Abstract
Induced pluripotent stem cells (iPSCs) can be formed from somatic cells by a defined set of genetic factors; however, aberrant epigenetic silencing of the imprinted Dlk1-Dio3 gene cluster often hinders their developmental potency and ability to contribute to high-grade chimerism in mice. Here, we describe an approach that allows splenic B cells activated to undergo Ig heavy-chain (IgH) class-switch recombination (CSR) to be reprogrammed into iPSCs that contribute to high-grade chimerism in mice. Treatment of naïve splenic B cells in culture with anti-CD40 plus IL-4 induces IgH CSR from IgM to IgG1 and IgE. CSR leads to irreversible IgH locus deletions wherein the IgM-producing Cμ exons are permanently excised from the B-cell genome. We find that anti-CD40 plus IL-4–activated B cells produce iPSCs that are uniformly hypermethylated in the imprinted Dlk1-Dio3 gene cluster and fail to produce chimerism in mice. However, treatment of activated B cells with the methyltransferase inhibitor 5-aza-2′-deoxycytidine before and at early stages of reprogramming attenuates hypermethylation of the Dlk1-Dio3 locus in resultant iPSCs and enables them to form high-grade chimerism in mice. These conditions allowed us to produce chimeric mice in which all mature B cells were derived entirely from IgG1-expressing B-cell–derived iPSCs. We conclude that culture conditions of activated B cells before and at early stages of reprogramming influence the developmental potency of resultant iPSCs.
Enforced expression of Oct4, Klf4, Sox2, and c-myc (OKSM) in somatic cells can result in the formation of induced pluripotent stem cells (iPSCs), which possess characteristics of blastocyst-derived embryonic stem cells (ES cells) (1–4). Although the iPSC label is applied to somatic cell-derived self-renewing cells that display ES cell-like morphology and growth characteristics, further characterization must be done to define iPSC functional developmental potency (5–7). In this context, iPSCs can vary widely in level of functional potency, with only a minority of iPSCs able to support high-grade chimerism and germline contribution in mice (8). Recent reports suggest that iPSCs may harbor epigenetic differences compared with ES cells that retard developmental potency (8). For example, recent work showed that iPSCs with methylation-induced Dlk1-Dio3 gene cluster silencing contribute poorly to chimerism in mice, whereas iPSCs with an active Dlk1-Dio3 region produce high-grade chimeras (9). Expression of the Meg3 gene (also known as Gtl2), located within the Dlk1-Dio3 locus, inversely correlates with the CpG methylation status of this locus and therefore serves as an indirect marker for iPSC functional quality in terms of developmental potency (9). In this context, iPSC clones that express elevated Meg3 levels (defined by >80% of ES cell levels) referred to as Meg3on clones exhibit low CpG methylation and high functional quality, whereas Meg3off iPSCs contribute poorly to mouse chimerism in general (9).
Another factor influencing reprogramming potential is the developmental stage of somatic cells, with less-differentiated cells in some cases being more amenable to epigenetic reprogramming (10). In this regard, enforced expression of the four standard OKSM factors can reprogram progenitor B cells more efficiently than mature naïve (i.e., IgM+ IgD+) B cells (10). Additionally, iPSCs derived from progenitor B cells through standard OKSM-mediated reprogramming have been shown to more readily contribute to high-grade chimerism compared with iPSCs derived from mature naïve B cells (10, 11). However, reprogramming of B cells representing developmental stages beyond the mature naïve B-cell stage has not been reported previously.
Products of the Rag1 and Rag2 genes are required for assembly of component variable (V), diversity (D), and joining (J) gene segments (“VDJ recombination”) to form IgH and T-cell receptor (TCR) variable region exons (12, 13). Because productive assembly of IgH or TCR variable region exons is required for the development of B and T cells, respectively, mice deficient in either Rag1 or Rag2 have no mature B or T cells due to a block in lymphocyte development at the early progenitor stage owing to inability to undergo V(D)J recombination (14, 15). Upon activation by antigen in peripheral lymphoid organs, mature B cells may undergo IgH class-switch recombination (CSR). CSR is a process in which the IgH μ constant region exons (Cμ) are deleted and replaced by one of several sets of downstream constant region exons (CHs; e.g., Cγ, Cε, and Cα), thereby allowing for the formation of other Ig classes (e.g., IgG, IgE, or IgA). CSR occurs within switch (S) regions, which are 1- to 10-kb sequences located 5′ to each set of CHs (16). The activation-induced cytidine deaminase (AID) enzyme initiates both CSR and the related process of somatic hypermutation (SHM) of Ig variable-region exons via cytidine deamination activity. During CSR, AID activity leads to DNA double-strand breaks (DSBs) in a donor S region (Sμ) upstream of Cμ and in a downstream acceptor S region; these DSBs then are joined so that Cμ is irreversibly deleted and replaced with one of the downstream CHs (17).
Each S region is preceded by a promoter and noncoding exon termed an “I” exon (18). Different forms of activation and/or cytokines provided by helper T cells or other cells can direct AID and, as a result, CSR to a particular target S region by specifically stimulating transcription from upstream I region promoters (16, 18). In this regard, stimulation of cultured splenic IgM+ B cells with anti-CD40 plus IL-4, mimicking in vivo activation by T helper type 2 (TH2) T cells, induces B-cell plasmablast differentiation and class switching to IgG1 and IgE (19, 20). Though anti-CD40 plus IL-4 treatment theoretically can lead to direct CSR from Cμ to either Cγ1 or Cε, direct CSR to Cε occurs less frequently than to Cγ1 in mature B cells (20–24). In this regard, various studies have shown that IgE switching largely occurs through a sequential CSR mechanism in which activated B cells first switch from IgM to IgG1 via direct CSR between Sμ and Sγ1 followed by switching to IgE via a “second step” recombination via breaking and joining of the newly made hybrid Sμ/Sγ1 and Sε (25–29). IgG1+ B-cell intermediates in sequential CSR have been proposed to be a required part of the development of high-affinity IgE responses in vivo (29, 30); however, the mechanisms regulating CSR from IgG1 to IgE remain poorly understood, partly because of the difficulty in isolating IgG1+ B-cell intermediates. In this context, the ability to reprogram IgG1+ B cells into iPSCs could allow the development of stable IgG1+ mouse models for studying second-step IgG1-to-IgE switching.
We now describe culture conditions that allow us to generate IgG1+ B-cell–derived iPSCs with sufficient developmental potency to produce chimeric animals harboring mature T and B cells derived entirely from IgG1-switched iPSCs and show that these B cells can be activated to switch from IgG1 to IgE.
Results
OKSM-Mediated Reprogramming of Activated B Cells Results in iPSCs Derived from IgH Class-Switched B Cells.
To develop an approach to reprogram IgH class-switched B cells, we used mice harboring a transgenic reprogramming system in which the OKSM reprogramming factors are expressed in a doxycycline-inducible fashion (31). We activated splenic B cells with anti-CD40 and IL-4 for 4 d to induce IgH class switching before addition of doxycycline to increase the probability of obtaining iPSC clones from IgH class-switched B cells. Cells were then plated on irradiated mouse embryonic fibroblasts (MEFs) before colonies with ES cell-like morphology and growth characteristics became apparent after 10–12 d of doxycycline treatment. We picked 72 colonies with ES cell-like morphology for analysis, and of these, 59 clones grew to be stable doxycycline-independent iPSC clones.
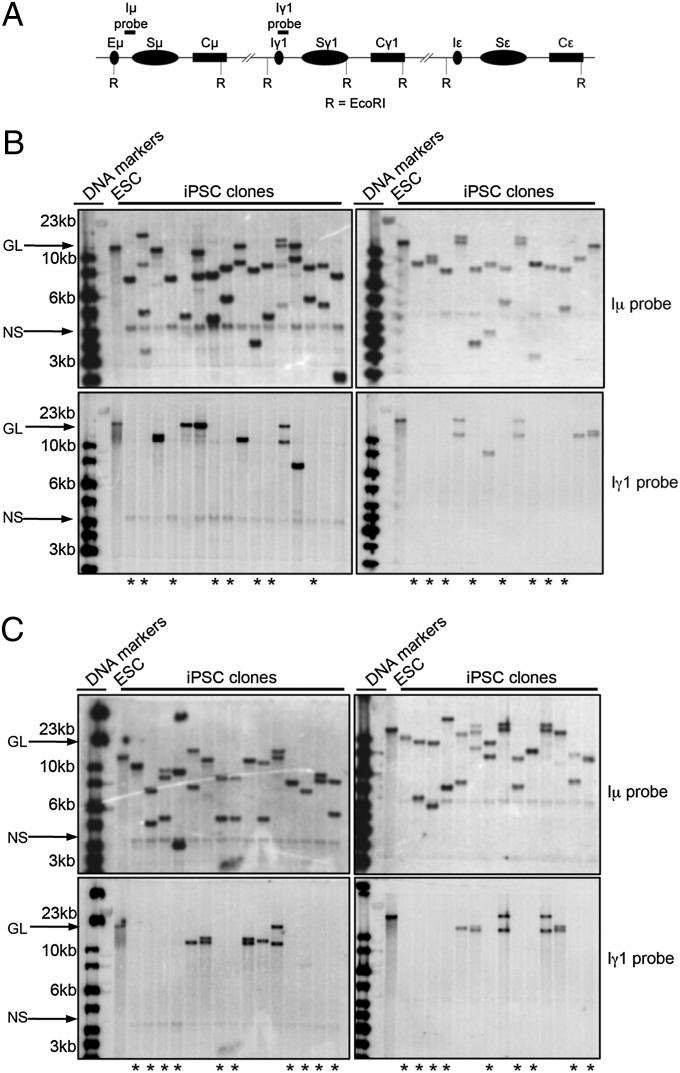
To determine if iPSC clones were indeed derived from B cells that have been activated for CSR, we characterized them by Southern blotting EcoRI-digested DNA with probes that flank both sides of Sμ. AID activity in B cells stimulated to undergo CSR induces multiple DSBs within a given S region (32–35), which may be relegated, joined to another DSB in the same S region, or ligated to a downstream S region to affect CSR. Rejoining DSBs within an S region, accompanied by resection or joining two DSBs within the same S region, can cause internal switch deletions (ISDs), which often are large enough to be observed via Southern blotting (33, 36). Therefore, a probe recognizing the 5′ end of Sμ (i.e., Iμ probe), which hybridizes to an EcoRI fragment that contains the entire Sμ region (Fig. 1A), will show different-sized restriction fragments compared with germline configuration if the B-cell had undergone either Sμ ISD or CSR to a downstream CH region. A probe 3′ to Sμ (Iγ1 probe) hybridizing to a separate EcoRI fragment (Fig. 1A) will be absent in cells that have undergone CSR to a downstream CH region, because intervening DNA in between S regions that have undergone CSR is excised in the process (18).
Fig. 1.
IgH class-switched B cells can be reprogrammed to iPSCs. (A) A schematic of the IgH constant region. EcoRI (R) restriction sites and locations of the Iμ and Iγ1 probes are indicated. (B and C) Southern blot analysis of EcoRI-digested genomic DNA extracted from ES cells and iPSC clones reprogrammed from day 4 anti-CD40/IL-4–stimlulated B cells hybridized to an Iμ (Upper) or Iγ1 (Lower) probe. Both Iμ- and Iγ1-hybridizing germline (GL) bands defined by EcoRI-digested ES cell DNA are indicated with an arrow and labeled. Signals likely representing nonspecific (NS) background bands are likewise indicated. The first two lanes contain DNA size markers, and DNA sizes are indicated in kilobases on the left. Lanes containing prominent Iμ-hybridizing bands different from GL indicate alleles that have undergone ISD or CSR. Lanes with asterisks (*) show no bands hybridizing to the Iγ1 probe, indicating that these clones have likely undergone CSR to either Cγ1 or to other downstream CHs.
We observed that nearly every iPSC clone demonstrated different-sized Iμ probe-reactive bands compared with germline configuration, suggesting that these clones were derived from B cells that had expressed AID and had undergone either ISD or CSR events (Fig. 1 B and C, Upper). We also observed that Iγ1 probe-reactive restriction fragments were absent in 37 of 59 clones (Fig. 1 B and C, Lower), indicating that at least 63% of these iPSC clones derived from B cells that had undergone CSR. The Iγ1 probe-reactive bands of sizes differing from germline could signify other AID-mediated recombination events such as Iγ1 ISD. Another probe hybridizing to Cε revealed that at least 11 clones had two clear bands (representing two alleles) diverging from germline configuration, suggesting that some iPSCs derived from B cells had undergone CSR to IgE (Fig. S1).
5-Aza-2′-Deoxycytidine Treatment of Activated B Cells at Early Stages of Reprogramming Allows for the Development of Meg3on iPSCs.
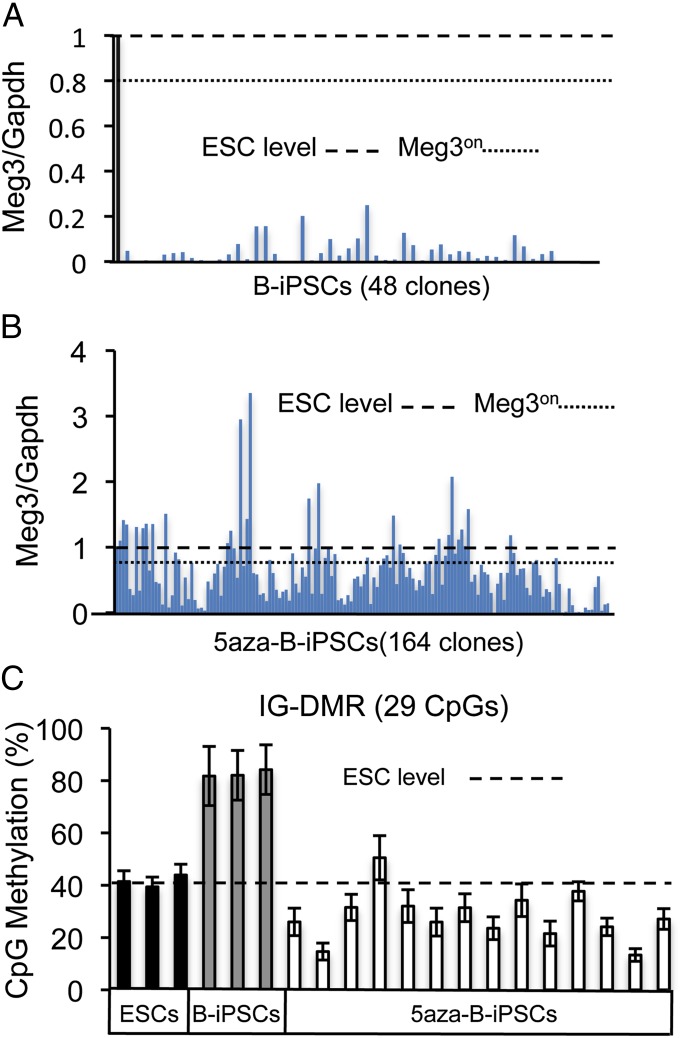
Given the predictive ability of iPSC Meg3 expression to identify iPSCs of high developmental potency (9), we assessed Meg3 levels by quantitative PCR. The TC1 and CJ7 ES cells, which showed nearly identical Meg3 expression levels, were used to compare with iPSC clones. We observed that all iPSC colonies derived from activated B cells (B-iPSCs) had Meg3 expression levels well below that of ES cell controls (Meg3off; Fig. 2A), which suggests low developmental potency (9). Because Meg3off status in general correlates with Dlk-Dio3 locus hypermethylation and poor iPSC developmental potency (9), we hypothesized that treatment of B cells with the methyltransferase inhibitor 5-aza-2′-deoxycytidine (5aza) may give rise to iPSCs with ES cell-like Meg3 expression levels and Dlk-Dio3 locus methylation status, perhaps resulting in improved iPSC quality. In this regard, previous attempts using 5aza during late stages of reprogramming have shown increased efficiency of iPSC generation, but these studies did not demonstrate contribution of 5aza-treated iPSCs to chimerism in mice (37, 38). We therefore used a similar 5aza treatment method; but in our approach, we provided a 2-d treatment course of 5aza beginning 1 d before induction of the OKSM reprogramming factors. We rationalized that the toxic effects of a global methyltransferase inhibition may be ameliorated during reprogramming by the reprogramming process itself and/or by allowing several days of selection against potential toxicities associated with genome-wide hypomethylation during the reprogramming process.
Fig. 2.
Treatment of activated B cells before reprogramming with 5aza leads to Meg3on B-cell–derived iPSCs with attenuated IG-DMR hypermethylation. (A and B) Quantitative PCR analysis of Meg3 expression in ES cell and iPSC clones derived from day 4 anti-CD40/IL-4–stimlulated B cells normalized to Gapdh expression. The iPSCs were derived without 5aza (B-iPSCs) (A) or with a 2-d 5aza treatment at the beginning of reprogramming (5aza-B-iPSCs) (B). Y axis indicates values relative to ES cell Meg3 expression. The black bar indicates ES cell Meg3 expression. ES cell expression is also indicated by the dashed horizontal line. The dotted horizontal line indicates 80% ES cell Meg3 expression, which is the threshold we used to define Meg3on status. (C) Degree of CpG DNA methylation at the IG-DMR in ES cell clones (black bars), untreated B-cell–derived iPSCs (gray bars), and 5aza-treated Meg3on iPSC clones (white bars) as measured by bisulfite sequencing. Dashed line indicates the average ES cell level of IG-DMR CpG methylation. Error bars indicate SDs of methylation status of the 29 CpGs within the IG-DMR.
We observed that this early 5aza treatment approach given to day 4 anti-CD40 plus IL-4–activated B cells resulted in doxycycline-independent iPSCs (5aza-B-iPSCs) that were morphologically indistinguishable from B-iPSCs (Fig. S2). In terms of Meg3 expression, Meg3 qPCR assays showed that 51 of a total of 165 5aza-B-iPSC clones were Meg3on (Fig. 2B). Because Meg3 expression is used here as a surrogate for Dlk-Dio3 DNA methylation status, we used bisulfite sequencing to measure the DNA methylation within this locus, specifically at the intergenic differentially methylated region (IG-DMR), which has been established as a useful test to assess iPSC potency (9, 39, 40). As expected, we find that our Meg3on 5aza-B-iPSC clones showed decreased IG-DMR CpG methylation compared with B-iPSCs (Fig. 2C).
5aza iPSCs Contribute to Mouse Chimerism and Can Make “All-IgG1” Mice in the Context of Rag2−/− Blastocyst Complementation.
It has previously been shown that iPSCs derived from mature naïve B cells by standard OKSM reprogramming contribute poorly to mouse chimerism (10, 11). It has also been previously shown that Meg3off iPSCs in general display reduced developmental potency and contribute poorly to mouse chimerism (9, 39, 40). Given these prior studies, we predicted that our B-iPSCs, which are uniformly Meg3off and derived via standard OKSM reprogramming, would display low developmental potency. To test this notion, we evaluated developmental potency of three independent B-iPSC clones in the context of Rag-deficient blastocyst complementation (RDBC). RDBC involves injection of selected ES cells into blastocysts from Rag2-deficient mice, leading to the generation of somatic chimeras harboring mature T and B cells derived entirely from the injected ES cells (41). Thus, we used RDBC to test iPSC developmental potency by assaying for the presence of mature B and T cells by FACS analysis of surface markers for B cells (B220) and T cells (Thy1.2) in peripheral blood of resultant chimeras. We found that consistent with the Meg3off status of B-iPSCs, neither B nor T cells could be detected in the peripheral blood of B-iPSC Rag2−/− chimeras from all three independent B-iPSCs (Table 1 and Fig. S3A).
Table 1.
Results of RDBC assay
| Injected clone | Live-born | T cell chimerism | T + B cell chimerism |
| B-iPSC1 | 6 | 0 | 0 |
| B-iPSC2 | 6 | 0 | 0 |
| B-iPSC3 | 6 | 0 | 0 |
| 5aza-B-iPSC1 | 15 | 0 | 7 |
| 5aza-B-iPSC2 | 12 | 0 | 6 |
| 5aza-B-iPSC3 | 5 | 3 | 0 |
| 5aza-B-iPSC4 | 12 | 6 | 0 |
| 5aza-B-iPSC5 | 9 | 1 | 3 |
The total number of live-born mice and number of T and T + B-cell chimerism resulting from Rag2−/− blastocyst complementation with the indicated iPSC clones are shown. The presence of T and T + B-cell chimerism was determined by FACS analysis of Thy1.2 (T-cell)- and B220 (B-cell)-expressing cells in peripheral blood as shown in Fig. S3.
To test the effect of 5aza treatment on developmental potency of iPSCs reprogrammed from activated B cells, we assayed five different 5aza-B-iPSCs by RDBC. Notably, all five Meg3on 5aza-B-iPSCs injected for RDBC gave rise to mouse chimerism, with three giving rise to T + B cells in peripheral blood, and two giving rise only to detectable T cells (Table 1 and Fig. S3B). We also found that injected 5aza-B-iPSCs into C57BL/6 blastocysts frequently give rise to high-grade (>80%) chimerism as determined by percentage of agouti (iPSC-derived) coat color (Fig. S4). Of eight 5aza-B-iPSCs tested, six had clear evidence of coat color chimerism with four displaying high-grade (>50% agouti) chimerism (Fig. S4B). Together, our findings suggest that 5aza treatment of activated B cells before reprogramming leads to Meg3on clones with developmental potency sufficient for B and T cell differentiation and high-grade coat color chimerism.
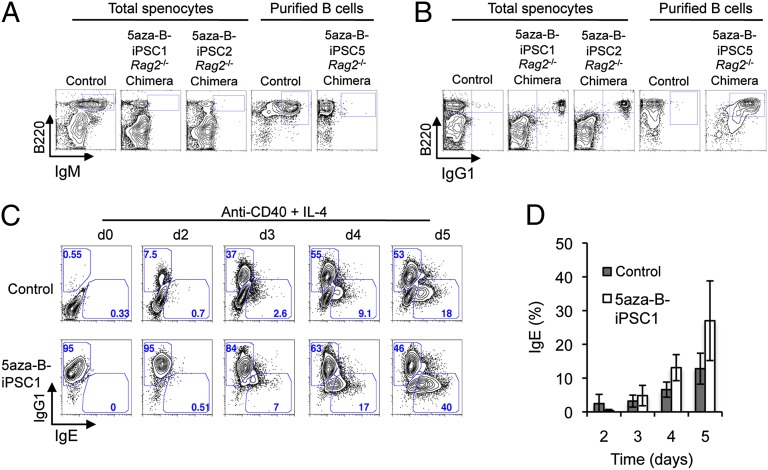
To further examine RDBC chimeric mice derived from the two independent 5aza-B-iPSC clones that gave T + B cells, we assayed splenic B cells for B220, as well as IgM and IgG1 by surface stains and flow cytometry. We observed that B cells from both clones were IgM− and IgG1+ (Fig. 3 A and B). These data indicate that the 5aza-B-iPSCs used to generate these chimeras were derived from B cells that had undergone CSR to IgG1, and that all mature B cells in these RDBC chimeras were iPSC derived. Further Southern blot analysis revealed that four of the five 5aza-B-iPSC clones used for RDBC had evidence of V(D)J rearrangements on both alleles, and that three of the five clones had undergone CSR on the nonproductive allele (Fig. S5). To determine whether the IgG1+ B cells derived from 5aza-B-iPSCs could be activated in culture for CSR to IgE, IgG1+ 5aza-B-iPSC–derived B cells and control IgM+ B cells from age-matched OKSM transgenic mice were treated with anti-CD40 plus IL-4, and IgE CSR was measured using a FACS-based approached we recently developed that involves removal of nonspecific surface IgE before detection of cytoplasmic IgE (20). We observed that 5aza-B-iPSC–derived IgG1+ B cells can be activated for switching to IgE similarly to control IgM+ B cells (Fig. 3 C and D). Notably, the overall kinetics of CSR to IgE also was similar in the 5aza-B-IPSC–derived IgG1+ B cells and the IgM+ control cells (Fig. 3D). Thus, the 5aza-B-iPSC–derived IgG1+ B cells can develop into naïve-like IgG1+ B cells that are functional in terms of their ability to be activated in culture to undergo further CSR reactions.
Fig. 3.
The 5aza-B-iPSCs can form all-IgG1 chimeric mice in Rag2−/− hosts, which can support further CSR to IgE. (A) FACS plots of splenocytes from 5aza-B-iPSC–complemented Rag2−/− chimeras stained for surface expression of the pan–B-cell marker B220 and IgM. For comparison, splenocytes from reprogrammable mice indicated as control were analyzed in parallel. Cells expressing both B220 and IgM are demarcated within FACS plots by a box in the upper right-hand corner. (B) FACS plots of splenocytes from control mice and 5aza-B-iPSC–complemented Rag2−/− chimeras stained for surface expression of B220 and IgG1. Cells expressing both B220 and IgG1 are shown by a box in the upper right-hand corner. (C) FACS analysis of day 0, 2, 3, 4, and 5 anti-CD40/IL-4–stimulated B cells from reprogrammable mice (control) or 5aza-B-iPSC–derived B cells stained for cytoplasmic IgG1 and IgE expression as described in the text. Gates within the plots demarcate IgG1+ and IgE+ B cells in the upper left and lower right portion of box, respectively. Numbers indicate percentage of cells within the gates. (D) Statistical analysis of three independent FACS analysis experiments for IgE switching of control and 5aza-B-iPSC–derived B cells activated with anti-CD40 plus IL-4 for the indicated time points. Shown are mean values ± SD of three independent experiments involving Rag2−/− iPSC chimeras derived from 5aza-B-iPSC#2.
Discussion
Our goal was to develop a method to produce IgH-switched B-cell–derived iPSCs that are able to support high-grade mouse chimeras to expand tools for the study of B-cell development and function. Given that tested iPSCs derived from activated B cells were Meg3off and did not support lymphocyte development in the setting of RDBC, we developed conditions using 5aza treatment of IgH class-switched B cells that allowed us to isolate Meg3on iPSCs that were able to form high-grade coat color chimerism. In addition, we found that several tested 5aza-B-IPSC cells derived from IgG1+ B cells were capable of generating all-IgG1 mice following injection into Rag2-deficient host blastocysts. Finally, we found that iPSC-derived all-IgG1 B cells can be efficiently activated to switch to IgE.
We have found that iPSCs derived from day 4-activated B cells from OKSM are uniformly Meg3off, and several tested failed to produce T or T + B-cell chimerism in Rag2−/− mice. Correspondingly, a contemporaneous study found that iPSCs derived from OKSM B cells taken directly from mice were uniformly Meg3off with reduced developmental potency (39). Similar to our findings, this study also found that attenuation of hypermethylation at the Dlk-Dio3 locus by treatment of B cells with ascorbic acid (39) resulted in Meg3on iPSCs with high developmental potential (39). In our studies, 5aza treatment of day 4-activated B cells to induce demethylation before iPSC generation enabled us to produce iPSC-derived B cells that had previously undergone CSR. Moreover, we found that these iPSC-derived IgG1+ B cells are functional in terms of the ability to undergo further activation and CSR to IgE in culture. Thus, our 5aza study, coupled with the ascorbic acid study (39), highlight the usefulness of developing reprogramming conditions aimed at attenuating Dlk-Dio3 locus hypermethylation during iPSC derivation, and show that different approaches to achieve this goal may be used.
Because our approach involved treatment of the day 4-activated B cells with 5aza before the onset of reprogramming, it may offer insights into the potential utility of epigenetic modification before and at early stages of reprogramming to positively influence resultant iPSCs. However, the detailed molecular mechanisms underlying this effect remain to be determined. Given that both loss of DNA methylation and de novo DNA methylation likely occur during the reprogramming process (42), 5aza treatment early during reprogramming may allow the reprogramming process to ameliorate potential negative effects of global hypomethylation. In this context, whether the reprogramming process provides a window for specific epigenetic remodeling not available in stable cycling iPSCs remains to be determined.
The irreversible genetic deletions associated with B-cell development and CSR provide a means to assess the CH configuration and clonality of activated B-cell–derived iPSCs. Furthermore, given that the process of CSR excises 100–200 kb of DNA between CHs, the derivation of B-cell models from IgH class-switched B-cell–derived iPSCs provides an approach to assess the potential role of sequences internal to the CH region in regulation of transcription, CSR and other processes during B-cell development and activation. Additionally, the ability to efficiently generate all-iPSC–switched B cells allows questions to be addressed regarding the functional consequences of expressing various IgH isotypes on B-cell development and function. In this regard, because IgG1+ B cells are often intermediates in normal sequential B-cell switching to IgE (20, 26–29), the creation of all-IgG1 chimeric mice provides a valuable tool for studies related to mechanisms regulating second-step switching from IgG1 to IgE and the stability of IgG1+ B cells.
An antigen-specific B-cell mouse model has been generated with somatic cell nuclear transfer technology (43). In that context, the approaches we describe here involving generation of iPSCs derived from activated, class-switched B cells followed by RDBC to generate mice in which all B cells derive from the clonally rearranged iPSC cells may provide a useful extension of that general method of generating monoclonal mice with selected antibody specificities. In particular, the RDBC approach could allow a more rapid method for initial screening for mice that make the desired antibody specificities and in the context of desired IgH isotypes. Though not yet demonstrated, we would expect that the high-level chimerism we have achieved with 5aza-B-iPSCs will eventually allow germline transmission of desired iPSC Ig genotypes after RDBC screening.
Materials and Methods
Mice, Cell Culture, and Reprogramming.
Doxycycline-inducible reprogrammable mice used in these experiments have been described previously (31) and were a gift from Konrad Hochedlinger (Massachusetts General Hospital, Boston, MA). All experiments with mice followed the protocols approved by the Children's Hospital Boston Animal Care and Use Committee. Splenic cells from reprogrammable mice were isolated by B220+ selection via magnetic columns (Miltenyi Biotech) and cultured in RPMI (Invitrogen) supplemented with L-glutamine, penicillin, streptomycin, and 15% (vol/vol) FBS together with anti-CD40 plus IL-4 to induce CSR to IgG1 and IgE as described (20). Fresh media was added to the cells on days 2, 3, and 4. After 4 d of culture, OKSM reprogramming factors were induced by the addition of 2 μg/mL doxycycline, and cells were transferred to ES cell medium consisting of DMEM (Invitrogen) in 15% FBS on gelatinized plates seeded with a feeder layer of irradiated MEFs for 10–12 d with medium exchanges every 2 d. Colonies with ES cell morphology were then picked and expanded in the absence of doxycycline for further analysis. For 5aza (Sigma) treatment, a 2.5-μM 5aza solution in ES cell medium was given to day 4-activated B cells 24 h before the addition of doxycycline, and another fresh 2.5 μM 5aza solution was provided at the time of doxycycline addition. 5aza was removed 24 h after doxycycline addition for a total 5aza treatment period of 48 h. Following 5aza removal, cells were incubated on MEFs with doxycycline for 10–12 d before ES cell-like colonies were picked and expanded in ES cell media without doxycycline to isolate stable iPSC clones. Media was exchanged every 2 d during reprogramming. Functional analysis of iPSCs was performed by injection of iPSCs into Rag2−/− or C57B/6 blastocysts after verifying normal karyotype.
Analysis of DNA Methylation.
IPSC clones were lysed in PK lysis buffer [100 mM Tris⋅HCl (pH 8.0), 5 mM EDTA, 0.2% SDS and 200 mM NaCl, 200 μg/mL proteinase K] followed by genomic DNA precipitation with isopropanol (50% vol/vol). After washing with 70% ethanol, DNA was resuspended in T low E buffer [10 mM Tris (pH 8.0), 0.1 mM EDTA]. DNA was bisulfite converted using the EpiTect Bisulfite Kit (Qiagen) and analyzed by EpigenDX using the ADS1452 (IG-DMR) assay.
RNA Isolation and Quantification.
Total RNA was extracted using the TRIzol method (Invitrogen) and reverse transcribed into cDNA using qScript (Quanta Biosciences). Meg3 and Gapdh transcripts were quantified using SYBR Green qPCR (Applied Biosystems) primers described elsewhere (9).
Southern Blot Analysis.
Genomic DNA (20 μg) was digested with EcoRI before separation on a 1% agarose gel and blotting to Hybond-XL membrane (Amersham Biosciences). Blots were hybridized to probes specific to DFL16, Iμ, Cμ, Iγ1, and Cε as described elsewhere (44), washed, and put on phosphorimaging cassettes for exposure.
Supplementary Material
Acknowledgments
We thank Drs. Hidde Ploegh, Juan Lafaille, and David Schatz for critical review of the manuscript. We thank K. Hochedlinger and M. Stadtfeld for the doxycycline-inducible reprogrammable OKSM transgenic mice and for technical advice in the reprogramming procedure. This work was supported by National Institutes of Health Grants AI077595 and AI020047 (to F.W.A.) and K08AI89972 (to D.R.W.). D.R.W. was also supported by an award from the American Academy of Allergy, Asthma & Immunology and CSL Behring and holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund. F.W.A. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1210286109/-/DCSupplemental.
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 3.Maherali N, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Wernig M, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 5.Maherali N, Hochedlinger K. Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;3:595–605. doi: 10.1016/j.stem.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol. 2007;25:1177–1181. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- 7.Daley GQ, et al. Broader implications of defining standards for the pluripotency of iPSCs. Cell Stem Cell. 2009;4:200–201, author reply 202. doi: 10.1016/j.stem.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol. 2011;13:497–505. doi: 10.1038/ncb0511-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stadtfeld M, et al. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465:175–181. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eminli S, et al. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nat Genet. 2009;41:968–976. doi: 10.1038/ng.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanna J, et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung D, Alt FW. Unraveling V(D)J recombination; insights into gene regulation. Cell. 2004;116:299–311. doi: 10.1016/s0092-8674(04)00039-x. [DOI] [PubMed] [Google Scholar]
- 13.Matthews AG, Oettinger MA. RAG: A recombinase diversified. Nat Immunol. 2009;10:817–821. doi: 10.1038/ni.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinkai Y, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 15.Mombaerts P, et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhuri J, et al. Evolution of the immunoglobulin heavy chain class switch recombination mechanism. Adv Immunol. 2007;94:157–214. doi: 10.1016/S0065-2776(06)94006-1. [DOI] [PubMed] [Google Scholar]
- 17.Yan CT, et al. IgH class switching and translocations use a robust non-classical end-joining pathway. Nature. 2007;449:478–482. doi: 10.1038/nature06020. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhuri J, Alt FW. Class-switch recombination: Interplay of transcription, DNA deamination and DNA repair. Nat Rev Immunol. 2004;4:541–552. doi: 10.1038/nri1395. [DOI] [PubMed] [Google Scholar]
- 19.Bacharier LB, Geha RS. Molecular mechanisms of IgE regulation. J Allergy Clin Immunol. 2000;105:S547–S558. doi: 10.1016/s0091-6749(00)90059-9. [DOI] [PubMed] [Google Scholar]
- 20.Wesemann DR, et al. Immature B cells preferentially switch to IgE with increased direct Sμ to Sε recombination. J Exp Med. 2011;208:2733–2746. doi: 10.1084/jem.20111155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snapper CM, Finkelman FD, Paul WE. Differential regulation of IgG1 and IgE synthesis by interleukin 4. J Exp Med. 1988;167:183–196. doi: 10.1084/jem.167.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung S, Siebenkotten G, Radbruch A. Frequency of immunoglobulin E class switching is autonomously determined and independent of prior switching to other classes. J Exp Med. 1994;179:2023–2026. doi: 10.1084/jem.179.6.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purkerson JM, Isakson PC. Independent regulation of DNA recombination and immunoglobulin (Ig) secretion during isotype switching to IgG1 and IgE. J Exp Med. 1994;179:1877–1883. doi: 10.1084/jem.179.6.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bottaro A, et al. S region transcription per se promotes basal IgE class switch recombination but additional factors regulate the efficiency of the process. EMBO J. 1994;13:665–674. doi: 10.1002/j.1460-2075.1994.tb06305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siebenkotten G, Esser C, Wabl M, Radbruch A. The murine IgG1/IgE class switch program. Eur J Immunol. 1992;22:1827–1834. doi: 10.1002/eji.1830220723. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida K, et al. Immunoglobulin switch circular DNA in the mouse infected with Nippostrongylus brasiliensis: Evidence for successive class switching from mu to epsilon via gamma 1. Proc Natl Acad Sci USA. 1990;87:7829–7833. doi: 10.1073/pnas.87.20.7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandler R, Finkelman FD, Levine AD, Snapper CM. IL-4 induction of IgE class switching by lipopolysaccharide-activated murine B cells occurs predominantly through sequential switching. J Immunol. 1993;150:407–418. [PubMed] [Google Scholar]
- 28.Hodgkin PD, Castle BE, Kehry MR. B cell differentiation induced by helper T cell membranes: Evidence for sequential isotype switching and a requirement for lymphokines during proliferation. Eur J Immunol. 1994;24:239–246. doi: 10.1002/eji.1830240138. [DOI] [PubMed] [Google Scholar]
- 29.Erazo A, et al. Unique maturation program of the IgE response in vivo. Immunity. 2007;26:191–203. doi: 10.1016/j.immuni.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong H, Dolpady J, Wabl M, Curotto de Lafaille MA, Lafaille JJ. Sequential class switching is required for the generation of high affinity IgE antibodies. J Exp Med. 2012;209:353–364. doi: 10.1084/jem.20111941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stadtfeld M, Maherali N, Borkent M, Hochedlinger K. A reprogrammable mouse strain from gene-targeted embryonic stem cells. Nat Methods. 2010;7:53–55. doi: 10.1038/nmeth.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen S, et al. AID is required to initiate Nbs1/gamma-H2AX focus formation and mutations at sites of class switching. Nature. 2001;414:660–665. doi: 10.1038/414660a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boboila C, et al. Alternative end-joining catalyzes class switch recombination in the absence of both Ku70 and DNA ligase 4. J Exp Med. 2010;207:417–427. doi: 10.1084/jem.20092449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catalan N, et al. The block in immunoglobulin class switch recombination caused by activation-induced cytidine deaminase deficiency occurs prior to the generation of DNA double strand breaks in switch mu region. J Immunol. 2003;171:2504–2509. doi: 10.4049/jimmunol.171.5.2504. [DOI] [PubMed] [Google Scholar]
- 35.Schrader CE, et al. Mutations occur in the Ig Smu region but rarely in Sgamma regions prior to class switch recombination. EMBO J. 2003;22:5893–5903. doi: 10.1093/emboj/cdg550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang T, et al. Downstream class switching leads to IgE antibody production by B lymphocytes lacking IgM switch regions. Proc Natl Acad Sci USA. 2010;107:3040–3045. doi: 10.1073/pnas.0915072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huangfu D, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 38.Mikkelsen TS, et al. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454:49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stadtfeld M, et al. Ascorbic acid prevents loss of Dlk1-Dio3 imprinting and facilitates generation of all-iPS cell mice from terminally differentiated B cells. Nat Genet. 2012;44:398–405, S1–S2. doi: 10.1038/ng.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carey BW, et al. Reprogramming factor stoichiometry influences the epigenetic state and biological properties of induced pluripotent stem cells. Cell Stem Cell. 2011;9:588–598. doi: 10.1016/j.stem.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Lansford R, Stewart V, Young F, Alt FW. RAG-2-deficient blastocyst complementation: An assay of gene function in lymphocyte development. Proc Natl Acad Sci USA. 1993;90:4528–4532. doi: 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dougan SK, et al. IgG1+ ovalbumin-specific B-cell transnuclear mice show class switch recombination in rare allelically included B cells. Proc Natl Acad Sci USA. 2012;109:13739–13744. doi: 10.1073/pnas.1210273109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dudley DD, et al. Internal IgH class switch region deletions are position-independent and enhanced by AID expression. Proc Natl Acad Sci USA. 2002;99:9984–9989. doi: 10.1073/pnas.152333499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.