Abstract
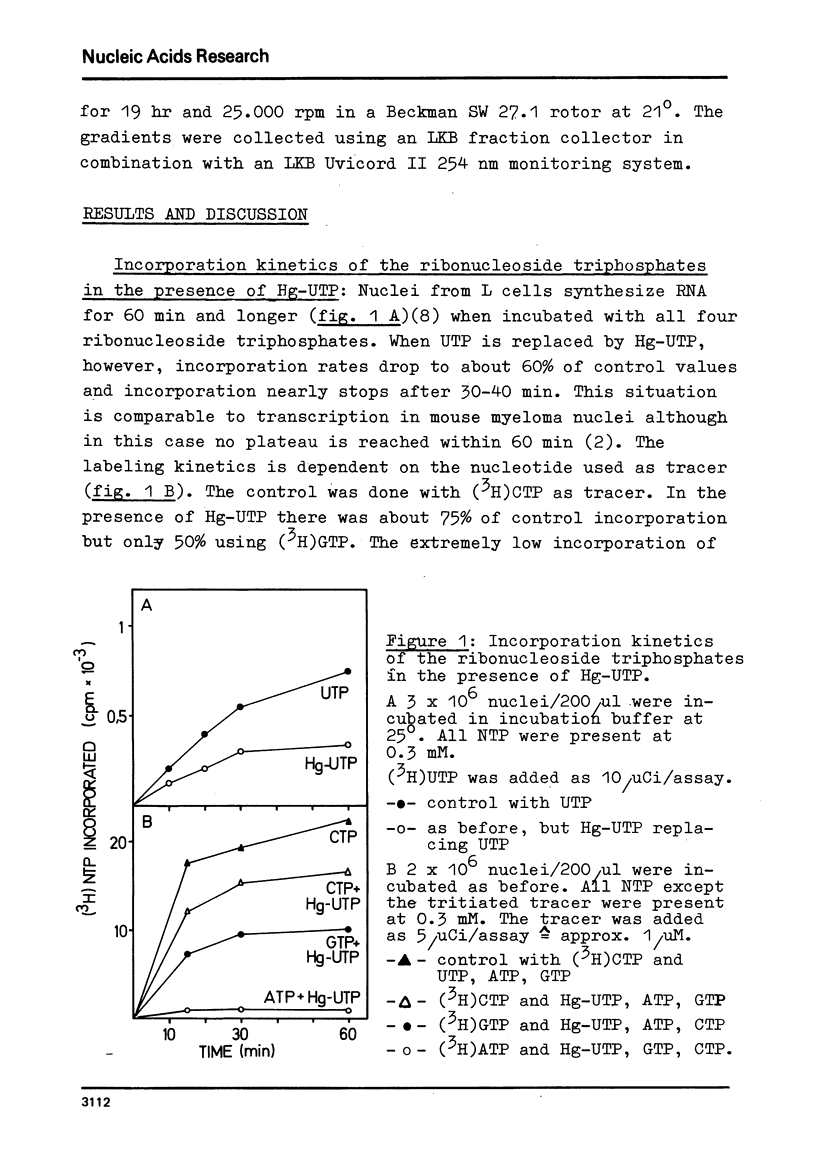
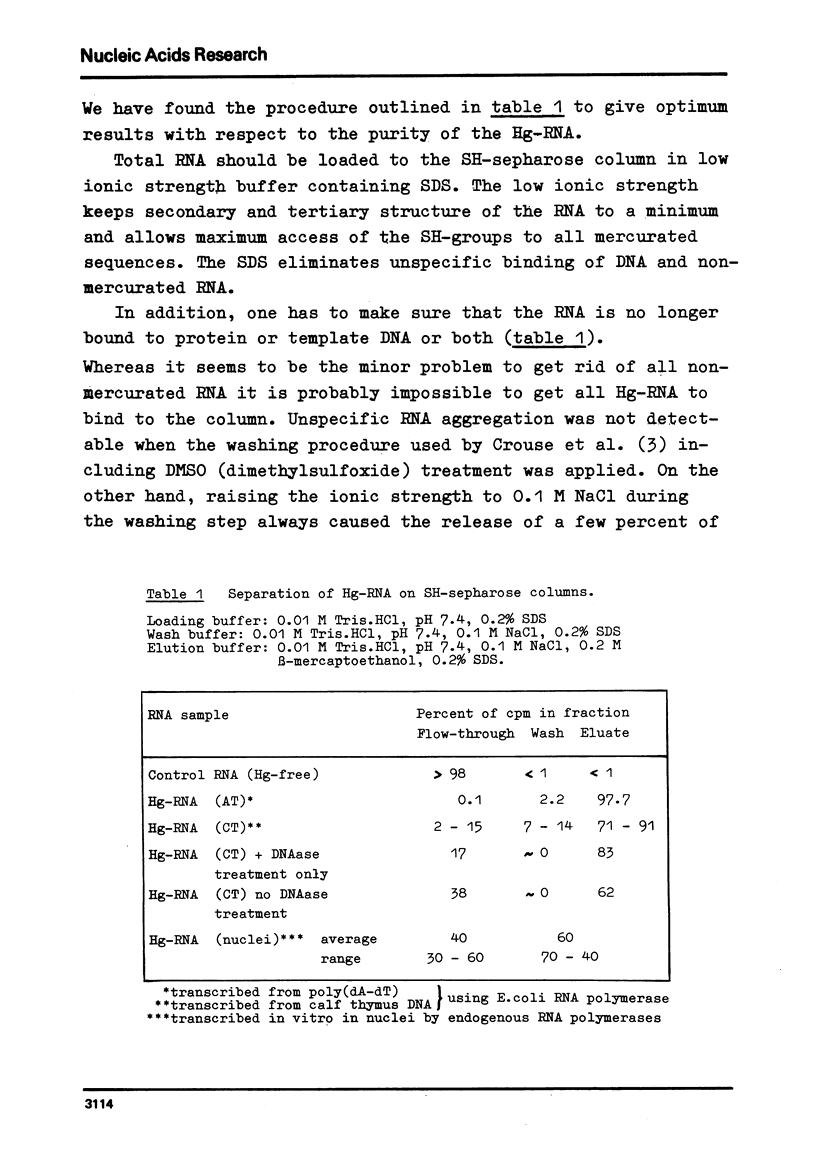
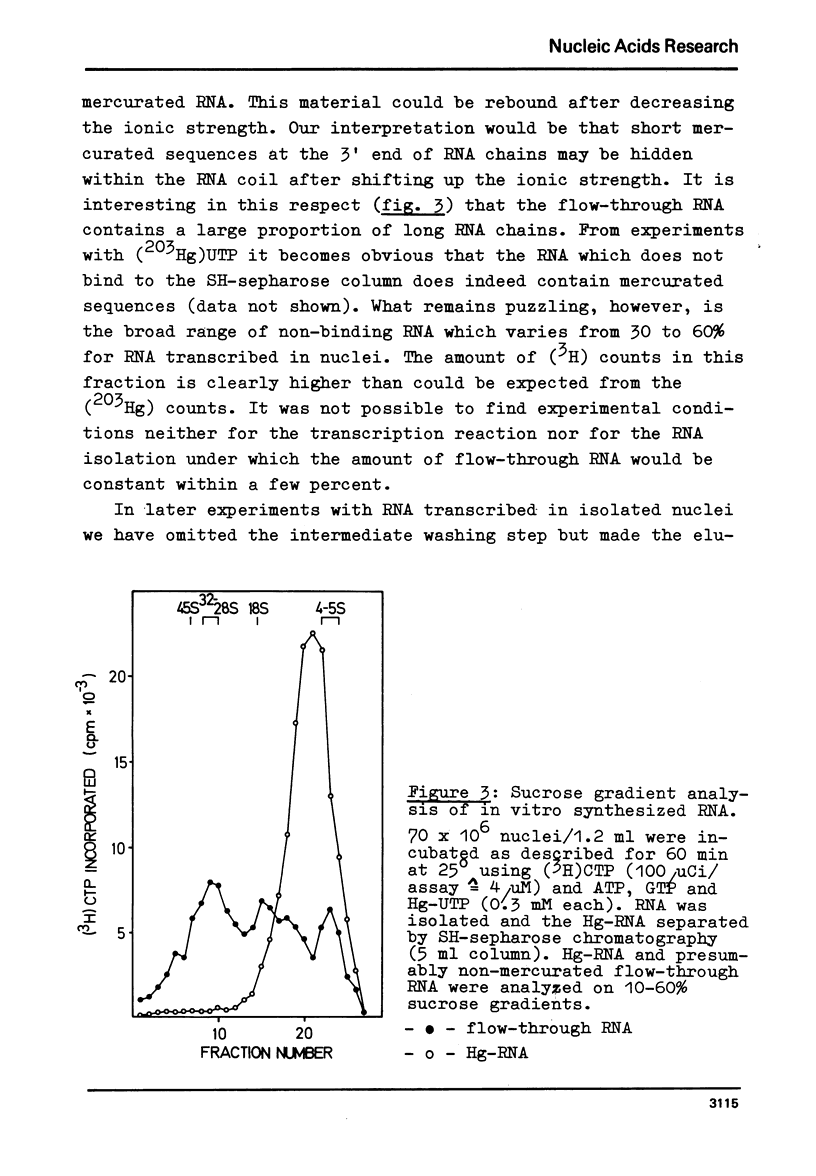
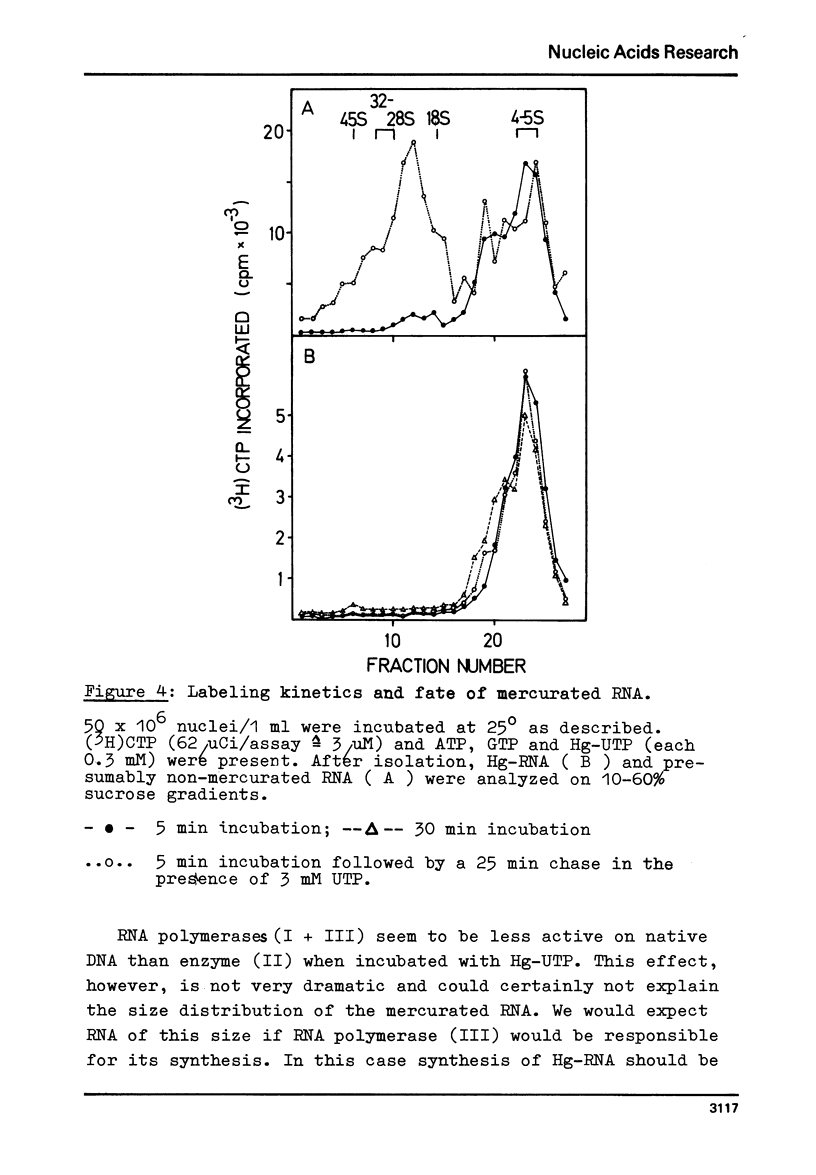
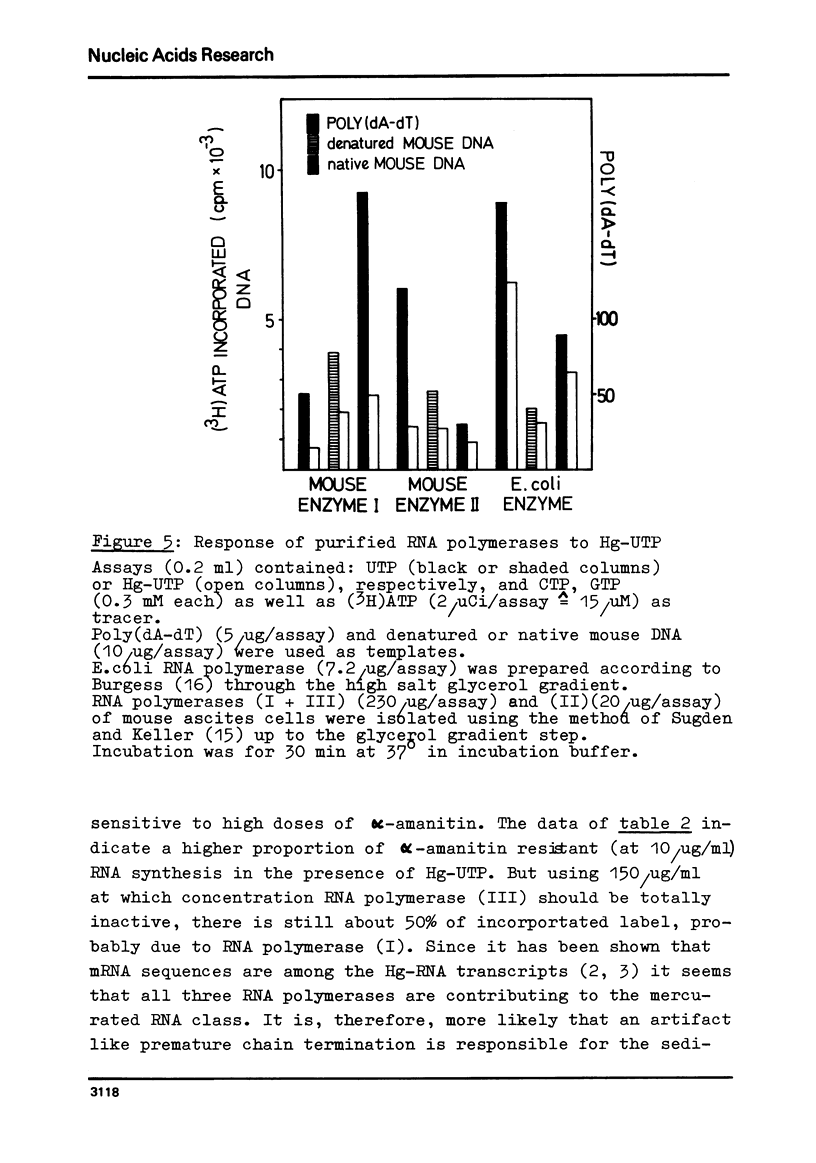
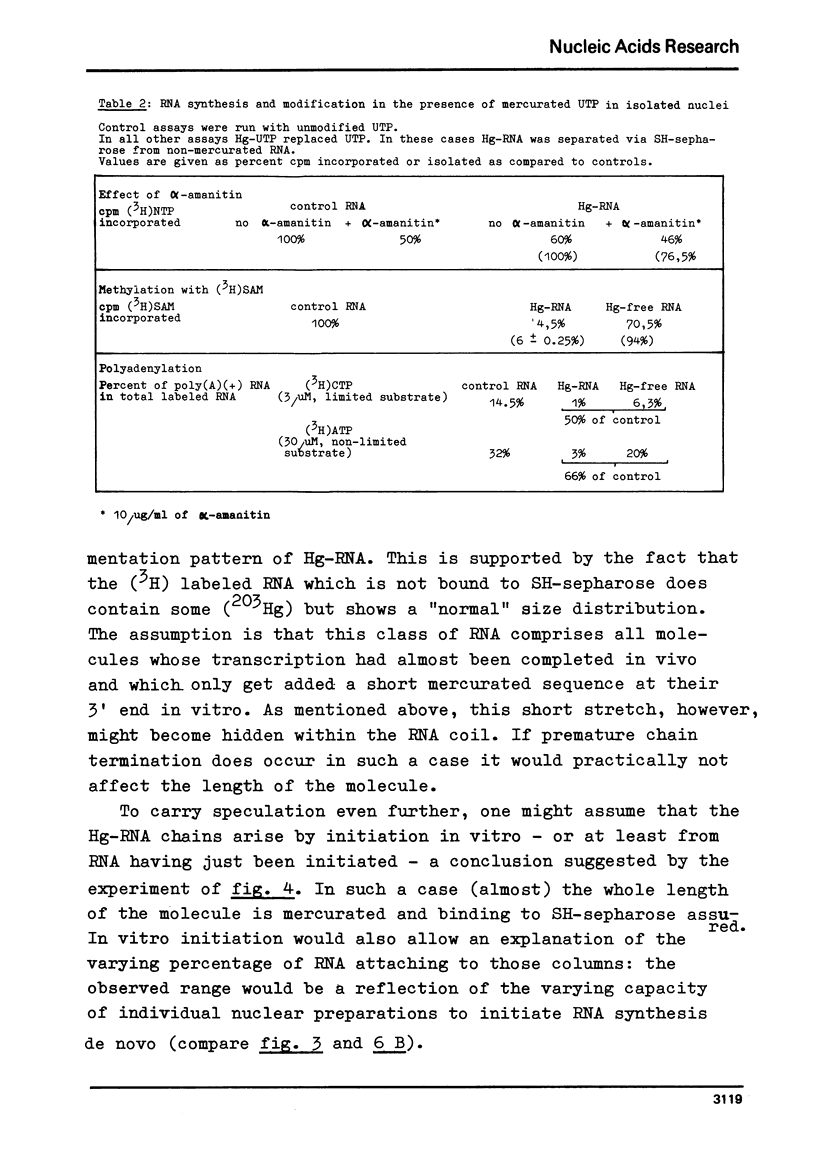
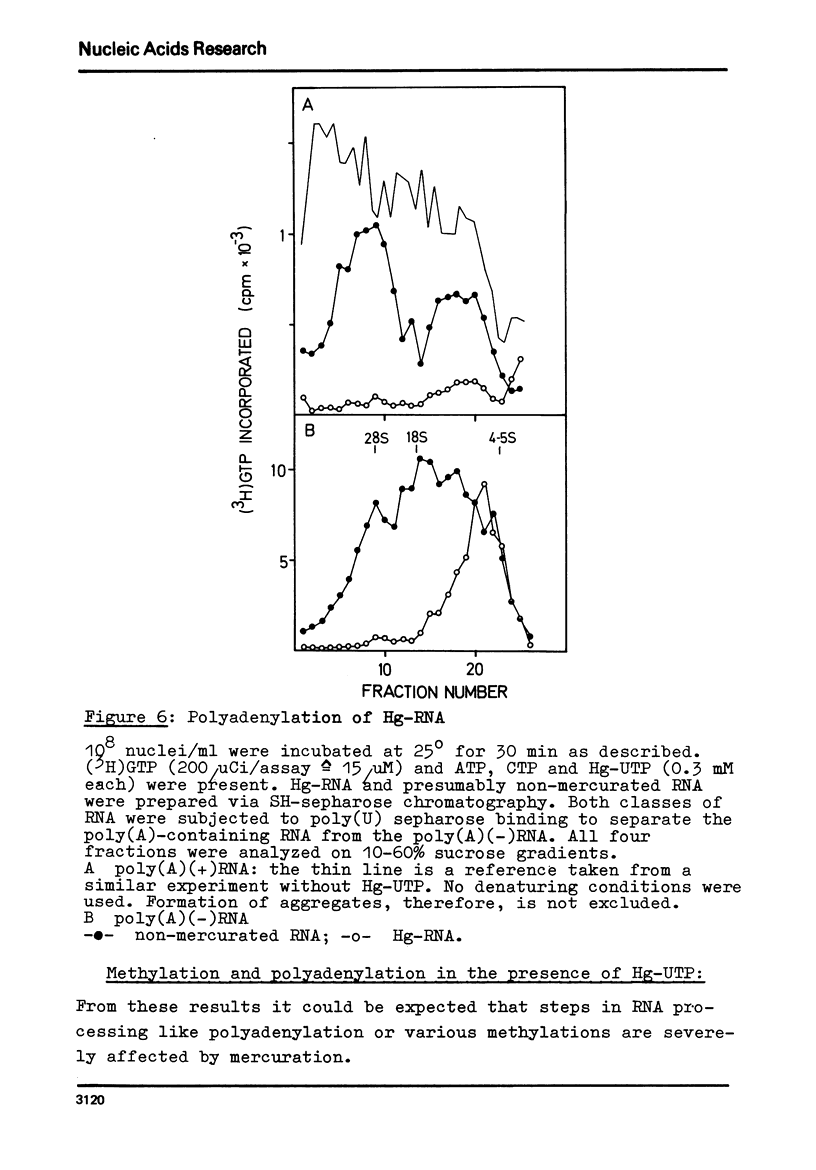
Mercurated pyrimidine nucleotides have been used to study RNA synthesis and processing in isolated nuclei from mouse L cells. 5-mercuridine triphosphate (5-Hg-UTP) or 5-Hg-CTP are accepted as substrates by the purified RNA polymerases (I + III) and (II) from mouse cells, respectively, as well as by the enzymes still bound to the nuclear chromatin. In nuclei, RNA synthesis in the presence of Hg-UTP is reduced to 60-70% of a control. 30-60% of RNA labeled in vitro with (3H)UTP in isolated nuclei is not retained on sulfhydryl sepharose columns. Sucrose gradient analysis reveals a size distribution of the non-bound RNA similar to non-mercurated control RNA. Hg-RNA is found in a single peak from 4-10S. Chase experiments indicate that this RNA is the original transcript. It is argued that Hg-nucleotides may cause premature chain termination. Methylation of RNA in vitro by S-adenosyl methionine ((3H)SAM) is reduced to 75% of controls in the presence of Hg-UTP. Only 6% of the methyl groups appear in Hg-RNA. Polyadenylation is reduced as well. 15% of poly(A) (+)RNA are found in control assays whereas only 1% of Hg-RNA carries a poly(A) end added in vitro. These results limit the use of mercurated nucleotides for studies of nuclear RNA synthesis and processing.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Crouse G. F., Fodor J. B., Doty P. In vitro transcription of chromatin in the presence of a mercurated nucleotide. Proc Natl Acad Sci U S A. 1976 May;73(5):1564–1567. doi: 10.1073/pnas.73.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Dale R. M., Livingston D. C., Ward D. C. The synthesis and enzymatic polymerization of nucleotides containing mercury: potential tools for nucleic acid sequencing and structural analysis. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2238–2242. doi: 10.1073/pnas.70.8.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., Martin E., Livingston D. C., Ward D. C. Direct covalent mercuration of nucleotides and polynucleotides. Biochemistry. 1975 Jun 3;14(11):2447–2457. doi: 10.1021/bi00682a027. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Ward D. C. Mercurated polynucleotides: new probes for hybridization and selective polymer fractionation. Biochemistry. 1975 Jun 3;14(11):2458–2469. doi: 10.1021/bi00682a028. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Groner Y., Hurwitz J. Synthesis of RNA containing a methylated blocked 5' terminus by HeLa nuclear homogenates. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2930–2934. doi: 10.1073/pnas.72.8.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W. R. Poly adenylic acid synthesis in vitro in isolated HeLa cell nuclei and whole cell homogenates,. Cell. 1974 Jul;2(3):197–204. doi: 10.1016/0092-8674(74)90094-4. [DOI] [PubMed] [Google Scholar]
- Perry R. P. Processing of RNA. Annu Rev Biochem. 1976;45:605–629. doi: 10.1146/annurev.bi.45.070176.003133. [DOI] [PubMed] [Google Scholar]
- Smith M. M., Huang R. C. Transcription in vitro of immunoglobulin kappa light chain genes in isolated mouse myeloma nuclei and chromatin. Proc Natl Acad Sci U S A. 1976 Mar;73(3):775–779. doi: 10.1073/pnas.73.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., Keller W. Mammalian deoxyribonucleic acid-dependent ribonucleic acid polymerases. I. Purification and properties of an -amanitin-sensitive ribonucleic acid polymerase and stimulatory factors from HeLa and KB cells. J Biol Chem. 1973 Jun 10;248(11):3777–3788. [PubMed] [Google Scholar]


