Abstract
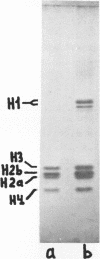
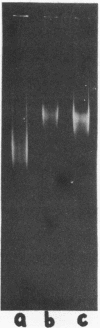
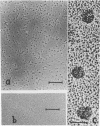
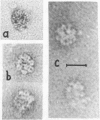
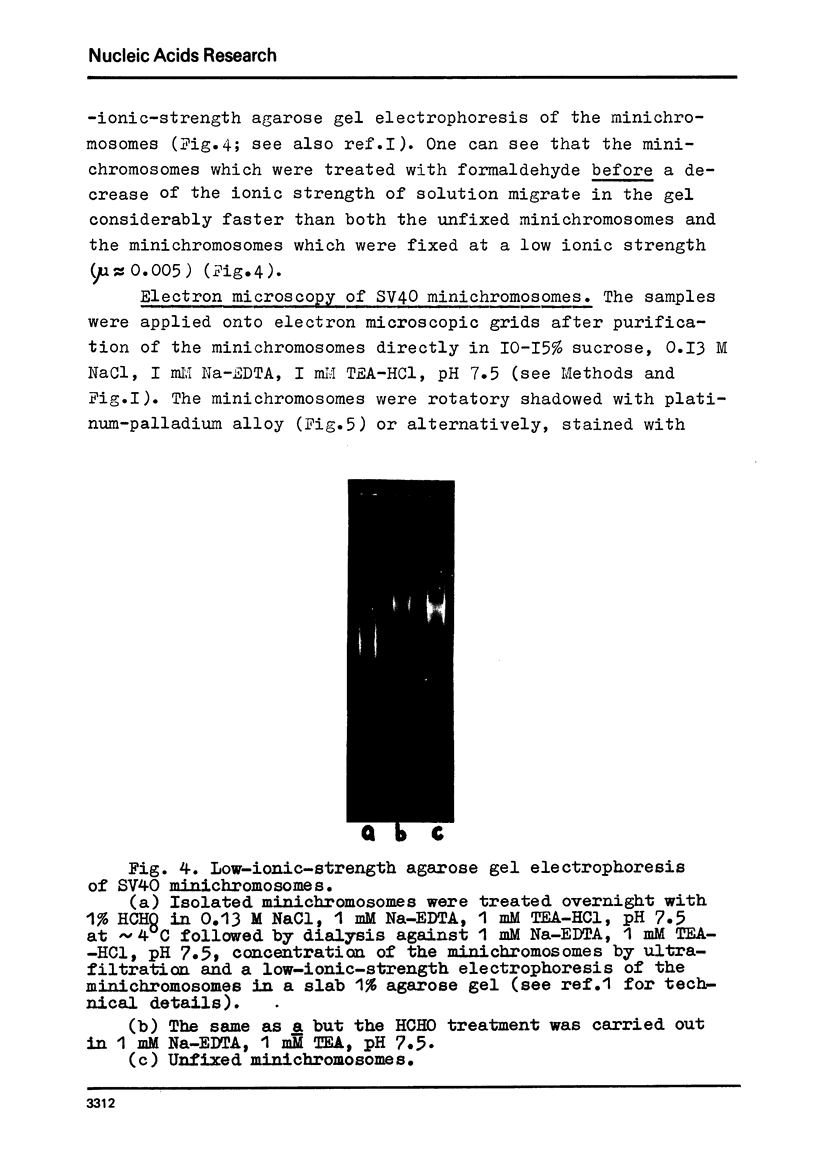
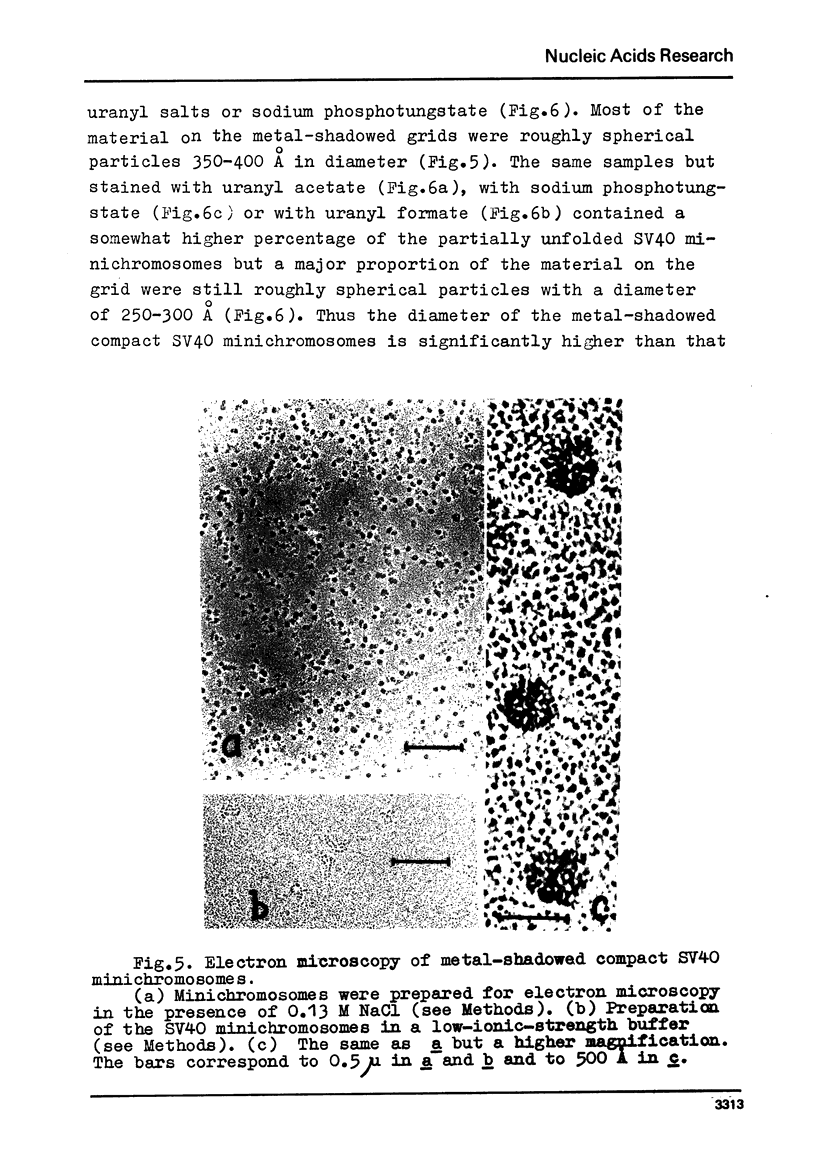
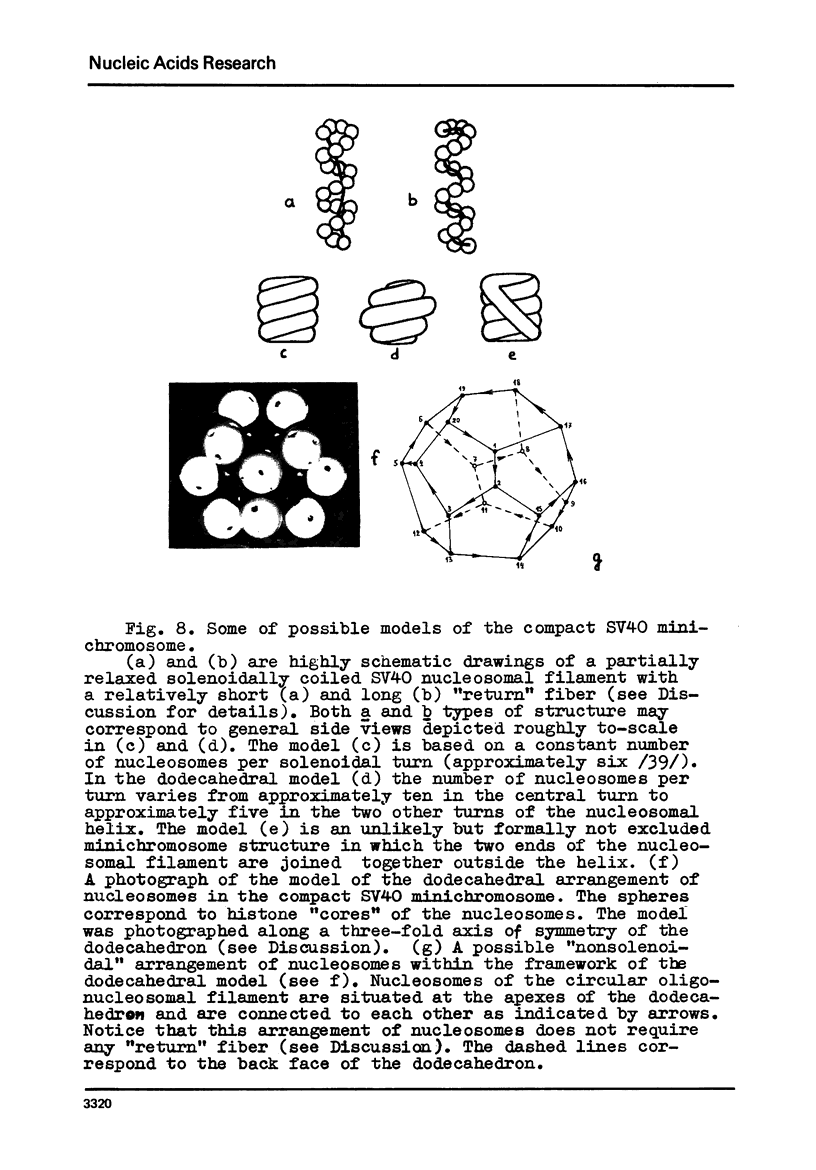
We report two new findings bearing on the "supranucleo-somal" level of the structure of the Simian Virus 40 minichromosome. I) Isolated SV40 minichromosome which contains all five histones including HI/I/ exists in solution under approximately physiological ionic conditions as a compact roughly spherical particle approximately 300 A in diameter which is capable of fitting within the virus capsid. In spite of such a compact conformation of the minichromosome individual nucleosomes can be readily visualized within the particle. Compact state of SV40 minichromosome depends on both the presence of histone HI and maintenance of approximately physiological ionic strength of solution (micron approximately 0.15). Removal of HI results in a conversion of the compact minichromosomes into an extended (circular beaded) structure. 2) The compact form of the SV40 minichromosome in contract to its circular beaded form is virtually completely resistant to staphylococcal nuclease, strongly suggesting that in particular nuclease-sensitive parts of the internucleosomal DNA regions are not exposed on the outside of the compact SV40 minichromosome. On the other hand, DNase I which is known to attack both inter-and intranucleosomal DNA in the chronatin /2,3/ readily digests the compact form of the SV40 minichromosome. Possible models of the compact minichromosome and implications for higher order structures of the cellular chromatin are discussed.
Full text
PDF




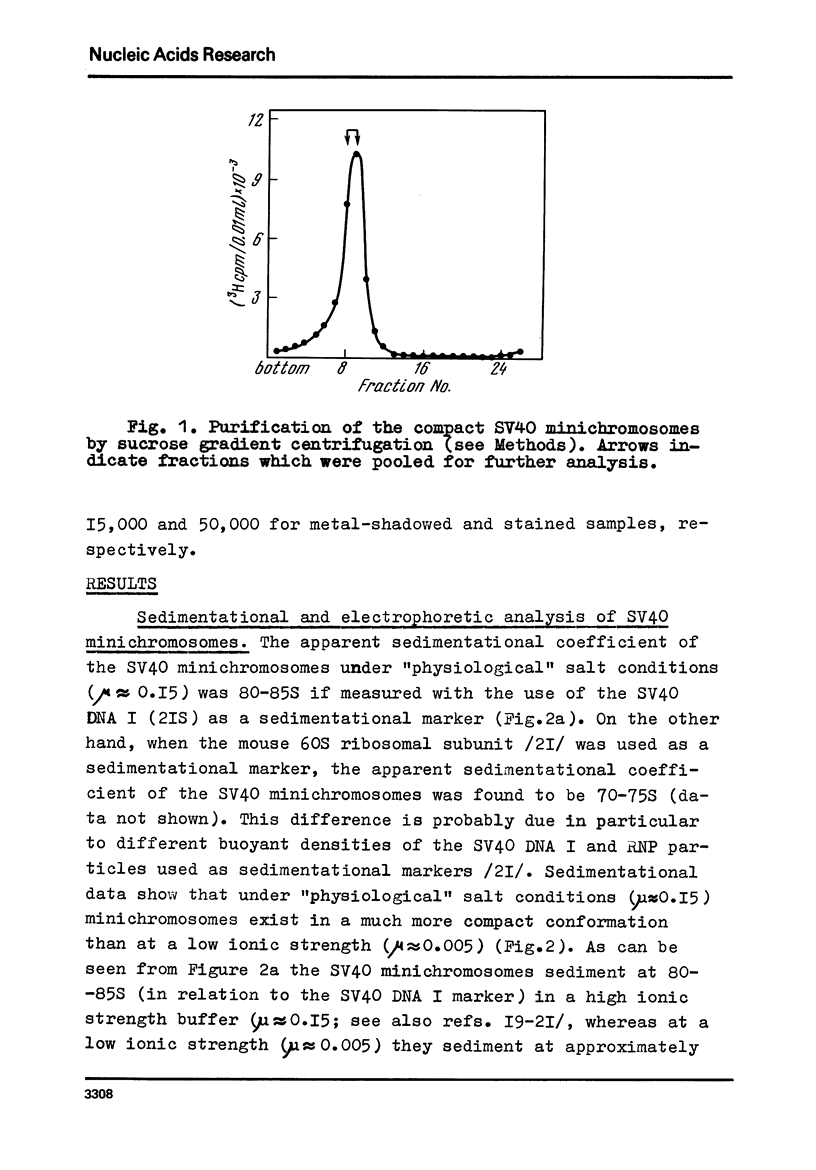
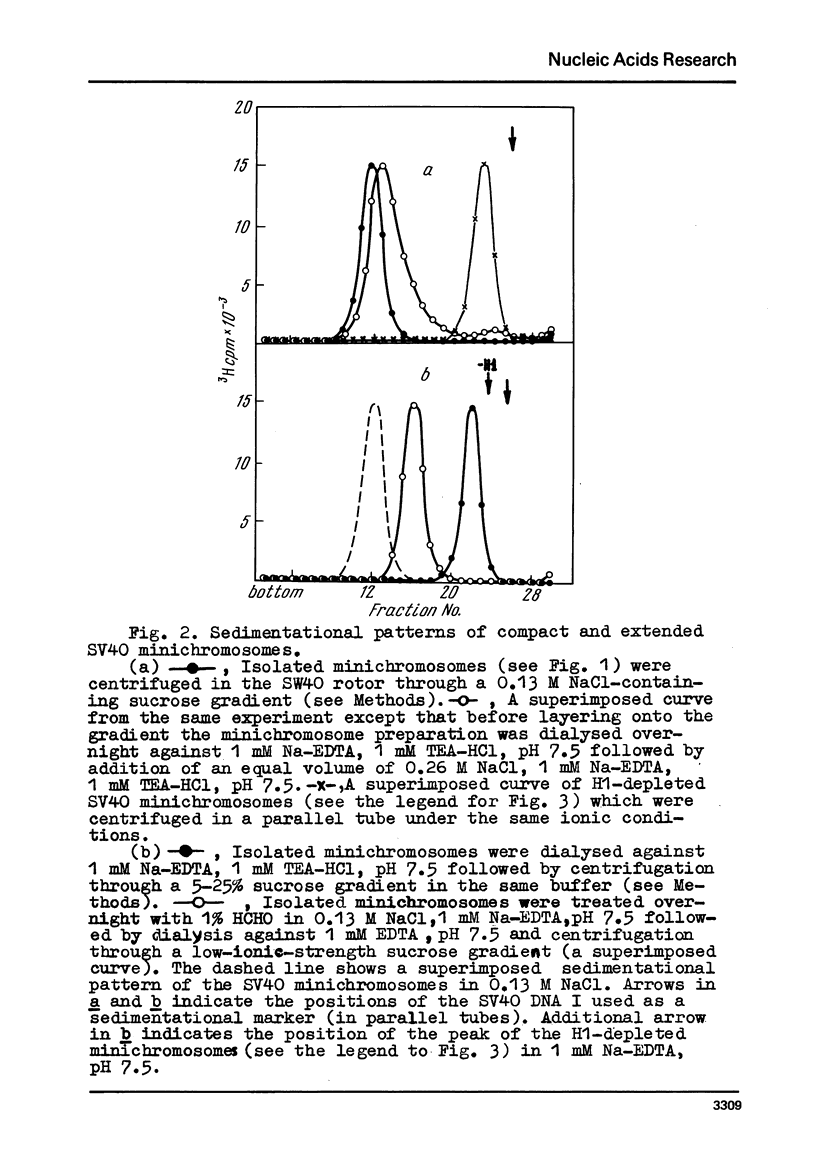













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. P., Boseley P. G., Bradbury E. M., Ibel K. The subunit structure of the eukaryotic chromosome. Nature. 1975 Jan 24;253(5489):245–249. doi: 10.1038/253245a0. [DOI] [PubMed] [Google Scholar]
- Bellard M., Oudet P., Germond J. E., Chambon P. Subunit structure of simian-virus-40 minichromosome. Eur J Biochem. 1976 Nov 15;70(2):543–553. doi: 10.1111/j.1432-1033.1976.tb11046.x. [DOI] [PubMed] [Google Scholar]
- Benyajati C., Worcel A. Isolation, characterization, and structure of the folded interphase genome of Drosophila melanogaster. Cell. 1976 Nov;9(3):393–407. doi: 10.1016/0092-8674(76)90084-2. [DOI] [PubMed] [Google Scholar]
- Christiansen G., Landers T., Griffith J., Berg P. Characterization of components released by alkali disruption of simian virus 40. J Virol. 1977 Mar;21(3):1079–1084. doi: 10.1128/jvi.21.3.1079-1084.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. H. Linking numbers and nucleosomes. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2639–2643. doi: 10.1073/pnas.73.8.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther R. A., Amos L. A. Three-dimensional image reconstructions of some small spherical viruses. Cold Spring Harb Symp Quant Biol. 1972;36:489–494. doi: 10.1101/sqb.1972.036.01.062. [DOI] [PubMed] [Google Scholar]
- Fey G., Hirt B. Fingerprints of polyoma virus proteins and mouse histones. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):235–241. doi: 10.1101/sqb.1974.039.01.030. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Woodhead L., Johns E. W. The presence of high mobility group non-histone chromatin proteins in isolated nucleosomes. FEBS Lett. 1977 Jan 15;73(1):85–88. [PubMed] [Google Scholar]
- Greil W., Igo-Kemenes T., Zachau H. G. Nuclease digestion in between and within nucleosomes. Nucleic Acids Res. 1976 Oct;3(10):2633–2644. doi: 10.1093/nar/3.10.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D. Chromatin structure: deduced from a minichromosome. Science. 1975 Mar 28;187(4182):1202–1203. doi: 10.1126/science.187.4182.1202. [DOI] [PubMed] [Google Scholar]
- Griffith J., Dieckmann M., Berg P. Electron microscope localization of a protein bound near the origin of simian virus 40 DNA replication. J Virol. 1975 Jan;15(1):167–172. doi: 10.1128/jvi.15.1.167-172.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller W. Determination of the number of superhelical turns in simian virus 40 DNA by gel electrophoresis. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4876–4880. doi: 10.1073/pnas.72.12.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake R. S., Barban S., Salzman N. P. Resolutions and identification of the core deoxynucleoproteins of the simian virus 40. Biochem Biophys Res Commun. 1973 Sep 18;54(2):640–647. doi: 10.1016/0006-291x(73)91471-x. [DOI] [PubMed] [Google Scholar]
- Lerman L. S. Chromosomal analogues: long-range order in psi-condensed DNA. Cold Spring Harb Symp Quant Biol. 1974;38:59–73. doi: 10.1101/sqb.1974.038.01.009. [DOI] [PubMed] [Google Scholar]
- Meinke W., Hall M. R., Goldstein D. A. Proteins in intracellular simian virus 40 nucleoportein complexes: comparison with simian virus 40 core proteins. J Virol. 1975 Mar;15(3):439–448. doi: 10.1128/jvi.15.3.439-448.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans D., Danna K. J. Specific origin in SV40 DNA replication. Nat New Biol. 1972 Apr 19;236(68):200–202. doi: 10.1038/newbio236200a0. [DOI] [PubMed] [Google Scholar]
- Noll M. Internal structure of the chromatin subunit. Nucleic Acids Res. 1974 Nov;1(11):1573–1578. doi: 10.1093/nar/1.11.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins A. L., Olins D. E. Spheroid chromatin units (v bodies). Science. 1974 Jan 25;183(4122):330–332. doi: 10.1126/science.183.4122.330. [DOI] [PubMed] [Google Scholar]
- Olins A. L., Senior M. B., Olins D. E. Ultrastructural features of chromatin nu bodies. J Cell Biol. 1976 Mar;68(3):787–793. doi: 10.1083/jcb.68.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudet P., Gross-Bellard M., Chambon P. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell. 1975 Apr;4(4):281–300. doi: 10.1016/0092-8674(75)90149-x. [DOI] [PubMed] [Google Scholar]
- Salzman N. P., Fareed G. C., Sebring E. D., Thoren M. M. The mechanism of SV40 DNA replication. Cold Spring Harb Symp Quant Biol. 1974;38:257–265. doi: 10.1101/sqb.1974.038.01.029. [DOI] [PubMed] [Google Scholar]
- Shaw B. R., Herman T. M., Kovacic R. T., Beaudreau G. S., Van Holde K. E. Analysis of subunit organization in chicken erythrocyte chromatin. Proc Natl Acad Sci U S A. 1976 Feb;73(2):505–509. doi: 10.1073/pnas.73.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shure M., Vinograd J. The number of superhelical turns in native virion SV40 DNA and minicol DNA determined by the band counting method. Cell. 1976 Jun;8(2):215–226. doi: 10.1016/0092-8674(76)90005-2. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Felsenfeld G. Pancreatic DNAase cleavage sites in nuclei. Cell. 1977 Mar;10(3):537–547. doi: 10.1016/0092-8674(77)90040-x. [DOI] [PubMed] [Google Scholar]
- Su R. T., DePamphilis M. L. In vitro replication of simian virus 40 DNA in a nucleoprotein complex. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3466–3470. doi: 10.1073/pnas.73.10.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Holde K. E., Sahasrabuddhe C. G., Shaw B. R. A model for particulate structure in chromatin. Nucleic Acids Res. 1974 Nov;1(11):1579–1586. doi: 10.1093/nar/1.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Chumackov P. M., Georgiev G. P. Minichromosome of simian virus 40: presence of histone HI. Nucleic Acids Res. 1976 Aug;3(8):2101–2113. doi: 10.1093/nar/3.8.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Georgiev G. P. Heterogeneity of chromatin subunits in vitro and location of histone H1. Nucleic Acids Res. 1976 Feb;3(2):477–492. doi: 10.1093/nar/3.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V. Studies on chromatin. IV. Evidence for a toroidal shape of chromatin subunits. Mol Biol Rep. 1975 Oct;2(3):247–254. doi: 10.1007/BF00356995. [DOI] [PubMed] [Google Scholar]