Abstract
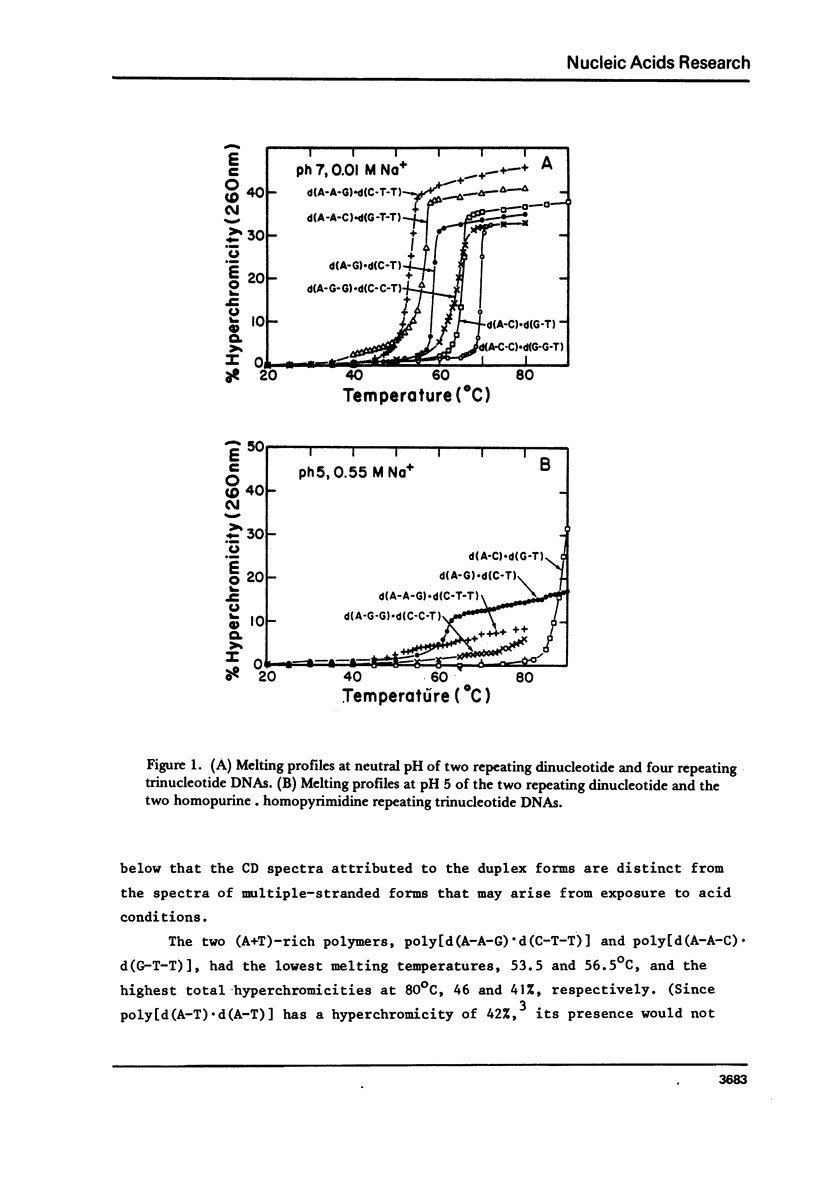
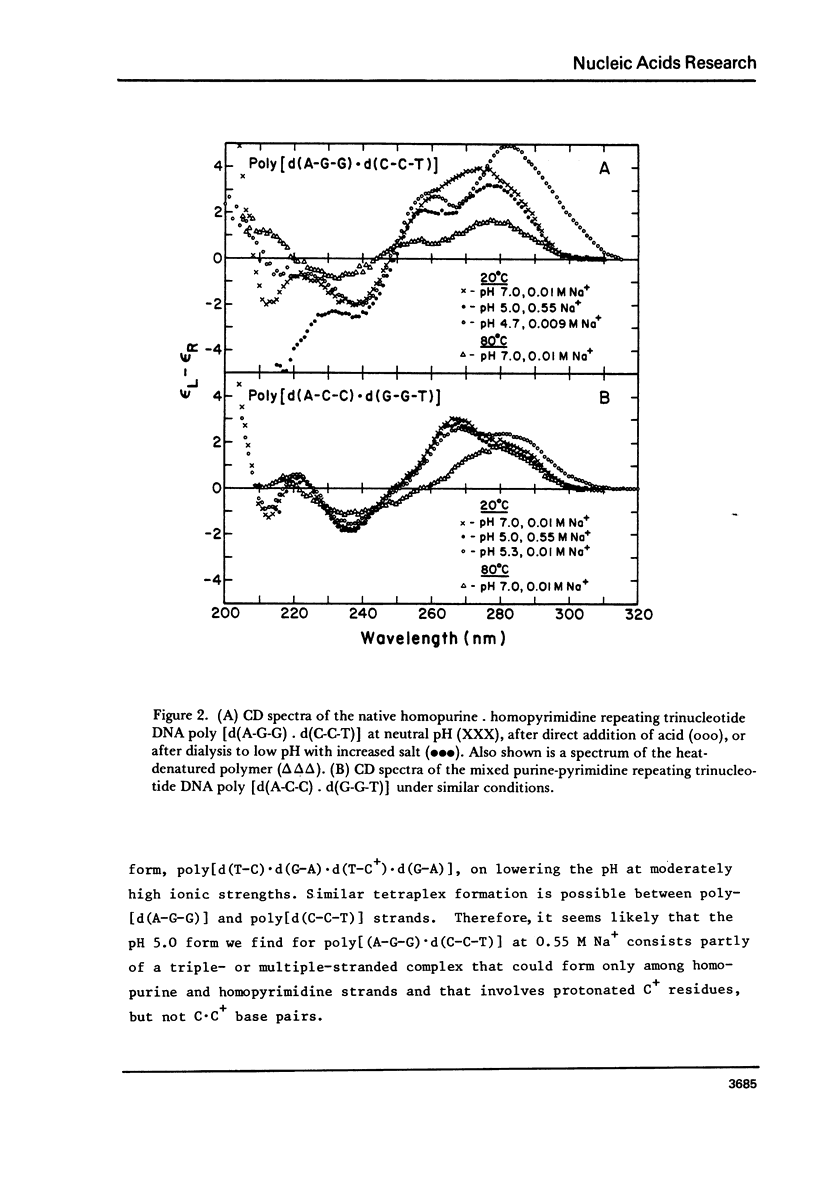
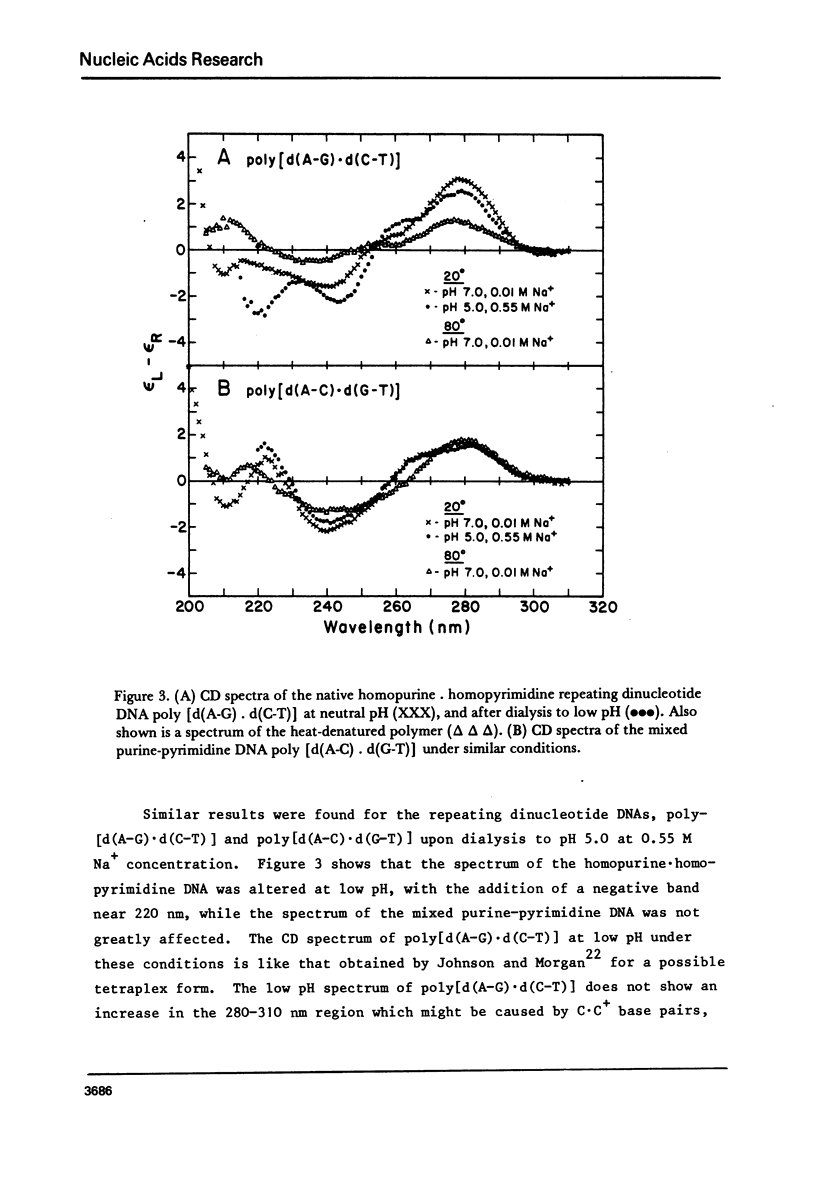
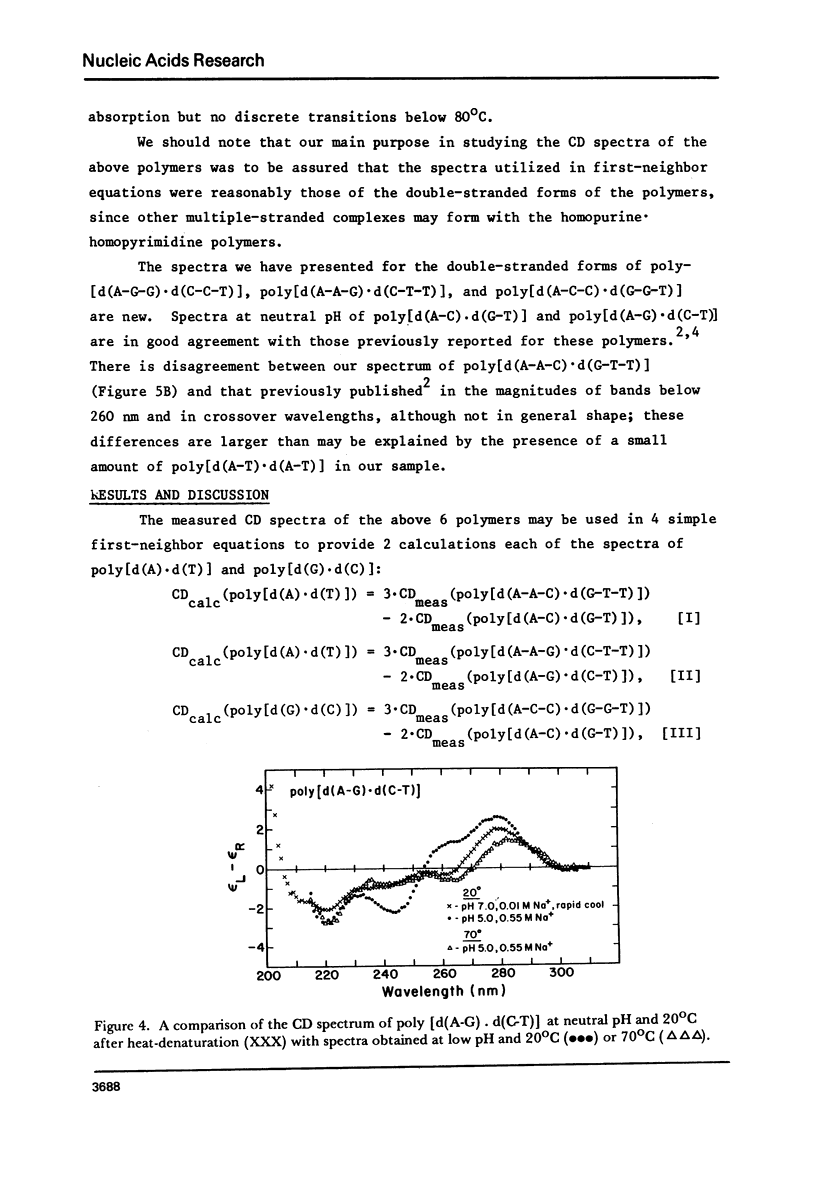
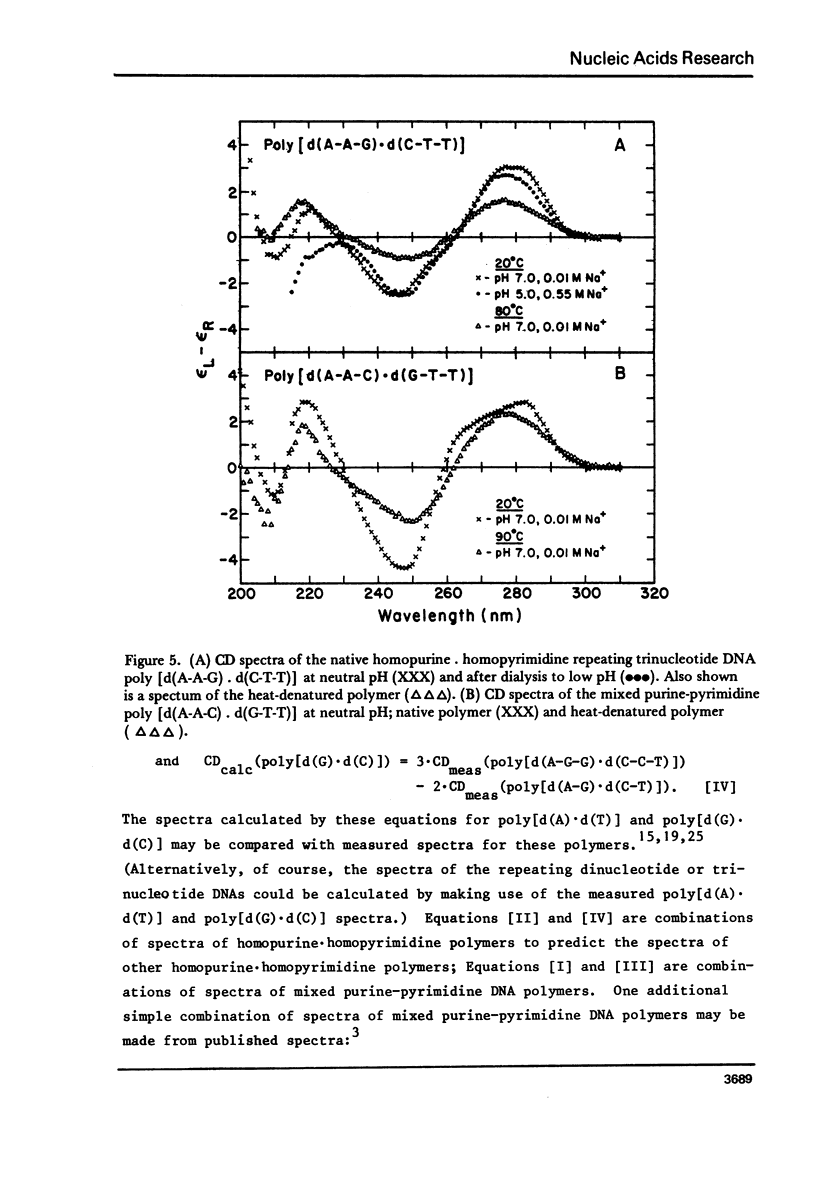
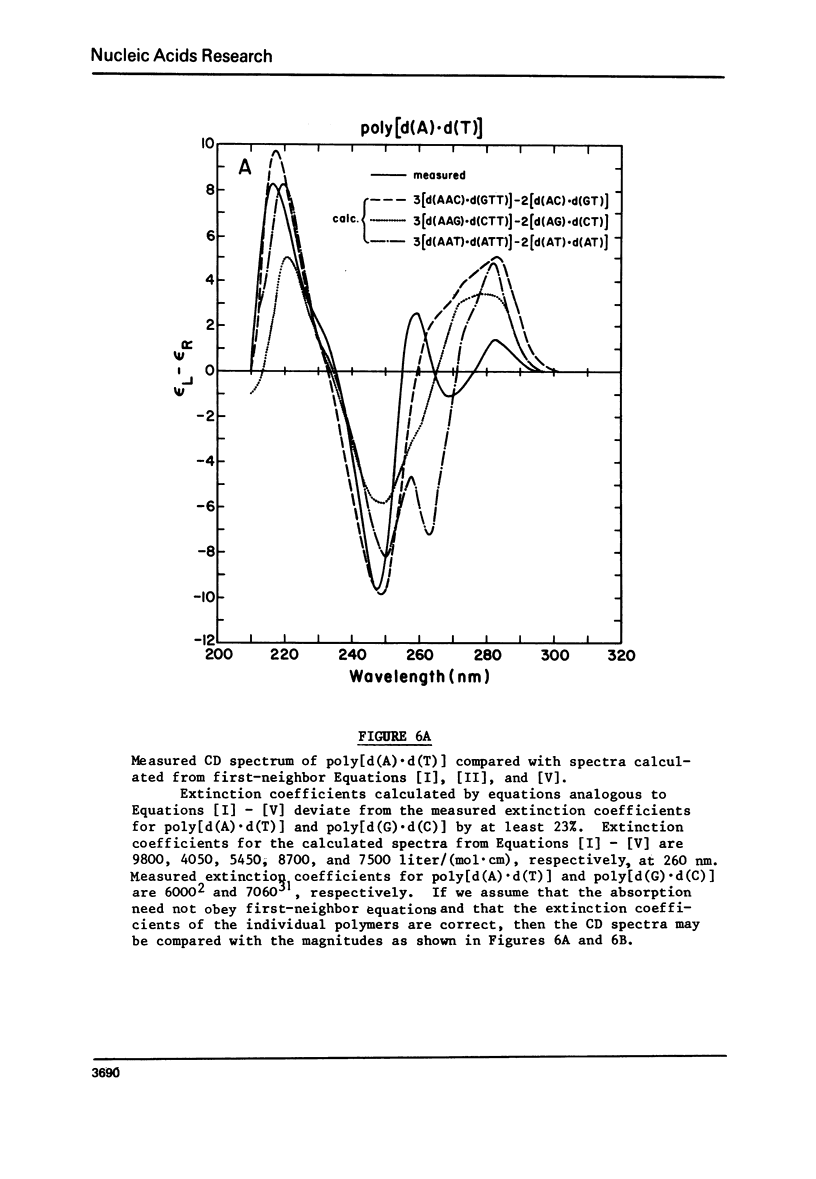
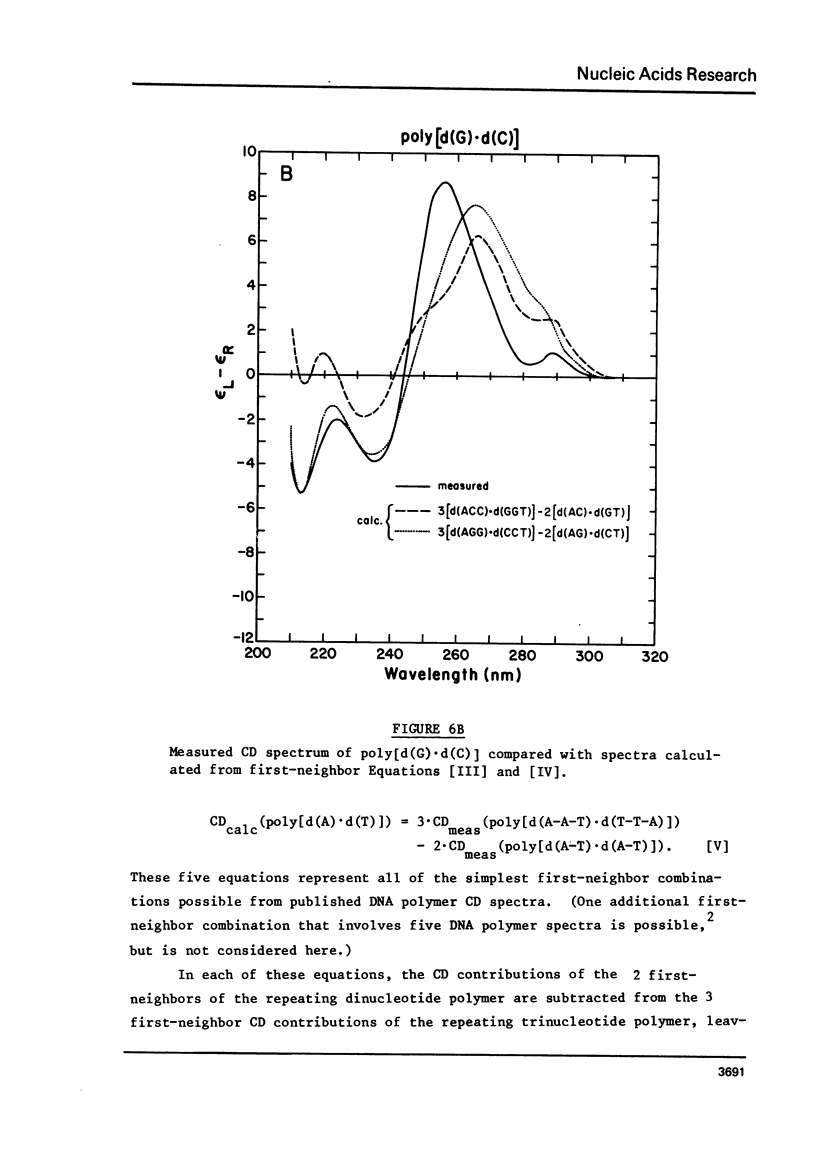
We have obtained the ultraviolet circular dichroism spectra of two repeating trinucleotide DNAs, poly [d(A-G-G).d(C-C-T)] and poly[d(A-A-G).d(C-T-T)], that have all purines on one strand and all pyrimidines on the other. These spectra, together with spectra of other synthetic polymers, can be combined to give 3 first-neighbor calculations of the spectrum of poly[d(A).d(T)] and 2 first-neighbor calculations of the spectrum of poly [d(G).d(C)]. The results show (1) that first-neighbor calculations utilizing only spectra of homopurine.homopyrimidine DNA sequences are no more accurate than are similar calculations that involve spectra of mixed purine-pyrimidine sequences, demonstrating that double-stranded homopurine.homopyrimidine sequences do not obviously belong to a special class of secondary conformations, and (2) that the wavelength region above 250 nm in the CD spectra of synthetic DNAs is least predictable from first-neighbor equations, probably because this region is especially sensitive to sequence-dependent conformational differences.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen F. S., Gray D. M., Roberts G. P., Tinoco I., Jr The ultraviolet circular dichroism of some natural DNAs and an analysis of the spectra for sequence information. Biopolymers. 1972;11(4):853–879. doi: 10.1002/bip.1972.360110410. [DOI] [PubMed] [Google Scholar]
- Arnott S., Arnott S. The sequence dependence of circular dichroism spectra of DNA duplexes. Nucleic Acids Res. 1975 Sep;2(9):1493–1502. doi: 10.1093/nar/2.9.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott S., Selsing E. Structures for the polynucleotide complexes poly(dA) with poly (dT) and poly(dT) with poly(dA) with poly (dT). J Mol Biol. 1974 Sep 15;88(2):509–521. doi: 10.1016/0022-2836(74)90498-7. [DOI] [PubMed] [Google Scholar]
- Cech C. L., Tinoco I., Jr Circular dichroism calculations for double-stranded polynucleotides of repeating sequence. Biopolymers. 1977 Jan;16(1):43–65. doi: 10.1002/bip.1977.360160105. [DOI] [PubMed] [Google Scholar]
- Gray D. M. A circular dichroism study of poly dG, poly dC, and poly dG:dC. Biopolymers. 1974;13(10):2087–2102. doi: 10.1002/bip.1974.360131011. [DOI] [PubMed] [Google Scholar]
- Gray D. M., Hamilton F. D., Vaughan M. R. The analysis of circular dichroism spectra of natural DNAs using spectral components from synthetic DNAs. Biopolymers. 1978 Jan;17(1):85–106. doi: 10.1002/bip.1978.360170107. [DOI] [PubMed] [Google Scholar]
- Gray D. M., Ratliff R. L. Circular dichroism evidence for G-U and G-T base pairing in poly[r(G-U)] and poly[d(G-T)]. Biopolymers. 1977 Jun;16(6):1331–1342. doi: 10.1002/bip.1977.360160613. [DOI] [PubMed] [Google Scholar]
- Gray D. M., Ratliff R. L. Circular dichroism spectra of poly[d(AC):d(GT)], poly[r(AC):r(GU)], and hybrids poly[d(AC):r(GU)] and poly[r(AC):d(GT)] in the presence of ethanol. Biopolymers. 1975 Mar;14(3):487–498. doi: 10.1002/bip.1975.360140305. [DOI] [PubMed] [Google Scholar]
- Gray D. M., Ratliff R. L., Williams D. L. The circular dichroism of poly d(AAT):d(ATT) and poly r(AAU):r(AUU). A test of the first-neighbor hypothesis. Biopolymers. 1973 Jun;12(6):1233–1245. doi: 10.1002/bip.1973.360120604. [DOI] [PubMed] [Google Scholar]
- Gray D. M., Taylor T. N., Lang D. Dehydrated circular DNA: circular dichroism of molecules in ethanolic solutions. Biopolymers. 1978 Jan;17(1):145–157. doi: 10.1002/bip.1978.360170111. [DOI] [PubMed] [Google Scholar]
- Greve J., Maestre M. F., Levin A. Circular dichroism of adenine and thymine containing synthetic polynucleotides. Biopolymers. 1977 Jul;16(7):1489–1504. doi: 10.1002/bip.1977.360160709. [DOI] [PubMed] [Google Scholar]
- Greve J., Maestre M. F., Moise H., Hosoda J. Circular dichroism study of the interaction between T4 gene 32 protein and polynucleotides. Biochemistry. 1978 Mar 7;17(5):887–893. doi: 10.1021/bi00598a022. [DOI] [PubMed] [Google Scholar]
- INMAN R. B., BALDWIN R. L. HELIX--RANDOM COIL TRANSITIONS IN DNA HOMOPOLYMER PAIRS. J Mol Biol. 1964 Apr;8:452–469. doi: 10.1016/s0022-2836(64)80003-6. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Minyat E. E., Frank-Kamenetskii M. D., Schyolkina A. K. The B to A transition of DNA in solution. J Mol Biol. 1974 Aug 25;87(4):817–833. doi: 10.1016/0022-2836(74)90086-2. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Schyolkina A. K., Poletayev A. I. Different conformations of double-stranded nucleic acid in solution as revealed by circular dichroism. Biopolymers. 1973;12(1):89–110. doi: 10.1002/bip.1973.360120109. [DOI] [PubMed] [Google Scholar]
- Johnson D., Morgan A. R. Unique structures formed by pyrimidine-purine DNAs which may be four-stranded. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1637–1641. doi: 10.1073/pnas.75.4.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge R. Nucleic acids and polynucleotides. J Cell Physiol. 1969 Oct;74(2 Suppl):1–20. doi: 10.1002/jcp.1040740403. [DOI] [PubMed] [Google Scholar]
- Maestre M. F., Wang J. C. Circular dichroism of superhelical DNA. Biopolymers. 1971 Jun;10(6):1021–1030. doi: 10.1002/bip.360100608. [DOI] [PubMed] [Google Scholar]
- Marck C., Guschlbauer W. A simple method for the computation of first neighbour frequencies of DNAs from CD spectra. Nucleic Acids Res. 1978 Jun;5(6):2013–2031. doi: 10.1093/nar/5.6.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marck C., Thiele D. Poly(dG).poly(dC) at neutral and alkaline pH: the formation of triple stranded poly(dG).poly(dG).poly(dC). Nucleic Acids Res. 1978 Mar;5(3):1017–1028. doi: 10.1093/nar/5.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A. R., Coulter M. B., Flintoff W. F., Paetkau V. H. Enzymatic synthesis of deoxyribonucleic acids with repeating sequences. A new repeating trinucleotide deoxyribonucleic acid, d(T-C-C)n-d(G-G-A)n. Biochemistry. 1974 Apr 9;13(8):1596–1603. doi: 10.1021/bi00705a007. [DOI] [PubMed] [Google Scholar]
- Morgan A. R., Wells R. D. Specificity of the three-stranded complex formation between double-stranded DNA and single-stranded RNA containing repeating nucleotide sequences. J Mol Biol. 1968 Oct 14;37(1):63–80. doi: 10.1016/0022-2836(68)90073-9. [DOI] [PubMed] [Google Scholar]
- Ratliff R. L., Hoard D. E., Hayes F. N., Smith D. A., Gray D. M. Preparation and properties of the repeating sequence polymers d(A-I-C)n-d(I-C-T)n and d(A-G-C)n-d(G-C-T)n. Biochemistry. 1976 Jan 13;15(1):168–176. doi: 10.1021/bi00646a026. [DOI] [PubMed] [Google Scholar]
- Tunis-Schneider M. J., Maestre M. F. Circular dichroism spectra of oriented and unoriented deoxyribonucleic acid films--a preliminary study. J Mol Biol. 1970 Sep 28;52(3):521–541. doi: 10.1016/0022-2836(70)90417-1. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E., Grant R. C., Shortle B. E., Cantor C. R. Physicochemical studies on polydeoxyribonucleotides containing defined repeating nucleotide sequences. J Mol Biol. 1970 Dec 28;54(3):465–497. doi: 10.1016/0022-2836(70)90121-x. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Luck G. Conformation and reactivity of DNA. VI. Circular dichroism studies of salt-induced conformational changes of DNAs of different base composition. Biochim Biophys Acta. 1974 Aug 15;361(1):11–32. [PubMed] [Google Scholar]


