY domain (413 to 593 in phyA; 444 to 624 in phyB). The four small rectangles indicate the chromophores.
(B) Diagram of 16 phyA/phyB chimeric proteins. The phyA and phyB molecules were divided into four parts, and their respective sequences were shuffled between phyA and phyB. Numbers shown on the AAAA and BBBB sequence denote the amino acid positions of the borders. The four rectangles indicate the chromophores.
(C) Immunoblot detection of the phyA/phyB chimeric phytochromes with a mouse monoclonal anti-GFP antibody in etiolated seedlings of representative transgenic lines. Five micrograms of crude protein extract was loaded in each lane.
For convenience, each chimeric phytochrome is referred to by four letters (Figure 1B). For example, BAAA represents the chimeric phytochrome in which the N-PAS phyB sequence was fused to the rest of the phyA sequence. In addition, the expression (A/B) was introduced to denote that the respective part can be either a phyA or phyB sequence. For example, A(A/B)A(A/B) collectively refers to four chimeric phytochromes with a phyA sequence in parts 1 and 3 (i.e., AAAA, AAAB, ABAA, and ABAB).
To examine their physiological activities, these chimeric phytochromes were fused to green fluorescent protein (GFP) and expressed under the control of the 35S viral promoter in a phyA phyB double mutant background in Arabidopsis. As expected from the similarity between phyA and phyB sequences, all of the chimeric proteins were successfully expressed (Figure 1C). However, a dilution series analysis (see Supplemental Figure 1 online) demonstrated that expression levels were lower than that of endogenous phyA for some constructs (Figure 2C; see Supplemental Figure 2 online). We circumvented this problem by comparing the responses in multiple lines with different expression levels ranging from 0.5 to 2.5 units relative to endogenous phyA. Exceptionally, the highest expression was 0.25 units for BBBA.
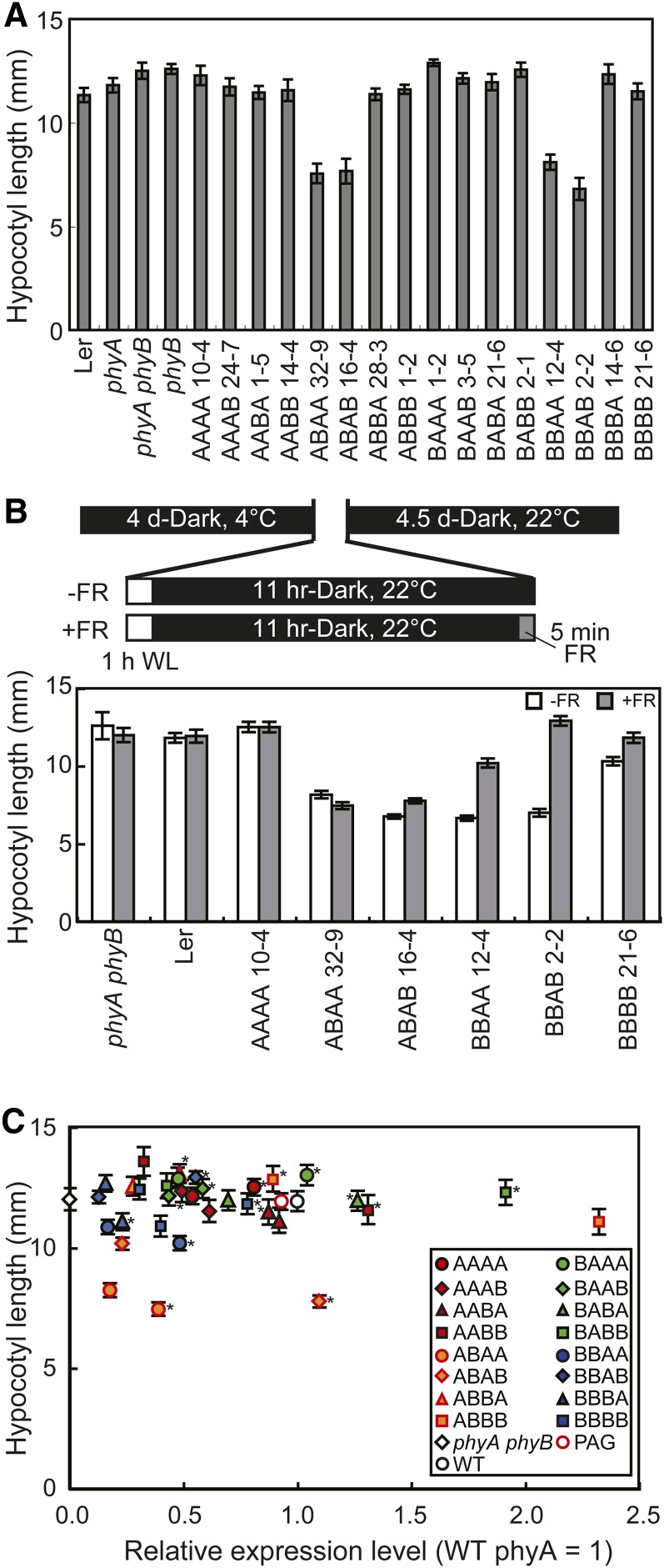
Figure 2.
Hypocotyl Lengths of Arabidopsis Seedlings Expressing phyA/phyB Chimeric Proteins in the Dark.
(A) Hypocotyl lengths of seedlings grown in the dark for 5 d. Data are the means ± se (n = 25). Ler, Landsberg erecta.
(B) The effects of an FR pulse on the dark phenotype. Schematic representations of the growth conditions (top) and the hypocotyl lengths of seedlings (bottom) are shown. Seeds were irradiated with white light for 1 h to synchronize germination and then kept in the dark for 11 h. To eliminate residual Pfr, seeds were treated with a 5-min FR pulse (18 μmol/m2/s) and returned to the dark. Hypocotyl lengths were determined after 4.5 d (n = 25; mean ± se). WL, white light.
(C) The relationship between hypocotyl length in the dark (ordinate) and protein expression levels (abscissa) in independent transgenic lines (see Supplemental Figure 2 online for enlarged views). Expression levels were estimated by densitometric analysis of the immunoblots (see Supplemental Figure 1 online) and are expressed in units relative to endogenous phyA. Data are the means ± se (n = 25). Asterisks indicate the lines chosen as representative, as shown in Figure 1. WT, the wild type.
phyA/phyB Chimeric Phytochromes in Darkness
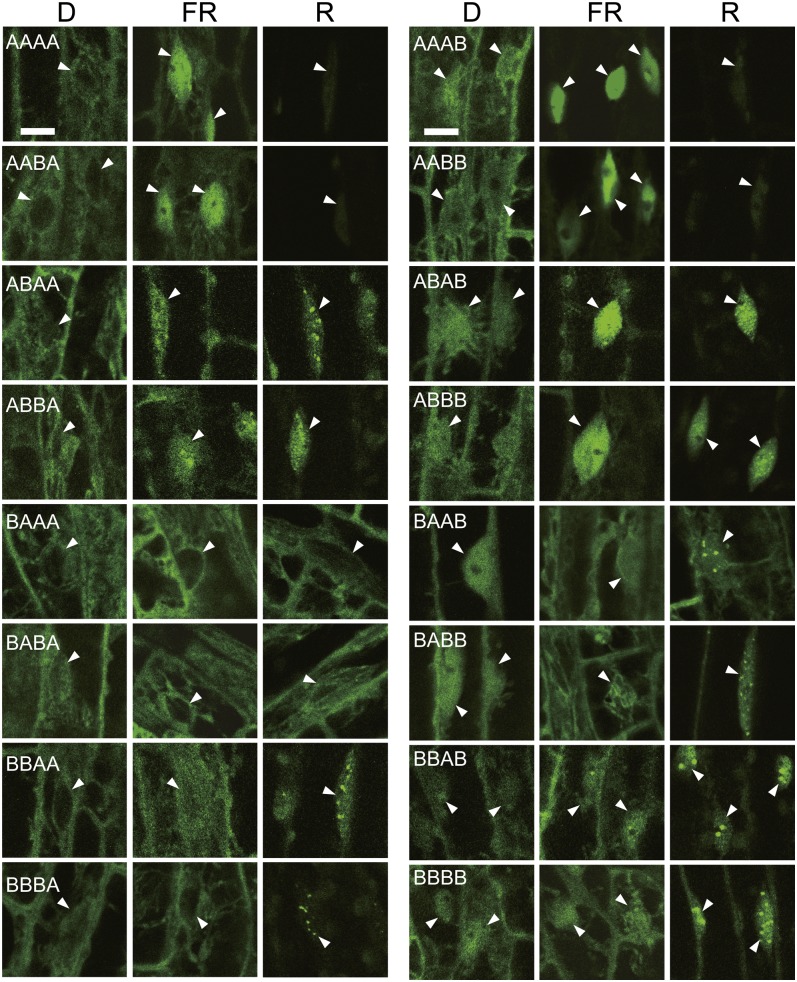
The intracellular distribution of the chimeric phytochromes fused to GFP was observed in dark-grown seedlings under a confocal laser scanning microscope (Figure 3). As previously reported, uniform BBBB fluorescence was observed in both the cytoplasm and the nucleus (Oka et al., 2008), whereas AAAA was detected exclusively in the cytoplasm (Kircher et al., 1999; Kim et al., 2000; Toledo-Ortiz et al., 2010). All of the remaining 14 chimeric phytochromes exhibited either phyA- or phyB-like distribution patterns, depending entirely on the part 4 (C-terminal) sequence. Quantification of GFP fluorescence in the cytoplasm and the nucleus further supported this view (see Supplemental Figure 3 online). Hence, the phyA C-terminal moiety is responsible for the strict exclusion of phyA from the nucleus in darkness.
Figure 3.
Subcellular Localization of phyA/phyB Chimeric Phytochromes in the Dark under Continuous FR (18 μmol/m2/s) or under Continuous R (5.5 μmol/m2/s).
Three-day-old, dark-grown seedlings were treated with FR or R for 24 h before observation. The epidermis in the hook regions of seedlings was observed using a confocal laser scanning microscope. Arrowheads indicate the nuclei. D, dark. Bars = 10 μm.
The morphogenic phenotype was then observed in etiolated seedlings grown under complete darkness (Figure 2A). Consistent with previous reports (Boylan and Quail, 1991; Wagner et al., 1996b; Yamaguchi et al., 1999; Genoud et al., 2008), neither AAAA nor BBBB exhibited visible phenotypes in this condition. Likewise, most of the chimeric phytochromes, with the exception of ABA(A/B) and BBA(A/B) (see below), exhibited no phenotype. To further analyze these four chimeric phytochromes, imbibed seeds were treated with an FR light pulse before germination (Leivar et al., 2008b) (Figure 2B). For BBA(A/B), this treatment effectively diminished the short hypocotyl phenotype, indicating that preexisting Pfr in the seeds affected subsequent seedling development in those lines. However, the ABA(A/B) phenotypes were not affected by the treatment. Hence, ABA(A/B) may exhibit a true dark phenotype, albeit weakly. The same finding was observed for cotyledon opening in these two lines (see Supplemental Figure 4 online).
To confirm the above observation, multiple lines with various expression levels of the chimeric phytochromes were observed for each construct (Figure 2C). As expected, the chimeric phytochromes exhibited normal hypocotyl lengths, regardless of their expression levels, with the exceptions of ABA(A/B). Interestingly, the short phenotypes of ABA(A/B) appeared to be partially dose dependent (Figure 2C; see Supplemental Figure 2 online), further supporting the notion that the phenotype was indeed caused by the introduced chimeric phytochrome.
Nuclear Accumulation of Chimeric Phytochromes under Continuous FR
Relatively rapid nuclear accumulation of phyA-GFP is observed under continuous FR (Kircher et al., 1999; Kim et al., 2000). By contrast, nuclear levels of phyB-GFP do not increase under such conditions (Yamaguchi et al., 1999). We examined whether FR treatment increased nuclear GFP signals in our chimeric transgenic lines. Seedlings were grown for 3 d in darkness, treated with FR for 24 h, and observed under a confocal laser scanning microscope (Figure 3; see Supplemental Figure 3 online). Interestingly, all of the chimeric phytochromes with the phyA N-PAS sequence had increased nuclear signals under FR, whereas the signals barely increased in those with phyB N-PAS. Hence, the phyA sequence in part 1 (N-PAS) is necessary and sufficient for FR-induced nuclear accumulation; this finding is consistent with the report that a phyA N-terminal fragment encompassing N-PAS and GAF accumulates in the nucleus under FR (Viczián et al., 2012).
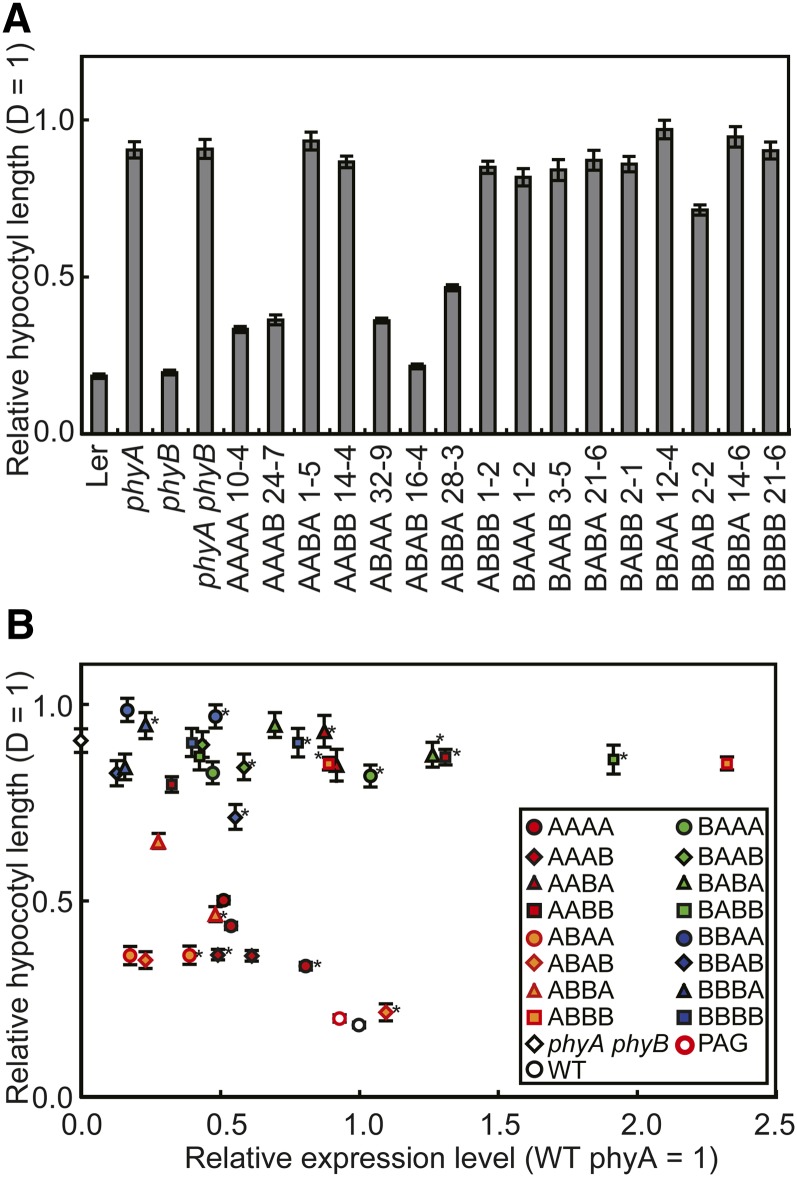
Inhibition of Hypocotyl Elongation under Continuous FR
phyA exclusively mediates FR-HIR, which triggers various photomorphogenic responses (Quail et al., 1995). To examine this activity in the chimeric phytochromes, seedlings were grown under continuous FR for 5 d, and their hypocotyl lengths were determined (Figure 4A). All chimeric phytochromes with the phyB N-PAS sequence failed to show the response with the exception of a notably weak response in BBAB. This result is not surprising because these phytochromes fail to accumulate in the nucleus under FR (Figure 3). However, nuclear accumulation alone was not sufficient for the response. Indeed, AAB(A/B) and ABBB, which accumulate in the nucleus under FR (Figure 3), were as tall as the parental phyA phyB mutant (Figure 4A).
Figure 4.
Hypocotyl Lengths of Arabidopsis Seedlings Expressing phyA/phyB Chimeric Proteins under Continuous FR.
(A) Hypocotyl lengths of seedlings grown under continuous FR (18 μmol/m2/s) for 5 d. The hypocotyl lengths are presented relative to the dark (D). Data are the means ± se (n = 25). Ler, Landsberg erecta.
(B) The relationship between hypocotyl length under continuous FR (ordinate) and protein expression levels (abscissa) in independent transgenic lines (see Supplemental Figure 5 online for enlarged views). Expression levels were estimated by densitometric analysis as for Figure 2C and are expressed in units relative to endogenous phyA. The hypocotyl lengths are presented relative to dark. Data are the means ± se (n = 25). Asterisks indicate the lines chosen as representative, as shown in Figure 1. WT, the wild type.
We then examined the relationship between the hypocotyl response and the expression levels for each construct (Figure 4B; see Supplemental Figure 5 online). First, those constructs with the phyB N-PAS sequence [AAB(A/B) and ABBB] failed to respond to FR regardless of expression levels. The responsiveness to FR varied in other lines. Specifically, AAAA and ABBA exhibited moderate responses in a dose-dependent manner, whereas ABA(A/B) exhibited stronger responses, even at ∼0.25 unit. It remained unclear to which group AAAB belonged because of the lack of a low expresser. We also examined the fluence rate/response relationship in the above five transgenic lines, but no significant difference was observed, except that ABAA was somewhat more sensitive to light than the others (see Supplemental Figure 6 online).
Although the above result is slightly complicated, the phyA sequences in part 3 (PHY), like that in part 1 (N-PAS), appears to play an important role in conferring competence to hypocotyl FR-HIR. Indeed, all four A(A/B)A(A/B) chimeric phytochromes exhibited the response, whereas three out of four A(A/B)B(A/B) phytochromes failed to do so (see Supplemental Table 1 online). As an exception, the phyA sequence in part 4 (C-terminal) appeared to play a similar role in ABBA.
R-Induced Degradation of phyA/phyB Chimeric Proteins
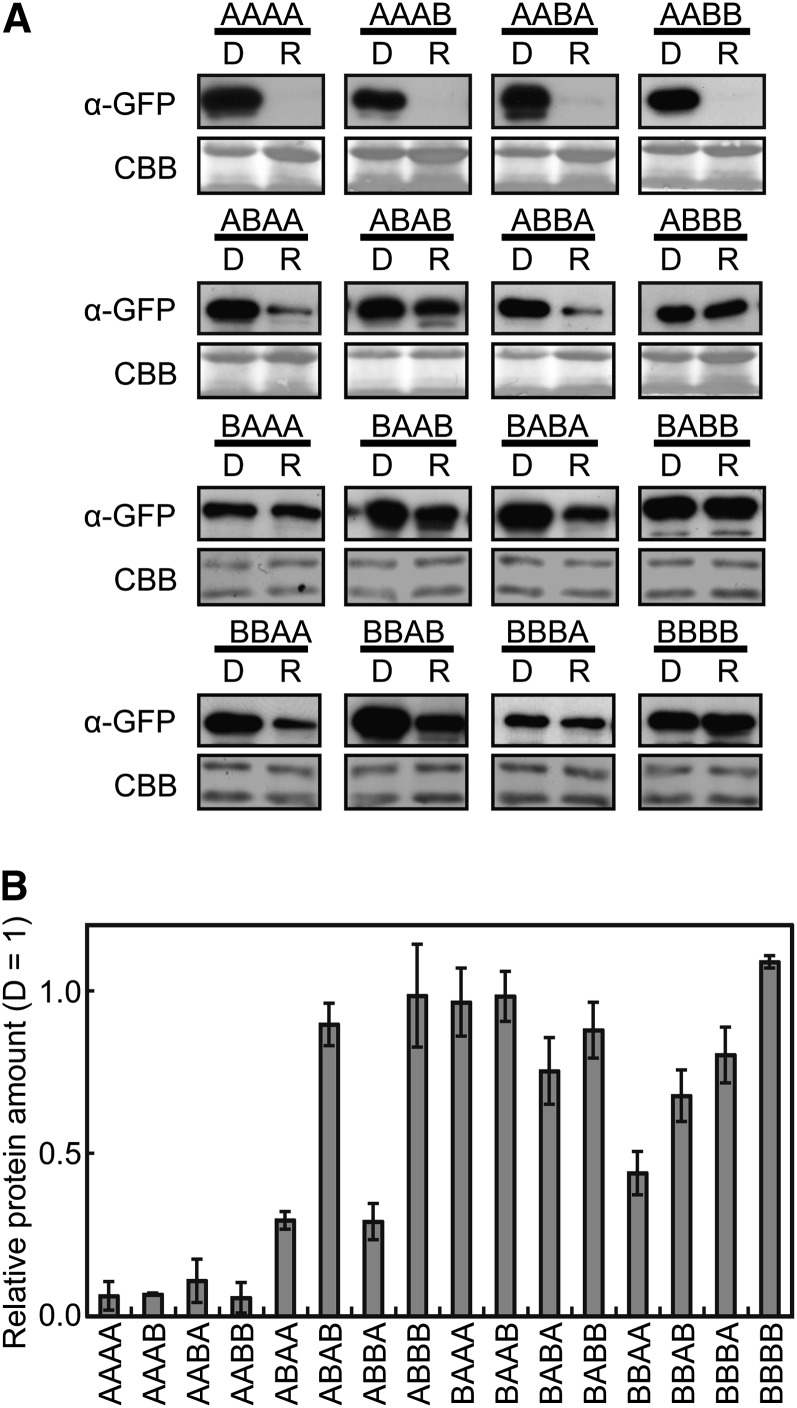
phyA protein is degraded rapidly upon conversion from Pr to Pfr in vivo (Jabben et al., 1989a, 1989b). Hence, we examined the stability of the chimeric phytochromes under continuous R. Seedlings were grown for 4 d in darkness and subjected to R treatment for 24 h before immunoblot detection (Figure 5A). The relative chimeric protein levels were further quantified with reference to the dark-grown, wild-type samples prepared in a dilution series (Figure 5B).
Figure 5.
Degradation of phyA/phyB Chimeric Proteins under Continuous R.
(A) Immunoblot detection of the phyA/phyB chimeric proteins in seedlings treated with continuous R. Three-day-old, dark-grown seedlings were kept in the dark (D) or exposed to R (8.5 μmol/m2/s) for 24 h. The blots were probed with anti-GFP monoclonal antibodies (top). Five micrograms of total protein was loaded in each lane. To confirm equal protein loading, the same blots were subjected to Coomassie blue (CBB) staining (bottom).
(B) Expression levels were estimated by densitometric analysis of the immunoblots using a dilution series. The data are presented relative to the dark levels. Data are the means ± se (n = 3).
As shown in Figure 5, all four AA(A/B)(A/B) phytochromes degraded almost completely under continuous R. By contrast, a substantial signal was detected after treatment in the remaining 12 phytochromes, although partial degradation was observed in some lines, such as ABAA, ABBA, and BBAA. Hence, both parts 1 (N-PAS) and 2 (GAF) are important for complete degradation under R. The same conclusion was obtained by observing GFP fluorescence (Figure 3). In addition, pairwise comparison between chimeric phytochromes that shared the same N-terminal moiety (for example, ABAA and ABAB) revealed that the phyA C terminus destabilized the phytochrome in some pairs (Figure 5). Hence, part 4 (the C-terminal) might be involved in light-dependent degradation in some chimeric contexts.
Nuclear Accumulation of phyA/phyB Chimeric Proteins under Continuous R
phyB-GFP accumulates and forms speckles, which have been proposed to be the sites of protein degradation and signal transduction (Chen et al., 2010), in the nucleus after prolonged irradiation with R (Kircher et al., 1999; Yamaguchi et al., 1999). By contrast, phyA-GFP becomes undetectable under R, due to rapid light-induced degradation (see above). We examined which chimeric phytochromes accumulate in the nucleus and form speckles under R at 10 μmol/m2/s (Figure 3). Consequently, most of the phytochromes, with the exception of those that were degraded completely under R, exhibited accumulation and speckle formation. However, the nuclear levels remained low in BA(A/B)A, regardless of their stability under R (see Supplemental Figure 7 online).
Inhibition of Hypocotyl Elongation under Continuous R
phyB but not phyA is the primary photoreceptor mediating the hypocotyl response under continuous R (Quail et al., 1995). To assess this response, seedlings were grown under continuous R at 3.3 μmol/m2/s, and their hypocotyl lengths were determined (Figure 6). Four AA(A/B)(A/B) phytochromes (including AAAA), all of which were almost completely degraded under R (Figure 5), and two BA(A/B)A phytochromes that failed to accumulate and form speckles in the nucleus (Figure 3), responded to R only weakly (Figure 6A). It is noteworthy that this weak response was observed even in AAAA; this response is probably due to the constitutive viral 35S promoter used to express the chimeric phytochromes in these lines. In contrast with the above six lines, the remaining lines exhibited clear responses to various extents.
Figure 6.
Hypocotyl Lengths of Arabidopsis Seedlings Expressing Chimeric Phytochromes under R.
(A) Hypocotyl lengths of seedlings grown under R (3.3 μmol/m2/s) for 5 d. The hypocotyl lengths are presented relative to the dark (D). Data are the means ± se (n = 25). Ler, Landsberg erecta.
(B) The relationship between hypocotyl length under R (ordinate) and protein expression levels (abscissa) in independent transgenic lines (see Supplemental Figure 8 online for enlarged views). Expression levels were estimated by densitometric analysis as for Figure 2C and are expressed in units relative to endogenous phyB. The hypocotyl lengths are presented relative to D. Data are the means ± se (n = 25). WT, the wild type.
We then examined the relationship between the hypocotyl response and the expression level for each construct (Figure 6B; see Supplemental Figure 8 online). It should be noted that the endogenous level of phyB is much lower than that of phyA in darkness (Sharrock and Clack, 2002). Our own analysis demonstrated that the latter was ∼30 times higher than the former (see Supplemental Figures 1A to 1C online). As expected, expression levels attained by the 35S promoter in the lines discussed in this study were much higher than that of endogenous phyB. Accordingly, the response appeared to be saturated for most of the constructs. As an exception, ABBA exhibited a strong dependence on expression level for unknown reasons.
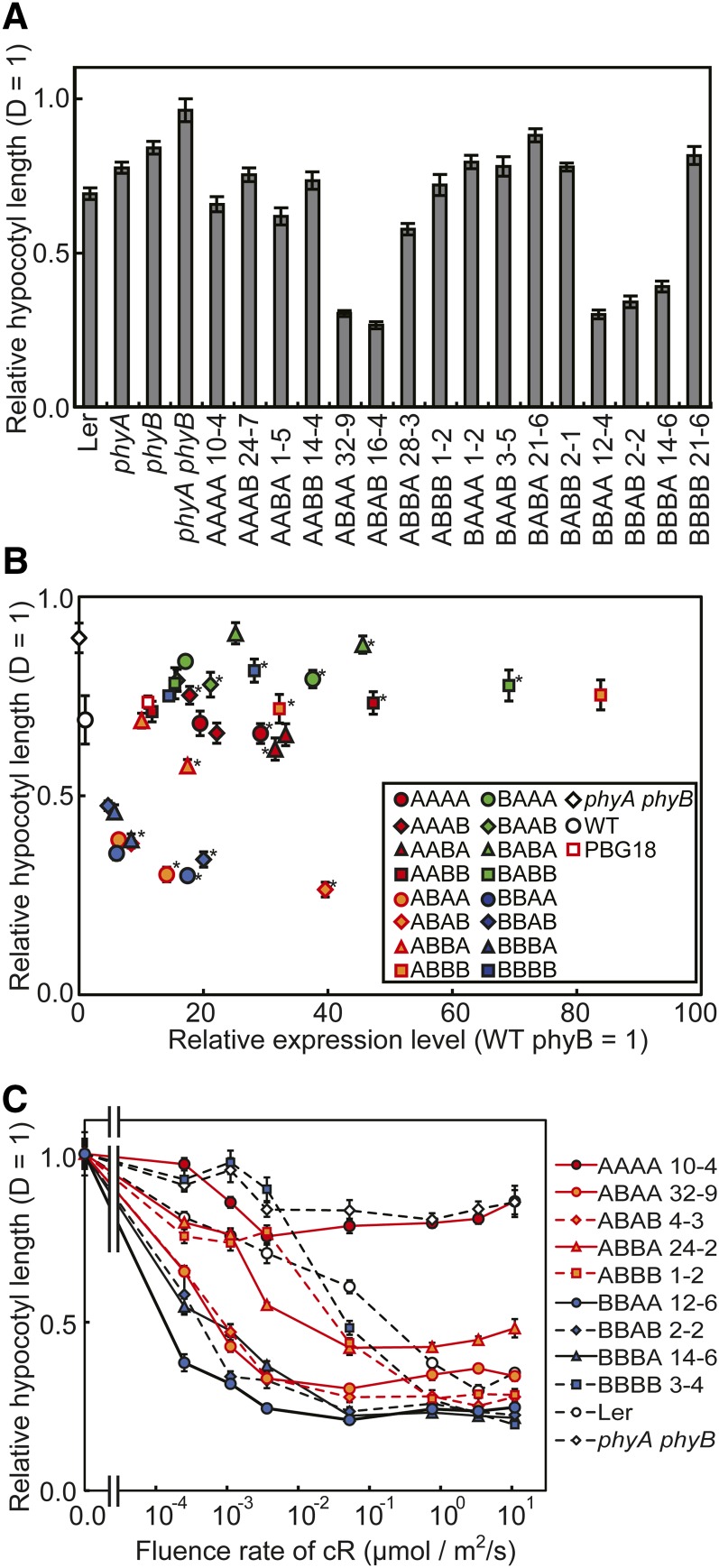
Sensitivities to R
We further compared the chimeric phytochromes with respect to sensitivity to R. First, hypocotyl lengths were determined under weak R (0.005 μmol/m2/s) (Figure 7A). Under this condition, BBBB barely exhibited any response. By contrast, strong responses were observed in ABA(A/B), BBA(A/B), and BBBA. In other words, these five phytochromes are substantially sensitized to R. It is worth noting that all four (A/B)BA(A/B) phytochromes were included in this category. The relationship between hypocotyl response and expression level was then examined under the same light condition (Figure 7B; see Supplemental Figure 9 online). Unlike the responses to R at 3.3 μmol/m2/s (Figure 6B), dose dependency of the response was observed, especially in the range below 10 units.
Figure 7.
Hypocotyl Lengths of Arabidopsis Seedlings Expressing Chimeric Phytochromes under Weak R.
(A) Hypocotyl lengths of seedlings grown under weak R (0.005 μmol/m2/s) for 5 d. The hypocotyl lengths are presented relative to the dark (D). Data are the means ± se (n = 25). Ler, Landsberg erecta.
(B) The relationship between hypocotyl length under weak R (ordinate) and protein expression levels (abscissa) in independent transgenic lines (see Supplemental Figure 9 online for enlarged views). Expression levels were estimated as for Figure 2C and are expressed in units relative to endogenous phyB. The hypocotyl lengths are presented relative to the dark. Data are the means ± se (n = 25). WT, the wild type.
(C) Fluence rate response curves for the inhibition of hypocotyl elongation under continuous R (cR). Seedlings were grown for 5 d under various fluence rates of R. The hypocotyl lengths are presented relative to D. Data are the means ± se (n = 25).
To compare the extents of sensitization, fluence rate/response curves were determined for eight lines that exhibited clear responses to R at 3.3 μmol/m2/s (Figure 7C; see Supplemental Figure 10 online). Although several of these lines exhibited complex fluence rate/response relationships, they were roughly classified into two types. ABA(A/B), BBA(A/B), and BBBA exhibited intense responses, even below 0.001 μmol/m2/s, and among them, BBAA exhibited the highest responsiveness and sensitivity to R. By contrast, the curves for ABB(A/B) were comparable to those for the wild type and BBBB.
The above results demonstrate that substitution of the phyB sequence with that of phyA in certain parts of the protein results in a substantial increase in sensitivity to R. Part 3 (PHY) was shown to be particularly important (see Supplemental Table 1 online). Among the 12 chimeric phytochromes that were not degraded completely under R (Figure 5), four of the six phytochromes with phyA PHY exhibited intense responses to weak R (Figure 7). The remaining two phytochromes with phyA PHY, namely, BAA(A/B), exhibited only weak or almost no responses. This finding was probably observed because phyB N-PAS and phyA GAF were combined in BAA(A/B) (see Discussion).
DISCUSSION
Intracellular Localization of phyA/phyB Chimeric Proteins
The chimeric phytochromes exhibited either phyA-like or phyB-like localization patterns in darkness, depending on the C-terminal sequence (Figure 3; see Supplemental Table 1 online). To exclude the possibility that nuclear signals detected in darkness represented residual Pfr, the seeds were treated with FR before observation; however, the signal was not reduced. In addition, even AA(A/B)B, the Pfrs of which were completely degraded under R (Figure 5), were detected in the nucleus. Hence, chimeric phytochromes containing the phyB C terminus appeared to be partially localized in the nucleus, even in the Pr form.
It is not surprising that N-PAS is involved in nuclear accumulation under FR. It is well established that the nuclear localization facilitators FHY1 and FHL play a critical role in this process (Hiltbrunner et al., 2005, 2006; Rösler et al., 2007; Genoud et al., 2008). The N-terminal fragment of phyA, which binds FHY1 in yeast cells (Hiltbrunner et al., 2005), accumulates in the nucleus under continuous FR (Pfeiffer et al., 2009; Wolf et al., 2011), whereas the missense mutation in the N-terminal extension impairs FR-induced nuclear accumulation (Sokolova et al., 2012). This result is also consistent with the recent model in which the association/dissociation of the phyA-FHY1/FHL complex plays an important role in FR-HIR (Rausenberger et al., 2011).
In contrast with FR, all of the light-stable phytochromes, except BA(A/B)A, translocated into the nucleus and formed speckles under continuous R (Figure 3; see Supplemental Table 1 online). This result is not surprising because both phyA and phyB accumulate in the nucleus in response to R without the aid of FHY1/FHL (Hiltbrunner et al., 2005; Müller et al., 2009). It is intriguing that the shuffling of domains between phyA and phyB did not impair the light-induced nuclear localization promoted by R. It has been proposed that the nuclear localization signal in the C-terminal domain is masked by GAF-PHY in darkness but is exposed under R (Chen et al., 2005). Hence, the intramolecular interaction between the GAF-PHY and C-terminal domains appears to be highly conserved between phyA and phyB.
Biological Activities of Chimeric Phytochromes in Darkness
It is widely accepted that the Pr phytochromes have no biological activity (Mancinelli, 1994). Consistently, the present chimeric phytochromes did not affect hypocotyl elongation in darkness with the exception of ABA(A/B). These phytochromes caused partial photomorphogenesis in darkness, even if the residual Pfr in seeds was eliminated by a pulse of FR (Figure 2B). Hence, these phytochromes might be partially activated even in the Pr form, as is the case with certain mutant forms of phytochrome (Su and Lagarias, 2007). However, another possibility exists. The phyB GAF sequence stabilized the chimeric phytochrome under R, whereas the phyA PHY sequence increased light sensitivity (see below) in ABA(A/B). Consequently, VLFR, which cannot be canceled by a pulse of FR (Mancinelli, 1994), might be highly exaggerated in ABA(A/B). In any case, ABA(A/B) could have been less stable in darkness because of their constitutive activity. However, this result was not likely because the mRNA level matched the protein level in those lines well (see Supplemental Figure 11 online).
In addition to ABA(A/B), BBA(A/B) exhibited partial photomorphogenesis in darkness when the FR pretreatment was absent (Figure 2B). It is intriguing here that the apparent nuclear levels of those phytochromes did not correlate with the extent of the responses observed. Although (A/B)BAA was strictly excluded from the nucleus, their responses were comparable to those of their counterparts containing the phyB C terminus. Hence, this response might be saturated at a low level of nuclear phytochrome, as is the case with the VLFR. Alternatively, (A/B)BAA might elicit the response outside the nucleus, although the mechanism remains unknown.
Biological Activities of Chimeric Phytochromes under FR
A certain level of expression is required to assess the physiological activities of the introduced phytochromes. Fortunately, AAAA exhibited a clear response to FR at 0.5 units (the endogenous phyA = 1) (Figure 4B; see Supplemental Figure 5 online), and the expression exceeded this level for most of the constructs (Figure 2C; see Supplemental Figure 2 online). However, the expression level was lower than 0.25 units in BBBA. Nevertheless, we reasoned that BBBA was incompetent for the response. First, the dose–response curves for AAAA (in the range of 0.5 to 1.0 units) and ABBA (in the range of 0.25 to 0.5 units) were smoothly connected (Figure 4B; see Supplemental Figure 5 online), suggesting that ABBA was as active as AAAA. By contrast, BBBA at 0.25 units totally failed to exhibit the response. Second, a previous report showed that oat/rice AAAB but not rice/oat BBBA mediated the hypocotyl response to FR (Wagner et al., 1996a).
The nuclear accumulation of phyA is necessary but not sufficient for FR-HIR (Genoud et al., 2008). Consistently, all of the chimeric phytochromes that failed to accumulate in the nucleus (Figure 3) did not exhibit the response (Figure 4; see Supplemental Table 1 online). More importantly, involvement of the PHY domain in FR-HIR was demonstrated (Figure 4; see Supplemental Table 1 online). It is intriguing that deletion and amino acid substitutions in PHY result in faster dark reversions of Pfr to Pr (Oka et al., 2004, 2008). In addition, some amino acid substitutions in the PHY domain alter the sensitivity of phytochrome to light (Kretsch et al., 2000; Maloof et al., 2001; Ádám et al., 2011). Hence, the PHY domain might modify the activity of phytochrome, even though it is somewhat distant from the core N-PAS/GAF domains (Essen et al., 2008; Yang et al., 2008) (see below).
Although the association/dissociation of the phyA-FHY1/FHL complex and rapid degradation of phyA Pfr have been proposed to be important components of FR-HIR (Rausenberger et al., 2011), this result cannot be fully explained by this model. As already discussed above, nuclear localization of chimeric phytochromes with N-PAS phyA fit well with this model. Although AAB(A/B) accumulated in the nucleus under FR and degraded efficiently under R (Figures 3 and 5), they failed to show the hypocotyl FR-HIR (Figure 4). Hence, involvement of the PHY domain, which probably contributes to the response by increasing the sensitivity of phytochrome to light, should be considered to fully understand FR-HIR.
Biological Activities of Chimeric Phytochromes under R
Although phyA can mediate short-term responses to R, a long-term response is barely observed because of light-induced degradation (Parks and Spalding, 1999). Consistently, all of AA(A/B)(A/B), which were fully degraded under R (Figures 3 and 5), failed to show the hypocotyl response under R (Figure 6; see Supplemental Table 1 online). It is less clear why BA(A/B)A failed to exhibit the response. As discussed below, a combination of phyB N-PAS and phyA GAF might not function properly. It is worth noting that nuclear speckle formation, which is proposed to be a prerequisite for the response (Chen et al., 2003), was not observed in BA(A/B)A (Figure 3). However, some phyB fragments elicit intense physiological responses without forming speckles (Matsushita et al., 2003; Oka et al., 2004; Palágyi et al., 2010).
Interestingly, four out of six phytochromes that were stable under R and contained the phyA PHY sequence responded to R with much greater sensitivity than BBBB in regards to the inhibition of hypocotyl elongation (Figure 7; see Supplemental Table 1 online). This effect cannot be explained simply by the altered degradation rates of Pfr because the PHY sequence did not affect the stability in a consistent manner (Figure 5). Rather, phyA PHY appears to increase the intrinsic sensitivity of phytochrome. It is not clear why BAA(A/B) failed to respond to weak R (Figure 7). As mentioned above, the BA combination in parts 1 and 2 might not function properly.
R-Induced Degradation of phyA/phyB Chimeric Proteins
Similar to nuclear accumulation under continuous FR, N-PAS was important for light-dependent degradation (Figure 5; see Supplemental Table 1 online). Furthermore, the GAF domain was equally important in determining the stability under R. Consistent with this view, missense mutations that reduce the degradation rate of phyA under R have been found within these domains (Weller et al., 2004; Dieterle et al., 2005; Han et al., 2010). In addition, pairwise comparison revealed that the phyA sequence in the C-terminal moiety partially reduces stability in some cases (Figure 5B).
phyA is degraded by the proteasome pathway in the presence of light (Jabben et al., 1989a, 1989b). COP1 has been shown to bind to the C-terminal moiety of phytochromes to promote ubiquitination (Seo et al., 2004). This study demonstrated that AA(A/B)B is degraded as effectively as AA(A/B)A under R. Hence, the phyB C-terminal sequence should be recognized by COP1, which is consistent with the results of previous binding experiments (Yang et al., 2001; Seo et al., 2004). However, this view raises a question as to how only phyA is rapidly degraded. Specific ubiquitination sites may reside in the N-PAS and/or GAF domains in phyA that are degraded under R.
Structural Implications
Although the three-dimensional structure of the C-terminal moiety of phytochrome has not yet been determined, the entire N-terminal moieties encompassing the PAS, GAF, and PHY domains of bacterial phytochromes (Cph1 and BphP) have been determined (Wagner et al., 2005; Essen et al., 2008; Yang et al., 2008). In those structures, the GAF domain constitutes a binding pocket for the chromophore. Interestingly, an unusual light-sensing knot is formed between PAS and GAF (Wagner et al., 2005). Furthermore, a truncated form of phyB comprising only N-PAS and GAF is capable of transducing the signal in the nucleus (Oka et al., 2004; Palágyi et al., 2010). Accordingly, this structure has been proposed to be involved in the interaction of phytochrome with its signaling partners, the PIFs (Kikis et al., 2009).
With respect to this work, a question arises as to whether N-PAS and GAF domains derived from different phytochromes can form a functional light-sensing knot. Based on our results, the combination of the phyA N-terminal and the phyB GAF regions was fully functional. However, the opposite combination might be less functional because all four of the BA(A/B)(A/B) phytochromes, especially BA(A/B)A, exhibited weak or nonexistent responses to light in all experiments (see Supplemental Table 1 online). It would be important to examine their spectral activities and affinities to PIFs in future studies.
Interestingly, a tongue-like structure protrudes from the PHY domain to the vicinity of the chromophore pocket residing in the GAF domain in bacterial phytochromes (Essen et al., 2008; Yang et al., 2008) (see Supplemental Figure 12 online). The PHY and C-terminal moieties are not essential for signaling activity in the nucleus (Matsushita et al., 2003; Oka et al., 2004; Palágyi et al., 2010). Nevertheless, amino acid substitutions in the tongue-like structure alter the photosensitivity and/or Pfr stability of phytochrome (Kretsch et al., 2000; Maloof et al., 2001; Oka et al., 2008; Ádám et al., 2011). Hence, the difference in the tongue structure between phyA and phyB might be a key to understanding how the phyA PHY sequence mechanistically increases the sensitivity of phytochrome.
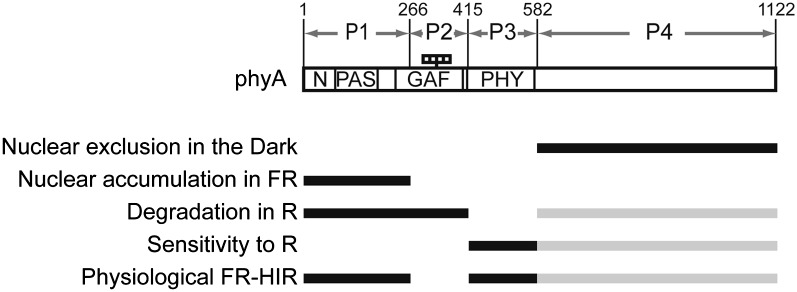
The Modular Structure of phyA
Our results illustrate that the structural requirements for distinct properties of phyA are separable (Figure 8). In fact, ABA(A/B) were stabilized under R, due to the phyB sequence in part 2 (GAF) (Figure 5). Consequently, a clear hypocotyl response was observed under R (see below) (Figure 6). Nevertheless, the phyA sequences in parts 1 (N-PAS) and 3 (PHY) enabled them to exhibit FR-HIR (Figure 4). Hence, we could design phytochromes that respond both to R and FR. Conversely, AAB(A/B) were physiologically incompetent because they responded neither to R nor FR due to their instability under R and lower light sensitivity. Hence, a few structural modules, each of which confers different properties, appear to be assembled on the phyA molecule to incorporate phyA-specific properties (Figure 8). It should be noted that the N-PAS region is responsible for both nuclear accumulation and degradation in our model (Figure 8). However, the determinants for those properties might be identified in distinct parts within this region in future work.
Figure 8.
The Structural Basis for Each phyA-Specific Function.
N, N-terminal extension (1 to 78); PAS, PAS domain (79 to 185); GAF, GAF domain (218 to 402); PHY, PHY domain (413 to 593). The four small rectangles in the figure indicate the chromophores. The most and second-most important components of each phyA-specific function are indicated by black and gray lines, respectively.
It is intriguing to find that a comparative analysis between divergent phyA and phyB sequences has revealed fundamental amino acid substitutions that distinguish phyA from phyB within the PAS, light-sensing knot, and tongue regions (Mathews, 2010; Nagatani, 2010). As discussed above, those regions are linked to distinct properties of phyA (Figure 8). Hence, these amino acid substitutions might have played crucial roles during the evolution of phyA, and this property should be experimentally tested in future studies.
METHODS
Plant Materials and Growth Conditions
The wild-type Arabidopsis thaliana used in this study was the Landsberg erecta accession. The mutants used were phyA-201 (Nagatani et al., 1993), phyB-5 (Reed et al., 1993), and the phyA-201 phyB-5 double mutant (Reed et al., 1994). The PAG line in the phyA-201 background and the PBG18 line in the phyB-5 background, in which phyA- and phyB-GFP were expressed under the control of the 35S promoter, respectively, have been described elsewhere (Yamaguchi et al., 1999; Toledo-Ortiz et al., 2010). The preparation of transgenic Arabidopsis expressing the phyA/phyB chimeric proteins is described below.
Seeds were surface sterilized and sown on 0.6% agar plates containing Murashige and Skoog (MS) medium. The plates were cultured in the dark at 4°C for 72 h and subsequently irradiated with continuous white light for 1 h at 22°C to induce seed germination. The plates were later placed under various light conditions as specified in the figure legends. For hypocotyl length measurements, the seedlings were grown on MS agar plates for 5 d at 22°C and were pressed gently onto the surface of agar medium before photographs were taken. Hypocotyl lengths were determined using NIH image software.
Plasmid Construction and Plant Transformation
To construct the phyA/phyB chimeric proteins, each PHYA fragment (part 1, 1 to 798; part 2, 799 to 1243; part 3, 1244 to 1746; and part 4, 1747 to 3366) and PHYB fragment (part 1, 1 to 900; part 2, 901 to 1338; part 3, 1339 to 1839; and part 4, 1840 to 3516) was amplified by PCR and cloned into the pTYB2 vector. To combine these fragments, StuI (the 3′ end of part 1 and the 5′ end of part 2), BsiWI (the 3′ end of part 2 and the 5′ end of part 3), AflII (the 3′ end of part 3 and the 5′ end of part 4), and ClaI (the 3′ end of part 4) restriction sites were introduced at the sites shown in parentheses without amino acid substitutions. The primer pairs for each part are as follows: for PHYA part 1, 5′-GGGGGATCCATGTCAGGCTCTAGGCCGACTCAGTCCTCT-3′ and 5′-TGCAGGCCTAGATAAGGCTCCAGCCCAGGT-3′; for PHYA part 2, 5′-TCTAGGCCTGCATTATCCTGCCACCGACAT-3′ and 5′-CTGCGTACGCAAAATGTTCTTCTCCACCAT-3′; for PHYA part 3, 5′-TTGCGTACGCAGACACTCTTGTGCGATATG-3′ and 5′-TTCCTTAAGATAAGTTGCAAGGAGTGTATG-3′; for PHYA part 4, 5′-TATCTTAAGGAATGCTTTCAAGGATAGTGA-3′ and 5′-CCCATCGATCTTGTTTGCTGCAGCGAGTTCCGCAGTGAT-3′; for PHYB part 1, 5′-GGGTCTAGAATGGTTTCCGGAGTCGGGGGTAGTGGCGGT-3′ and 5′-GCAGGCCTATATAAGGCTCTAAATCATCTC-3′; for PHYB part 2, 5′-TATAGGCCTGCATTATCCTGCTACTGATAT-3′ and 5′-CTGCGTACGCAAAACGCGTTTCTCTGACAT-3′; for PHYB part 3, 5′-TTGCGTACGCAGACACTGTTATGTGATATG-3′ and 5′-TCTCTTAAGATAAGCTGGAGCGAGTGAATC-3′; for PHYB part 4, 5′-TATCTTAAGAGACTCTTTTAAAGAATCTGA-3′ and 5′-CCCATCGATATATGGCATCATCAGCATCATGTCACCACT-3′.
Each part was reciprocally combined at the respective restriction sites on pTYB2 to construct the chimeric phytochromes. The phyB fragment in PBG (Matsushita et al., 2003) was replaced with chimeric phytochrome fragments at the 5′ XbaI and 3′ ClaI restriction sites. The fusion sequences of the chimeric phytochromes and GFP were inserted between the constitutive cauliflower mosaic virus 35S promoter and the nopaline synthase terminator of pPZP211/35S-nosT, which is derived from pPZP211 (Hajdukiewicz et al., 1994). The phyA-201 phyB-5 double mutant was used as the host and transformed using the Agrobacterium tumefaciens–mediated floral dip method (Clough and Bent, 1998). Transformed plants were selected on MS medium containing 25 mg/mL kanamycin and 166 mg/mL claforan (Hoechst).
Immunochemical Experiments
For immunoblot analysis, 3-d-old seedlings were grown in the dark or exposed to continuous R (5.5 μmol/m2/s) for 24 h at 22°C. Protein extraction, SDS-PAGE, protein blotting, and immunodetection were performed as described by Yamaguchi et al. (1999). The monoclonal antibodies used were mAA1 and mBA1, which are specific to phyA and phyB, respectively (Shinomura et al., 1996). Anti-GFP monoclonal antibody was obtained from Nacalai Tesque.
Light Sources
White light was obtained from fluorescent tubes (FLR40SW/M-B; Hitachi). Red light was produced by a combination of red fluorescent tubes (FL20S/R-F; National) and a 3-mm-thick, red plastic plate (Shinkolite A102; Mitsubishi). FR light was produced using a combination of fluorescent tubes (FL20S FR-74; Toshiba) and a 3-mm methacrylic plate (Dalaglass A-900; Asahi Chemical Industry). The fluence rates and spectral qualities were measured using an optical power meter (model LI-1000; Li-Cor) and a spectroradiometer (USR-40V; USHIO), respectively.
Microscopy Analysis
For microscopy, transgenic seedlings were grown on MS agar plates for 3 d at 22°C in the dark. All manipulation was performed under dim green light. For observation, seedlings were kept in the dark or exposed to continuous FR (18 μmol/m2/s) or R (5.5 μmol/m2/s). Samples were scanned only three times with a laser scanning microscope (FV300+BX60; Olympus) to eliminate any effect from the excitation laser light on the subcellular localization of the chimeric phytochromes. GFP fluorescence was observed using laser excitation at 543 nm. To quantitate fluorescence, Olympus Fluoview and NIH Image J software were used. Olympus images recorded at different photomultiplier tube settings were normalized with reference to a standard curve prepared with a standard sample.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: PHYA, At1g09570; and PHYB, At2g18790.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Immunoblot Detection of Endogenous phyA, Endogenous phyB, phyA-GFP (PAG and AAAA), and phyB-GFP (PBG and BBBB).
Supplemental Figure 2. The Relationship between Hypocotyl Length in the Dark and Protein Expression Levels.
Supplemental Figure 3. Nuclear Accumulation of Chimeric Phytochromes under cFR.
Supplemental Figure 4. Cotyledon Separation in the Dark.
Supplemental Figure 5. The Relationship between Hypocotyl Length under Continuous FR and Protein Expression Levels.
Supplemental Figure 6. Fluence Rate Response Curves for the Inhibition of Hypocotyl Elongation under cFR.
Supplemental Figure 7. Nuclear Accumulation of Chimeric Phytochromes under cR.
Supplemental Figure 8. The Relationship between Hypocotyl Length under R and Protein Expression Levels.
Supplemental Figure 9. The Relationship between Hypocotyl Length under Weak R and Protein Expression Levels.
Supplemental Figure 10. Fluence Rate Response Curves for the Inhibition of Hypocotyl Elongation under cR.
Supplemental Figure 11. Protein Levels of ABAA and ABAB Are Roughly Correlative with Those mRNA Levels in the Dark.
Supplemental Figure 12. Alignment of phyA, phyB, and Bacterial PHY Domain Sequences.
Supplemental Table 1. Summary of the Action of Chimeric Phytochromes.
Supplementary Material
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas (No. 17084002), a Grant-in-Aid for the Global Centers of Excellence Program “Formation of a strategic base for biodiversity and evolutionary research: from genome to ecosystem” (A06), a Grant-in-Aid for Scientific Research on Innovative Areas (No. 22120002) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and a Grant-in-Aid for Scientific Research (B) (No. 21370020) from the Japan Society for the Promotion of Science to A.N.
AUTHOR CONTRIBUTIONS
Y. Oka, Y. Ono, G.T.-O., N.M., and A.N. designed the research. Y. Oka, Y. Ono, G.T.-O., K.K., M.M., and A.N. performed the experiments. Y. Oka, Y. Ono, G.T.-O., and A.N. analyzed the data. Y. Oka and A.N. wrote the article.
Glossary
- R
red light
- FR
far-red light
- VLFR
very low fluence response
- FR-HIR
far-red high irradiance response
- GFP
green fluorescent protein
- MS
Murashige and Skoog
References
- Abe H., Takio K., Titani K., Furuya M. (1989). Amino-terminal amino acid sequences of pea phytochrome II fragments obtained by limited proteolysis. Plant Cell Physiol. 30: 1089–1097 [Google Scholar]
- Ádám E., Hussong A., Bindics J., Wüst F., Viczián A., Essing M., Medzihradszky M., Kircher S., Schäfer E., Nagy F. (2011). Altered dark- and photoconversion of phytochrome B mediate extreme light sensitivity and loss of photoreversibility of the phyB-401 mutant. PLoS ONE 6: e27250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sady B., Ni W., Kircher S., Schäfer E., Quail P.H. (2006). Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol. Cell 23: 439–446 [DOI] [PubMed] [Google Scholar]
- Bauer D., Viczián A., Kircher S., Nobis T., Nitschke R., Kunkel T., Panigrahi K.C., Adám E., Fejes E., Schäfer E., Nagy F. (2004). Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell 16: 1433–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan M.T., Quail P.H. (1991). Phytochrome a overexpression inhibits hypocotyl elongation in transgenic Arabidopsis. Proc. Natl. Acad. Sci. USA 88: 10806–10810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs W.R., Christie J.M., Salomon M. (2001). Phototropins: A new family of flavin-binding blue light receptors in plants. Antioxid. Redox Signal. 3: 775–788 [DOI] [PubMed] [Google Scholar]
- Cashmore A.R., Jarillo J.A., Wu Y.J., Liu D. (1999). Cryptochromes: Blue light receptors for plants and animals. Science 284: 760–765 [DOI] [PubMed] [Google Scholar]
- Chen M., Galvão R.M., Li M., Burger B., Bugea J., Bolado J., Chory J. (2010). Arabidopsis HEMERA/pTAC12 initiates photomorphogenesis by phytochromes. Cell 141: 1230–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Schwab R., Chory J. (2003). Characterization of the requirements for localization of phytochrome B to nuclear bodies. Proc. Natl. Acad. Sci. USA 100: 14493–14498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Tao Y., Lim J., Shaw A., Chory J. (2005). Regulation of phytochrome B nuclear localization through light-dependent unmasking of nuclear-localization signals. Curr. Biol. 15: 637–642 [DOI] [PubMed] [Google Scholar]
- Clack T., Mathews S., Sharrock R.A. (1994). The phytochrome apoprotein family in Arabidopsis is encoded by five genes: The sequences and expression of PHYD and PHYE. Plant Mol. Biol. 25: 413–427 [DOI] [PubMed] [Google Scholar]
- Clough R.C., Jordan-Beebe E.T., Lohman K.N., Marita J.M., Walker J.M., Gatz C., Vierstra R.D. (1999). Sequences within both the N- and C-terminal domains of phytochrome A are required for PFR ubiquitination and degradation. Plant J. 17: 155–167 [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Debrieux D., Fankhauser C. (2010). Light-induced degradation of phyA is promoted by transfer of the photoreceptor into the nucleus. Plant Mol. Biol. 73: 687–695 [DOI] [PubMed] [Google Scholar]
- Dieterle M., Bauer D., Büche C., Krenz M., Schäfer E., Kretsch T. (2005). A new type of mutation in phytochrome A causes enhanced light sensitivity and alters the degradation and subcellular partitioning of the photoreceptor. Plant J. 41: 146–161 [DOI] [PubMed] [Google Scholar]
- Essen L.O., Mailliet J., Hughes J. (2008). The structure of a complete phytochrome sensory module in the Pr ground state. Proc. Natl. Acad. Sci. USA 105: 14709–14714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoud T., Schweizer F., Tscheuschler A., Debrieux D., Casal J.J., Schäfer E., Hiltbrunner A., Fankhauser C. (2008). FHY1 mediates nuclear import of the light-activated phytochrome A photoreceptor. PLoS Genet. 4: e1000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdukiewicz P., Svab Z., Maliga P. (1994). The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 25: 989–994 [DOI] [PubMed] [Google Scholar]
- Han Y.J., Kim H.S., Kim Y.M., Shin A.Y., Lee S.S., Bhoo S.H., Song P.S., Kim J.I. (2010). Functional characterization of phytochrome autophosphorylation in plant light signaling. Plant Cell Physiol. 51: 596–609 [DOI] [PubMed] [Google Scholar]
- Hiltbrunner A., Tscheuschler A., Viczián A., Kunkel T., Kircher S., Schäfer E. (2006). FHY1 and FHL act together to mediate nuclear accumulation of the phytochrome A photoreceptor. Plant Cell Physiol. 47: 1023–1034 [DOI] [PubMed] [Google Scholar]
- Hiltbrunner A., Viczián A., Bury E., Tscheuschler A., Kircher S., Tóth R., Honsberger A., Nagy F., Fankhauser C., Schäfer E. (2005). Nuclear accumulation of the phytochrome A photoreceptor requires FHY1. Curr. Biol. 15: 2125–2130 [DOI] [PubMed] [Google Scholar]
- Hisada A., Hanzawa H., Weller J.L., Nagatani A., Reid J.B., Furuya M. (2000). Light-induced nuclear translocation of endogenous pea phytochrome A visualized by immunocytochemical procedures. Plant Cell 12: 1063–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq E., Al-Sady B., Hudson M., Kim C., Apel K., Quail P.H. (2004). Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science 305: 1937–1941 [DOI] [PubMed] [Google Scholar]
- Huq E., Al-Sady B., Quail P.H. (2003). Nuclear translocation of the photoreceptor phytochrome B is necessary for its biological function in seedling photomorphogenesis. Plant J. 35: 660–664 [DOI] [PubMed] [Google Scholar]
- Huq E., Quail P.H. (2002). PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J. 21: 2441–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabben M., Shanklin J., Vierstra R.D. (1989a). Red light-induced accumulation of ubiquitin-phytochrome conjugates in both monocots and dicots. Plant Physiol. 90: 380–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabben M., Shanklin J., Vierstra R.D. (1989b). Ubiquitin-phytochrome conjugates. Pool dynamics during in vivo phytochrome degradation. J. Biol. Chem. 264: 4998–5005 [PubMed] [Google Scholar]
- Jang I.C., Henriques R., Seo H.S., Nagatani A., Chua N.H. (2010). Arabidopsis PHYTOCHROME INTERACTING FACTOR proteins promote phytochrome B polyubiquitination by COP1 E3 ligase in the nucleus. Plant Cell 22: 2370–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kami C., Lorrain S., Hornitschek P., Fankhauser C. (2010). Light-regulated plant growth and development. Curr. Top. Dev. Biol. 91: 29–66 [DOI] [PubMed] [Google Scholar]
- Khanna R., Huq E., Kikis E.A., Al-Sady B., Lanzatella C., Quail P.H. (2004). A novel molecular recognition motif necessary for targeting photoactivated phytochrome signaling to specific basic helix-loop-helix transcription factors. Plant Cell 16: 3033–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikis E.A., Oka Y., Hudson M.E., Nagatani A., Quail P.H. (2009). Residues clustered in the light-sensing knot of phytochrome B are necessary for conformer-specific binding to signaling partner PIF3. PLoS Genet. 5: e1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim L., Kircher S., Toth R., Adam E., Schäfer E., Nagy F. (2000). Light-induced nuclear import of phytochrome-A:GFP fusion proteins is differentially regulated in transgenic tobacco and Arabidopsis. Plant J. 22: 125–133 [DOI] [PubMed] [Google Scholar]
- Kircher S., Gil P., Kozma-Bognár L., Fejes E., Speth V., Husselstein-Muller T., Bauer D., Adám E., Schäfer E., Nagy F. (2002). Nucleocytoplasmic partitioning of the plant photoreceptors phytochrome A, B, C, D, and E is regulated differentially by light and exhibits a diurnal rhythm. Plant Cell 14: 1541–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher S., Kozma-Bognar L., Kim L., Adam E., Harter K., Schafer E., Nagy F. (1999). Light quality-dependent nuclear import of the plant photoreceptors phytochrome A and B. Plant Cell 11: 1445–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretsch T., Poppe C., Schäfer E. (2000). A new type of mutation in the plant photoreceptor phytochrome B causes loss of photoreversibility and an extremely enhanced light sensitivity. Plant J. 22: 177–186 [DOI] [PubMed] [Google Scholar]
- Leivar P., Monte E., Al-Sady B., Carle C., Storer A., Alonso J.M., Ecker J.R., Quail P.H. (2008a). The Arabidopsis phytochrome-interacting factor PIF7, together with PIF3 and PIF4, regulates responses to prolonged red light by modulating phyB levels. Plant Cell 20: 337–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Monte E., Oka Y., Liu T., Carle C., Castillon A., Huq E., Quail P.H. (2008b). Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr. Biol. 18: 1815–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Tepperman J.M., Monte E., Calderon R.H., Liu T.L., Quail P.H. (2009). Definition of early transcriptional circuitry involved in light-induced reversal of PIF-imposed repression of photomorphogenesis in young Arabidopsis seedlings. Plant Cell 21: 3535–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain S., Allen T., Duek P.D., Whitelam G.C., Fankhauser C. (2008). Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 53: 312–323 [DOI] [PubMed] [Google Scholar]
- Maloof J.N., Borevitz J.O., Dabi T., Lutes J., Nehring R.B., Redfern J.L., Trainer G.T., Wilson J.M., Asami T., Berry C.C., Weigel D., Chory J. (2001). Natural variation in light sensitivity of Arabidopsis. Nat. Genet. 29: 441–446 [DOI] [PubMed] [Google Scholar]
- Mancinelli A.L. (1994). The physiology of phytochrome action. In Photomorphogenesis in Higher Plants, 2nd ed, R.E. Kendrick and G.H.M. Kronenberg, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 211–269
- Mathews S. (2006). Phytochrome-mediated development in land plants: Red light sensing evolves to meet the challenges of changing light environments. Mol. Ecol. 15: 3483–3503 [DOI] [PubMed] [Google Scholar]
- Mathews S. (2010). Evolutionary studies illuminate the structural-functional model of plant phytochromes. Plant Cell 22: 4–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita T., Mochizuki N., Nagatani A. (2003). Dimers of the N-terminal domain of phytochrome B are functional in the nucleus. Nature 424: 571–574 [DOI] [PubMed] [Google Scholar]
- Montgomery B.L., Lagarias J.C. (2002). Phytochrome ancestry: Sensors of bilins and light. Trends Plant Sci. 7: 357–366 [DOI] [PubMed] [Google Scholar]
- Müller R., Fernández A.P., Hiltbrunner A., Schäfer E., Kretsch T. (2009). The histidine kinase-related domain of Arabidopsis phytochrome a controls the spectral sensitivity and the subcellular distribution of the photoreceptor. Plant Physiol. 150: 1297–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani A. (2010). Phytochrome: Structural basis for its functions. Curr. Opin. Plant Biol. 13: 565–570 [DOI] [PubMed] [Google Scholar]
- Nagatani A., Reed J.W., Chory J. (1993). Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 102: 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff M.M., Fankhauser C., Chory J. (2000). Light: An indicator of time and place. Genes Dev. 14: 257–271 [PubMed] [Google Scholar]
- Ni M., Tepperman J.M., Quail P.H. (1998). PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 95: 657–667 [DOI] [PubMed] [Google Scholar]
- Ni M., Tepperman J.M., Quail P.H. (1999). Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature 400: 781–784 [DOI] [PubMed] [Google Scholar]
- Oh E., Kang H., Yamaguchi S., Park J., Lee D., Kamiya Y., Choi G. (2009). Genome-wide analysis of genes targeted by PHYTOCHROME INTERACTING FACTOR 3-LIKE5 during seed germination in Arabidopsis. Plant Cell 21: 403–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E., Yamaguchi S., Hu J., Yusuke J., Jung B., Paik I., Lee H.S., Sun T.P., Kamiya Y., Choi G. (2007). PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 19: 1192–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E., Yamaguchi S., Kamiya Y., Bae G., Chung W.I., Choi G. (2006). Light activates the degradation of PIL5 protein to promote seed germination through gibberellin in Arabidopsis. Plant J. 47: 124–139 [DOI] [PubMed] [Google Scholar]
- Oka Y., Matsushita T., Mochizuki N., Suzuki T., Tokutomi S., Nagatani A. (2004). Functional analysis of a 450-amino acid N-terminal fragment of phytochrome B in Arabidopsis. Plant Cell 16: 2104–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y., Matsushita T., Mochizuki N., Quail P.H., Nagatani A. (2008). Mutant screen distinguishes between residues necessary for light-signal perception and signal transfer by phytochrome B. PLoS Genet. 4: e1000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palágyi A., Terecskei K., Adám E., Kevei E., Kircher S., Mérai Z., Schäfer E., Nagy F., Kozma-Bognár L. (2010). Functional analysis of amino-terminal domains of the photoreceptor phytochrome B. Plant Physiol. 153: 1834–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E., Kim J., Lee Y., Shin J., Oh E., Chung W.I., Liu J.R., Choi G. (2004). Degradation of phytochrome interacting factor 3 in phytochrome-mediated light signaling. Plant Cell Physiol. 45: 968–975 [DOI] [PubMed] [Google Scholar]
- Parks B.M., Quail P.H. (1993). hy8, a new class of arabidopsis long hypocotyl mutants deficient in functional phytochrome A. Plant Cell 5: 39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks B.M., Spalding E.P. (1999). Sequential and coordinated action of phytochromes A and B during Arabidopsis stem growth revealed by kinetic analysis. Proc. Natl. Acad. Sci. USA 96: 14142–14146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A., Kunkel T., Hiltbrunner A., Neuhaus G., Wolf I., Speth V., Adam E., Nagy F., Schäfer E. (2009). A cell-free system for light-dependent nuclear import of phytochrome. Plant J. 57: 680–689 [DOI] [PubMed] [Google Scholar]
- Quail P.H., Boylan M.T., Parks B.M., Short T.W., Xu Y., Wagner D. (1995). Phytochromes: Photosensory perception and signal transduction. Science 268: 675–680 [DOI] [PubMed] [Google Scholar]
- Rausenberger J., Tscheuschler A., Nordmeier W., Wüst F., Timmer J., Schäfer E., Fleck C., Hiltbrunner A. (2011). Photoconversion and nuclear trafficking cycles determine phytochrome A’s response profile to far-red light. Cell 146: 813–825 [DOI] [PubMed] [Google Scholar]
- Reed J.W., Nagatani A., Elich T.D., Fagan M., Chory J. (1994). Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 104: 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J.W., Nagpal P., Poole D.S., Furuya M., Chory J. (1993). Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5: 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösler J., Klein I., Zeidler M. (2007). Arabidopsis fhl/fhy1 double mutant reveals a distinct cytoplasmic action of phytochrome A. Proc. Natl. Acad. Sci. USA 104: 10737–10742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H.S., Watanabe E., Tokutomi S., Nagatani A., Chua N.H. (2004). Photoreceptor ubiquitination by COP1 E3 ligase desensitizes phytochrome A signaling. Genes Dev. 18: 617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock R.A., Clack T. (2002). Patterns of expression and normalized levels of the five Arabidopsis phytochromes. Plant Physiol. 130: 442–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock R.A., Quail P.H. (1989). Novel phytochrome sequences in Arabidopsis thaliana: Structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 3: 1745–1757 [DOI] [PubMed] [Google Scholar]
- Shen H., Zhu L., Castillon A., Majee M., Downie B., Huq E. (2008). Light-induced phosphorylation and degradation of the negative regulator PHYTOCHROME-INTERACTING FACTOR1 from Arabidopsis depend upon its direct physical interactions with photoactivated phytochromes. Plant Cell 20: 1586–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Khanna R., Carle C.M., Quail P.H. (2007). Phytochrome induces rapid PIF5 phosphorylation and degradation in response to red-light activation. Plant Physiol. 145: 1043–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Kim K., Kang H., Zulfugarov I.S., Bae G., Lee C.H., Lee D., Choi G. (2009). Phytochromes promote seedling light responses by inhibiting four negatively-acting phytochrome-interacting factors. Proc. Natl. Acad. Sci. USA 106: 7660–7665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Park E., Choi G. (2007). PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J. 49: 981–994 [DOI] [PubMed] [Google Scholar]
- Shinomura T., Nagatani A., Hanzawa H., Kubota M., Watanabe M., Furuya M. (1996). Action spectra for phytochrome A- and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 93: 8129–8133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova V., Bindics J., Kircher S., Ádám E., Schäfer E., Nagy F., Viczián A. (2012). Missense mutation in the amino terminus of phytochrome A disrupts the nuclear import of the photoreceptor. Plant Physiol. 158: 107–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. (2000). Phytochromes and light signal perception by plants—An emerging synthesis. Nature 407: 585–591 [DOI] [PubMed] [Google Scholar]
- Su Y.S., Lagarias J.C. (2007). Light-independent phytochrome signaling mediated by dominant GAF domain tyrosine mutants of Arabidopsis phytochromes in transgenic plants. Plant Cell 19: 2124–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepperman J.M., Hudson M.E., Khanna R., Zhu T., Chang S.H., Wang X., Quail P.H. (2004). Expression profiling of phyB mutant demonstrates substantial contribution of other phytochromes to red-light-regulated gene expression during seedling de-etiolation. Plant J. 38: 725–739 [DOI] [PubMed] [Google Scholar]
- Tepperman J.M., Hwang Y.S., Quail P.H. (2006). phyA dominates in transduction of red-light signals to rapidly responding genes at the initiation of Arabidopsis seedling de-etiolation. Plant J. 48: 728–742 [DOI] [PubMed] [Google Scholar]
- Tepperman J.M., Zhu T., Chang H.S., Wang X., Quail P.H. (2001). Multiple transcription-factor genes are early targets of phytochrome A signaling. Proc. Natl. Acad. Sci. USA 98: 9437–9442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Ortiz G., Kiryu Y., Kobayashi J., Oka Y., Kim Y., Nam H.G., Mochizuki N., Nagatani A. (2010). Subcellular sites of the signal transduction and degradation of phytochrome A. Plant Cell Physiol. 51: 1648–1660 [DOI] [PubMed] [Google Scholar]
- Viczián A., Ádám E., Wolf I., Bindics J., Kircher S., Heijde M., Ulm R., Schäfer E., Nagy F. (2012). A short amino-terminal part of Arabidopsis phytochrome a induces constitutive photomorphogenic response. Mol. Plant 5: 629–641 [DOI] [PubMed] [Google Scholar]
- Vierstra R.D. (1994). Phytochrome degradation. In Photomorphogenesis in Higher Plants, 2nd ed, R.E. Kendrick and G.H.M. Kronenberg, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 141–162
- Wagner D., Fairchild C.D., Kuhn R.M., Quail P.H. (1996a). Chromophore-bearing NH2-terminal domains of phytochromes A and B determine their photosensory specificity and differential light lability. Proc. Natl. Acad. Sci. USA 93: 4011–4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D., Koloszvari M., Quail P.H. (1996b). Two small spatially distinct regions of phytochrome B are required for efficient signaling rates. Plant Cell 8: 859–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J.R., Brunzelle J.S., Forest K.T., Vierstra R.D. (2005). A light-sensing knot revealed by the structure of the chromophore-binding domain of phytochrome. Nature 438: 325–331 [DOI] [PubMed] [Google Scholar]
- Weller J.L., Batge S.L., Smith J.J., Kerckhoffs L.H., Sineshchekov V.A., Murfet I.C., Reid J.B. (2004). A dominant mutation in the pea PHYA gene confers enhanced responses to light and impairs the light-dependent degradation of phytochrome A. Plant Physiol. 135: 2186–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam G.C., Johnson E., Peng J., Carol P., Anderson M.L., Cowl J.S., Harberd N.P. (1993). Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell 5: 757–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf I., Kircher S., Fejes E., Kozma-Bognár L., Schäfer E., Nagy F., Adám E. (2011). Light-regulated nuclear import and degradation of Arabidopsis phytochrome-A N-terminal fragments. Plant Cell Physiol. 52: 361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi R., Nakamura M., Mochizuki N., Kay S.A., Nagatani A. (1999). Light-dependent translocation of a phytochrome B-GFP fusion protein to the nucleus in transgenic Arabidopsis. J. Cell Biol. 145: 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.Q., Tang R.H., Cashmore A.R. (2001). The signaling mechanism of Arabidopsis CRY1 involves direct interaction with COP1. Plant Cell 13: 2573–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Kuk J., Moffat K. (2008). Crystal structure of Pseudomonas aeruginosa bacteriophytochrome: Photoconversion and signal transduction. Proc. Natl. Acad. Sci. USA 105: 14715–14720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Stojkovic E.A., Kuk J., Moffat K. (2007). Crystal structure of the chromophore binding domain of an unusual bacteriophytochrome, RpBphP3, reveals residues that modulate photoconversion. Proc. Natl. Acad. Sci. USA 104: 12571–12576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky M.J., Casal J.J., Whitelam G.C. (1995). Phytochrome A, phytochrome B and HY4 are involved in hypocotyl growth-responses to natural radiation in Arabidopsis: Weak de-etiolation of the phyA mutant under dense canopies. Plant Cell Environ. 18: 788–794 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.