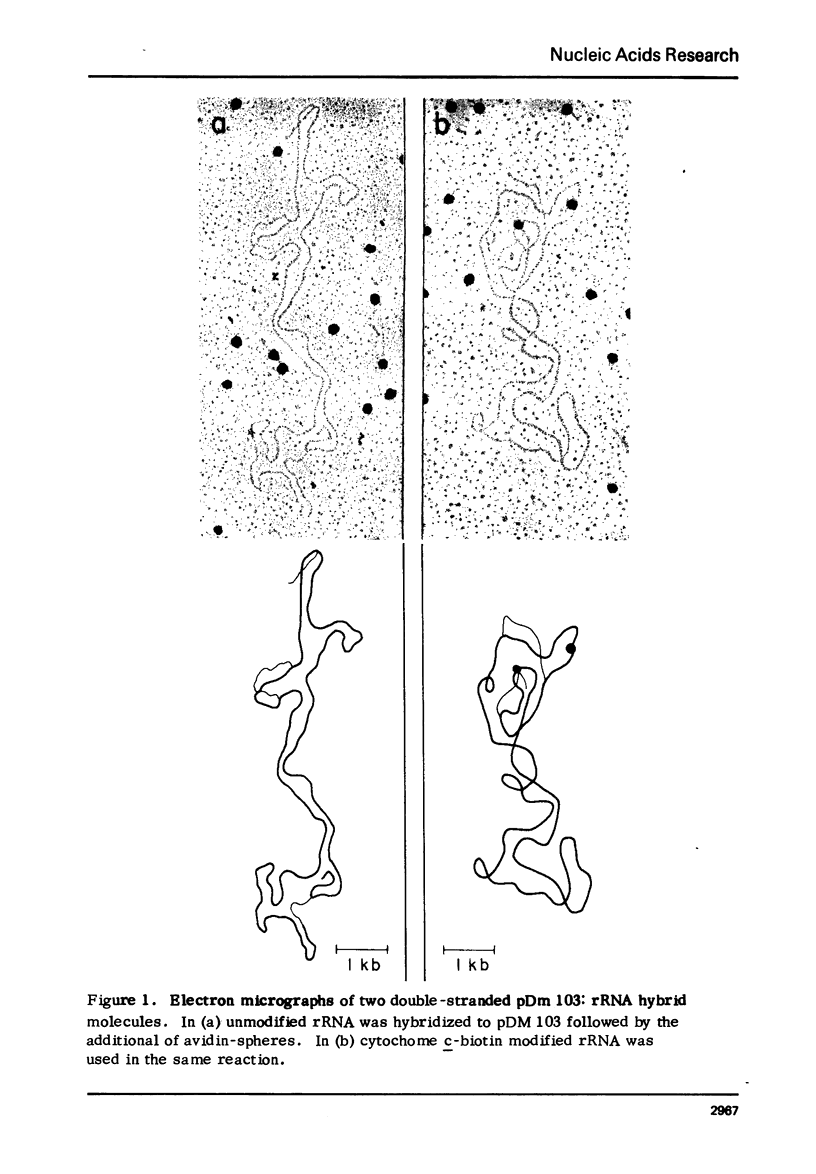
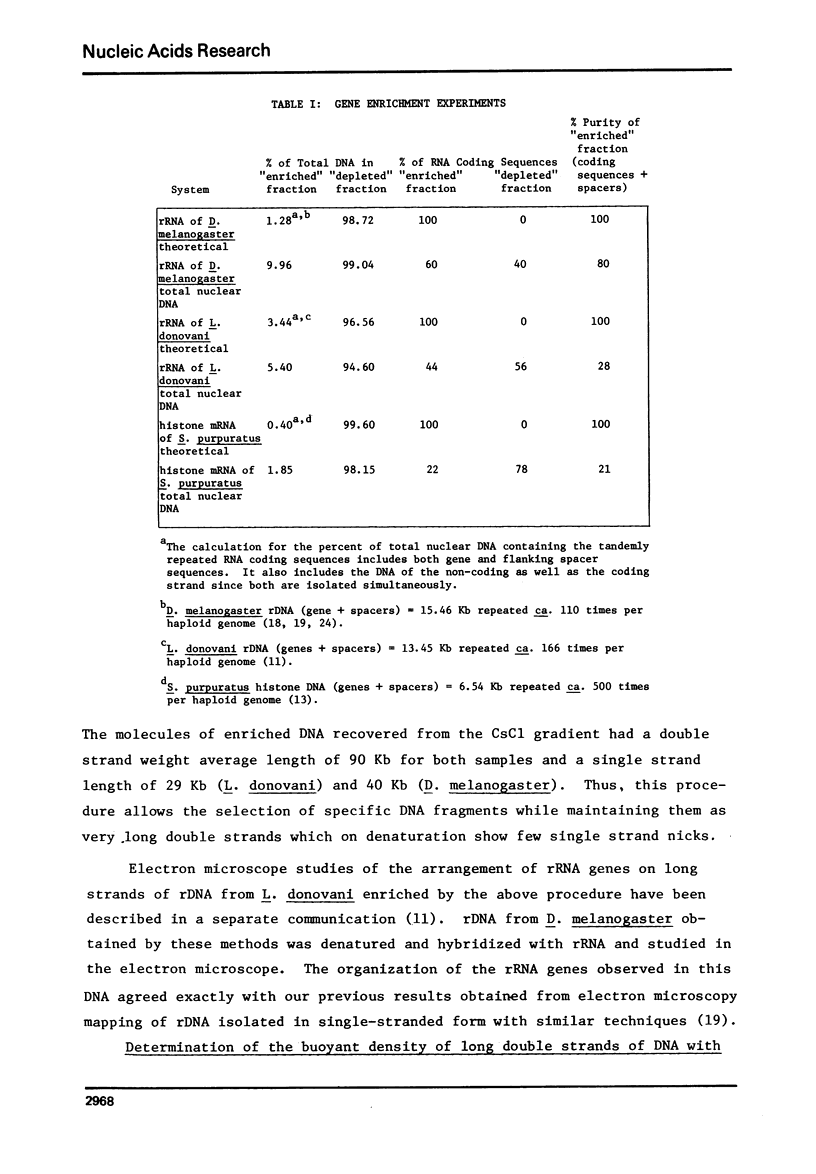
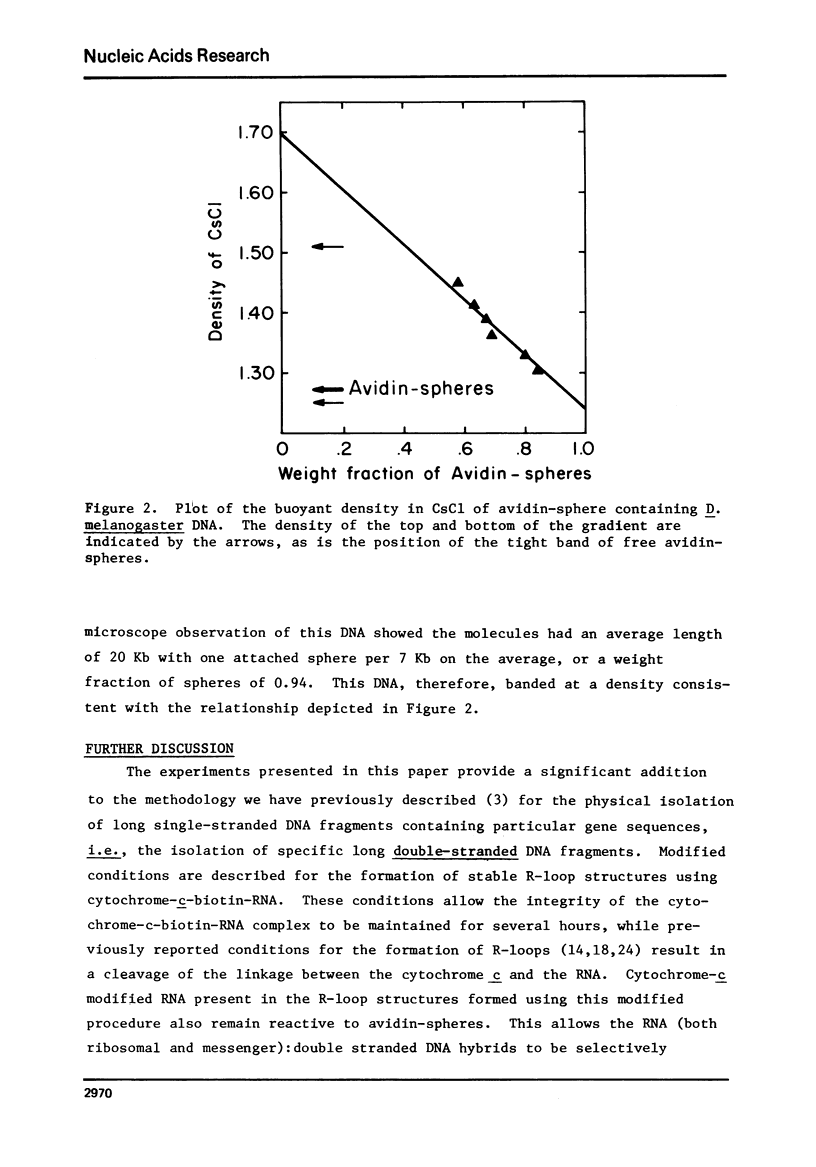
Abstract
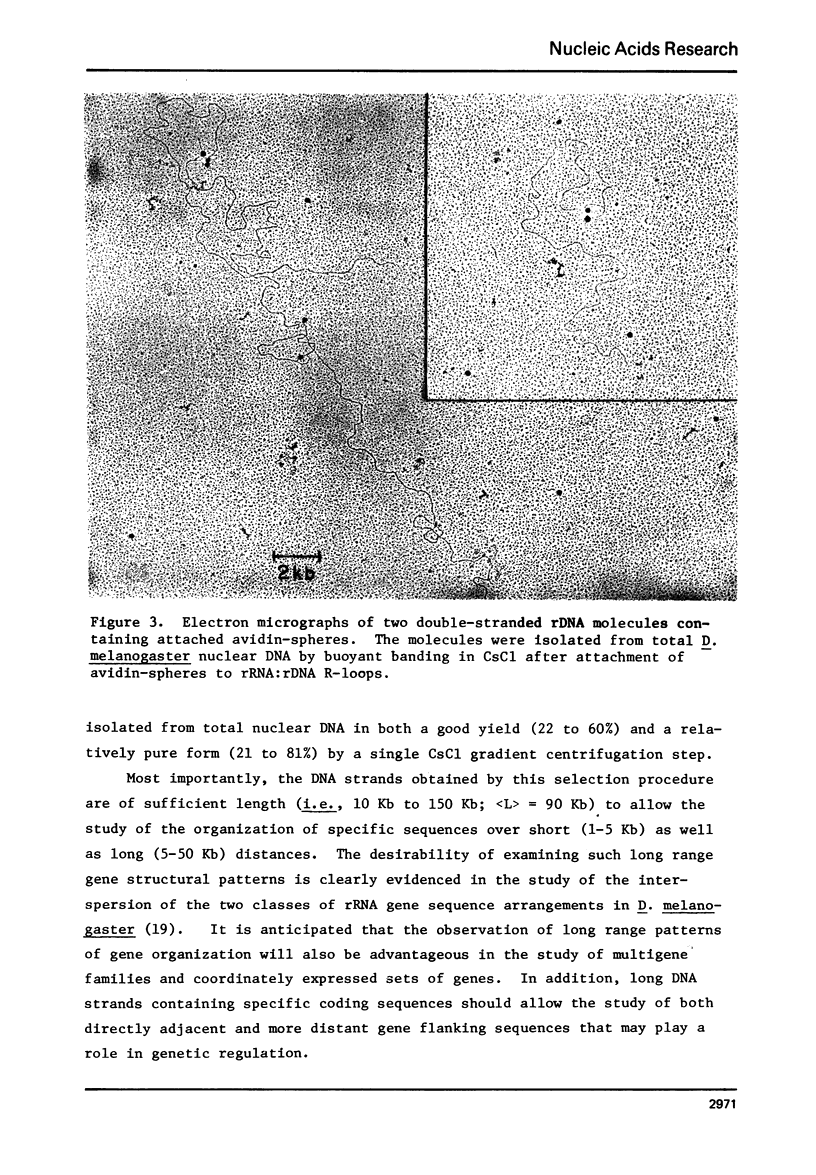
A method of enriching for long double-stranded segments of eukaryotic DNA carrying particular genes is described. A purified RNA coded for by the gene is covalently attached to biotin via the protein, cytochrome c. This modified RNA is hybridized to total nuclear, double-stranded DNA under conditions that allow the formation of R-loops. Avidin, which has a high affinity for biotin, is covalently attached to polymer spheres. The complexes of avidin-spheres with DNA:RNA-biotin R-loop hybrids band in CsCl at a much lower bouyant density than does free DNA. This density is a function of the length of DNA coupled per avidin-sphere. This method was used to prepare very long double-strands of DNA highly enriched in the coding sequences for the large rRNAs of D. melanogaster and L. donovani and the histone mRNAs of S. purpuratus.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnstiel M., Speirs J., Purdom I., Jones K., Loening U. E. Properties and composition of the isolated ribosomal DNA satellite of Xenopus laevis. Nature. 1968 Aug 3;219(5153):454–463. doi: 10.1038/219454a0. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Weber C. S. Gene linkage by RNA-DNA hybridization. I. Unique DNA sequences homologous to 4 s RNA, 5 s RNA and ribosomal RNA. J Mol Biol. 1968 Jun 28;34(3):661–680. doi: 10.1016/0022-2836(68)90188-5. [DOI] [PubMed] [Google Scholar]
- Fouts D. L., Manning J. E., Wolstenholme D. R. Physicochemical properties of kinetoplast DNA from Crithidia acanthocephali. Crithidia luciliae, and Trypanosoma lewisi. J Cell Biol. 1975 Nov;67(2PT1):378–399. doi: 10.1083/jcb.67.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover D. M., White R. L., Finnegan D. J., Hogness D. S. Characterization of six cloned DNAs from Drosophila melanogaster, including one that contains the genes for rRNA. Cell. 1975 Jun;5(2):149–157. doi: 10.1016/0092-8674(75)90023-9. [DOI] [PubMed] [Google Scholar]
- Kedes L. H., Chang A. C., Houseman D., Cohen S. N. Isolation of histone genes from unfractionated sea urchin DNA by subculture cloning in E. coli. Nature. 1975 Jun 12;255(5509):533–538. doi: 10.1038/255533a0. [DOI] [PubMed] [Google Scholar]
- Kedes L. H., Cohn R. H., Lowry J. C., Chang A. C., Cohen S. N. The organization of sea urchin histone genes. Cell. 1975 Nov;6(3):359–369. doi: 10.1016/0092-8674(75)90185-3. [DOI] [PubMed] [Google Scholar]
- Kourilsky P., Mercereau O., Gros D., Tremblay G. Hybridization of filters with competitor DNA in the liquid phase in a standard and a micro-assay. Biochimie. 1974;56(9):1215–1221. doi: 10.1016/s0300-9084(74)80014-3. [DOI] [PubMed] [Google Scholar]
- Manning J. E., Hershey N. D., Broker T. R., Pellegrini M., Mitchell H. K., Davidson N. A new method of in situ hybridization. Chromosoma. 1975 Nov 24;53(2):107–117. doi: 10.1007/BF00333039. [DOI] [PubMed] [Google Scholar]
- Manning J. E., Schmid C. W., Davidson N. Interspersion of repetitive and nonrepetitive DNA sequences in the Drosophila melanogaster genome. Cell. 1975 Feb;4(2):141–155. doi: 10.1016/0092-8674(75)90121-x. [DOI] [PubMed] [Google Scholar]
- Manning J., Pellegrini M., Davidson N. A method for gene enrichment based on the avidin-biotin interaction. Application to the Drosophila ribosomal RNA genes. Biochemistry. 1977 Apr 5;16(7):1364–1370. doi: 10.1021/bi00626a020. [DOI] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W., Vinograd J. EQUILIBRIUM SEDIMENTATION OF MACROMOLECULES IN DENSITY GRADIENTS. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):581–588. doi: 10.1073/pnas.43.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini M., Manning J., Davidson N. Sequence arrangement of the rDNA of Drosophila melanogaster. Cell. 1977 Feb;10(2):213–214. doi: 10.1016/0092-8674(77)90215-x. [DOI] [PubMed] [Google Scholar]
- Raleigh E. A., Davis R. W. Determination of DNA concentration by electron microscopy. Anal Biochem. 1976 May 7;72:460–467. doi: 10.1016/0003-2697(76)90554-6. [DOI] [PubMed] [Google Scholar]
- Serwer P. Buoyant density sedimentation of macromolecules in sodium iothalamate density gradients. J Mol Biol. 1975 Mar 5;92(3):433–448. doi: 10.1016/0022-2836(75)90290-9. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas M., White R. L., Davis R. W. Hybridization of RNA to double-stranded DNA: formation of R-loops. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2294–2298. doi: 10.1073/pnas.73.7.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. The structural organization of ribosomal DNA in Drosophila melanogaster. Cell. 1977 Feb;10(2):193–212. doi: 10.1016/0092-8674(77)90214-8. [DOI] [PubMed] [Google Scholar]
- White R. L., Hogness D. S. R loop mapping of the 18S and 28S sequences in the long and short repeating units of Drosophila melanogaster rDNA. Cell. 1977 Feb;10(2):177–192. doi: 10.1016/0092-8674(77)90213-6. [DOI] [PubMed] [Google Scholar]