Abstract
Asthma, one of the most prevalent diseases affecting people worldwide, is a chronic respiratory disease characterized by heightened airway inflammation, airway hyperresponsiveness and airflow obstruction in response to specific triggers. While the specific mechanisms responsible for asthma are not well understood, changing environmental factors associated with urban lifestyles may underlie the increased prevalence of the disorder. Vitamin D is of particular interest in asthma since vitamin D concentrations decrease with increased time spent indoors, decreased exposure to sunlight, less exercise, obesity, and inadequate calcium intake. Additionally, a growing body of literature suggests that there is a relationship between vitamin D status and respiratory symptoms, presumably through immunomodulatory effects of vitamin D. This review discusses vitamin D as it relates to asthma across the age spectrum, with a focus on human studies.
Keywords: vitamin D, asthma, asthma prevalence, children, inflammation
Introduction
Asthma, one of the most prevalent diseases affecting people worldwide, is a chronic respiratory disease characterized by heightened airway inflammation, airway hyperresponsiveness and airflow obstruction in response to specific triggers (Fig. 1). Common symptoms include chest tightness, wheezing, cough and difficulty breathing, which are commonly treated with two different classes of medications: inhaled corticosteroids, used as a daily controller medication, and β-adrenergic agonists, which are used to induce bronchodilation.1 While the specific mechanisms responsible for asthma are poorly understood, in part due to the marked heterogeneity of the disorder in both adults2 and children,3 numerous aberrant immune responses are clearly associated with the disorder.4 For example, T-helper cell type-2 (TH2) cytokines, such as interleukin (IL)-4, IL-5, and IL-13, are upregulated in the asthmatic airway and are associated with increased eosinophilia,5,6 mast cell degranulation7 and increased levels of immunoglobulin E (IgE).6,7 Impairment of immunogenic tolerance, along with complex interactions between cells and inflammatory mediators, ultimately promotes airway injury in a process commonly referred to as airway “remodeling.”8 This process involves smooth muscle hypertrophy, epithelial goblet-cell hyperplasia and permanent deposition of airway extracellular matrix proteins which may ultimately increase airflow obstruction and the respiratory symptom burden of the disorder.9
Figure 1.
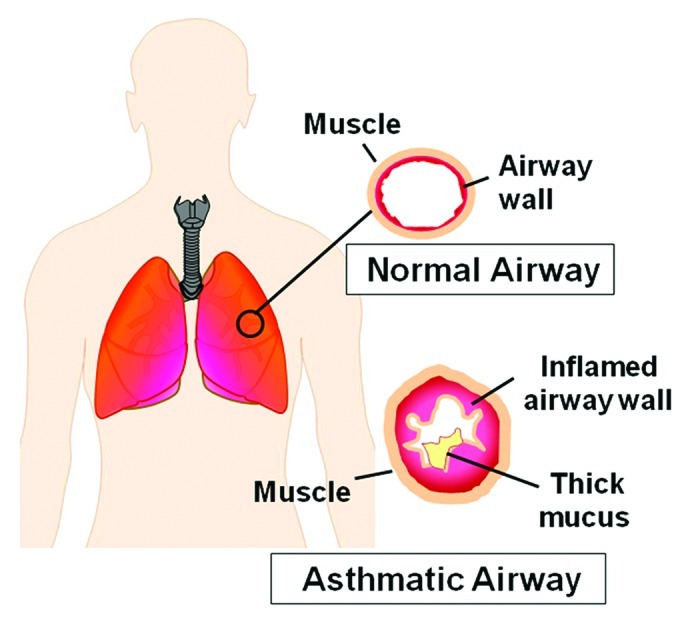
Airway inflammation associated with asthma.
Within industrialized Westernized countries, the prevalence of asthma has steadily increased by 25% to 75% per decade since 1960, causing it to become a major public health concern.10-12 The increased prevalence of asthma may be influenced by changing environmental factors associated with urban lifestyles. Indeed, numerous dietary hypotheses have been proposed.13-15 Among the nutrients and antioxidants included in this hypothesis, vitamin D is of particular interest since vitamin D concentrations decrease with increased time spent indoors, decreased exposure to sunlight, less exercise, obesity, and inadequate calcium intake.16 Additionally, a growing body of literature suggests that there is a relationship between vitamin D status and asthma-related respiratory symptoms,17-22 presumably through the immunomodulatory effects of vitamin D.23,24 This brief review discusses vitamin D as it relates to asthma across the age spectrum, with a focus on emerging human studies over the past decade. A literature review was performed for relevant publications spanning from 2002 to present. Publications were limited to those indexed on PubMed and written in English. Searches were made for “vitamin D” and “asthma,” excluding in vitro and animal studies. Relevant articles identified from this search are discussed below.
Vitamin D Overview
Vitamin D, a fat-soluble nutrient, is a secosteroid hormone which is widely recognized as a modulator of calcium absorption and bone health and further regulates neuromuscular function, cellular differentiation, insulin secretion, and blood pressure.25,26 Vitamin D also plays an important role in immune regulation through interactions between 1,25-dihydroxyvitamin D and vitamin D receptors (VDRs). VDRs are expressed on a variety of airway immune cells, where they function as classic nuclear steroid hormone receptors and ultimately regulate the transcription of numerous genes associated with inflammation and immunomodulation.27 Vitamin D also plays an important role in respiratory infection by facilitating Toll-like receptor signaling through increased synthesis of human cathelicidin antimicrobial peptide,28-30 which is cleaved to generate the active cationic peptide, LL-37.31,32 Vitamin D also exerts direct effects on target cells independent of gene transcription33 and may therefore be of relevance to airway inflammatory disorders.34 While vitamin D can suppress IL-1735 and IL-4-mediated expression of IL-13,35,36 it can also shift the Th1/Th2 balance toward Th2 dominance.37 These contradictory actions may be due to the direct actions of vitamin D on CD4+ T cells to promote an IL-10-secreting T-regulatory population.38
Biosynthesis of vitamin D
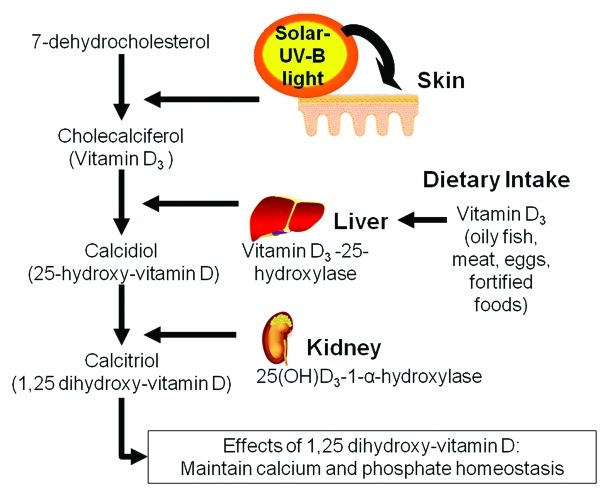
Biosynthesis of vitamin D begins primarily with absorption of solar ultravoilet-B (UVB) radiation and secondarily via consumption of vitamin D-rich and fortified foods, such as oily fish, fortified grains and dairy products.26 Following exposure to UVB radiation, 7-dehydrocholesterol within the skin is converted to previtamin D3 (Fig. 2), and eventually to the prohormone vitamin D3, which is also referred to as cholecalciferol. Vitamin D3 is then hydroxylated within the liver by 25-hydroxylase to 25-hydroxyvitamin D3 (25[OH]D), the major circulating metabolite of vitamin D. Under parathyroid control, 25(OH)D is hydroxylated by 1-α-hydroxylase in the kidney to its final and biologically active form, 1,25-dihydroxyvitamin D (1,25[OH]2D3), a key regulator of calcium and phosphate homeostasis. 1,25(OH)2D3, also referred to as calcitriol, is then transported throughout the body via the blood to various cells types that express VDRs, where gene expression is induced.24,39 Because the majority of vitamin D metabolism occurs in this skin, several factors are thought to influence vitamin D skin metabolism, including age, body fat, the level of skin melanin, and other variables such as latitude, the amount and degree of sun exposure, and use of sunscreen products with UV protection.40 These factors have been previously associated with vitamin D deficiency in epidemiologic studies.41-43
Figure 2.
Biosynthesis of vitamin D.
Vitamin D thresholds
Serum concentrations of 25(OH)D are considered to be a biomarker and indicator of vitamin D status.44,45 Although vitamin D insufficiency has traditionally been defined as a serum 25(OH)D concentration of 20 to 29 ng/mL,46 a panel of experts from the Institute of Medicine recently suggested that vitamin D insufficiency be redefined as a serum 25(OH)D concentration less than 20 ng/mL.47 This recommendation was based on inconclusive evidence for the threshold of 29 ng/mL and evidence for adverse skeletal effects at thresholds less than 20 ng/mL.47 However, this revised definition of vitamin D insufficiency has generated significant controversy48,49 and thus there is presently no universally accepted definition for “optimal” 25(OH)D concentrations independent of musculoskeletal health.
Maternal/Infant Vitamin D Exposure and Childhood Respiratory Symptoms
The rapid increase in asthma prevalence within Westernized countries is unlikely solely due to genetics. Instead, a number of environmental exposures may also alter the rate of asthma development.50-54 Of these modifiable environmental exposures, the role of diet in asthma is of particular interest13,55 since recent studies suggest that the development of asthma in children may be due to in utero exposure to nutrients from the mother’s diet.56 Given the role of vitamin D in inflammation and immunomodulation, several studies have focused on in utero vitamin D exposure and subsequent asthma in children. While some studies quantified maternal dietary intake of vitamin D during the final trimester of pregnancy, others measured vitamin D levels within the plasma and whole blood of pregnant women (Table 1). Several of these studies noted an inverse relationship between maternal vitamin D intake and the risk of subsequent wheezing in young infants and preschool children.58,61,62,64 However, vitamin D intakes varied considerably between geographic locations, from 548 IU/day in the United States58 and 260 IU/day in Finland62 to 248 and 137 IU/day in Japan64 and Scotland,61 respectively. It is also important to note that associations between prenatal vitamin D exposure, wheezing and subsequent asthma have not been consistent across studies. Whereas Devereux et al.61 did observe a negative association between vitamin D intake and wheezing in preschool children, no associations between vitamin D intake and asthma were noted in children 5 y of age. Similarly, in a Finnish birth cohort, infants supplemented with vitamin D during the first year of life were not more likely to have asthma in early adulthood.67 Rather, in that study67 and another,68 the prevalence of allergic sensitization was greater at age 6 and age 31 y in those subjects who received regular vitamin D supplementation during infancy.
Table 1. Studies of vitamin D exposure in early life and respiratory outcomes.
| Author | Year | Country | Study Design | Sample Size | Age of children at assessment | Determination of Vitamin D status | Association between vitamin D and respiratory outcomes |
|---|---|---|---|---|---|---|---|
| Belderbos57 |
2011 |
Netherlands |
Prospective cohort |
156 neonates |
Birth to 1 y |
Maternal food-frequency questionnaire and cord blood 25(OH)D |
Lower 25(OH)D concentrations associated with an increased risk of respiratory syncytial virus infection |
| Camargo58 |
2007 |
United States |
Prospective cohort |
1194 mother-child dyads |
3 y |
Maternal food-frequency questionnaire |
Highest quartile of vitamin D intake during pregnancy associated with a lower risk of recurrent wheezing |
| Camargo59 |
2011 |
New Zealand |
Prospective cohort |
922 newborns |
Birth to 5 y |
Cord blood 25(OH)D |
Higher 25(OH)D associated with a decreased risk of respiratory infection and wheezing but no associations with childhood asthma noted |
| Carroll60 |
2011 |
Canada |
Cross-sectional |
340 mother-infant dyads |
5–29 weeks |
Maternal whole blood 25(OH)D |
Increasing maternal 25(OH)D associated with decreased odds of asthma in mothers but no associations with infantile wheezing noted |
| Devereux61 |
2007 |
Scotland |
Prospective cohort |
2000 pregnant women, 1212 children |
5 y |
Maternal food-frequency questionnaire |
Highest and lowest maternal vitamin D intakes associated with lower risk of wheezing |
| Erkkola62 |
2009 |
Finland |
Prospective cohort |
1669 mother-child dyads |
5 y |
Maternal food-frequency questionnaire |
Higher maternal vitamin D intake associated with lower risk of asthma and allergic rhinitis |
| Gale63 |
2008 |
UK |
Prospective cohort |
466 mothers and 178 children |
9 mo and 9 y |
Maternal serum 25(OH)D |
Maternal vitamin D levels > 75 nmol/L associated with increased risk of atopic dermatitis and asthma |
| Miyake64 |
2010 |
Japan |
Prospective cohort |
763 mother-child dyads |
16–24 mo |
Maternal food-frequency questionnaire |
Higher maternal vitamin D intake associated with a decreased risk of wheeze and atopic dermatitis |
| Morales65 |
2012 |
Spain |
Prospective cohort |
1724 children |
12 mo, 4–6 y |
Maternal plasma 25(OH)D |
Increased maternal 25(OH)D associated with a decreased risk of lower respiratory infections but not wheezing or asthma |
| Rothers66 | 2011 | United States | Prospective cohort | 219 children | Birth to 5 y | Cord blood 25(OH)D | Both low and high cord blood 25(OH)D associated with increased aeroallergen sensitization but not with allergic rhinitis or asthma |
In other birth cohort studies where 25(OH)D concentrations were measured in the plasma and whole blood of pregnant women, median 25(OH)D concentrations ranged from approximately 20 to 29.5 ng/mL.60,63,65 A 50% decrease in the odds of maternal asthma was further observed with every 35 nmol/L increase in 25(OH)D levels, suggesting a possible link between vitamin D status and asthma during pregnancy.60 However, similar to the studies of maternal vitamin D intake, no consistent relationships between 25(OH)D concentrations and respiratory symptoms during childhood have been observed. For example, while Carroll et al.60 failed to find an association between maternal 25(OH)D concentrations and the development of respiratory infections in their offspring, Morales et al.65 found that circulating maternal 25(OH)D concentrations were associated with a lower risk of lower respiratory tract infections during infancy. However, again, no associations between maternal 25(OH)D and wheezing and asthma in childhood were noted.65
To further delineate the role of vitamin D and childhood allergic disease, several studies have also examined concentrations of 25(OH)D in cord blood from newborn infants. In a recent cohort study based in the United States, cord blood 25(OH)D concentrations below 20 ng/ml were associated with increased IgE levels and aeroallergen sensitization.66 Two similar birth cohorts in New Zealand59 and the Netherlands57 also found that children with lower concentrations of cord-blood 25(OH)D had an increased risk of respiratory viral infection and wheezing during infancy. However, in these studies, there were no associations between cord-blood 25(OH)D concentrations and subsequent asthma at 5 y of age.59,66
Collectively, these studies suggest that the benefits of vitamin D during early life may be limited to its effects on respiratory infections and viral-induced wheezing and not asthma per se.17 However, although vitamin D may have important roles in immune regulation during early life, excessive vitamin D exposure may also be associated with adverse health outcomes. For example, Rothers et al.66 found that both low (< 20 ng/mL) and high (> 40 ng/mL) cord blood 25(OH)D concentrations were associated with increased IgE levels and aeroallergen sensitization. This finding is similar to other studies where an increased risk of allergic sensitization was noted in infants who received vitamin D supplementation.67,68 Likewise, Gale et al.63 noted that maternal 25(OH)D concentrations greater than 75 nmol/L (30 ng/mL) were associated with 5-fold increased odds of asthma at 9 y of age in the exposed children. However, it is important to note that a large percentage of children were lost to follow-up, and thus the resulting sample of mothers had higher concentrations of 25(OH)D and were older, less likely to smoke during pregnancy, and more educated.63 Therefore while there may be therapeutic window for vitamin D supplementation in early life, further studies are needed to more clearly understand the risks of high vitamin D intake and bioavailability during the early childhood years.
Vitamin D in School-Aged Children with Asthma
Several recent studies have shown associations between vitamin D status and asthma outcomes in school-age children (Table 2), although there is considerable variation between the study populations. Of particular interest is the finding of racial disparities in vitamin D status. Due to higher levels of melanin, African Americans have the highest prevalence of vitamin D deficiency, with the highest prevalence in those living in urban environments.43,76 At the same time, asthma is also more prevalent in African American children.10,11 Only one study to date has focused on vitamin D status in school-aged African American asthmatic children. In this cross-sectional case-control study, Freishtat et al.72 demonstrated that 25(OH)D concentrations were ~54% lower in African American children with asthma when compared with African American children without asthma (23.1 vs. 40 ng/ml, respectively). However, 25(OH)D concentrations in the non-asthmatic group were significantly higher than previously-reported average values in African American children.21 The Childhood Asthma Management Program study, which consisted of a racially diverse population, also found that 24% of vitamin D deficient subjects were African American, compared with only 7% in the vitamin D sufficient group.69 The factors responsible for the racial disparity in vitamin D status are unclear and warrant further study.
Table 2. Studies of vitamin D status in school-age children with asthma.
| Author | Year | n (% male) |
Age (years) | Study Design | Sample | Outcome Measures | Findings |
|---|---|---|---|---|---|---|---|
| Brehm41 |
2009 |
616 (60) |
8.7 |
Cross-sectional |
Mild-to-severe persistent asthma |
Asthma exacerbations |
Increased 25(OH)D associated with reduced hospitalization, reduced anti-inflammatory medication use and reduced airway hyperresponsiveness |
| Brehm69 |
2010 |
1024 (60) |
8.9 |
Prospective cohort |
Mild-to-moderate persistent asthma |
Hospitalization or emergency department visit |
Baseline 25(OH)D levels < 30 ng/mL associated with higher odds of hospitalization or emergency department over 4 y |
| Chinellato70 |
2011 |
75 (60) |
9.6 |
Cross-sectional |
Well-controlled and poorly controlled asthma |
Spirometry, asthma control |
Positive correlations noted between 25(OH)D and asthma control |
| Chinellato71 |
2011 |
45 (60) |
10 |
Cross-sectional |
Intermittent asthma |
Lung function and airway hyperresponsiveness |
Lower serum 25(OH)D associated with decreased lung function and increased airway hyperresponsiveness with exercise |
| Freishtat72 |
2010 |
92 (63) |
11.1 |
Cross-sectional |
African Americans with and without asthma |
Physician-diagnosed asthma |
Decreased 25(OH)D in asthmatics vs. controls |
| Majak73 |
2011 |
48 (67) |
11.5 |
Randomized, double-blind, parallel arm clinical trial |
Newly diagnosed asthma |
Asthma exacerbations |
Fewer exacerbations in children with vitamin D3 supplementation added to inhaled budesonide |
| Searing74 |
2010 |
100 (64) |
7 |
Cross-sectional |
Moderate to severe persistent asthma |
Corticosteroid use and airflow limitation |
Decreased 25(OH)D associated with lower lung function and higher corticosteroid requirements |
| Urashima75 | 2010 | 217 (57) | 10.0 | Randomized, double-blind clinical trial | Schoolchildren (allcomers), subgroup with physician-diagnosed asthma | Asthma exacerbations | Reduced risk of asthma exacerbations in the subgroup with asthma after vitamin D3 supplementation |
Other cross-sectional studies of vitamin D status have also examined the associations between 25(OH)D concentrations and asthma control in school-age children. In both North American and Costa Rican populations, Brehm et al.41,69 found that 25(OH)D insufficiency was associated with increased total IgE concentrations, eosinophil counts, airway hyperresponsiveness, and increased symptoms and exacerbations. Similarly, in Italian studies, asthma control, lung function, and airway hyperresponsiveness were positively correlated with serum levels of 25(OH)D.70,71 Searing et al.74 also noted lower lung function, increased corticosteroid requirements, and increased aeroallergen sensitization in children as a function of decreased 25(OH)D concentrations. While these studies show intriguing associations between vitamin D insufficiency (or deficiency) and asthma control, the cross-sectional nature of the 25(OH)D measurement and outcome assessment prevents causal interpretation. For example, reverse causation may be responsible for the findings, in that children with more poorly controlled asthma may be less likely to go outdoors and therefore may have lower 25(OH)D concentrations as a result of their illness.
Given the limitations associated with these epidemiologic studies, randomized controlled trials of vitamin D are needed in children to better understand the causal relationship between vitamin D status and respiratory outcomes such as asthma control in this population. However, few such trials have been performed. In one recent randomized, double-blind, placebo-controlled trial, Urashima et al.75 compared 1,200 IU/day of vitamin D3 vs. placebo administered to Japanese schoolchildren (all comers) age 6–15 y during the winter months. Although the primary outcome was the incidence of influenza infection, sub-analyses within the group of children with existing asthma were also performed with asthma exacerbations as a secondary outcome. In these sub-analyses, vitamin D3 supplementation was associated with a decreased risk of exacerbations compared with placebo, with a relative risk of 0.17.75 Similarly, in a more recent randomized, double-blind 6-mo trial where 800 µg of inhaled budesonide was administered daily to a small group of 48 children with asthma in the presence or absence of 500 IU/day of vitamin D3, children receiving supplemental vitamin D did not have a reduction in 25(OH)D during the winter months and also had a reduced risk of asthma exacerbations triggered by acute respiratory viral infections.73 These initial findings suggest that vitamin D supplementation may have therapeutic benefit children with asthma, although additional randomized, double-blind, placebo-controlled studies are needed.
Vitamin D in Adults with Asthma
In adults, the recommended dietary intake for vitamin D ranges from 600 to 800 IU daily.47 However, despite the large variety of foods supplemented with vitamin D, a large proportion of the adult population remains vitamin D deficient,77,78 perhaps due in part to inadequate sun exposure and the rising prevalence of obesity which can lead to decreased vitamin D bioavailability.79 Similar to the studies in school age children, a number of cross-sectional studies in adults have also demonstrated associations between vitamin D status and asthma control (Table 3). Using data from the National Health and Nutrition Examination Survey, Keet et al.81 found that serum 25(OH)D concentrations were inversely related to current wheezing and asthma in subjects 6 y and older across the lifespan, such that a 10 ng/mL decline in 25(OH)D was associated with 20% increased odds of wheezing and 8% increased odds of asthma. Furthermore, in those subjects with current asthma, lower 25(OH)D concentrations were associated with increased odds of asthma exacerbations and healthcare utilization in the preceding year.81 Other studies have also noted associations between decreased serum 25(OH)D concentrations and decreased lung function, increased airway hyperresponsiveness to methacholine, and blunted glucocorticoid responsiveness.80,82,84 However, in a recent case-control study of obese subjects with and without asthma, no associations between vitamin D status and asthma prevalence were noted.83 The cross-sectional nature of these studies should again be emphasized since causation cannot be determined due to potential confounding of the study results. Ultimately, randomized controlled trials of vitamin D are needed in adults with asthma to determine the causal associations between vitamin D and asthma and to develop an evidence base for treatment. In the first known clinical trial focused on the role of vitamin D in allergic disease, Rappaport et al.85 found that supplementation with 60,000 to 30,000 IU of vitamin D2 per day resulted in significant relief of asthma and hay fever symptoms in more than 96% of patients suffering from asthma and seasonal allergies. Thus, vitamin D may have therapeutic effectiveness in adults with asthma, but more studies are clearly needed.
Table 3. Studies of vitamin D status in adults with asthma.
| Author | Year | n (% male) |
Age (years) | Study Design | Sample | Outcome Measures | Findings |
|---|---|---|---|---|---|---|---|
| Black80 |
2005 |
14,091 (55) |
> 20 y |
Cross-sectional |
General US population |
Lung function |
Higher serum 25(OH)D associated with increased lung function |
| Keet81 |
2011 |
6857 (49) |
23.6 |
Cross-sectional |
General US population |
Wheeze, history of asthma, and asthma exacerbation |
Higher serum 25(OH)D associated with decreased odds of current wheezing and asthma as well as emergency visits for asthma and asthma exacerbations |
| Li82 |
2011 |
435 (38) |
48.57 |
Cross-sectional |
Newly diagnosed asthmatics |
Lung function, total serum IgE |
Higher 25(OH)D associated with greater lung function with no associations noted for IgE |
| Oren83 |
2008 |
290 (26) |
43 |
Cross-sectional |
Obese patients |
Asthma and allergic rhinitis |
25(OH)D deficiency (< 25 ng/mL) associated with increased odds of atopic dermatitis but no associations with asthma |
| Sutherland84 | 2010 | 54 (43) | 38.3 | Cross-sectional | Persistent asthma | Lung function, airway hyper-responsiveness, glucocorticoid responsiveness | Decreased 25(OH)D associated with decreased lung function, increased airway hyperresponsiveness, and reduced glucocorticoid responses |
Genetics of Vitamin D and Asthma
Some studies have focused on genetic associations between vitamin D and asthma in larger populations. Of these, five studies have explored the relationship between VDR polymorphisms and asthma (Table 4).87-91 While three of the studies found associations between several VDR polymorphisms and asthma,87-89 others found no such association.90,91 However, in the study by Vollmert et al.,90 only one single SNP was examined as opposed to multiple variables. Additionally, all of the studies except Saadi et al.89 included family-based cohorts.
Table 4. Genetic studies of vitamin D and asthma.
| Author | Year | n | Location/ Population | Outcome Measures | Findings |
|---|---|---|---|---|---|
| Bossé86 |
2009 |
1,064 |
Québec, Canada/ French-Canadian families |
Genotype of genes involved in the vitamin D pathway |
SNPs in IL10, CYP24A1, CYP2R1, IL1RL1 and CD86 were associated with asthma and atopy |
| Poon87 |
2004 |
1,139 |
Quebec |
VDR genetic variants in family-based cohorts |
Six allelic varients were associated with asthma, while four varients were associated with atopy |
| Raby88 |
2004 |
582 |
United States/ Multi-center |
Twenty-eight loci in 7 positional candidates were genotyped |
VDR polymorphism demonstrated significant transmission distortion |
| Saadi89 |
2009 |
1,090 |
Jinan China/ Han population |
SNPs in the VDR |
Only one marker showed a significant association with asthma. Haplotype analysis of the VDR polymorphisms showed a significant association with asthma |
| Vollmert90 |
2004 |
32 |
Munich, Germany/ German |
SNPS in the VDR and integrin β7 |
None of the SNPs were associated with asthma |
| Wjst91 |
2005 |
951 |
Munich, Germany/ Germany and Sweden |
13 SNPs in the VDR |
In unaffected siblings, allele in the 5′ region was undertransmitted while two other alleles in the 3′ terminal region were overtransmitted |
| Wjst92 | 2006 | 947 | Munich, Germany/ Germany and Sweden | Serum levels of vitamin D and genotyping of DNA single base variants | At least one positive SNP with a transmission disequilibrium of for asthma or total IgE and vitamin D was found in several genes |
Only a handful of studies have evaluated the link between asthma and polymorphisms in other genes involved in vitamin D synthesis, bioavailability and metabolism. In one study, modest associations were noted between several genes in the vitamin D pathway and asthma and allergic sensitization in populations enriched for subjects with current asthma.86 A separate study of asthmatics also found haplotype associations for CYP24A1, the primary enzyme associated with calcidiol metabolism, where a 5-point haplotype was associated with asthma, allergic sensitization, and vitamin D metabolites in asthmatic subjects.92 While these preliminary results suggest that genetic components may account for vitamin D status in subjects with asthma, other studies are needed to determine tease out the genetic vs. environmental underpinnings of vitamin D status in this population.
Conclusion
There is a growing interest in the role of vitamin D in asthma and respiratory disorders across the lifespan. While several observational studies and a handful of small clinical trials suggest that vitamin D may be beneficial in asthma and wheezing disorders resulting from respiratory viral infections, findings are not consistent. These studies also differ significantly in terms of the populations studied, the timing of the vitamin D intake assessments and the 25(OH)D sampling, the 25(OH)D thresholds used for determining insufficiency, and the respiratory outcomes of interest. Because of the limitations of observational studies which include confounding by indication, definitive recommendations for vitamin D in subjects with asthma across the lifespan cannot be made. Ultimately, randomized, double-blind, placebo-controlled studies are needed in asthma populations to sort out causal relationships between vitamin D status and outcomes and to develop recommendations for treatment. Further mechanistic studies are also needed to understand the mechanisms by which vitamin D exerts its effects both in healthy individuals and subjects with chronic inflammatory disorders such as asthma.
Acknowledgments
Supported in part by NIH RO1 NR012021.
Glossary
Abbreviations:
- 25(OH)D
25-hydroxyvitamin D3
- IgE
immunoglobulin E
- IL
interleukin
- TH2
T-helper cell type-2
- UVB
ultraviolet-B
- VDR
vitamin D receptor
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/20434
References
- 1.National Asthma Education and Prevention Program Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 2.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–23. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, et al. National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol. 2011;127:382–9.e1-13. doi: 10.1016/j.jaci.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holt PG, Strickland DH. Interactions between innate and adaptive immunity in asthma pathogenesis: new perspectives from studies on acute exacerbations. J Allergy Clin Immunol. 2010;125:963–72, quiz 973-4. doi: 10.1016/j.jaci.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 6.Pope SM, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI, et al. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J Allergy Clin Immunol. 2001;108:594–601. doi: 10.1067/mai.2001.118600. [DOI] [PubMed] [Google Scholar]
- 7.Bradding P. The role of the mast cell in asthma: a reassessment. Curr Opin Allergy Clin Immunol. 2003;3:45–50. doi: 10.1097/00130832-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Broide DH. Immunologic and inflammatory mechanisms that drive asthma progression to remodeling. J Allergy Clin Immunol. 2008;121:560–70, quiz 571-2. doi: 10.1016/j.jaci.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies DE. The role of the epithelium in airway remodeling in asthma. Proc Am Thorac Soc. 2009;6:678–82. doi: 10.1513/pats.200907-067DP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980-2007. Pediatrics. 2009;123(Suppl 3):S131–45. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 11.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005-2009. Natl Health Stat Report. 2011:1–14. [PubMed] [Google Scholar]
- 12.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, et al. Centers for Disease Control and Prevention (CDC) National surveillance for asthma--United States, 1980-2004. MMWR Surveill Summ. 2007;56:1–54. [PubMed] [Google Scholar]
- 13.Allan K, Devereux G. Diet and asthma: nutrition implications from prevention to treatment. J Am Diet Assoc. 2011;111:258–68. doi: 10.1016/j.jada.2010.10.048. [DOI] [PubMed] [Google Scholar]
- 14.Nurmatov U, Devereux G, Sheikh A. Nutrients and foods for the primary prevention of asthma and allergy: systematic review and meta-analysis. J Allergy Clin Immunol. 2011;127:724–33. e1-30. doi: 10.1016/j.jaci.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Willers SM, Devereux G, Craig LC, McNeill G, Wijga AH, Abou El-Magd W, et al. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax. 2007;62:773–9. doi: 10.1136/thx.2006.074187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mason RS, Sequeira VB, Gordon-Thomson C. Vitamin D: the light side of sunshine. Eur J Clin Nutr. 2011;65:986–93. doi: 10.1038/ejcn.2011.105. [DOI] [PubMed] [Google Scholar]
- 17.Ginde AA, Mansbach JM, Camargo CA., Jr Vitamin D, respiratory infections, and asthma. Curr Allergy Asthma Rep. 2009;9:81–7. doi: 10.1007/s11882-009-0012-7. [DOI] [PubMed] [Google Scholar]
- 18.Herr C, Greulich T, Koczulla RA, Meyer S, Zakharkina T, Branscheidt M, et al. The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir Res. 2011;12:31. doi: 10.1186/1465-9921-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–5. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 20.Mak G, Hanania NA. Vitamin D and asthma. Curr Opin Pulm Med. 2011;17:1–5. doi: 10.1097/MCP.0b013e3283411440. [DOI] [PubMed] [Google Scholar]
- 21.Mansbach JM, Ginde AA, Camargo CA., Jr Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics. 2009;124:1404–10. doi: 10.1542/peds.2008-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandhu MS, Casale TB. The role of vitamin D in asthma. Ann Allergy Asthma Immunol. 2010;105:191–9, quiz 200-2, 217. doi: 10.1016/j.anai.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Chambers ES, Hawrylowicz CM. The impact of vitamin D on regulatory T cells. Curr Allergy Asthma Rep. 2011;11:29–36. doi: 10.1007/s11882-010-0161-8. [DOI] [PubMed] [Google Scholar]
- 24.Chishimba L, Thickett DR, Stockley RA, Wood AM. The vitamin D axis in the lung: a key role for vitamin D-binding protein. Thorax. 2010;65:456–62. doi: 10.1136/thx.2009.128793. [DOI] [PubMed] [Google Scholar]
- 25.Borradale D, Kimlin M. Vitamin D in health and disease: an insight into traditional functions and new roles for the ‘sunshine vitamin’. Nutr Res Rev. 2009;22:118–36. doi: 10.1017/S0954422409990102. [DOI] [PubMed] [Google Scholar]
- 26.LoPiccolo MC, Lim HW. Vitamin D in health and disease. Photodermatol Photoimmunol Photomed. 2010;26:224–9. doi: 10.1111/j.1600-0781.2010.00524.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, et al. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol. 2005;19:2685–95. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]
- 28.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–77. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 29.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 30.Schauber J, Dorschner RA, Coda AB, Büchau AS, Liu PT, Kiken D, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–11. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sørensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS, Hiemstra PS, et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–9. doi: 10.1182/blood.V97.12.3951. [DOI] [PubMed] [Google Scholar]
- 32.Tjabringa GS, Rabe KF, Hiemstra PS. The human cathelicidin LL-37: a multifunctional peptide involved in infection and inflammation in the lung. Pulm Pharmacol Ther. 2005;18:321–7. doi: 10.1016/j.pupt.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Marcinkowska E. A run for a membrane vitamin D receptor. Biol Signals Recept. 2001;10:341–9. doi: 10.1159/000046902. [DOI] [PubMed] [Google Scholar]
- 34.Litonjua AA. Childhood asthma may be a consequence of vitamin D deficiency. Curr Opin Allergy Clin Immunol. 2009;9:202–7. doi: 10.1097/ACI.0b013e32832b36cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang J, Zhou R, Luger D, Zhu W, Silver PB, Grajewski RS, et al. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J Immunol. 2009;182:4624–32. doi: 10.4049/jimmunol.0801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pichler J, Gerstmayr M, Szépfalusi Z, Urbanek R, Peterlik M, Willheim M. 1 alpha,25(OH)2D3 inhibits not only Th1 but also Th2 differentiation in human cord blood T cells. Pediatr Res. 2002;52:12–8. doi: 10.1203/00006450-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Matheu V, Bäck O, Mondoc E, Issazadeh-Navikas S. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J Allergy Clin Immunol. 2003;112:585–92. doi: 10.1016/S0091-6749(03)01855-4. [DOI] [PubMed] [Google Scholar]
- 38.Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116:146–55. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80(Suppl):1717S–20S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 40.Lange NE, Litonjua A, Hawrylowicz CM, Weiss S. Vitamin D, the immune system and asthma. Expert Rev Clin Immunol. 2009;5:693–702. doi: 10.1586/eci.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brehm JM, Celedón JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179:765–71. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009;169:626–32. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001-2004. Pediatrics. 2009;124:e362–70. doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heaney RP, Horst RL, Cullen DM, Armas LA. Vitamin D3 distribution and status in the body. J Am Coll Nutr. 2009;28:252–6. doi: 10.1080/07315724.2009.10719779. [DOI] [PubMed] [Google Scholar]
- 45.Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr. 2008;87:1087S–91S. doi: 10.1093/ajcn/87.4.1087S. [DOI] [PubMed] [Google Scholar]
- 46.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 47.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res. 2011;26:455–7. doi: 10.1002/jbmr.328. [DOI] [PubMed] [Google Scholar]
- 49.Holick MF. The IOM D-lemma. Public Health Nutr. 2011;14:939–41. doi: 10.1017/S1368980011000590. [DOI] [PubMed] [Google Scholar]
- 50.Freeman NC, Schneider D, McGarvey P. Household exposure factors, asthma, and school absenteeism in a predominantly Hispanic community. J Expo Anal Environ Epidemiol. 2003;13:169–76. doi: 10.1038/sj.jea.7500266. [DOI] [PubMed] [Google Scholar]
- 51.Gilmour MI, Jaakkola MS, London SJ, Nel AE, Rogers CA. How exposure to environmental tobacco smoke, outdoor air pollutants, and increased pollen burdens influences the incidence of asthma. Environ Health Perspect. 2006;114:627–33. doi: 10.1289/ehp.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinez FD. The origins of asthma and chronic obstructive pulmonary disease in early life. Proc Am Thorac Soc. 2009;6:272–7. doi: 10.1513/pats.200808-092RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller RL, Ho SM. Environmental epigenetics and asthma: current concepts and call for studies. Am J Respir Crit Care Med. 2008;177:567–73. doi: 10.1164/rccm.200710-1511PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Searing DA, Rabinovitch N. Environmental pollution and lung effects in children. Curr Opin Pediatr. 2011;23:314–8. doi: 10.1097/MOP.0b013e3283461926. [DOI] [PubMed] [Google Scholar]
- 55.Kim JH, Ellwood PE, Asher MI. Diet and asthma: looking back, moving forward. Respir Res. 2009;10:49. doi: 10.1186/1465-9921-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller RL. Prenatal maternal diet affects asthma risk in offspring. J Clin Invest. 2008;118:3265–8. doi: 10.1172/JCI37171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belderbos ME, Houben ML, Wilbrink B, Lentjes E, Bloemen EM, Kimpen JL, et al. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics. 2011;127:e1513–20. doi: 10.1542/peds.2010-3054. [DOI] [PubMed] [Google Scholar]
- 58.Camargo CA, Jr., Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85:788–95. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Camargo CA, Jr., Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, et al. New Zealand Asthma and Allergy Cohort Study Group Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127:e180–7. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 60.Carroll KN, Gebretsadik T, Larkin EK, Dupont WD, Liu Z, Van Driest S, et al. Relationship of maternal vitamin D level with maternal and infant respiratory disease. Am J Obstet Gynecol. 2011;205:215. e1-7. doi: 10.1016/j.ajog.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85:853–9. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- 62.Erkkola M, Kaila M, Nwaru BI, Kronberg-Kippilä C, Ahonen S, Nevalainen J, et al. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clin Exp Allergy. 2009;39:875–82. doi: 10.1111/j.1365-2222.2009.03234.x. [DOI] [PubMed] [Google Scholar]
- 63.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, et al. Princess Anne Hospital Study Group Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62:68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miyake Y, Sasaki S, Tanaka K, Hirota Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. Eur Respir J. 2010;35:1228–34. doi: 10.1183/09031936.00100609. [DOI] [PubMed] [Google Scholar]
- 65.Morales E, Romieu I, Guerra S, Ballester F, Rebagliato M, Vioque J, et al. INMA Project Maternal vitamin D status in pregnancy and risk of lower respiratory tract infections, wheezing, and asthma in offspring. Epidemiology. 2012;23:64–71. doi: 10.1097/EDE.0b013e31823a44d3. [DOI] [PubMed] [Google Scholar]
- 66.Rothers J, Wright AL, Stern DA, Halonen M, Camargo CA., Jr Cord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization in children from Tucson, Arizona. J Allergy Clin Immunol. 2011;128:1093–9. e1-5. doi: 10.1016/j.jaci.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hyppönen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, et al. Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann N Y Acad Sci. 2004;1037:84–95. doi: 10.1196/annals.1337.013. [DOI] [PubMed] [Google Scholar]
- 68.Bäck O, Blomquist HK, Hernell O, Stenberg B. Does vitamin D intake during infancy promote the development of atopic allergy? Acta Derm Venereol. 2009;89:28–32. doi: 10.2340/00015555-0541. [DOI] [PubMed] [Google Scholar]
- 69.Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, et al. Childhood Asthma Management Program Research Group Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol. 2010;126:52–8.e5. doi: 10.1016/j.jaci.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chinellato I, Piazza M, Sandri M, Peroni D, Piacentini G, Boner AL. Vitamin D serum levels and markers of asthma control in Italian children. J Pediatr. 2011;158:437–41. doi: 10.1016/j.jpeds.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 71.Chinellato I, Piazza M, Sandri M, Peroni DG, Cardinale F, Piacentini GL, et al. Serum vitamin D levels and exercise-induced bronchoconstriction in children with asthma. Eur Respir J. 2011;37:1366–70. doi: 10.1183/09031936.00044710. [DOI] [PubMed] [Google Scholar]
- 72.Freishtat RJ, Iqbal SF, Pillai DK, Klein CJ, Ryan LM, Benton AS, et al. High prevalence of vitamin D deficiency among inner-city African American youth with asthma in Washington, DC. J Pediatr. 2010;156:948–52. doi: 10.1016/j.jpeds.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Majak P, Olszowiec-Chlebna M, Smejda K, Stelmach I. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. 2011;127:1294–6. doi: 10.1016/j.jaci.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 74.Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol. 2010;125:995–1000. doi: 10.1016/j.jaci.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–60. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 76.Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin Pediatr (Phila) 2007;46:42–4. doi: 10.1177/0009922806289311. [DOI] [PubMed] [Google Scholar]
- 77.Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–43. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 78.O’Donnell S, Cranney A, Horsley T, Weiler HA, Atkinson SA, Hanley DA, et al. Efficacy of food fortification on serum 25-hydroxyvitamin D concentrations: systematic review. Am J Clin Nutr. 2008;88:1528–34. doi: 10.3945/ajcn.2008.26415. [DOI] [PubMed] [Google Scholar]
- 79.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 80.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–8. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 81.Keet CA, McCormack MC, Peng RD, Matsui EC. Age- and atopy-dependent effects of vitamin D on wheeze and asthma. J Allergy Clin Immunol. 2011;128:414–16.e5. doi: 10.1016/j.jaci.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li F, Peng M, Jiang L, Sun Q, Zhang K, Lian F, et al. Vitamin D deficiency is associated with decreased lung function in Chinese adults with asthma. Respiration. 2011;81:469–75. doi: 10.1159/000322008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oren E, Banerji A, Camargo CA., Jr Vitamin D and atopic disorders in an obese population screened for vitamin D deficiency. J Allergy Clin Immunol. 2008;121:533–4. doi: 10.1016/j.jaci.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 84.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010;181:699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rappaport BZ, Reed CI, Hathaway ML, Struck HC. The treatment of hay fever and asthma with viosterol of high potency. J Allergy. 1934;5:541–53. doi: 10.1016/S0021-8707(34)90130-1. [DOI] [Google Scholar]
- 86.Bossé Y, Lemire M, Poon AH, Daley D, He JQ, Sandford A, et al. Asthma and genes encoding components of the vitamin D pathway. Respir Res. 2009;10:98. doi: 10.1186/1465-9921-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Poon AH, Laprise C, Lemire M, Montpetit A, Sinnett D, Schurr E, et al. Association of vitamin D receptor genetic variants with susceptibility to asthma and atopy. Am J Respir Crit Care Med. 2004;170:967–73. doi: 10.1164/rccm.200403-412OC. [DOI] [PubMed] [Google Scholar]
- 88.Raby BA, Lazarus R, Silverman EK, Lake S, Lange C, Wjst M, et al. Association of vitamin D receptor gene polymorphisms with childhood and adult asthma. Am J Respir Crit Care Med. 2004;170:1057–65. doi: 10.1164/rccm.200404-447OC. [DOI] [PubMed] [Google Scholar]
- 89.Saadi A, Gao G, Li H, Wei C, Gong Y, Liu Q. Association study between vitamin D receptor gene polymorphisms and asthma in the Chinese Han population: a case-control study. BMC Med Genet. 2009;10:71. doi: 10.1186/1471-2350-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vollmert C, Illig T, Altmuller J, Klugbauer S, Loesgen S, Dumitrescu L, et al. Single nucleotide polymorphism screening and association analysis–exclusion of integrin beta 7 and vitamin D receptor (chromosome 12q) as candidate genes for asthma. Clin Exper Allergy. 2004;34:1841–50. doi: 10.1111/j.1365-2222.2004.02047.x. [DOI] [PubMed] [Google Scholar]
- 91.Wjst M. Variants in the vitamin D receptor gene and asthma. BMC Genet. 2005;6:2. doi: 10.1186/1471-2156-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wjst M, Altmüller J, Faus-Kessler T, Braig C, Bahnweg M, André E. Asthma families show transmission disequilibrium of gene variants in the vitamin D metabolism and signalling pathway. Respir Res. 2006;7:60. doi: 10.1186/1465-9921-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]