Abstract
Since allogeneic stem cell transplantation (SCT) represents an intensive curative treatment for high-risk malignancies, its failure to prevent relapse leaves few options for successful salvage treatment. While many patients have a high early mortality from relapse, some respond and have sustained remissions, and a minority has a second chance of cure with appropriate therapy. The prognosis for relapsed hematological malignancies after SCT depends on four factors: the time elapsed from SCT to relapse (with relapses occurring within 6 months having the worst prognosis), the disease type (with chronic leukemias and some lymphomas having a second possibility of cure with further treatment), the disease burden and site of relapse (with better treatment success if disease is treated early), and the conditions of the first transplant (with superior outcome for patients where there is an opportunity to increase either the alloimmune effect, the specificity of the antileukemia effect with targeted agents or the intensity of the conditioning in a second transplant). These features direct treatments toward either modified second transplants, chemotherapy, targeted antileukemia therapy, immunotherapy or palliative care.
Keywords: bone marrow transplant, donor lymphocyte infusion, graft-versus-leukemia, relapse, second transplant
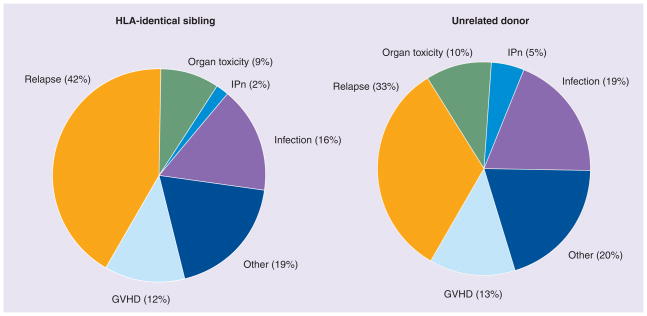
The last decade has witnessed a reduction in non-relapse mortality following allogeneic stem cell transplantation (SCT) for leukemia, because of a greater availability of suitable donors, modulation of conditioning regimen intensity and advances in supportive care. Relapse of the original malignancy after SCT now remains the most frequent cause of treatment failure and mortality. Approximately 40–45% of recipients of HLA-identical siblings and approximately 35% of recipients of unrelated donor transplants will relapse with their original malignancy (Figure 1) [1]. In the future, relapse is likely to become an even greater challenge, as improved nontransplant treatments make SCT increasingly reserved for the most stubborn malignancies, and SCT is extended using reduced-intensity regimens to older patients who tend to have more aggressive disease. The classical approach to controlling disease by SCT relied upon high-intensity conditioning and modulation of the balance between the linked graft-versus-host disease (GVHD)/graft-versus-malignancy (GVM) effect and relapse by varying T-lymphocyte immunosuppression. Current clinical approaches now de-emphasize the role of conditioning intensity, while exploiting selective T-lymphocyte-mediated GVM effects, administration of alloreactive natural killer (NK) cells, incorporation of noncytotoxic novel agents and maintenance strategies. These developments widen the options for treatment of relapse post-SCT, and may improve the prospect of survival. In this article, we list the mechanisms precipitating relapsed leukemia after SCT, characterize patterns and risk factors for relapse, and outline treatment approaches and results in specific relapse situations. Last, we review new treatment strategies, which should, in the future, both improve outcome for relapsed patients and further reduce the risk of relapse after SCT.
Figure 1. Causes of death after allogeneic stem cell transplantation reported to Center for International Blood and Marrow Transplant Research 2002–2007.
GVHD: Graft-versus-host disease; IPn: Interstitial pneumonitis.
Why do patients relapse after allogeneic SCT?
Allogeneic SCT is designed as a curative treatment for hematological malignancies (HMs), and is used in patients who are unlikely to achieve control of their disease with other less-intensive treatments. Since SCT is often the treatment of last resort, the diseases selected for treatment are more likely to be the most resistant to curative attempts. Indeed, a simple categorization of HMs at transplantation dichotomizes those with early disease (e.g., acute leukemia in first complete remission [CR1], myelodysplastic syndrome [MDS], intermediate-1 risk by International Prognostic Scoring Schema, and chronic myeloid leukemia [CML] in chronic phase [CP]) as standard risk (relapse rate of 20% or less). Remaining patients with more advanced disease, termed ‘high risk’, have a relapse rate of 40–80%. SCT is a platform that can bring two therapeutic modalities together to eradicate HMs – antileukemia treatment with chemotherapy, radiotherapy or other modalities (e.g., tyrosine kinase inhibitors [TKIs] and antibodies), and the powerful GVM or graft-versus-leukemia (GVL) effect of the allograft, mediated through donor-derived T cells, NK cells and possibly antibodies [2]. Disease relapse can occur early after transplant if the initial conditioning regimen is insufficient to bring the HM to a level at which a GVM effect can be brought in time to prevent recurrence, or because an effective GVM effect is never established. Relapse can also occur after a period of effective GVM if the immune system weakens or becomes tolerant to the residual disease, or the disease undergoes immune escape through clonal selection of immune-resistant progenitors. It should also be borne in mind that, occasionally, leukemia can recur in donor cells as a de novo event, masquerading as a relapse [3,4]. Mechanisms of relapse are derived largely from anecdotal accounts and small patient series.
Leukemia escape
Effective immunotherapy creates the setting for acquisition of somatic mutations that lead to immune evasion as the mechanism for tumor relapse [5]. A small study of six patients, where leukemia cryopreserved before SCT was available to be compared with leukemia at relapse, found a diversity of downregulation of costimulatory molecules and MHC class I, and acquired resistance to NK cell cytotoxicity [6]. More recently, Vago et al. studyed patients relapsing after haploidentical SCT, and found that, in five out of 17 relapses, the leukemia had deleted the mismatched MHC HLA class I and II haplotype, indicating an escape mechanism from the powerful cytotoxic effect of HLA-mismatched donor T cells [7]. A similar finding of acquired uniparental disomy on the short arm of chromosome 6 as a mechanism for leukemia escape has been confirmed by Villalobos et al. [8]. These observations suggest that leukemic escape from both NK and T-cell control is not uncommon after SCT.
Failure of immune control
There are several anecdotal accounts of patients with CML entering remission on withdrawal of immunosuppression and relapsing again when immunosuppression was instituted to treat GVHD and numerous accounts of GVL effects, only initiating on withdrawal of immunosuppression [9,10]. Immune recovery correlates strongly with protection from relapse. Lymphocyte counts (and, in particular, NK cell counts) above the median in the first month after SCT are associated with significantly lower relapse risks in acute myeloid leukemia (AML) [11,12]. Soon after transplant, there is a period of ‘immune intolerance’, when host antigen-presenting cells (APCs) stimulate alloresponses in the donor. Later, the establishment of donor APCs tolerize donor immune cells, and may reduce the GVL potential [13,14]. Levels of regulatory T cells rise rapidly after SCT [15], and may also limit GVL. Some of the factors determining relapse have been traced back to the stem cell donor: donors with particular NK immunoglobulin-like receptor groups can protect against relapse, while donors with higher Treg levels confer less GVHD, and it is possible this may also reduce the GVL effect [15] [McIver Z, Pers. Comm]. The tumor load itself can determine the T-cell response – if the leukemia antigen burden is too high, T cells may become exhausted and express inhibitory molecules, such as programmed death-1 (PD-1), which, upon engagement by PD-1 ligand, reduce proliferation and cytotoxicity of antigen-reactive T cells [16]. Conversely, if antigen levels are too low, memory T-cell populations become quiescent.
Microenvironment & sanctuary sites
Extramedullary relapse presenting as granulocytic sarcomas/chloromas in the retroperitoneum, kidney, brain and bone, and other diverse sites, is a relatively common form of leukemic relapse after SCT [17]. At least initially, the bone marrow can remain in CR. T-cell homing is determined by a range of selectin molecules, ‘addressins’, which direct the T cell to specific tissues, and such relapse may occur because of sanctuary sites not patrolled by antileukemic T cells [18].
Intrinsic features of individual leukemias
Since GVL takes weeks to become fully established, the degree to which GVL can control leukemia after SCT depends on the growth kinetics of the leukemia. Thus, in general, chronic leukemias, and some lymphomas, are more susceptible to GVL because of their slower pace of proliferation. It is also clear that, for GVL to be effective, the leukemic progenitors must be targeted. For example, in AML, pioneering studies modeling leukemia in severe combined immunodeficient mice show that alloreactive T-cell clones can eliminate leukemia-initiating cells, which represent only a minority of the leukemia population [19]. As illustrated previously, immune pressure constantly forces residual leukemia cells to undergo clonal escape. Therefore, the propensity of the malignancy to mutate is critically important for the risk of relapse. Although there is a slower pace of relapse for CML, the general pace of relapse remains very similar, with an almost exponential fall in relapse risk in the first 6–12 months after transplant.
Transplant factors
Table 1 lists the variables in the transplant that predispose to relapse representing either nonimmune factors of the conditioning regimen, and immunomediated factors affecting GVL.
Table 1.
Transplant factors affecting rate of relapse.
| Low risk | Intermediate risk | High risk |
|---|---|---|
| Age (acute leukemias) | ||
| Young | Older | |
| Disease at transplant | ||
| Complete remission | Partial remission | Refractory |
| Donor | ||
| Mismatched (male–female) | Matched | Auto/identical twins other combinations |
| Conditioning | ||
| Non-myeloablative | Reduced-intensity conditioning | Fully myeloabaltive |
| Graft-versus-host disease prophylaxis | ||
| None | Reduced | Combinations, Campath® |
| Chimerism | ||
| Full | Donor | |
Treatment of relapse after SCT
Treatment at relapse presents huge challenges. As mentioned previously, the conditions at relapse may involve inadequate initial disease control, immune failure or a leukemia that has clonally escaped. In addition, the patient’s performance status and resilience to further cytotoxic or immune therapy is reduced by the tissue damage induced by the conditioning, post-transplant infections and GVHD. While the immune compartment remains fully donor chimeric at relapse, the marrow reserve of donor hematopoietic function may be compromised or, in the case of early relapse, not even fully established. Thus, the recipient’s ability to withstand further treatment is compromised. It is important to observe, however, that most patients who experience relapse (being a selected population who have already accepted the risks of SCT) are anxious to try further attempts of leukemia control or eradication. Since relapse treatment is best regarded as a salvage approach, its management tends to be on an individual basis. Some patients receive only palliative, supportive care. Where possible, patients are given a donor lymphocyte infusion (DLI) with or without chemotherapy [20], and some are offered a second SCT. Challenges and opportunities for relapse treatment approaches are summarized in Table 2.
Table 2.
Challenges and opportunities for relapse after hematopoietic stem cell transplantation.
| Therapeutic constraints after transplant | Opportunities after transplant |
|---|---|
| Poor hematopoietic reserve limits the use of cytotoxic drugs | Established donor immune system (platform for cellular immunotherapy) |
| Impaired performance status | Induction of graft-versus-leukemia by immunosuppression taper |
| Transplant as an exclusion for clinical trial access to investigational agents | Homeostatic expansion of immune cells: donor lymphocyte infusion, ex vivo expanded cytotoxic T-cell lymphoma and natural killer cells |
| Intrinsic resistance malignancy | Vaccination strategies |
Principles of management of post-transplant relapse
A rational approach to the management of relapse is based upon taking into consideration five factors, which determine the realistic objectives of treatment.
First transplant
Examination of the features of the first SCT will identify whether further treatment can improve upon the disease control by either nonimmunological or immunological means. For example, patients who have received a reduced-intensity conditioning (RIC) SCT, if they have a suitable performance status, might benefit from more-intensive myeloablative therapy, requiring stem cell rescue. After second transplantation, the occurrence of chronic GVHD is the main factor determining improved survival and freedom from relapse [21]. Factors that favor the development of chronic GVHD are reduced immunosuppression, selection of a less than fully matched donor, use of peripheral blood as the stem cell source [22], and T-replete transplantation. Other specific examples are illustrated in Table 3.
Table 3.
Conditions that allow opportunities for successful salvage treatment of relapse.
| Feature of improved initial transplant | Improved strategy | Rationale |
|---|---|---|
| Conditioning | ||
| Reduced intensity | More intensive conditioning, alternative chemotherapy agents | Increase tumor kill |
| Myeloablative | Add disease-targeted specific agents (e.g., Mylotarg®) | |
| Transplant | ||
| T-cell-depleted stem cell transplant | Donor lymphocyte infusion, T-replete second transplant | Increase graft-versus-myeloma/graft-versus-leukemia |
| Marrow transplant | Peripheral blood progenitor cells | |
| Fully matched siblings | Alternative donor (HLA matched/mismatched) | |
| Post transplant | ||
| Full graft-versus-host disease prophylaxis | Reduced or no prophylaxis IFN-α or granulocyte–macrophage colony-stimulating factor |
Increase graft-versus-myeloma/graft-versus-leukemia, immune stimulation, increase antigenicity of hematological malignancies |
| No maintenance | Demethylating agents, interferon maintenance with antileukemia drugs | Increase disease control |
| Standard treatment | Investigational immunotherapy: vaccines, leukemia-specific T or natural killer cells Investigational targeted therapies: tyrosine kinase inhibitors, monoclonal antibodies |
Increase graft-versus-myeloma/graft-versus-leukemia Synergy with graft-versus-myeloma/graft-versus-leukemia |
Disease type
Broadly, the outcome for relapse varies according to three disease categories. Acute leukemias and MDS represent diseases with the poorest prognosis, typically relapsing rapidly after transplant and being weakly controlled by GVL mechanisms. CML represents a special case, presenting unique opportunities to plan curative treatment approaches. Indolent diseases, such as lymphoma and chronic lymphocytic leukemia (CLL) are also susceptible to GVL effects, again raising greater possibilities of long-term disease control.
Age of the patient
In general, as might be anticipated, younger age (<20 years) is a favorable factor for outcome, notably after a second SCT [23–25].
Interval between transplant & relapse
Large retrospective analyses identify the cut point between early and late relapse as between 6 and 12 months after SCT, with earlier relapses having significantly worse outcomes. This relates to the time available for tissue repair, with relapses occurring late after SCT having a greater choice of sustainable treatments and a greater chance of responding to treatment [21,23,26–28]. For example, the Center for International Blood and Marrow Transplant Research reported a 5-year probability of relapse, transplantation-related mortality (TRM) and overall survival (OS) of 42, 30 and 28%, respectively, in 279 patients with acute and chronic leukemia, provided with a second SCT for relapse after HLA-identical sibling transplantation. Relapse beyond 6 months and younger age were the major factors affecting survival [24]. In a study of 307 patients treated by the Seattle group and given some form of active treatment for relapse, 2-year OSs for early (<100 days), intermediate (100–200 days) or late (>200 days) recurrence were 3, 9 and 19%, respectively [29].
Type of relapse
Incipient relapse, detectable by increasing mixed chimerism [30], multiparameter flow for residual leukemia [31] or by sensitive techniques for molecular disease markers [32–34], is favorable, offering more time for effective therapeutic strategies before the disease causes clinical problems. Surveillance strategies for detecting relapse are listed in Table 4. Low disease burden is associated with survival doubling after second SCT in one study [26]. Occasionally, isolated extramedullary relapse can be successfully treated by local radiotherapy or surgical removal.
Table 4.
Surveillance for relapse after stem cell transplantation.
| Method | Comments |
|---|---|
| Minimal residual disease by PCR | Sensitivity (10e-4 to 10e-6) but not applicable for every malignancy |
| Minimal residual disease by flow cytometry | Sensitivity (10e-2 to 10e-5) |
| Fluorescent in situ hybridization | Sensitivity (10e-2) but highly specific |
| Donor-recipient chimerism | Persistent residual host cells or reappearance signals relapse |
| Paraproteins (immunofixation electrophoresis and serum-free light chains) | For multiple myeloma |
| Morphology of marrow and blood | Insensitive |
| Serial scans (computed tomography and PET) | Useful for lymphoma and to identify extramedullary relapse |
Current approaches to the management of relapsed hematological malignancy
Acute leukemia & MDS
Patterns of relapse
The probability of acute leukemia or MDS relapsing is greatest in the first year after SCT, and half the relapses occur within 6 months of SCT. Less frequently, relapse occurs late, when it is often likely to manifest in the form of chloromas. Since relapse of these diseases is usually rapid, it is more often identified by blood changes (falling platelet count or appearance of blasts in the blood) than by minimal disease monitoring. However, relapse can sometimes be identified by falling marrow chimerism or, when measurable, by Wilms tumor gene (WT1) expression [34]. Once relapse manifests hematologically, the disease course for acute leukemia is typically, but not always, rapid. In MDS, relapse can be slower in pace, and the associated diminished marrow function can be mitigated by the quality of the coexisting donor hematopoiesis. Relapse of acute leukemia usually represents the re-emergence of a disease identical to that prior to SCT.
Management
Treatment approaches range from the simplest support and palliation to second attempts at cure with second transplants. Management is strongly dictated by the timing of the relapse. Patients relapsing within 6 months have median survivals of 6 months, with less than 5% of survivors beyond 1 year [21,35]. A sequential treatment approach for relapsed acute leukemia and MDS patients is to begin treatment with immunosupression withdrawal, introduce chemotherapy appropriate for the disease type and follow it with a DLI or, in selected cases, a second SCT. Alternatively, chemotherapy can be followed by a peripheral blood SCT, which accelerates hematological recovery, while providing GVL through the transfer of donor T cells. Whatever treatment approach is chosen, the overall outcome for patients with high-risk malignancies (mainly acute leukemia and MDS) is very poor, with median survivals of 6 months or less, and longer survivals of less than 25% [35]. It should be borne in mind that patients receiving this approach are a highly selected group, having an available donor and with good performance scores.
Modulating immunity
Although the GVL effect in acute leukemia and MDS is only modest, immunosuppression is usually withdrawn as a prelude to other treatment. Cytokine therapy to modulate GVL effects may hold promise – in a recent study of 81 acute lymphoblastic leukemia (ALL) and 72 AML relapsing patients, median post-relapse survival was 442 days for seven patients receiving IFN-α and granulocyte–macrophage colony-stimulating factor, compared with 51 days for chemotherapy recipients, 84 days for DLI and 303 days for second transplants [36].
Chemotherapy
The relative preservation of donor marrow in relapse post-SCT makes it possible to retreat acute leukemia and MDS with myelo-suppressive chemotherapy, and anticipate hematological recovery. However, in early relapse, especially if complicated by concurrent GVHD, relatively low-intensity chemotherapy is often the only safe approach. Nevertheless, patients with ALL often respond to combinations of vincristine and prednisone, with or without an anthra-cycline [37]. AML relapse can respond to low doses of etoposide, gemtuzumab ozogamicin or reduced-intensity fludarabine, cytosine arbinoside and idarubicin regimens [38]. The novel purine analog clofarabine is promising in both forms of acute leukemia relapse [39]. Newer targeted inhibitors continue to show promise [40], and imatinib with or without DLI may achieve sustained remission in Philadelphia chromosome-positive relapsed ALL [41,42]. Patients with MDS that advanced to acute leukemia can be treated in the same way, while those relapsing with less-advanced MDS may have a more indolent course, permitting clinical trial of MDS treatment agents, such as azacytidine, decitabine or lenalidomide [43–45].
Donor lymphocyte infusions
Donor lymphocyte infusions, given as a single treatment approach, carries only a modest chance of achieving a response [46], with a 2-year survival of 21 versus 9% for patients not receiving DLI [47]. Several analyses in MDS [48] and AML [49] suggest that any form of chemotherapy followed by DLI probably offers better outcome than chemotherapy alone, even in patients who only achieve partial disease control with chemotherapy.
Second SCT
Several recent studies report second SCT for relapsed leukemias [50,51]. Since only a minority of patients receive second SCT for acute leukemia and MDS, the selection favors relatively fit younger patients who have already responded to chemotherapy. Nevertheless, even in this highly elected group, outcome is disappointing, with less than 20% of patients surviving 6 months, and only a few long-term survivors. A recent study of 93 relapsed patients showed that second SCT was superior to chemotherapy (58 vs 14% 1-year survival), but this advantage was lost by 2 years owing to further relapse [52]. A recent study of 25 second allografts, mainly for relapsed acute leukemia and MDS, showed that remission could be achieved in all patients with subsequent relapse in 44%, and a 32% 18-month survival [53].
Extramedullary relapse
Chloromas are usually seen as late forms of relapsed acute leukemia and MDS. Although they may be isolated, they have a tendency to involve the marrow. Chloromas have a poor prognosis. They appear to be resistant to DLI, and can develop during GVHD, suggesting they occur in immune-privileged sites [54,55].
Chronic myeloid leukemia
Relapse of CML after SCT has unique features that set its management apart from other HMs. First, the ability to detect minimal residual disease (MRD) with regular blood monitoring by PCR for BCR-ABL1 makes early treatment possible. Second, CML, at least in CP, is especially sensitive to GVL, making DLI an effective and potentially curative treatment. Third, the use of TKIs after SCT can improve the chances of disease control. Last, CML tends to relapse very late after SCT, making aggressive treatments, including second transplant, more likely to be effective. Thus, despite the very small numbers of patients with CML undergoing SCT in the era of TKIs, there remains a legacy of patients transplanted in a previous decade who continue to present with relapse. It is worth remembering that patients currently being transplanted with CML tend to have TKI-intolerant or refractory disease, with different outcomes than legacy patients.
Patterns of CML relapse
In some patients (especially those who develop chronic GVHD), BCR-ABL1 message is lost within 6 months after SCT, resulting in permanent leukemia eradication. Others show fluctuating but stable low-level disease, which does not progress. Relapse is defined operationally as three consecutive positive and increasing BCR-ABL1 measurements. Patients transplanted in the accelerated phase (AP) or blast phase (BP) may also follow this pattern, but the interval between acquiring BCR-ABL1 positivity and overt hematological relapse can be very short, so that some patients will present in full hematological relapse or with chloromas.
Management of relapsed CML
Management follows a stepwise stratification from minimal intervention approaches to second transplants. In the absence of overt relapse, MRD monitoring can be performed every 6–12 weeks, following each intervention to document response.
Withholding immunosuppressive treatment
Patients relapsing with MRD who are still on immunosuppression can achieve remission (with or without development of GVHD) by tapering immunosuppression with ciclosporin/tacrolimus and steroids [10].
Donor lymphocyte infusion
Kolb’s first report of the use of DLI to achieve remission in CML in hematological relapse post-SCT introduced a new era in the treatment of all forms of leukemia relapsing after SCT [56]. However, only in CML has the use of DLI made such an impact on long-term disease-free survival [57,58]. Careful dose-escalation studies by MacKinnon identified a T-cell dose of 1 × 107 CD3 cells/kg as the threshold for achieving disease response, while having only limited risk of causing GVHD [59]. This dose is currently considered the standard for treating relapsed leukemias. DLI risks include GVHD and bone marrow aplasia. The development of aplasia, initially a puzzling complication, was subsequently found to be related to depletion of the reservoir of healthy donor stem cells [60]. Marrow aplasia can be prevented in patients in full hematological relapse by the transfusion of backup donor stem cells with DLI – simply achieved by a second transplant, limiting the T-cell dose to avoid GVHD. The probability of response of CML to DLI depends on the disease stage – MRD has a greater than 90% chance of achieving permanent cure, falling to 80% for patients with more overt CP disease, identified by chromosome analysis or blood count changes, and to less than 35% for relapse into AP or BP [61,62].
Role of TKIs
Some CML patients, relapsing late after SCT, were transplanted before TKIs were introduced, and other more recent transplants, typically in AP, may have received only imatinib. In these patients, a case can be made to treat relapse at any stage with a TKI that the patient has not previously been exposed to, and raises the question of whether TKIs might not, in any case, be preferable to DLI because of the lack of GVHD risk. Data shows that imatinib is, indeed, effective at controlling MRD, in both naive and imatinib-exposed patients [63]. There has been concern that all TKIs, notably dasatinib, carry the off-target risk of suppressing T-cell immunity and, therefore, working against GVL. This would argue against combining imatinib with DLI. In one retrospective study, however, the association of imatinib with DLI was found to be synergistic with patients who received both treatment approaches at relapse, having the most rapid progression to MRD negativity, a higher overall response rate, including higher efficacy when combined with IFN-α in treating full hematological relapse, and CML more advanced than CP. Thus, there seems to be no contraindication to treating all relapsed CML with combined DLI and TKI, and for using nilotinib or dasatinib as alternatives to imatinib in patients who were previously shown to be imatinib resistant. Dasatinib is the preferred TKI for intracranial disease because of its penetration into the CNS.
Other agents
IFN-α has efficacy in CML, and it is logical to incorporate it into treatment of more-advanced CML relapse, as described previously [10]. However, there are no large studies to confirm its efficacy in such patients.
Second SCT
In the case where simpler strategies have failed, or the relapsed CML is advancing despite DLI and TKI treatment, a second SCT may be considered. The opportunity for a cure of CML by a second SCT depends on whether a second SCT offers a better chance of disease control by improving on the GVL effect (e.g., T-replete vs T-depleted, full-intensity vs reduced-intensity and peripheral blood SCT vs marrow transplant treatment for second vs first SCT, respectively). Thus, depending on the nature of the first transplant, attempts to increase either the myeloablative or the GVL component can be made, always bearing in mind the increased risk of transplant-related mortality borne by amplifying either strategy. Implicit in decision making is the interval between first and second transplant. Second transplants beyond 5 years are justifiable, with the proviso that only one conditioning with total-body irradiation is possible. There are no recent data on second SCT for CML. Available data from retrospective analyses indicates a lymphoma-free survival in CML of 40% for second SCT in selected patients [64].
Management of chloromas
With an increasing proportion of CML patients being transplanted for more-advanced disease, extramedullary relapse is more common. This form of relapse carries a very poor prognosis. In a recent study of five extramedullary relapses in CML, treatment with local irradiation, TKI and chemotherapy achieved only transient responses [17].
Chronic malignancies (non-Hodgkin lymphoma, Hodgkin lymphoma, multiple myeloma, CLL & myelofibrosis)
Patterns of relapse
This group of disorders is mostly treated with R IC-based transplants, which reduces nonrelapse mortality but with an increased tendency to relapse [65,66]. Many patients will have persistent disease at the time of transplant, which may progress in the early post-transplant period. Additionally, with the possibility of post-transplant molecular MRD screening of JAK2V617F, early (molecular-level) relapse of myeloproliferative disorders bearing this mutation may be detected.
Immune manipulation
Immune manipulation by the withdrawal of immunosuppressive therapy or by DLI plays an important role after allogeneic transplant for this group of disorders for several reasons: intrinsic susceptibility to alloimmune effects, slower proliferative kinetics and favoring of RIC often results in a state of mixed chimerism, which requires conversion to full donor chimerism before a full GVM effect can be obtained. The existence of a GVM effect in lymphoproliferative disorders has been widely described and reviewed by Ringden et al. and Grigg et al. [67,68]. Clinically meaningful GVM effects have been well described in follicular, mantle cell, small lymphocytic, Hodgkin lymphomas and myeloma [69–71]. The evidence for potent GVM effects in diffuse large-cell lymphoma and Burkitt lymphoma is not convincing.
Donor lymphocyte infusion
Donor lymphocyte infusions are effective in myeloma relapsing after allogeneic transplant, with overall responses seen in two-thirds of patients, and strong association with GVHD [72]. One retrospective review showed responses in eight out of 13 patients, with a median time to response of 6 weeks [73]. Two responding patients developed fatal bone marrow aplasia. T-cell doses greater than 1 × 108/kg, as well as the occurrence of GVHD, were associated with response. In a different analysis of 25 patients with relapsed myeloma who received DLIs, ten patients experienced some benefit, but the majority developed grade 3–4 GVHD [74]. DLIs also exert a clinically significant impact against indolent non-Hodgkin lymphoma. In a series of 28 patients treated with escalating DLIs, the cumulative response rates after DLI to treat progressive disease and persistent mixed chimerism were 76.5 and 91.6%, respectively [75]. In another series of 17 patients who received DLIs for lymphoid malignancies, with or without prior chemotherapy, ten patients achieved CR, including three patients with CLL, four with mantle cell lymphoma, three with follicular non-Hodgkin lymphoma but none with aggressive non-Hodgkin lymphoma or Richters. The median CD3 cell dose to achieve CR for siblings was 2 × 107/kg [76]. JAK2-V617F-triggered preemptive DLIs for MRD or molecular relapses have been used, with success in myelofibrosis, and may be superior to salvage DLIs for clinical relapse [77].
Second transplants
The role of second transplants in lymphoproliferative disorders is less defined than in the acute leukemias. In the data reported by Shaw et al. for reduced-intensity second allogeneic transplants, the relapse rate at 1 year after the second transplant was lowest (18%) in the lymphoproliferative disorders, in contrast to more than 50% for the leukemias [21].
Novel agents
Improved understanding of molecular pathogenesis continues to yield novel noncytotoxic agents, such as imids (lenalidomide/thalidomide), sirolimus, proteasomal inhibitors (bortezomib), Jak-2 inhibtors and monoclonal antibodies, with great potential in the relapse setting. The utility of novel agents is not confined to relapse, but also for maintenance, and in combinatorial strategies with immune manipulation [78]. Maintenance strategies are most needed in myeloma, where the transition to RIC has lowered TRM at the cost of poor disease control [79].
New directions in relapse treatment
Fortunately, future prospects for treating relapse after SCT are improving through new chemotherapy agents, targeted therapies and developments in immunology. Although any single agent is unlikely to make breakthroughs in the treatment of relapsed leukemia, the judicious combinations of immune therapies with drugs that enhance malignant cell killing may prove extremely effective, even in the setting of salvage treatment. Box 1 illustrates the diverse armamentarium of approaches now available [80]. However, it should be noted that, although some of these agents are being used in conjunction with SCT, there are no substantial data to define their efficacy on the treatment of post-SCT relapse.
Box 1. New approaches for treating leukemic relapse after stem cell transplantation.
Cytotoxic chemotherapy: clofarabine and nelarabine
Epigenetic modulators: azacytidine and decitabine
Small-molecule-targeted inhibitors: tyrosine kinase inhibitors (e.g., imatinib), sirolimus, bortezomib and imids (e.g., lenalidomide)
Cell immunotherapy: natural killer cell infusion, improved donor lymphocyte infusions (selected T cells, additional cytokines and haploidentical), antigen-specific T cells recognizing minor histocompatibility antigens, tumor antigens and chimeric antigen receptors
Cytokines: IL-2, IL-15 and interferon
Antibodies: myeloid (anti-CD33), B cell (anti-CD20/22)
Vaccines: peptide, DNA and dendritic cell vaccines
New drugs
Perhaps the most promising cytotoxic agents are clofarabine and myelotarg, which are effective in refractory acute leukemias. The nucleoside analog azacytidine has both cytotoxic and demethylating activities, the latter potentially improving GVL by increasing antigen presentation on leukemia cells by upregulating expression of costimulatory molecules and tumor antigens. Azacytidine in combination with DLI is being explored in the treatment of relapsing MDS after SCT.
Targeted therapies
Targeted therapies have a promising future when used in conjunction with SCT, and could find a place in the treatment of relapse. Sirolimus has been exploited for direct suppression of B-cell proliferation, and is an attractive agent that can both control GVHD and provide lymphoma control [81,82]. The proteasome inhibitor bortezomib has an important potential for increasing NK-mediated GVL effects by upregulating TNF-related apoptosis-inducing ligands on the target, and its receptors on NK cells [83]. In B-cell malignancies, lenalidomide has potential to increase tumor killing by multiple mechanisms [84].
T-cell therapies
Investigators have explored a number of ways to manipulate DLIs to render them less likely to cause GVHD. These approaches include CD6 T-cell depletion [85], selective depletion of alloreactive T cells and insertion of a suicide gene into the transfused T cells [86,87]. An alternative approach has been to enhance GVL by selecting haploidentical donors to provide DLI. This protocol (NHBI, 09-H-0087) is currently open for patients relapsing early after SCT. A second line of research has been to identify and select T-cells specific for antigens expressed by leukemia cells (WT-1, PR1) or minor histocompatibility antigens (mHAgs). Falkenburg and colleagues were the first to show, proof of principle, that patients relapsing with CML who failed standard DLI could be put into remission (without GVHD) with donor T-cell lines expanded against the patients’ leukemia [88]. Subsequently, Riddell used defined mHAg-specific T-cell lines to successfully treat several patients with relapsed leukemia after SCT, and noted remissions (not always sustained), but cautioned against initial inflammatory responses from the T-cell infusions [89]. Improved techniques for selecting and expanding leukemia-specific mHAg-reacting T-cell lines from donor recipient pairs before transplant may make this strategy more generally applicable to relapsed patients [90]. Last, techniques that insert T-cell receptor trans-genes or chimeric single-chain variable fragment receptors, also known as chimeric antigen receptors, which can specifically redirect T cells to malignancies, would overcome HLA restriction and T-cell cloning limitations, to provide an ‘off-the-shelf ’ approach to adoptive T-cell immunotherapy [91].
NK cell therapies
Alloreacting NK cells represent an attractive source of GVL-reacting cells, which have the advantage of not causing GVHD. Pioneering work by Velardi and colleagues, and more recent observations, have defined the ‘rules of engagement’ between NK cells and their leukemia targets in both HLA-mismatched and HLA-matched donor–recipient pairs that make it possible to identify NK cells from donors which can confer strong GVL effects [92–94]. NK cells can be obtained by apheresis from healthy donors but the cell doses obtained are limiting. Methods to expand these cells have the advantage of increasing their cytotoxicity, as well as their numbers, and represent a practical source to treat relapsed patients with effector cells with enhanced GVL efficacy. Furthermore, NK cytotoxicity can be further enhanced by bortezomib [95]. In vivo expansion and function of endogenous or transfused NK cells may be further improved by IL-15, a critical growth factor, which is being developed for clinical trials [96]. Trials of NK cells in relapsed leukemia are ongoing.
Cytokines
Cytokines have several applications in the treatment of relapsed leukemia. IFN-α and -γ upregulated mHAgs on target cells and, together with IL-2, increase cytotoxicity of T and NK cells [97]. IFN-α is also antiproliferative for some leukemias [98]. GM-CSF has a similar ability to upregulate antigen presentation on the leukemia cell [99]. Several investigators have used DLIs in association with these cytokines.
Antibodies
Antibodies, such as CD20 and CD22 binding to malignant B cells, and linking of the CD16 Fcγ receptor on NK cells, can bind NK cells to the malignant cell. Beyond the two most widely used antibodies (CD20 rituximab and gemtuzumab ozogamicin) the role of antibodies in the treatment of post-SCT relapsed leukemia has been unexplored [100].
Vaccines
Several vaccines are being explored in the treatment of leukemia. They include peptide vaccines to WT1, PR1 and BCR-ABL and vaccine-modified dendritic cells [101,102]. Post-SCT, PR1- and WT1-specific T cells increase in the blood and marrow, making it a reasonable strategy to boost these T cells with vaccines, either to prevent or treat relapse. Approaches to combining vaccines with SCT have been reviewed recently [103].
Expert commentary
Leukemic relapse after SCT is the largest single cause of treatment failure. While management of relapse is largely a salvage approach, with a high probability of failure, it is important always to consider offering treatment; first, because patients usually ask for active treatment, second, because we need to learn the mechanisms of relapse in order to do better transplants, and third, because relapse with its high risk permits us to develop novel and untried treatments ethically, and may give the opportunity for breakthroughs that benefit leukemia patients outside the context of SCT. It is important to be aware of subgroups of patients with more favorable outcomes, and to avoid intensive treatments in patients where it is futile. Understanding the mechanisms underlying disease recurrence after SCT can point the way to making transplants more efficient at eradicating disease, while leukemic relapse offers an ethically justifiable opportunity to test novel immune and nonimmune strategies to control leukemia. Since prognosis is generally very poor, and its management is dependent on diverse factors – interval from first transplant, nature of disease and features of the first SCT – there is no consensus on the best approach for particular situations. Where reports exist, they are subject to a selection bias, for example, in patients receiving second SCT. The consensus view is that relapse is best treated by a combination of chemotherapy or, when possible, by leukemia-specific agents, such as imatinib, accompanied by boosting immune function with reduced immunosuppression, DLI or second transplant in highly selected patients.
Five-year view
In the next 5 years, some improvement in the understanding of leukemia relapse mechanisms should be forthcoming from an NCI-sponsored initiative on relapse after SCT, which will assemble biological data at relapse and promote trials of novel treatments [104,105]. These studies should pave the way to better first-line transplant treatments to prevent relapse occurring, while developing improved disease-specific treatments to increase the survival of relapsed patients.
Key issues.
Relapse is the most common cause of treatment failure after allogeneic stem cell transplantation (SCT) for hematological malignancies.
The prognosis depends on four factors: the time elapsed from SCT to relapse, the disease type, the disease burden and the conditions of the first transplant.
Minimal residual disease monitoring is important in providing appropriate treatments early; graft-versus-myeloma (GVM) effects work best when disease burden is low.
Current methods to treat relapse include immunomodulation (withdrawal of immunosuppression and donor lymphocyte infusions), salvage therapies (chemotherapy, epigenetic modulation, targeted inhibitors and antibodies) or second transplant (for selected indications).
Therapies in development exploit selective T-lymphocyte-mediated GVM effects, utilization of alloreactive natural killer cells, incorporation of noncytotoxic-targeted inhibitors and maintenance strategies.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
This work was supported (in part) by the Intramural Research Program of the NHLBI, NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: part I – CIBMTR summary slides. CIBMTR Newsletter (serial online) 2009;15(1):7–11. [Google Scholar]
- 2.Barrett AJ. Understanding and harnessing the graft-versus-leukaemia effect. Br J Haematol. 2008;142(6):877–888. doi: 10.1111/j.1365-2141.2008.07260.x. [DOI] [PubMed] [Google Scholar]
- 3.Murata M, Ishikawa Y, Ohashi H, et al. Donor cell leukemia after allogeneic peripheral blood stem cell transplantation: a case report and literature review. Int J Hematol. 2008;88(1):111–115. doi: 10.1007/s12185-008-0094-3. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz-Arguelles GJ, Ruiz-Arguelles A, Garces-Eisele J. Donor cell leukemia: a critical review. Leuk Lymphoma. 2007;48(1):25–38. doi: 10.1080/10428190601003462. [DOI] [PubMed] [Google Scholar]
- 5.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3(11):999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dermime S, Mavroudis D, Jiang YZ, Hensel N, Molldrem J, Barrett AJ. Immune escape from a graft-versus-leukemia effect may play a role in the relapse of myeloid leukemias following allogeneic bone marrow transplantation. Bone Marrow Transplant. 1997;19(10):989–999. doi: 10.1038/sj.bmt.1700778. [DOI] [PubMed] [Google Scholar]
- 7••.Vago L, Perna SK, Zanussi M, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361(5):478–488. doi: 10.1056/NEJMoa0811036. Unequivocal evidence for leukemia escape from immune control. [DOI] [PubMed] [Google Scholar]
- 8.Villalobos IB, Takahashi Y, Akatsuka Y, et al. Relapse of leukemia with loss of mismatched HLA resulting from uniparental disomy after haploidentical hematopoietic stem cell transplantation. Blood. 2010;115(15):3158–3161. doi: 10.1182/blood-2009-11-254284. [DOI] [PubMed] [Google Scholar]
- 9.Boatsman EE, Fu CH, Song SX, Moore TB. Graft-versus-leukemia effect on infant lymphoblastic leukemia relapsed after sibling hematopoietic stem cell transplantation. J Pediatr Hematol Oncol. 2009;32(2):e57–e60. doi: 10.1097/MPH.0b013e3181c6beef. [DOI] [PubMed] [Google Scholar]
- 10•.Elmaagacli AH, Beelen DW, Schaefer UW. A retrospective single centre study of the outcome of five different therapy approaches in 48 patients with relapse of chronic myelogenous leukemia after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1997;20(12):1045–1055. doi: 10.1038/sj.bmt.1701026. Highlights role of interferon in control of relapsed chronic myelogenous leukemia (CML) [DOI] [PubMed] [Google Scholar]
- 11.Savani BN, Mielke S, Rezvani K, et al. Absolute lymphocyte count on day 30 is a surrogate for robust hematopoietic recovery and strongly predicts outcome after T cell-depleted allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(10):1216–1223. doi: 10.1016/j.bbmt.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savani BN, Mielke S, Adams S, et al. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia. 2007;21(10):2145–2152. doi: 10.1038/sj.leu.2404892. [DOI] [PubMed] [Google Scholar]
- 13.Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285(5426):412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 14.Collin MP, Bogunovic M, Merad M. DC homeostasis in hematopoietic stem cell transplantation. Cytotherapy. 2007;9(6):521–531. doi: 10.1080/14653240701507314. [DOI] [PubMed] [Google Scholar]
- 15.Rezvani K, Mielke S, Ahmadzadeh M, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108(4):1291–1297. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 17.Ocheni S, Iwanski GB, Schafhausen P, et al. Characterisation of extramedullary relapse in patients with chronic myeloid leukemia in advanced disease after allogeneic stem cell transplantation. Leuk Lymphoma. 2009;50(4):551–558. doi: 10.1080/10428190902755513. [DOI] [PubMed] [Google Scholar]
- 18.Sackstein R. A revision of Billingham’s tenets: the central role of lymphocyte migration in acute graft-versus-host disease. Biol Blood Marrow Transplant. 2006;12(1 Suppl 1):2–8. doi: 10.1016/j.bbmt.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 20.Loren AW, Porter DL. Donor leukocyte infusions for the treatment of relapsed acute leukemia after allogeneic stem cell transplantation. Bone Marrow Transplant. 2008;41(5):483–493. doi: 10.1038/sj.bmt.1705898. [DOI] [PubMed] [Google Scholar]
- 21.Shaw BE, Mufti GJ, Mackinnon S, et al. Outcome of second allogeneic transplants using reduced-intensity conditioning following relapse of haematological malignancy after an initial allogeneic transplant. Bone Marrow Transplant. 2008;42(12):783–789. doi: 10.1038/bmt.2008.255. [DOI] [PubMed] [Google Scholar]
- 22•.Platzbecker U, Thiede C, Freiberg-Richter J, et al. Treatment of relapsing leukemia after allogeneic blood stem cell transplantation by using dose-reduced conditioning followed by donor blood stem cells and GM-CSF. Ann Hematol. 2001;80(3):144–149. doi: 10.1007/s002770000258. Use of peripheral blood stem cell rescue to treat relapsed leukemia. [DOI] [PubMed] [Google Scholar]
- 23.Al-Qurashi F, Ayas M, Al Sharif F, et al. Second allogeneic bone marrow transplantation after myeloablative conditioning analysis of 43 cases from single institution. Hematology. 2004;9(2):123–129. doi: 10.1080/10245330310001652509. [DOI] [PubMed] [Google Scholar]
- 24••.Eapen M, Giralt SA, Horowitz MM, et al. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone Marrow Transplant. 2004;34(8):721–727. doi: 10.1038/sj.bmt.1704645. Largest retrospective analysis of second transplants for relapse, identifying age and interval between first transplant and relapse as major risk factors. [DOI] [PubMed] [Google Scholar]
- 25•.Michallet M, Tanguy ML, Socie G, et al. Second allogeneic haematopoietic stem cell transplantation in relapsed acute and chronic leukaemias for patients who underwent a first allogeneic bone marrow transplantation: a survey of the Societe Francaise de Greffe de moelle (SFGM) Br J Haematol. 2000;108(2):400–407. doi: 10.1046/j.1365-2141.2000.01851.x. Large retrospective European study of relapse treatment for lymphoid malignancies identifying effective salvage treatment. [DOI] [PubMed] [Google Scholar]
- 26.Hosing C, Saliba RM, Shahjahan M, et al. Disease burden may identify patients more likely to benefit from second allogeneic hematopoietic stem cell transplantation to treat relapsed acute myelogenous leukemia. Bone Marrow Transplant. 2005;36(2):157–162. doi: 10.1038/sj.bmt.1705011. [DOI] [PubMed] [Google Scholar]
- 27.Mrsic M, Horowitz MM, Atkinson K, et al. Second HLA-identical sibling transplants for leukemia recurrence. Bone Marrow Transplant. 1992;9(4):269–275. [PubMed] [Google Scholar]
- 28.Michallet AS, Nicolini F, Furst S, et al. Outcome and long-term follow-up of alloreactive donor lymphocyte infusions given for relapse after myeloablative allogeneic hematopoietic stem cell transplantations (HSCT) Bone Marrow Transplant. 2005;35(6):601–608. doi: 10.1038/sj.bmt.1704807. [DOI] [PubMed] [Google Scholar]
- 29••.Mielcarek M, Storer BE, Flowers ME, Storb R, Sandmaier BM, Martin PJ. Outcomes among patients with recurrent high-risk hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2007;13(10):1160–1168. doi: 10.1016/j.bbmt.2007.06.007. Large single-center analysis of outcome for relapse identifying second transplant as a useful therapy for patients who relapse late after first transplant. [DOI] [PubMed] [Google Scholar]
- 30.Bader P, Kreyenberg H, Hoelle W, et al. Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: possible role for pre-emptive immunotherapy? J Clin Oncol. 2004;22(9):1696–1705. doi: 10.1200/JCO.2004.05.198. [DOI] [PubMed] [Google Scholar]
- 31.Diez-Campelo M, Perez-Simon JA, Perez J, et al. Minimal residual disease monitoring after allogeneic transplantation may help to individualize post-transplant therapeutic strategies in acute myeloid malignancies. Am J Hematol. 2009;84(3):149–152. doi: 10.1002/ajh.21340. [DOI] [PubMed] [Google Scholar]
- 32.Bacher U, Zander AR, Haferlach T, Schnittger S, Fehse B, Kroger N. Minimal residual disease diagnostics in myeloid malignancies in the post transplant period. Bone Marrow Transplant. 2008;42(3):145–157. doi: 10.1038/bmt.2008.185. [DOI] [PubMed] [Google Scholar]
- 33.Sramkova L, Muzikova K, Fronkova E, et al. Detectable minimal residual disease before allogeneic hematopoietic stem cell transplantation predicts extremely poor prognosis in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;48(1):93–100. doi: 10.1002/pbc.20794. [DOI] [PubMed] [Google Scholar]
- 34.Candoni A, Tiribelli M, Toffoletti E, et al. Quantitative assessment of WT1 gene expression after allogeneic stem cell transplantation is a useful tool for monitoring minimal residual disease in acute myeloid leukemia. Eur J Haematol. 2009;82(1):61–68. doi: 10.1111/j.1600-0609.2008.01158.x. [DOI] [PubMed] [Google Scholar]
- 35.Pollyea DA, Artz AS, Stock W, et al. Outcomes of patients with AML and MDS who relapse or progress after reduced intensity allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2007;40(11):1027–1032. doi: 10.1038/sj.bmt.1705852. [DOI] [PubMed] [Google Scholar]
- 36.Arellano ML, Langston A, Winton E, Flowers CR, Waller EK. Treatment of relapsed acute leukemia after allogeneic transplantation: a single center experience. Biol Blood Marrow Transplant. 2007;13(1):116–123. doi: 10.1016/j.bbmt.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 37•.Barrett AJ, Joshi R, Tew C. How should acute lymphoblastic leukaemia relapsing after bone-marrow transplantation be treated? Lancet. 1985;1(8439):1188–1191. doi: 10.1016/s0140-6736(85)92865-x. Efficacy of low-intensity treatment to control relapsed acute lymphoblastic leukemia. [DOI] [PubMed] [Google Scholar]
- 38.Abrahamsson J, Clausen N, Gustafsson G, et al. Improved outcome after relapse in children with acute myeloid leukaemia. Br J Haematol. 2007;136(2):229–236. doi: 10.1111/j.1365-2141.2006.06419.x. [DOI] [PubMed] [Google Scholar]
- 39•.Hijiya N, Gaynon P, Barry E, et al. A multi-center phase I study of clofarabine, etoposide and cyclophosphamide in combination in pediatric patients with refractory or relapsed acute leukemia. Leukemia. 2009;23(12):2259–2264. doi: 10.1038/leu.2009.185. Potential of clofarabine to treat relapsed acute leukemia after stem cell transplant (SCT) [DOI] [PubMed] [Google Scholar]
- 40.Safaian NN, Czibere A, Bruns I, et al. Sorafenib (Nexavar) induces molecular remission and regression of extramedullary disease in a patient with FLT3-ITD+ acute myeloid leukemia. Leuk Res. 2009;33(2):348–350. doi: 10.1016/j.leukres.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Tiribelli M, Sperotto A, Candoni A, Simeone E, Buttignol S, Fanin R. Nilotinib and donor lymphocyte infusion in the treatment of Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) relapsing after allogeneic stem cell transplantation and resistant to imatinib. Leuk Res. 2009;33(1):174–177. doi: 10.1016/j.leukres.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 42.Imahashi N, Tokunaga M, Nishiwaki S, Yanagisawa M, Ozawa Y, Miyamura K. Successful treatment with imatinib-combined chemotherapy for relapsed Philadelphia-positive acute lymphoblastic leukemia after allogeneic bone marrow transplantation. Rinsho Ketsueki. 2009;50(11):1612–1615. [PubMed] [Google Scholar]
- 43.Jabbour E, Giralt S, Kantarjian H, et al. Low-dose azacitidine after allogeneic stem cell transplantation for acute leukemia. Cancer. 2009;115(9):1899–1905. doi: 10.1002/cncr.24198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ravandi F, Kantarjian H, Cohen A, et al. Decitabine with allogeneic peripheral blood stem cell transplantation in the therapy of leukemia relapse following a prior transplant: results of a Phase I study. Bone Marrow Transplant. 2001;27(12):1221–1225. doi: 10.1038/sj.bmt.1703028. [DOI] [PubMed] [Google Scholar]
- 45.Ford CD, Asch J, Konopa K, Petersen FB. CR with lenalidomide in del(5)(q13q33) AML relapsing after allogeneic hematopoietic SCT. Bone Marrow Transplant. 2010;45(2):403–404. doi: 10.1038/bmt.2009.146. [DOI] [PubMed] [Google Scholar]
- 46•.Levine JE, Barrett AJ, Zhang MJ, et al. Donor leukocyte infusions to treat hematologic malignancy relapse following allo-SCT in a pediatric population. Bone Marrow Transplant. 2008;42(3):201–205. doi: 10.1038/bmt.2008.135. Identifies a small but significant benefit for donor leukocyte infusion as treatment for relapse in a pediatric population. [DOI] [PubMed] [Google Scholar]
- 47.Schmid C, Labopin M, Nagler A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007;25(31):4938–4945. doi: 10.1200/JCO.2007.11.6053. [DOI] [PubMed] [Google Scholar]
- 48•.Lubbert M, Bertz H, Wasch R, et al. Efficacy of a 3-day, low-dose treatment with 5-azacytidine followed by donor lymphocyte infusions in older patients with acute myeloid leukemia or chronic myelomonocytic leukemia relapsed afterallografting. Bone Marrow Transplant. 2009;45(4):627–632. doi: 10.1038/bmt.2009.222. Emerging role of demethylating agents in maintaining control of myelodysplastic syndrome after SCT. [DOI] [PubMed] [Google Scholar]
- 49.Levine JE, Braun T, Penza SL, et al. Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem-cell transplantation. J Clin Oncol. 2002;20(2):405–412. doi: 10.1200/JCO.2002.20.2.405. [DOI] [PubMed] [Google Scholar]
- 50••.Savani BN, Mielke S, Reddy N, Goodman S, Jagasia M, Rezvani K. Management of relapse after allo-SCT for AML and the role of second transplantation. Bone Marrow Transplant. 2009;44(12):769–777. doi: 10.1038/bmt.2009.300. Comprehensive up-to-date review of management approaches for acute myeloid leukemia relapsing after SCT. [DOI] [PubMed] [Google Scholar]
- 51•.Thakar MS, Forman SJ. ASH evidence-based guidelines: is there a role for second allogeneic transplant after relapse? Hematology Am Soc Hematol Educ Program. 2009:414–418. doi: 10.1182/asheducation-2009.1.414. Balanced review of treatment options for relapsed leukemia. [DOI] [PubMed] [Google Scholar]
- 52.Kurosawa S, Fukuda T, Tajima K, et al. Outcome of 93 patients with relapse or progression following allogeneic hematopoietic cell transplantation. Am J Hematol. 2009;84(12):815–820. doi: 10.1002/ajh.21555. [DOI] [PubMed] [Google Scholar]
- 53.Hartwig M, Ocheni S, Asenova S, et al. Second allogeneic stem cell transplantation in myeloid malignancies. Acta Haematol. 2009;122(4):185–192. doi: 10.1159/000253025. [DOI] [PubMed] [Google Scholar]
- 54.Tringali S, Vasta S, Scime R, Catania P, Cavallaro AM, Majolino I. Testicular relapse of AML during chronic graft-versus-host disease induced by donor leukocyte infusion. Haematologica. 1996;81(4):339–342. [PubMed] [Google Scholar]
- 55•.Szomor A, Passweg JR, Tichelli A, Hoffmann T, Speck B, Gratwohl A. Myeloid leukemia and myelodysplastic syndrome relapsing as granulocytic sarcoma (chloroma) after allogeneic bone marrow transplantation. Ann Hematol. 1997;75(5–6):239–241. doi: 10.1007/s002770050350. Detailed clinical presentations of extramedullary relapse after SCT. [DOI] [PubMed] [Google Scholar]
- 56.Kolb HJ, Mittermuller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76(12):2462–2465. [PubMed] [Google Scholar]
- 57.Chalandon Y, Passweg JR, Schmid C, et al. Outcome of patients developing GVHD after given to treat CML relapse: a study by the chronic leukemia working party of the EBMT. Bone Marrow Transplant. 2010;45(3):558–564. doi: 10.1038/bmt.2009.177. [DOI] [PubMed] [Google Scholar]
- 58.Raiola AM, Van Lint MT, Valbonesi M, et al. Factors predicting response and graft-versus-host disease after donor lymphocyte infusions: a study on 593 infusions. Bone Marrow Transplant. 2003;31(8):687–693. doi: 10.1038/sj.bmt.1703883. [DOI] [PubMed] [Google Scholar]
- 59•.Mackinnon S, Papadopoulos EB, Carabasi MH, et al. Adoptive immunotherapy evaluating escalating doses of donor leukocytes for relapse of chronic myeloid leukemia after bone marrow transplantation: separation of graft-versus-leukemia responses from graft-versus-host disease. Blood. 1995;86(4):1261–1268. Important dose-finding study that formed the basis for cell dose selection for treating relapsed CML at 1 × 107 CD3 cells/kg. [PubMed] [Google Scholar]
- 60.Keil F, Haas OA, Fritsch G, et al. Donor leukocyte infusion for leukemic relapse after allogeneic marrow transplantation: lack of residual donor hematopoiesis predicts aplasia. Blood. 1997;89(9):3113–3117. [PubMed] [Google Scholar]
- 61••.Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86(5):2041–2050. Comprehensive review of treatments describing different susceptibilities of acute and chronic hematological malignancies to control by donor lymphocyte infusions. [PubMed] [Google Scholar]
- 62.Collins RH, Jr, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15(2):433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 63•.Kantarjian HM, O’Brien S, Cortes JE, et al. Imatinib mesylate therapy for relapse after allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood. 2002;100(5):1590–1595. Describes the efficacy of tyrosine kinase inhibitors to control CML relapsing after SCT. [PubMed] [Google Scholar]
- 64.Barrett AJ, Locatelli F, Treleaven JG, Gratwohl A, Szydlo R, Zwaan FE. Second transplants for leukaemic relapse after bone marrow transplantation: high early mortality but favourable effect of chronic GVHD on continued remission. A report by the EBMT Leukaemia Working Party. Br J Haematol. 1991;79(4):567–574. doi: 10.1111/j.1365-2141.1991.tb08083.x. [DOI] [PubMed] [Google Scholar]
- 65.Crawley C, Iacobelli S, Bjorkstrand B, Apperley JF, Niederwieser D, Gahrton G. Reduced-intensity conditioning for myeloma: lower nonrelapse mortality but higher relapse rates compared with myeloablative conditioning. Blood. 2007;109(8):3588–3594. doi: 10.1182/blood-2006-07-036848. [DOI] [PubMed] [Google Scholar]
- 66.Hari P, Carreras J, Zhang MJ, et al. Allogeneic transplants in follicular lymphoma: higher risk of disease progression after reduced-intensity compared to myeloablative conditioning. Biol Blood Marrow Transplant. 2008;14(2):236–245. doi: 10.1016/j.bbmt.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ringden O, Karlsson H, Olsson R, Omazic B, Uhlin M. The allogeneic graft-versus-cancer effect. Br J Haematol. 2009;147(5):614–633. doi: 10.1111/j.1365-2141.2009.07886.x. [DOI] [PubMed] [Google Scholar]
- 68.Grigg A, Ritchie D. Graft-versus-lymphoma effects: clinical review, policy proposals, and immunobiology. Biol Blood Marrow Transplant. 2004;10(9):579–590. doi: 10.1016/j.bbmt.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 69•.Khouri IF, McLaughlin P, Saliba RM, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111(12):5530–5536. doi: 10.1182/blood-2008-01-136242. Effective chemotherapy control of relapsed lymphoma in the rituximab era. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Escalon MP, Champlin RE, Saliba RM, et al. Nonmyeloablative allogeneic hematopoietic transplantation: a promising salvage therapy for patients with non-Hodgkin’s lymphoma whose disease has failed a prior autologous transplantation. J Clin Oncol. 2004;22(12):2419–2423. doi: 10.1200/JCO.2004.09.092. [DOI] [PubMed] [Google Scholar]
- 71.Khouri IF, Lee MS, Saliba RM, et al. Nonablative allogeneic stem-cell transplantation for advanced/recurrent mantle-cell lymphoma. J Clin Oncol. 2003;21(23):4407–4412. doi: 10.1200/JCO.2003.05.501. [DOI] [PubMed] [Google Scholar]
- 72.Mehta J, Singhal S. Graft-versus-myeloma. Bone Marrow Transplant. 1998;22(9):835–843. doi: 10.1038/sj.bmt.1701459. [DOI] [PubMed] [Google Scholar]
- 73.Lokhorst HM, Schattenberg A, Cornelissen JJ, Thomas LL, Verdonck LF. Donor leukocyte infusions are effective in relapsed multiple myeloma after allogeneic bone marrow transplantation. Blood. 1997;90(10):4206–4211. [PubMed] [Google Scholar]
- 74.Salama M, Nevill T, Marcellus D, et al. Donor leukocyte infusions for multiple myeloma. Bone Marrow Transplant. 2000;26(11):1179–1184. doi: 10.1038/sj.bmt.1702685. [DOI] [PubMed] [Google Scholar]
- 75.Bloor AJ, Thomson K, Chowdhry N, et al. High response rate to donor lymphocyte infusion after allogeneic stem cell transplantation for indolent non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14(1):50–58. doi: 10.1016/j.bbmt.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 76•.Russell NH, Byrne JL, Faulkner RD, Gilyead M, Das-Gupta EP, Haynes AP. Donor lymphocyte infusions can result in sustained remissions in patients with residual or relapsed lymphoid malignancy following allogeneic haemopoietic stem cell transplantation. Bone Marrow Transplant. 2005;36(5):437–441. doi: 10.1038/sj.bmt.1705074. Efficacy of donor lymphocyte infusions to treat relapsed lymphoma. [DOI] [PubMed] [Google Scholar]
- 77.Kroger N, Alchalby H, Klyuchnikov E, et al. JAK2-V617F-triggered preemptive and salvage adoptive immunotherapy with donor-lymphocyte infusion in patients with myelofibrosis after allogeneic stem cell transplantation. Blood. 2009;113(8):1866–1868. doi: 10.1182/blood-2008-11-190975. [DOI] [PubMed] [Google Scholar]
- 78.Cavattoni I, Zabelina T, Ayuk F, et al. Pilot study of rituximab plus donor-lymphocyte infusion to prevent or treat relapse in B-cell lymphoma after allogeneic stem cell transplantation. Leuk Lymphoma. 51(1):146–148. doi: 10.3109/10428190903275594. [DOI] [PubMed] [Google Scholar]
- 79.Vesole DH, Zhang L, Flomenberg N, Greipp PR, Lazarus HM, Huff CA. A Phase II trial of autologous stem cell transplantation followed by mini-allogeneic stem cell transplantation for the treatment of multiple myeloma: an analysis of Eastern Cooperative Oncology Group ECOG E4A98 and E1A97. Biol Blood Marrow Transplant. 2009;15(1):83–91. doi: 10.1016/j.bbmt.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blair A, Goulden NJ, Libri NA, Oakhill A, Pamphilon DH. Immunotherapeutic strategies in acute lymphoblastic leukaemia relapsing after stem cell transplantation. Blood Rev. 2005;19(6):289–300. doi: 10.1016/j.blre.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 81.Armand P, Gannamaneni S, Kim HT, et al. Improved survival in lymphoma patients receiving sirolimus for graft-versus-host disease prophylaxis after allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning. J Clin Oncol. 2008;26(35):5767–5774. doi: 10.1200/JCO.2008.17.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82•.Cutler C, Kim HT, Hochberg E, et al. Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2004;10(5):328–336. doi: 10.1016/j.bbmt.2003.12.305. Important role of sirolimus in controlling lymphoma while preventing graft-versus-host disease. [DOI] [PubMed] [Google Scholar]
- 83.Lundqvist A, Abrams SI, Schrump DS, et al. Bortezomib and depsipeptide sensitize tumors to tumor necrosis factor-related apoptosis-inducing ligand: a novel method to potentiate natural killer cell tumor cytotoxicity. Cancer Res. 2006;66(14):7317–7325. doi: 10.1158/0008-5472.CAN-06-0680. [DOI] [PubMed] [Google Scholar]
- 84.Chanan-Khan AA, Cheson BD. Lenalidomide for the treatment of B-cell malignancies. J Clin Oncol. 2008;26(9):1544–1552. doi: 10.1200/JCO.2007.14.5367. [DOI] [PubMed] [Google Scholar]
- 85.Soiffer RJ, Weller E, Alyea EP, et al. CD6+ donor marrow T-cell depletion as the sole form of graft-versus-host disease prophylaxis in patients undergoing allogeneic bone marrow transplant from unrelated donors. J Clin Oncol. 2001;19(4):1152–1159. doi: 10.1200/JCO.2001.19.4.1152. [DOI] [PubMed] [Google Scholar]
- 86••.Bonini C, Ferrari G, Verzeletti S, et al. HSV–TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276(5319):1719–1724. doi: 10.1126/science.276.5319.1719. Describes new techniques to rapidly identify minor histocompatibility antigen-specific T cells for expansion and use in the adoptive T-cell therapy of relapse. [DOI] [PubMed] [Google Scholar]
- 87.Onodera M. Gene and cell therapy for relapsed leukemia after allo-stem cell transplantation. Front Biosci. 2008;13:3408–3414. doi: 10.2741/2935. [DOI] [PubMed] [Google Scholar]
- 88.Falkenburg JH, Wafelman AR, Joosten P, et al. Complete remission of accelerated phase chronic myeloid leukemia by treatment with leukemia-reactive cytotoxic T lymphocytes. Blood. 1999;94(4):1201–1208. [PubMed] [Google Scholar]
- 89.Warren EH, Fujii N, Akatsuka Y, et al. Therapy of relapsed leukemia after allogeneic hematopoietic cell transplant with T cells specific for minor histocompatibility antigens. Blood. 2010;115(19):3869–3878. doi: 10.1182/blood-2009-10-248997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bleakley M, Otterud BE, Richardt JL, et al. Leukemia-associated minor histocompatibility antigen discovery using T-cell clones isolated by in vitro stimulation of naive CD8+ T cells. Blood. 2010 doi: 10.1182/blood-2009-12-260539. blood-2009-2012-260539. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berry LJ, Moeller M, Darcy PK. Adoptive immunotherapy for cancer: the next generation of gene-engineered immune cells. Tissue Antigens. 2009;74(4):277–289. doi: 10.1111/j.1399-0039.2009.01336.x. [DOI] [PubMed] [Google Scholar]
- 92.Velardi A, Ruggeri L, Mancusi A, Aversa F, Christiansen FT. Natural killer cell allorecognition of missing self in allogeneic hematopoietic transplantation: a tool for immunotherapy of leukemia. Curr Opin Immunol. 2009;21(5):525–530. doi: 10.1016/j.coi.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 93.Cooley S, Trachtenberg E, Bergemann TL, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113(3):726–732. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stringaris K, Adams S, Uribe M, et al. Donor KIR genes 2DL5A, 2DS1 and 3DS1 are associated with a reduced rate of leukaemia relapse after HLA-identical sibling stem cell transplantation for acute myeloid leukaemia but not other haematological malignancies. Biol Blood Marrow Transplant. 2010 doi: 10.1016/j. bbmt.2010.03.004. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95•.Yong AS, Keyvanfar K, Hensel N, et al. Primitive quiescent CD34+ cells in chronic myeloid leukemia are targeted by in vitro expanded natural killer cells, which are functionally enhanced by bortezomib. Blood. 2009;113(4):875–882. doi: 10.1182/blood-2008-05-158253. Potential of combining bortezomib with natural killer cell therapy to control leukemic stem cells and treat relapsed myeloid leukemias. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Berger C, Berger M, Hackman RC, et al. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114(12):2417–2426. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu Y, Waller EK. Dichotomous role of interferon-γ in allogeneic bone marrow transplant. Biol Blood Marrow Transplant. 2009;15(11):1347–1353. doi: 10.1016/j.bbmt.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kujawski LA, Talpaz M. The role of interferon-α in the treatment of chronic myeloid leukemia. Cytokine Growth Factor Rev. 2007;18(5–6):459–471. doi: 10.1016/j.cytogfr.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 99.Waller EK. The role of sargramostim (rhGM-CSF) as immunotherapy. Oncologist. 2007;12(Suppl 2):22–26. doi: 10.1634/theoncologist.12-S2-22. [DOI] [PubMed] [Google Scholar]
- 100.Morris JC, Waldmann TA. Antibody-based therapy of leukaemia. Expert Rev Mol Med. 2009;11:e29. doi: 10.1017/S1462399409001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barrett AJ, Rezvani K. Translational mini-review series on vaccines: peptide vaccines for myeloid leukaemias. Clin Exp Immunol. 2007;148(2):189–198. doi: 10.1111/j.1365-2249.2007.03383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rezvani K, Barrett AJ. Characterizing and optimizing immune responses to leukaemia antigens after allogeneic stem cell transplantation. Best Pract Res Clin Haematol. 2008;21(3):437–453. doi: 10.1016/j.beha.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barrett J, Rezvani K. Immunotherapy: can we include vaccines with stem-cell transplantation? Nat Rev Clin Oncol. 2009;6(9):503–505. doi: 10.1038/nrclinonc.2009.115. [DOI] [PubMed] [Google Scholar]
- 104•.Miller JS, Warren EH, Vandenbrink MR, et al. NCI 1st International Workshop on the biology, prevention, and treatment of relapse after allogeneic hematopoietic stem cell transplantation: report from the committee on the biology underlying recurrence of malignant disease following allogeneic hsct: graft-versus-tumor/leukemia reaction. Biol Blood Marrow Transplant. 2010;16(5):565–586. doi: 10.1016/j.bbmt.2010.02.005. Describes a new ongoing initiative to understand and treat relapsed leukemia after SCT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105••.van den Brink MR, Porter DL, Giralt S, et al. Relapse after allogeneic hematopoietic cell therapy. Biol Blood Marrow Transplant. 2010;16(1 Suppl):S138–S145. doi: 10.1016/j.bbmt.2009.10.023. Up-to-date and comprehensive review of the mechanisms of post-transplant relapse and therapeutic challenges. [DOI] [PMC free article] [PubMed] [Google Scholar]