Abstract
The colonization, development and maturation of the newborn gastrointestinal tract that begins immediately at birth and continues for two years, is modulated by numerous factors including mode of delivery, feeding regime, maternal diet/weight, probiotic and prebiotic use and antibiotic exposure pre-, peri- and post-natally. While in the past, culture-based approaches were used to assess the impact of these factors on the gut microbiota, these have now largely been replaced by culture-independent DNA-based approaches and most recently, high-throughput sequencing-based forms thereof. The aim of this review is to summarize recent research into the modulatory factors that impact on the acquisition and development of the infant gut microbiota, to outline the knowledge recently gained through the use of culture-independent techniques and, in particular, highlight advances in high-throughput sequencing and how these technologies have, and will continue to, fill gaps in our knowledge with respect to the human intestinal microbiota.
Keywords: infant, gut microbiota, high-throughput sequencing, colonization, probiotics, prebiotics, antibiotics
Introduction
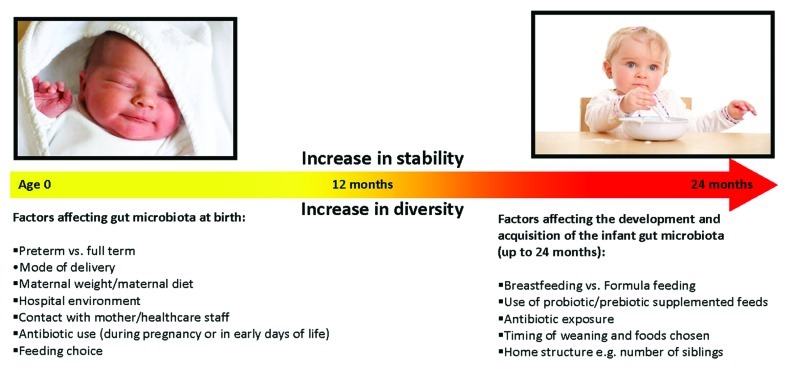
Following birth, the gut microbial composition undergoes remarkable alterations during the first two years of life. More specifically, the human gastrointestinal tract (GIT) changes from being initially sterile, to possessing an adult-like stable microbiome by the time the infant reaches 2 years of age.1 Despite being home to more than 1014 bacterial cells outnumbering the total amount of human cells in the body (1013),2 which contribute up to 60% of fecal mass, the human gut contains a surprisingly limited number of dominant phyla (i.e., Firmicutes and Bacteroidetes). A diverse number of factors contribute to the development of the gut microbiota and impact on the unique composition that each individual develops (Fig. 1).
Figure 1.
Factors contributing to changes in gut microbiota composition in the first 2 years of life.
The infant gut is initially an aerobic environment. However, through colonization the environment is altered, resulting in a reduction in oxygen levels thereby creating an environment suitable for the growth of anaerobes. The initial gut composition is simple, dynamic and very unstable and undergoes marked fluctuations.3 Nonetheless, evidence exists that the initial colonization influences subsequent immune system development by influencing intestinal morphology and gut-associated lymphoid tissue (GALT) function. Furthermore, more recently it has also been suggested that the gut microbiota play a significant role in the regulation of the immune system (something which will be returned to later).4 Thus, an altered gut microbiota composition can potentially predispose the infant to more frequent infections and allergic disease risk.5
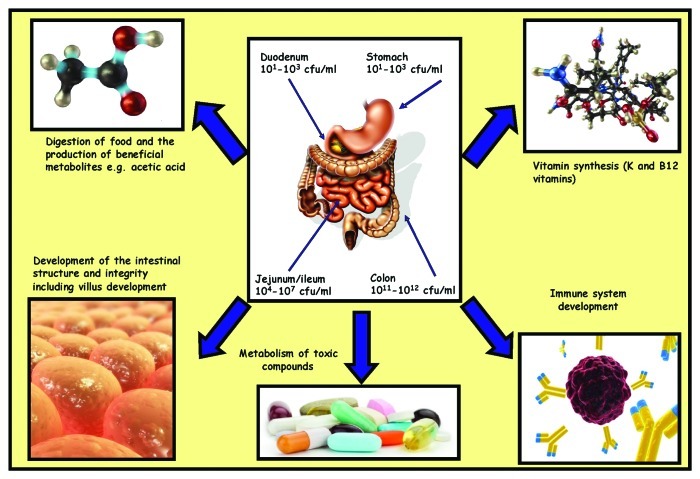
Given the enormity of the bacterial population present in the gut, it has been proposed that the gut bacterial microbiome be considered a “super organism.”6 While the composition of the species present within this microbiome is of great significance, the overall diversity of this population can also be of critical importance. In the majority of cases, the interaction between the bacteria and the human host can be regarded as mutualistic, in that both the bacteria and the host benefit from a mutual relationship. The vast array of functions which these bacteria are capable of is still being elucidated but many benefits have been well documented,7 and these functions can be divided into those which are metabolic, trophic or protective.7 The mechanisms by which these functions occur are outside the scope of this review, but examples of some of these diverse functions have been summarized in Figure 2.
Figure 2.
Location, concentrations and functions of human gut microbiota.
Advances in Techniques to Study Gut Microbiota
In the past, investigations into the infant gut microbiome were culture-based and therefore the insight provided was limited by a lack of knowledge with respect to the growth requirements of the majority of microbes present in the gut. Selection of the correct media, temperature, oxygen content and time for growth all impacted on the ability to generate accurate culture-based results. It has since been estimated that as little as 10–50% of the entire gut bacteria are easily cultured.8 With our increasing knowledge of the growth requirements of a vast number of microbes, as well as the availability of specialized culturing media, we can now successfully culture increasing numbers of different microbes, as recently demonstrated by Goodman et al.9 Culture-based approaches are still being employed in some studies [despite the availability of much more sophisticated and complex technologies, some of which will be outlined below (Table 1)], though most often in combination with culture-independent techniques such as fluorescent in situ hybridization (FISH) or flow cytometry (FCM). Despite these advances in culturing capabilities, this approach is still unsuitable for characterizing the microbiota as a whole, especially in complex environments such as the human gut.
Table 1. Techniques used to investigate the human gut microbiota; advantages, disadvantages and examples of use.
| Microflora associated characteristics |
Culture-dependent techniques |
Culture-independent techniques |
High-throughput sequencing | |
|---|---|---|---|---|
|
Technique description |
The use of characteristics associated with microbes e.g., SCFA production to identify if differences exist in the gut microbial populations between different subject groups |
Use of selective media to culture specific microorganisms or species of microorganisms e.g., Man Rogosa Sharpe (MRS) media for lactobacilli growth |
Identify bacteria through isolation and amplification of bacterial DNA e.g., 16S rRNA gene. Includes: PCR, DGGE, TGGE, qPCR, dot blot hybridization, FISH, flow cytometry |
Sequencing based approaches used to rapidly identify bacteria using bacterial DNA as template e.g., 454, Illumina, SoLID, Ion torrent |
|
History of use |
To date has been predominantly used as an initial population screen or in epidemiological studies |
Historically, the most frequently used approach to identify bacteria present in various environments |
Increasingly popular in past 2 decades with increasing availability of computer based technologies and software programs |
Became commercially available at the beginning of the 21st century and becoming increasingly popular ever since |
|
Advantages |
Simple Inexpensive Suitable as an initial screen to test a novel hypothesis Useful for large population screens e.g., in epidemiology studies |
Quick Inexpensive Limited skill required Limited equipment needed Useful as the initial screen before more detailed investigations |
Relatively inexpensive Relatively simple More detailed results achievable |
Less biased results Very detailed information Bacterial profile in complex environments e.g., gut microbiota can be identified Huge phylogenetic information provided Relatively quick |
|
Disadvantages |
Provides limited information No bacterial species identification possible |
Up to 90% of bacteria non-culturable Provides limited information Need prior knowledge of bacteria to screen for Requires further tests for species identification |
Prone to PCR bias Requires more sophisticated equipment and training on their use May need several methods in combination to get appropriate level of details in results |
Extremely expensive Data handling requirements are significant Requires training on sample preparation and machine use and experience of interpreting results |
|
Examples of studies efficiently using this technique |
References 10–12 |
References 13, 14 |
Reference 15 |
References 2, 16 |
| Future use in infant gut microbiota research | Most likely to be used to test novel hypotheses and to be followed up with more detailed techniques | Likely to become infrequently used and to be mainly used in combination with and verified by newer technologies |
Likely to remain popular in the coming decade, but decrease thereafter as increased availability and use of sequencing approaches occurs | Increased use since the year 2000 as cost is decreasing and likely to become the main approach used in the future |
Another approach that has been taken has involved the study of differences in the composition or presence/absence of microflora associated characteristics (MACs) between different subject groups. The concept of MACs was first proposed in 1978,17 and some examples include mucin degradation, conversion of cholesterol to coprostanol and inactivation of tryptic activity. One of the most commonly studied MACs are short chain fatty acids (SCFAs). These are a sub-group of fatty acids that contain six or fewer carbons on their aliphatic side chain. They include acetic, butyric and propionic acids and are produced as a result of fermentation of dietary fiber by bacteria in the large intestine. Differences in bacteria populations could result in alterations in the type and amount of fecal SCFAs present. The comparison of MACs has been a major component of studies investigating the contribution of the microbiome in, for example, coeliacs relative to controls,10,18 probiotic or antibiotic treated infants/children compared with controls11,19 and even to identify changes in gut microbiota-related functionality due to allergic disease.12,20 While MACs are useful as a tool for screening large populations, such as in epidemiological studies, they are most useful when supplemented with detailed insights into gut microbial composition.
Due to the limitations associated with culture-based approaches, researchers began to develop and utilize culture-independent, DNA-based approaches to gain such detailed insights. There are a variety of such DNA-based approaches available. Among the most popular of those employed initially were temperature gradient gel electrophoresis (TGGE) and denaturing gradient gel electrophoresis (DGGE).21 These systems work by the separation of amplicons [often of the 16S ribosomal RNA gene (rRNA)] based on their GC content, to reveal distinctive patterns. The 16S gene allows phylogenetic identification of the bacteria present, as this gene is present in all prokaryotes and contains conserved and variable regions, which facilitate amplicon generation and differentiation.22 These techniques are rapid and provide an overview of the composition of microbial populations. Downstream analysis to identify specific components of the population can be facilitated by band excision and sequencing, however, despite this, these approaches usually only provide limited phylogenetic information and, as with all PCR based strategies, can be subject to PCR bias. Dot-blot hybridization technologies have also been utilized to investigate the infant gut microbiota.23 In this case, RNA is isolated, immobilized and assessed qualitatively and quantitatively using oligonucleotide-labeled probes. This approach is not subject to PCR bias, but the resolution of results can be limited, and it is focused on specific populations rather than the microbiota as a whole. Furthermore, results depend on the ability to first generate reference sequences to facilitate the design of probes. Similarly, FISH approaches have been used and have also provided valuable information, but, as with dot-blot hybridization, the results are again focused on specific populations and reference sequence generation is again required.24,25 Quantitative PCR (qPCR) is now also frequently used, which measures the accumulation of products through measurement of fluorescently labeled primers or probes.26 Studies have also employed several techniques in combination, such as dot-blot hybridization together with qPCR to allow for the quantification of bacterial numbers as well as identification of the different species present.27 Other studies have used qPCR and FISH in combination, yielding significantly more detailed and enlightening results.28
The next step in the evolution of culture-independent technologies involved the use of phylogenetic microarrays.3 Microarrays are similar to the previously described approaches, but are more advantageous in that they allow hybridization of greater numbers of sequences to the one slide, thus allowing extensive data generation from the one read. Briefly, the sequences are attached to the glass slide, using a robotic arrayer. These sequences are fluorescently labeled and their expression can be measured using a fluorescence assay.29 Thus it is clear that a shift in gut microbiota research has occurred in recent decades, to focusing more specifically on the bacterial 16S rRNA gene.30,31
As highlighted by an extensive review in 2008,30 the investigation into the gastrointestinal microbiota has moved into the “metagenomic era,” with increasing numbers of studies employing DNA sequencing-based techniques. Sequencing of the 16S rRNA gene has the advantage of providing the gene sequence itself (and, thus, valuable information regarding the identity of microbes present) rather than the indirect, and less accurate, information provided by DGGE and TGGE. When performed on a larger scale, DNA sequencing can reveal detailed information relating to the overall microbial population in a particular environment e.g., the human gut which contrasts with targeted approaches such as dot-blotting, FISH, qPCR and, to a lesser extent, phylogenetic microarrays. The earliest sequencing-based approaches were based on cloning of full length 16S rRNA genes into a plasmid, its introduction into a host (most often Escherichia coli, E. coli), followed by conventional, capillary-based, Sanger sequencing thereof. While this technique allows the identification of bacterial species, it is slow and expensive. This process can take up to 3 weeks from the generation of purified DNA to the generation of results.32 Today the focus has shifted to high-throughput sequencing which, because of the scale at which sequence data are generated, provides a greater insight into the precise composition of the microbiota present.31 High-throughput sequencing technologies (also known as next generation sequencing), such as those supplied by Roche/454 and Illumina, have been used extensively for gut microbiota-related studies. The Roche/454 pyrosequencing approach is based on sequencing by synthesis. For 16S sequencing, purified DNA is used to generate an amplicon library which then undergoes an emulsion based clonal PCR. This PCR uses beads coated in oligonucleotides, which are specific to adaptor sequences attached to the amplicons. Following bead recovery and enrichment, the amplicon-coated beads are added to a picotiter plate and sequencing ensues. Sequencing involves an enzymatic reaction and, as each nucleotide is sequentially added, pyrophosphate is released and ATP is subsequently generated. This then enables the conversion of luciferin and the emission and detection of photons of light.32 For Illumina sequencing, single stranded DNA fragments are generated with oligo-ligated adaptors attached. These are then attached to a glass flow cell, onto which oligonucleotides complementary to the adaptor region of the amplicons are attached. Heating and cooling cycles follow, after which incubation with reagents and a polymerase to hybridize the DNA fragments to the oligonucleotides occurs. The flow cell, when placed into a cassette, is then sequenced and the incorporation of the nucleotides (each of which is fluorescently labeled) is measured using imaging technologies.33 However, these techniques also have their own inherent limitations. In the case of amplicons, they are prone to PCR bias. One also requires extensive bioinformatic capabilities to handle the vast amount of bioinformatic data generated and the associated platforms are expensive to purchase and run. In addition to this, while these technologies provide valuable information with respect to the proportions of different populations present, qPCR is often required to generate absolute quantification data.
While amplicon-based 16S compositional sequencing has been most frequently used for human studies, another option is shotgun sequencing whereby the metagenomic DNA (i.e., all of the DNA from the microbial population) is first fragmented into short lengths and sequenced randomly.34,35 This approach involves the sequencing of random fragments of DNA rather than specifically targeted regions and provides valuable information regarding the functional potential and, to some degree, the identity of the microbes present in a particular niche and, if performed on a sufficiently large enough scale, entire genomic sequences can be generated.36,37 As with target-specific approaches, shotgun sequencing has also benefited enormously from the availability of high-throughput sequencing technologies.2,38-40
Other high-throughput sequencing technologies have, or will shortly, emerge. Examples include the Ion torrent,41 SOLid (Applied Biosystems),42 SMRT (Pacific Biosystems),43 and nano pore sequencers.44,45 These techniques aim to provide longer, or greater numbers of reads, more rapidly and/or at a lower cost. While the exact mechanisms, advantages, disadvantages and differences between these new culture-independent techniques are outside the scope of this review (and are covered extensively in other reviews cited herein), it is worth noting that these technologies will undoubtedly revolutionize the way in which we study the human gut microbiota in the future. Indeed, in the past few years alone, these high-throughput sequencing technologies have already been employed to study the gut microbiota associated with different diseases including, but not limited to, diabetes,46 Crohn disease,47 irritable bowel syndrome,48 cancer49,50 and obesity38,51,52 and to investigate the effects of diet16,39 and antibiotics53 on the gut microbiota.
Thus, it is clear that in the past 15 years researchers have progressed from relying heavily on culture-based approaches to utilizing sophisticated high-throughput sequencing technologies to investigate the microbial world within us. Before proceeding, it should also be noted that while there has been enormous progress made, one limitation that still remains with respect to studying the gut microbiota, is accessing a representative bacterial sample to study. Most frequently, the composition of the gut microbiota of infants is assessed following the collection of stool samples and the extraction of DNA. However, there are limitations to this approach, as fecal samples are most representative of the bacteria present in the lower colon but less representative of the bacteria of the stomach and upper intestine. However, despite this limitation, fecal samples are very useful with respect to identifying the majority of bacteria present in the colon, which is where the preponderance of intestinal bacteria reside (due to transit time, pH, nutrient availability, etc.), and, in the absence of other alternatives, fecal-based assessments remain the approach of choice. This review will focus on the infant gut microbiota development, based on results generated using culture-independent approaches and will highlight how the results generated using these different technologies compare with those generated using older approaches.
Shaping the Early Intestinal Microbiota: Effect of Mode of Delivery
Infants undergo rapid colonization during delivery and in the first few hours following birth. Initially the infant is colonized by aerobes, followed by facultative anaerobes and, as the oxygen level is diminished, strict anaerobes predominate.54-56 Some of the earliest colonizers include E. coli and enterococci, and, once the oxygen has been consumed, they are followed by strict anaerobes including bifidobacteria, Bacteroides and Clostridium spp.1,57 However, while these general patterns of colonization occur, colonization of the infant’s gut is altered by birth mode. Infants born vaginally are colonized with vaginal and fecal microbes from their mother, and this has been shown to result in a strong maternal signature, which contrasts with the microbiota of caesarean born infants.1 It is generally accepted that infants born by caesarean section have no access to the mother’s microbiota, although there have been suggestions that the swallowing of amniotic fluid allows some colonization of the infant’s gut in utero.58 Caesarean delivered infants are instead colonized by microbes from the environment, such as those from healthcare staff, wards and other infants. A recent study of 9 women and their 10 infants (i.e., including one set of twins) was completed using high-throughput sequencing (Roche/454) of the variable 2 (V2) region of the bacterial 16S rRNA gene.59 The authors sequenced 34 samples from the mother and 46 from their infants, resulting in 157,915 partial 16S sequences. The study found that there was a strong vertical transmission of vaginal microbes from the mother to the infant when birth was by vaginal delivery, resulting in a dominant number of lactobacilli within hours of birth. In contrast, in the gut of caesarean delivered infants there was a strong presence of maternal skin microbes, with staphylococci being dominant in these infants.59 This study advances our understanding of the relationship between the mother’s microbiota and that of her infant and highlights the benefits of employing high-throughput sequencing for such purposes.
Other DNA-based studies have also been completed that support the aforementioned results. A study of over 1,000 infants in the Netherlands examined, using qPCR, the potential of over 16 factors to alter the composition of the infant gut microbiota at age 1 month.60 When the gut microbiota of infants that were vaginally born was compared with those born by caesarean section, it was apparent that the latter group had 100-fold lower bifidobacteria and Bacteroides fragilis numbers. In addition, birth by caesarean delivery was also associated with a 100-fold increased colonization with Clostridium difficile. C. difficile is a Gram positive spore-forming anaerobic pathogen, which has been shown to be capable of producing toxins and is frequently a cause of diarrhea and colitis.61-63 Notably, a follow-on study by the same group found a positive association between mode of delivery, the gut microbiota and atopy risk.64 Another such study focused on the microbial composition of even younger infants (i.e., 3 days old) (n = 46).65 The TGGE- and DGGE-based approaches employed again highlighted the strong impact of delivery mode on the microbial composition, with vaginally born, exclusively breastfed infants, having the highest bifidobacteria levels and lowest C. difficile counts of all infants.65 Surprisingly, although subject to bias and the inherent limitations outlined previously, culture-based approaches have revealed similar trends in that, when Adlerberth et al. examined over 300 infants across three European cohorts, they found that caesarean delivered infants are colonized with greater numbers of clostridia and Klebsiella and decreased E. coli, bifidobacteria and Bacteroides compared with vaginally delivered infants.66
A FISH-based study of 168 one month old Finnish infants provided a somewhat different set of results in that the Clostridium, Bacteroides and Lactobacillus populations in the gut were found to be similar in both vaginally and caesarean born infants.67 Notably, however, it was again apparent that bifidobacteria were greatly impacted upon by delivery mode, with a 1,300-fold higher level observed in infants born vaginally. This is significant as bifidobacteria, along with lactobacilli, are the microorganisms most frequently employed as probiotics. Bifidobacteria were first characterized in the period 1899–1900 and, since then, have been shown to predominate in vaginally delivered, breastfed infants. The health promoting properties of specific lactobacilli and bifidobacteria have been reported and include the combat of diarrhea, increasing resistance to pathogenic microorganisms, decreased occurrence of urinary, gastrointestinal and respiratory infections, alleviating lactose intolerance symptoms, reducing constipation and boosting immune functioning.68-70 However, as has been highlighted recently by EFSA (European Food Safety Authority), it is critical that the health claims pertaining to each specific strain are rigorously tested.71 Nonetheless, research to date does suggest that a delivery-mode mediated variation in the numbers and diversity of both lactobacilli and bifidobacteria occurs and considerable research has been performed with a view to determining the significance of these differences. Moving forward this area of research will benefit from more detailed investigations to determine precisely which populations are influenced by delivery modes and to establish which of these populations can be specifically associated with subsequent health-related impacts.
Culture-based studies have shown that the influence of delivery mode on the gut microbiota can persist for some time and thus may impact on the subsequent health of the infant.54 Recent culture-independent approaches have also shown this to be the case, once more showing the ability to verify the results of culture-based approaches using new culture-independent and high-throughput sequencing based technologies. More specifically, a FISH-based study of 60 children at age 7, in which 31 had been born by caesarean section and 29 vaginally,56 revealed that vaginally born infants had increased levels of clostridia compared with those delivered by caesarean section. The authors reported that lower clostridia levels appear in those infants being treated for asthma at age 7, while healthy children had higher numbers of clostridia, thereby highlighting a potential long-term consequence of the impact of delivery mode on the gut microbiota. A recent birth cohort supports these gut microbiota findings of a long-term consequence on health due to delivery mode.72 The study examined the association between caesarean delivery and the subsequent risk of being obese at age 23–25 years. After controlling for sex, birth weight, activity, income, smoking and maternal factors (schooling and smoking during the pregnancy), it was revealed that those born by caesarean section had a 58% increased risk of obesity compared with vaginally born infants, thus highlighting the long-term effects of a factor that impacts on the infant’s gut microbiota.72 This topic has also been the focus of a recent review.73
Effect of Early Feeding Regime
The impact of feeding choice, i.e., breastfeeding vs. formula feeding, and weaning on the infant gut microbiota has also been investigated. The World Health Organization (WHO) recommends exclusive breastfeeding of all infants up to 6 months of age and continued supplemented breastfeeding up to 12 months of age.74 Despite this, there are large variations in the rates of breastfeeding from one country to another. Presently, Scandinavian countries have some of the highest levels of breastfeeding with, for example, recent data for Norway suggesting that 96% of infants are breastfed at birth, of which 84% are exclusively breastfed.75 In comparison to these high levels, many developed countries have much lower rates of breastfeeding, with recent Irish data suggesting that rates of exclusive breastfeeding currently stand at just 47% at hospital discharge and drop to between 6.5 and 9.4% of women partially breastfeeding during the first 6 months of the infant’s life.76 In the USA, the 2007 National Immunization Survey found that at 3 months of age, just 33% of infants were exclusively breastfed, and that this level falls further at 6 mo to just 13% being exclusively breastfed.77
Breastfeeding is accepted as being highly beneficial to both mothers and infants.78 Breastmilk is a nutritious food for the newborn, the composition of which varies in response to the infant’s changing nutritional requirements and age. In addition to containing the appropriate nutrients for the growing infant, breastmilk can have a significant impact on the gut microbial composition by virtue of being a source of prebiotics (non-digestible food ingredients that beneficially effect the host by selectively stimulating the growth of one or a limited number of bacteria in the colon), lactoferrin (an antimicrobial protein) and lysozyme (an enzyme found naturally in milk, tears and sweat that is capable of digesting the cell walls of bacteria).79 Thus, the constituents of milk may play a determining role in the gut microbial composition and development. Significantly, there are considerable differences in the oligosaccharide composition of human breastmilk and cows’ milk although it has been revealed that the addition of prebiotics to cows’ milk based infant formulas can reduce these differences somewhat.80 The ability of prebiotics to modulate the infant gut microbiota in a manner similar to that associated with breastmilk will be discussed in greater depth later in this review.
Thirty years ago, Stark and Lee pioneered the research into the influence of different approaches to feeding on the infant gut microbiota.57 This culture-based study paved the way for the more recent investigations and was notable in that it revealed that bifidobacteria levels varied greatly depending on feeding method, with breastfed infants having higher bifidobacteria levels compared with formula fed controls. Although it is now apparent that the feeding regime is not the sole determinant of the levels of bifidobacteria and lactobacilli in the infant gut,1 it is clear that feeding does have a crucial impact. The findings of this initial culture-based study by Stark and Lee,57 have been corroborated by several more recent culture-independent studies and reviews, which confirm that bifidobacteria are more dominant (and, in at least some cases, more diverse) in the gut of breastfed infants.60,81-84 These studies have also revealed that E. coli and clostridia counts, including C. difficile, are lower in breastfed infants than those fed infant formula. Notably, when formula was supplemented with oligosaccharides it resulted in greater bifidobacteria counts in the fecal samples of the associated infants than was present in samples provided by the unsupplemented control group.60 Thus, in this case and as is often the case, the use of culture-independent techniques has resulted in the validation of earlier culture-dependent studies but has also significantly advanced our understanding of the broader consequences of feeding method choice with respect to the infant gut microbiota. Thus, while culture-based approaches are rapid and relatively straightforward and therefore are useful as preliminary investigations, the more advanced techniques provide us with the greatest insight into the complex interaction between feeding method and gut microbiota.
Other patterns have also been noted in that a review by Adlerberth and Wold (2009) noted trends toward higher levels of Lactobacillus rhamnosus in partially breastfed infants compared with weaned infants, observed that staphylococci are also more common in breastfed infants, while also establishing that higher levels of Klebsiella and Nitrobacteria are seen in formula fed infants.1 Fallani et al. have reported that Bacteroides were dominant in the gut microbial population of 6 week old formula fed infants82 and it has also been noted that the microbiota of formula fed infants is, in general, more diverse than that of their breastfed counterparts.81 Perhaps most notably of all, it has also been established that the consumption of breastmilk can significantly reduce the risk of necrotizing enterocolitis (NEC) (by 3- to 10-fold) in infants relative to those who are formula fed.85
In addition to the fact that the composition of milk consumed influences infant gut microbial composition, it has also been claimed that breastmilk contains microbes such as staphylococci, streptococci, lactobacilli, micrococci and bifidobacteria86 and thus may be a direct source of the lactobacilli and bifidobacteria that become established in the infant gut.87,88 These earlier studies have been supported by a 2010 study that provided further evidence of the role of breastmilk-associated microbes in the establishment of lactic acid bacteria (LAB; which includes the lactobacilli) and bifidobacteria in the immature infant gut.89 In addition to the benefits of nutrients, oligosaccharides and, perhaps, microbes present in breastmilk on the infant gut microbiota, the antimicrobial impact of lactoferrin, as alluded to earlier, may be beneficial. In 2009, a review found that oral treatment with lactoferrin reduced the incidence of sepsis and NEC in very low birth weight (VLBW) infants (usually including infants 1–1.5 kg in weight). Significant reductions in sepsis and NEC were apparent when lactoferrin was supplemented along with the probiotic L. rhamnosus GG.90
The introduction of solid foods (recommended by the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) to occur not before 17 weeks of age and no later than 26 weeks),91 is also known to induce alterations in the gut composition of infants.55,92 Koenig and colleagues performed high-throughput sequencing of fecal DNA from one infant over a 2.5 year period.92 The study employed 454-pyrosequencing to generate 318,620 16S rRNA gene sequences from 60 samples and over 500,000 metagenomic sequences from 12 samples. The study identified so called “steps” at which dramatic alterations occurred in the infant’s GIT microbiota, which they found could be attributed to a significant life event.92 An example of this was “Step 3,” which took place around days 170–290, when the introduction of formula and peas to the diet of the previously exclusively breastfed infant resulted in a significant increase in Bacteroidetes. Overall, the study found that the introduction of solid foods was associated with an increase in Bacteroidetes and Firmicutes. It was also again suggested that by 2.5 years the gut microbiota closely resembled that of an adult. The impact of weaning has also been investigated by others. In one case, this involved a study of 605 children from five European countries.15 The infants were examined 4 weeks after weaning commenced and were compared with the same infants prior to weaning, with results being generated using FISH and flow cytometry. The study found Bifidobacterium, the Clostridium coccoides group and Bacteroides to predominate after weaning but it was noted that the relative proportions of these were affected by the approach to pre-weaning feeding i.e., infants who had been breastfed had higher levels of bifidobacteria and decreased Bacteroides compared with infants who had been formula fed prior to weaning. The authors noted that despite weaning having a noticeable modifying effect on the gut microbiota of infants, other modulating factors such as mode of delivery, continued to exert measurable effects during the weaning period.15 Roger and colleagues have also reported an increase in the diversity of bifidobacteria corresponding to the introduction of solid foods.84
Finally, an alternative approach to the investigation of the impact of diet was demonstrated by De Filippo et al. In a study which compared diet, and its effects on the gut microbiota, of children (aged 1–6 years) from Europe (n = 15) compared with those from rural Africa (n = 14).16 The African diet was low in fat and protein from animal sources and was high in fiber and starch and these children also differed in that they were breastfed up to 2 y of age. The authors performed pyrosequencing of the V5 and V6 hyper-variable regions of the bacterial 16S rRNA gene and generated 438,219 gene sequences, corresponding to 15,111 sequences per sample. The study found that the lack of diversity in the western diet, and its over-reliance on nutrient dense, processed and refined foods, appears to have an effect on the gut microbial composition. More specifically, the western diet was associated with a reduced microbial diversity, with the European gut microbiota containing higher proportions of Firmicutes and Proteobacteria and lower proportions of Bacteroidetes and Actinobacteria.16
Despite the increased knowledge gained in recent years, it is clear that there is a need to more closely investigate the gut microbial composition of breast and formula fed infants as well as the effects of weaning and other diet-related issues. Investigations are also required to determine the duration of such effects and the short- and long-term impact that they have on infant health. It is anticipated that high-throughput sequencing technologies will provide significant clarity in this regard.
Impact of Family Structure
Though studied to a lesser extent, a factor that is emerging as a possible contributor to the composition of an infant’s gut microbiota is family structure. One relevant concept is known as the “sibling effect,” which is an adjunct to the hygiene hypothesis and postulates that allergic disease is lower in children from larger families.93 However, this theory remains controversial, particularly as the studies, which have been performed to test this theory have been performed in a myriad of different ways. Thus, to date, definitive evidence of the effects of family size, structure and birth order has yet to be established. Nonetheless, as part of the ALLERGYFLORA study,66 it was found (using culture-based approaches) that infants without older siblings had increased proportions of non-E. coli enterobacteria as well as clostridia in the gut, but also had a lower anaerobe to facultative anaerobe ratio, resembling that of Caesarean delivered infants.66 In 2006, Penders et al. had reported the presence of greater bifidobacteria concentrations in infants with older siblings than those without.60 While family order and the environment have been implicated in allergic disease development,94,95 the link between these effects and the composition of the intestinal microbiota requires further investigation. Further culture-independent studies are needed, for example, to identify if the gut microbiota of infants without older siblings is significantly different from that of other infants and if this predisposes them to later health risks. By providing answers to such questions, it will then become possible to address problems that might previously have been overlooked, by positively influencing the gut microbiota (e.g., through probiotics use). As has been shown in the previous sections, the results of culture-based approaches are often verified by newer approaches, though these new technologies are advantageous as they provide a more detailed and less biased insight into such complex interactions between environmental factors and the gut microbiota of infants. Thus, there is an obvious opportunity to employ sequencing approaches to identify the gut microbiota of these infants and its relationship to health outcomes.
Effect of Maternal Weight/Diet
The WHO released startling figures in 2010, which were updated in 2011, relating to the state of the world’s obesity crisis.96 The statistics showed that obesity levels have doubled since 1980 and that, as of 2008, 200 million men and 300 million women were obese. Childhood obesity was also highlighted, with 43 million children under 5 years being obese in 2010. Surprisingly, 65% of the world’s populations now live in countries where more deaths occur due to obesity rather than being underweight.96 Obesity appears to be a vicious cycle, as an obese mother is more likely to have an obese infant, who in turn has an increased risk of becoming an obese adult.97 Work by Gordon, Cani and others have shown that obesity is influenced by the microbial composition of the gut.21,52 The effects of childhood obesity on the composition of the child’s gut microbiota have recently been studied.98 This culture-independent study (comparing FISH and flow cytometry in combination, to results from microscopic detection and qRT-PCR) examined participants (n = 25) and controls (n = 24) at birth, at 3, 6, 12, 18 and 24 months and again when aged 4 and 7 years (at which time BMI was calculated). The controls (i.e., normal weight children) were matched for birth mode, gestational age, probiotic treatment, breastfeeding duration, antibiotic treatment, atopic disease prevalence and cohort at age 7 years. Fecal samples were analyzed and it was shown that children who were classified as being of normal weight had, and continued to have, higher levels of bifidobacteria than those who were, or who became, obese. They also noted lower Staphylococcus aureus levels in normal weight infants. This therefore provides a further indicator for the role of gut microbiota in obesity development and highlights the possibility of modulating disease risk through the alteration of the gut microbiota.
A recent study has taken the alternative approach of investigating if a mother’s weight before or during pregnancy could impact on her infant’s gut microbiota.28 The results from this 2010 study showed that infants of overweight mothers tended to be overweight or heavier at birth than those of normal weight mothers, while also revealing that overweight mothers had infants with decreased numbers of gut bacteria from the Bacteroides-Prevotella group at age one month, but had higher levels of Clostridium histolyticum in their gut at age 6 months. Similar results were observed among infants whose mothers underwent significant weight gain during pregnancy. The gut microbiota of the offspring of overweight mothers contained higher Clostridium leptum, lower Clostridium perfringens and higher S. aureus levels than that of the infants of normal weight mothers. In contrast, at 6 mo bifidobacteria counts were higher in the infants of normal weight mothers than in those of overweight mothers. While this culture-independent study provides intriguing evidence of the effect that obese mothers have on their infant’s gut microbiota, research in this area still remains limited and additional culture-independent studies are required. There is an opportunity to apply high-throughput sequencing approaches to this area of research to compare the microbial profile of the mother at birth, and at later time points, with that of her infant and to track the changes in gut microbiota and the weight profile of both. There is a clear opportunity to exploit these technologies to considerably advance our knowledge of this complex interaction between gut microbiota and weight. Finally, it has also been shown that a mother can modulate her infant’s gut microbiota through the consumption of probiotics99 or the use of antibiotics during pregnancy.100 The impact of probiotics and antibiotics on the infant’s gut microbial population will be addressed later in this review.
Probiotics
The word probiotic is derived from the Latin “pro” meaning for and from the Greek “biotic” meaning living. Having undergone numerous alterations101-103 since the first proposed definition in 1965,104 today the most generally accepted, and most widely used definition, is that provided by the Food and Agricultural Organization (FAO) who define probiotics as “live microorganisms which, when consumed in adequate amounts as part of food, confer a health benefit on the host”.105 Additionally, criteria have been proposed to allow for a more systematic identification of probiotics and these have been outlined by a review in 2007.106 To date, representatives of the lactobacilli have been most extensively studied with a view to their use as probiotics.107
The issue of the health benefits associated with the consumption of a probiotic has been the focus of great attention in recent years. Since 2006, EFSA has implemented regulations pertaining to nutrition and health claims, including claims relating to probiotics. They have outlined that with respect to health claims relating to the ability of a probiotic to modulate the gut microbiota positively, they expect that the changes induced should have a specific health benefit, such as a reduction in specific (potentially) pathogenic microorganisms within the gut, which is clearly related to the consumption of the product under investigation.108 They do not, however, support the claim that increased levels of bifidobacteria or lactobacilli are beneficial to overall health per se, due to a lack of specific scientific evidence to support such a claim. Thus, in many cases, further evidence is needed to prove the role of specific probiotic strains in the gut and thus allow health claims relating to them. EFSA also require all scientific documents presented in the dossier supporting the health claim to specifically relate to the species and strain of probiotic microorganisms being examined. Thus, while there is considerable evidence supporting the role of some probiotics in gut microbiota modulation (as discussed below), care needs to be taken when making associated health claims.
Given the recent EFSA rulings, it is not surprising that the specific mechanisms by which probiotics exert beneficial health effects on the host continues to be the focus of much attention.109-111 There are several proposed modes of action including the production of bacteriocins and other antimicrobials which inhibit other bacteria or the alteration of immune function, possibly through altered GALT function or through a physical enhancement of the mucosal barrier function.6 Indeed, the specific mechanism(s) involved will vary depending on the specific strain administered, further highlighting the importance of assessing each probiotic strain individually. Regardless of the precise mechanism(s) involved, a vast array of data exists relating to the beneficial impact of probiotics on host health.102,107,112 However, for the purpose of this review, the focus will be confined to the benefits to infant gut microbiota and subsequent health. With respect to healthy, full term infants, this review has previously outlined the transition that the infant gut undergoes from being initially sterile to having a composition that is relatively stable and resembles that of an adult by 2 years of age. It is during this initial transition phase that probiotics may be most beneficial. It is also notable, however, that in many cases the proposed benefit has related to increasing levels of lactobacilli and bifidobacteria in the gut, which, as highlighted above, is not accepted as a health claim by EFSA.
Given the ongoing debate regarding the significance of the ability of a probiotic to alter the composition of the gut microbiota (other than alterations in levels of specific pathogens), we have presented just a few examples to highlight the considerable degree to which some probiotics can bring about change. In one instance, a randomized control trial (RCT) examining the effects of supplementing the diet of infants with Bifidobacterium breve Bb12 for the first 28 days of life showed, using culturing techniques, that gut colonization patterns were altered compared with those of infants in the placebo group.113 As one might expect, B. breve colonization commenced more quickly in these infants but, in addition, after 6 weeks Lactobacilllus colonization rapidly increased. In contrast, Enterobacteriaceae decreased over the supplementation period in treated infants compared with controls.113 Investigations have also taken place to determine if probiotic administration to pregnant mothers affects the gut microbiota of their infants. In 2006 a study investigated (using qPCR) the impact of probiotic administration of L. rhamnosus GG to pregnant mothers on the gut microbiota of their infants.99 The probiotic was fed to 29 mothers 2–4 weeks prior to delivery and up to 3 weeks after delivery, while the control group consisted of the infants of 24 mothers not in receipt of probiotics. Results showed that supplementation of the mother’s diet with the probiotic had a significant impact on the infants’ gut microbial composition, i.e., significantly increased bifidobacteria numbers and diversity in these infants at day 5 and a trend toward increased B. breve levels at age 3 weeks, relative to the controls. Thus, probiotics have the potential to have a significant impact regardless of whether they are administered to the mother during pregnancy or directly to the infant, via supplemented formula after birth. A recent study was conducted using qPCR and flow cytometry coupled with FISH (FCM-FISH) to analyze the fecal microbiota of infants in Finland and Germany who received perinatal probiotic treatment.114 This study of over 150 infants found that the perinatal administration of probiotics did impact on the gut microbiota of the infants, but also found that the consequences depended on the feeding method employed (either breastfed or formula fed) as well as the microbiota present in the infant’s gut prior to probiotic administration. This area of research lends itself perfectly to further investigation through high-throughput sequencing, which will provide information with respect to the impact of these probiotics on gut microbes other than bifidobacteria and lactobacilli.
In recent years there has been an increase in allergies and atopic diseases, which have paralleled a corresponding decrease in infectious diseases. In 1976 John Gerrard first proposed the hygiene hypothesis,115 although it was not until 1989, when David P. Strachen published his paper in the BMJ which focused on hay fever, hygiene and household size that the hygiene hypothesis really began to gain scientific interest.93 Strachen’s paper suggested that decreased exposure to environmental challenges in early life, due to improved sanitation and hygiene practices, resulted in the reduced exposure of the immature immune system to the challenges necessary to develop tolerance and resistance to everyday environmental challenges e.g., dust, pollen, etc. Additionally, in 1997, the hygiene hypothesis was extended to incorporate the relationship between gut microbiota and immune regulation.116 This hypothesis is still being debated and studied today. However, given the knowledge we have of the influential role that gut microorganisms play in the establishment, maturation and regulation of the infant immune system, studies have once more returned to the hygiene hypothesis to determine if alterations in the gut microbial composition could result in alterations in the development of the immune system, which result in an altered allergy risk. In 2003, Bourlioux et al. reviewed the evidence up to that point which related to the role of the intestinal microbiota in immune function.117 The authors reminded us that alterations in the ratio of T helper 1/T helper 2 cells can have adverse consequences for the host i.e., increased Th2 levels result in an increased risk of allergy, while increased Th1 levels increases autoimmune disease risk e.g., diabetes mellitus. Studies have shown that having lower counts of bifidobacteria and atopy risk are associated and it has been proposed that bifidobacteria alter the level of Th2 development and inhibit the Th2 type response.118 Similarly, it has also been revealed that, in children with allergic parents, higher levels of lactobacilli in early life did reduce the risk of allergy development at age 5 years.119 It has also been suggested that the beneficial roles of specific commensal bacteria in allergic disease prevention may be due to alterations in the immune regulation process. The regulatory role that gut microbes play in the immune system has been convincingly demonstrated through studies involving gnotobiotic mice as well as human trials and, most recently, it was also shown to influence secretory IgA levels and subsequent allergic symptom development.4 Such a role of gut microbes in the regulation of the immune system would help explain why it is not only Th2 mediated allergic diseases, but also Th1 associated illnesses such as Type 1 diabetes, which are increasing globally.120
Following on from findings such as these, scientists have investigated the potential to favorably alter the infant gut microbiota in early life to decrease allergic disease risk. Notably, several studies have shown benefits in treating atopic children with probiotics and thus, modulation of the infant gut could potentially reduce the risk of them becoming allergic to environmental stimuli. The proposed regulatory role of gut microbes would occur predominantly during infancy and this may also explain why the effects of probiotics are more clearly observed in infants than in adults. This review will now summarize a number of relevant studies that address this topic.
In a study published in 2002, L. rhamnosus GG was provided to pregnant women who had a family history of atopic diseases.121 The supplement was consumed for the last 4 weeks of pregnancy and throughout the breastfeeding period, until 3 months after the birth of the infant. The study found that the risk of the infant developing eczema was significantly reduced, i.e., 15% compared with 47% incidence in the control group up to 2 years of age. A subsequent study again involved supplementation with L. rhamnosus GG but, on this occasion, L. rhamnosus LC705 (DSM 7061), B. breve Bb99 (DSM 13692) and Propionibacterium freudenreichii ssp shermanii JS (DSM 7076) were also provided to expectant mothers who had a family history of atopy.122 Once born, these infants also received this combination of probiotics, plus galactooligosaccharides (prebiotics). The authors found that compared with controls, probiotic treatment reduced the frequency of IgE associated (atopic) diseases, with an odds ratio (OR) of 0.71; 95% CI: 0.5–1.00, though not significantly. Probiotic treatment did significantly reduced the risk of eczema with an OR of 0.74; CI:0.55–0.98, p < 0.035. The authors also noted the frequent colonization of bifidobacteria and lactobacilli in the gut of supplemented infants. In 2005, a study of 230 infants (aged 1.4–11.9 months) investigated the use of probiotics in the reduction in the symptoms of atopic eczema/dermatitis.123 Unlike the previous studies in which mothers received probiotics, this study specifically investigated the effect of directly treating the infants with probiotics. Treatment was either with Lactobacillus GG (LGG), LGG in combination with three other probiotics or a placebo. Participants were randomized into the three groups and treated for 4 weeks. Although the authors noted that symptoms improved, they did so in all three groups and only a non-significant improvement was observed when the probiotic treated group was compared with the control groups. The study found there to be potential for probiotics with respect to decreasing symptoms in IgE sensitized individuals but showed little benefit in non-IgE sensitized infants.
Despite the fact that, as noted above, some positive outcomes have been reported, a 2007 review of this topic concluded that the studies to date are conflicting and inconclusive124 and a Cochrane meta-analysis of the effects of probiotics in the treatment of eczema found no significant benefit of probiotic treatment.125 In a recent paper on this topic evidence of the benefits of providing probiotics in order to prevent atopic eczema was quite convincing.126 However, the authors did agree that weaker associations have been shown between probiotics and other atopic diseases.126 The inconsistent findings to date most likely reflect differences with respect to the probiotic strains employed in the studies reviewed. It is apparent that large RCTs involving infants are needed to investigate fully and to specifically determine the benefits of treatment with specific probiotics in the context of allergy and atopic diseases. Use of high-throughput sequencing of fecal samples from affected vs. unaffected individuals could be employed to determine if differences in symptoms are due to altered gut microbial compositions. Given that the hypothesis is that probiotic treatment alters gut microbiota, thus reducing allergy risk, one would presume that it is only a matter of time before culture-independent strategies are employed to investigate the link between probiotic use, alterations to the gut microbiota and subsequent impacts on allergy. It is also notable that the studies to date have often been limited to the use of lactobacilli as probiotics and thus the inclusion of other genera or the use of strains in combination may also be beneficial. While there has been a focus on the impact of specific strains, a consistent observation across many studies is the reduced microbial diversity in the gut of allergic infants.127 This reduction in diversity and allergic status relates well to the research on the association between early antibiotic exposure, the accompanying reduction in gut microbiota diversity and subsequent allergic disease risk.128,129
While debate continues as to the specific beneficial health effects of many probiotics, one area where more convincing evidence exists is with respect to NEC. NEC, though first characterized over 100 years ago, still remains a poorly understood disease. The condition is characterized by abdominal distension, bleeding of the intestines and ulcer formation.85,130 There has been considerable interest in the use of probiotics to prevent NEC by normalizing the intestinal microbiota of preterm infants, i.e., trying to change its composition to resemble that of healthy, full term infants.131,132 Notably, trials using animal models of NEC have shown the benefits of introducing probiotic supplemented diets.133,134 In one instance the animals, which were fed 109 organisms/animal/day, had significantly reduced cases of NEC with just 7/24 in the treatment group suffering from NEC, compared with 19/27 in the control group. Corresponding human studies have also been completed.135 In one case, the benefits of feeding Lactobacillus acidophilus in combination with B. infantis (no strain details provided) to infants was tested.136 This large, year long, study of over 1,000 infants revealed that the cases of NEC, as well as the mortality rates in the treated group, were reduced compared with the controls. In 2005, a trial was conducted to examine the effects of some probiotics and NEC prevention but in low birth weight infants.137 The study found that a reduction in NEC cases in treated infants occurred with a reduction in the incidence of NEC from 17% in controls to 4% in the treated group. They noted that levels of clinically significant NEC (classified as Bell Stage 2 or 3) in the treated group (1/72 i.e., 1%) were statistically significantly reduced compared with the control group (10/73 i.e., 14%). Several meta-analysis and systematic reviews have been conducted on this topic to date138-140 and they have provided support for the use of probiotics in preterm infants to prevent NEC. Despite this, questions relating to what changes occur in the gut microbiota composition of NEC affected infants as well as the changes that occur following probiotic treatment remain unanswered and this is one knowledge gap that lends itself to the utilization of modern DNA based approaches. It is anticipated that in the future, in addition to assessing the ability of different strains to prevent NEC, attention will also begin to focus on unraveling the specific mechanism(s) via which probiotics can prevent this disease.
Some studies have also been performed to investigate the potential benefits of using probiotics to prevent or treat antibiotic associated diarrhea (AAD). The concept is that probiotics could temporarily colonize the gut, to compensate for the collateral damage to the gut microbiota resulting from antibiotic use, thus reducing the risk of diarrhea due to altered digestion and absorption. It is estimated that between 8 and 30% of children suffer from AAD.141,142 Two systematic reviews on this topic concluded that when probiotics and antibiotics were co-administered, AAD risk was reduced.143,144 However, these reviews were based mainly on studies in adults. Cremonini and colleagues also highlighted the lack of RCTs, especially with respect to infants and noted that generalizations could not be made about probiotic effects, as different strains exerted different effects.143 The studies which have taken place which relate to children have provided conflicting outcomes. In 1990, a small study on children treated with L. acidophilus and Lactobacillus helveticus (administered prophylactically as Lactinex) found that they did not have a significant effect with respect to the prevention of AAD.145 In contrast in 2004, ESPGHAN concluded that there is promising evidence to suggest that some probiotics can contribute to the prevention of AAD.146 In a 2007 review, it was concluded that (based on 6 RCTs at the time), co-treatment with probiotics did result in a reduced risk of AAD compared with those who received antibiotics alone (28.5% to 11.9% reduction in risk).118 This meta-analysis found that the most significant beneficial effects occurred when Lactobacillus GG, Saccharomyces boulardii and Bifidobacterium lactis and Streptococcus thermophilus were administered. They did not however, see any significant beneficial effect from administering Lactobacillus acidophilus/Bifidobacterium infantis or L. acidophilus/Lactobacillus bulgaricus. This again further emphasizes the species and strain specific effects of probiotics and the need for rigorous testing of each proposed probiotic rather than making generalizations that all probiotics are beneficial to health. A recent Cochrane review on this topic, which also looked at the above mentioned species, again found evidence of a protective effect from concomitant treatment with probiotics during antibiotic therapy.181 However, once again the authors emphasized the species and strain specific effects that occurred and the need for further high quality studies on this topic before routine administration of probiotics to infants/children could be recommended. Thus, there is still a considerable gap in our knowledge in this area with respect to the specific impact of probiotic administration on the composition of the gut microbiota of infants in receipt of antibiotics. Further research is required to establish if temporary colonization by probiotics occurs, to identify which microbial populations are impacted on by antibiotic administration and probiotic supplementation, to assess the duration of microbiota-related changes and to definitively establish the merits of probiotic administration in such circumstances. Finally, there is a strong need to carry out further studies to assess the success with which probiotics can prevent C. difficile associated diarrhea (CDAD) in infants and children. This was also the conclusion of a meta-analysis on this topic.147
Prebiotics
In 1995, Gibson and Roberfroid defined a prebiotic as “a non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon and thus improves health.”148 Based on this definition a substance must escape digestion or degradation in the stomach and small intestine and reach the colon intact, where it must only act as a stimulant for the growth of beneficial bacteria, which must then result in a measurable benefit to the host. The substances that have received the greatest attention to date have been oligosaccharides. Oligosaccharides are composed of repeating sugar units (2–20 units generally) and they exist naturally in breastmilk at a level of 10–12 g/l.149 It is notable however that the human milk oligosaccharides present in human breastmilk have yet to be produced commercially, and instead it has been fructooligosaccharides (FOS) and galactooligosaccharides (GOS) which have been the most studied as potential prebiotics. In the past, lactulose was investigated with a view to its use as a potential prebiotic, however such investigations have become more limited due to associated laxative effects at high doses. Although FOS and GOS are naturally present in foods such as bananas, celery, chicory and artichoke,80 the amount present is too small to be beneficial and thus there is considerable interest in incorporating prebiotics into functional foods following their extraction from plant sources or synthesis thereof.150 This review will focus on the studies relating to the effect of prebiotics on infant health, through modulation of their gut microbiota.
Oligosaccharides are unusual in that they consist of a β-glycosidic bond, which is resistant to degradation in the human GIT, due to a lack of appropriate enzymes to digest this bond. They remain intact until they reach the colon where they are fermented by a subset of bacteria, which are capable of degrading this bond. This fermentation results in short chain fatty acids, primarily acetate, butyrate and propionate. On the basis of culture-based studies, it has been known for quite some time now that the gut microbes which benefit from supplementation with prebiotics, such as bifidobacteria, proliferate at the expense of other gut microbes including Bacteroides, clostridia or coliforms, thus resulting in what is being suggested as being a more favorable gut microbial composition.151 Indeed, most research to date has focused on the ability of prebiotics to increase bifidobacteria and, to a lesser extent, lactobacilli numbers. However, it has been suggested that moving forward the increase in the numbers of other bacteria such as Roseburia and Eubacterium needs consideration also.152 Furthermore, as highlighted previously in this review, details on the actual benefit to health from increased levels of specific populations of microorganisms are needed i.e., simply targeting an increase in the numbers of specific microbes cannot be employed as a health claim.
Interest in prebiotics has increased as a consequence of evidence of several potential benefits, including the possibility of decreased colon cancer risk,153 improved host resistance to pathogens, improved calcium absorption, altered blood lipids and altered immunological responses,154,155 the majority of which still require further testing before EFSA will fully approve these claims. However extensive investigations have occurred and have been of considerable value.156 To date GOS and FOS have been the most extensively studied as prebiotics for supplementation to infant formula. ESPGHAN have concluded that the inclusion of 0.8 g/100 ml of oligosaccharide (combination of 90% oligogalactosyl-lactose and 10% high molecular weight oligofructosyl-saccharose) in infant formulas poses no major risk to the infant.157 The review also showed evidence that 0.4 g/dL, 0.8 g/dL or 1 g/dL mix of 90:10 GOS:FOS ratio brought about a significant increase in fecal bifidobacteria levels. It appears that a combination of long chain and short chain FOS/GOS and the ratio they appear in plays an important role in the efficiency of the prebiotic and its ability to exert beneficial effects. Extensive studies have repeatedly shown that prebiotics increase bifidobacteria and lactobacilli levels158,159 and examples of these studies will be described below. Before proceeding, it should again be noted that lactobacilli and bifidobacteria constitute only a small proportion of the overall gut microbiota and future studies will need to investigate the global impact of prebiotics on the infant gut microbiota. Furthermore, as noted before, an increase in bifidobacteria and lactobacilli levels is not regarded as a valid health claim by EFSA.
In 2008, a review of studies investigating the impact of prebiotics on infant health was completed.160 The authors highlighted the benefits of consuming human milk oligosaccharides (HMOS) with respect to infant health. These included decreased incidence of gastroenteritis and respiratory infections.161 It was also noted that specific combinations of prebiotics, including short chain (sc) GOS/long chain (lc) FOS, increased bifidobacteria and lactobacilli levels to the extent that, in some cases, levels of these genera were comparable to those observed in the gut of breastfed infants.106,162-165 This impact was apparent despite the fact that these prebiotics had structures, which differed from those of HMOS. In one such study qPCR and FISH were used in combination to identify and quantify the bifidobacteria in fecal samples from infants fed GOS and FOS supplemented feeds.163 This study revealed significant increases in fecal bifidobacteria in treated infants compared with controls and once again highlighted the ability of prebiotic supplemented formula to mimic breastfeeding effects on the gut microbiota, thus corroborating the results from earlier culture-based studies. As this review has shown, newer studies are now employing culture-independent methods (e.g., FISH and qPCR in combination or separately) to investigate more specifically the effects of prebiotics on the gut microbiota of infants, which may lead to greater insights compared with those provided by earlier culture-based approaches. There is also however, an opportunity to use sequencing technologies to more accurately assess the effects of prebiotics on the gut microbiota (and not just the effects on bifidobacteria and lactobacilli levels).
Antibiotics
While probiotics and prebiotics can potentially modulate the infant’s gut microbiota in a positive manner, antibiotics can exert a detrimental effect on the infant’s commensal microbiota. The use of antibiotics has increased dramatically and consequently the effects of specific antibiotics on the gut microbiota of infants are a significant concern. A culture-based study in 1970 was one of the first to examine the effects of various antimicrobials on the gut microbial composition.166 The study suggested that the intestinal microbiota was altered to different degrees depending on the spectrum of specificity of the antimicrobial administered, the duration of treatment and the route of administration. Interestingly in this early study, and also in some infant related studies since, it has been shown that penicillin exerts a less significant effect (and in some cases no significant effect) on the gut microbiota13 relative to other antibiotics, once more stressing the need to investigate the effect of the different antimicrobials commonly prescribed during childhood in turn in order to determine the specific impact that they have on the gut microbiota. Since the initial Finegold et al. study highlighted the effects of antibiotics on the gut microbiota,166 several other culture-based studies have also supported these findings.13,167 Following on from these culture-based approaches, culture-independent approaches were completed and, in the majority of cases, they corroborated the results of the earlier studies while also providing an even greater insight.100 In 2009, a culture-independent study examining the effects of antibiotics on the infant gut microbiota in the early postnatal period was published.168 This study involved 26 infants, 5 of whom had been treated with antibiotics. Fecal samples were analyzed for the first 5 days of life and then monthly for 2 months. The impact on the gut microbiota was assessed using qPCR targeting the V1-V3 regions of the 16S rRNA gene. The study found that antibiotic treated infants (treated with cefalexin 50 mg/kg four times daily, for the first four days of life) had significantly lower bifidobacteria until 1 month of age and had increased Enterococcus levels compared with antibiotic free controls. The impact of antibiotic administration on colonization patterns has also been the subject of attention. In one instance this involved a study which focused on an infant in receipt of clavulanic acid and amoxicillin (Augmentin®) for 13 days, followed by trimethoprim and sulfamethoxazol (Bactrimel®) for 12 d.169 This study employed both culture-based and culture-independent techniques to examine the effects of antibiotics on gut microbial composition. The antibiotic treated infant had an extremely unstable microbiota up to the age of 1 month, with E. coli being dominant in the early colonization period. However, the most significant difference between the antibiotic treated infant and the controls was the apparent absence of gut bifidobacteria. Indeed, up to 5 months of age no bifidobacteria were detected, highlighting the prolonged effects of antibiotic treatment on some commensal bacteria.169 Following on from the previously outlined negative effect of antibiotic treatment on bifidobacteria populations, another study in 2010 showed that treatment of infants with parenteral antibiotics (a combination of ampicillin and gentamycin), administered within 48 hours of birth, reduced, but did not completely eliminate bifidobacteria, i.e., some bifidobacteria such as B. bifidum survived antibiotic treatment.170 At 8 weeks of age those who had been treated with antibiotics continued to have a less diverse population of bifidobacteria relative to controls. Recent reviews have indicated that the recovery of microorganisms after antibiotic administration can be delayed128 and that in some cases some bacteria (namely Bacteroides) may not re-establish.60 In a recent longitudinal study of 28,354 mother-child pairs in the Danish national birth cohort it was found that antibiotics administered in the first 6 months of life were positively associated with an increased obesity risk in children of normal weight mothers by the time the children reached age 7.171 Thus, the changes to the infant’s gut microbiota in the initial months of life could predispose the infant to chronic illness in later life.
The impact of administering antibiotics to expectant mothers with respect to the gut microbiota and/or health of their infants has also been investigated.172 Furthermore, in 2002, McKeever and colleagues examined the effect of maternal antibiotic use on the risk of allergic disease in their infants.94 The study was part of the general practice research database in the UK and included 24,690 children. The authors noted that three or more exposures to antibiotics during pregnancy was associated with an increased hazard ratio for asthma (1.36; 95% CI: 1.16–1.60), eczema (1.19; 95% CI: 1.02–1.39) and hay fever (1.33; 95% CI: 1.0–1.77) in the infant. The positive association between antibiotic use in the first year of the child’s life and subsequent asthma and allergy risk was also demonstrated in a 2009 study, when it was established among 193,412 children that such antibiotic use was associated with an increased risk of asthma and allergies at age 6 or 7.173 Previous studies have also supported this association between early life exposure to antibiotics and increased asthma and allergy risk.174,175 Russell and Murch have also reviewed the impact of peripartum antibiotics on the gut microbiota of infants and in turn, their health effects and have suggested that administration of peripartum antibiotics could alter the initial colonization of the infant gut, resulting in an alteration to the GALT and a shift toward Th2 differentiation. Such a shift is known to result in an increased risk of atopy.176 Furthermore, as highlighted previously in this review, changes to the gut microbiota in early life could affect the regulation of the immune system, which in turn could cause health effects. A complication in studying the effects of antibiotics and atopic disease is called “reverse causation.” The concept is that antibiotics may have preceded the atopic disease or may have been prescribed in response to symptoms. Thus, it is difficult to separate these to identify cause and effect.
While these studies demonstrate the negative effects of antibiotics on the gut microbiota, there is considerable merit in carrying out further investigations using the newer technologies available to us. Thus, by not having to select for specific microorganisms, as is the case when culture- or hybridization-based approaches are employed, one could generate an overall profile of the impact of antibiotics on infant gut microbiota. While the studies to date are significant, and it is notable that a recent meta-analysis of 18 studies also found a weak positive association between antibiotic use in infancy and asthma and wheeze risk (OR 1.27 95% CI: 1.12–1.43),177 there are a number of other studies, such as that by Celedon et al.178 that failed to reveal the existence of an association. Similarly in 2007, the Koala Birth Cohort Study of over 2,700 families, once more failed to demonstrate any association between antibiotic use and eczema or asthma risk.179 In contrast, the most recent publication on this topic found that the limited diversity in the gut microbiota of infants arising through antibiotic exposure before 1 months of age, was positively associated with atopic eczema risk by the age of 2 years.180 This again provides support for the theory that it may be reductions in the diversity of the gut microbiota (rather than in any particular species of bacteria), which results in an altered development and regulation of the immune system and subsequently results in long-term health consequences. Antibiotics by their very nature cause alterations to the microbiota present in the individual. However, the extent of the effects on beneficial microbiota, during and after the treatment period, needs to be examined further. As a consequence of the research on the concomitant use of probiotics and antibiotics and the observed benefits (such as decreased antibiotic associated diarrhea risk), the use of specific probiotics in conjunction with antibiotics to benefit the host by minimizing the negative effects on gut microbiota composition, may become even more prevalent in the future. New technologies such as high-throughput sequencing will also improve our ability to more accurately study the gut microbiota of antibiotic treated subjects in a less biased manner, than culture-dependent techniques. In particular, the impact of antibiotics on the GIT of preterm infants requires greater attention. Knowing the impact of different antibiotics on full-term and preterm infant’s gut microbiota acquisition and development could lead to the selection of particular antibiotics on the basis of their efficiency in dealing with the illness, while having a minimal impact on the infant’s microbiota.
Conclusions
The infant’s gut microbiota undergoes rapid and radical changes during the first 2 years of life. During this period, changes at both the phylum and species levels occur. The changes that occur are determined by the factors that have been outlined in this review. Some of the most influential factors appear to be mode of delivery, feeding practices and the use of probiotics and prebiotics to potentially modulate the infant gut in what appears to be a positive manner (though there is a need for further research to determine what constitutes a “normal healthy” gut microbiota). However, in contrast, antibiotics have clearly been shown to have detrimental and often prolonged effects. Thus, the first 2 years of the infant’s life may pose a unique window of opportunity, during which time the gut microbial composition can be positively modulated through diet and lifestyle factors. However, a number of questions remain. As scientists we must question what is the ideal composition of the infant gut microbiota? Do we know what core gut microbes will lead to the most favorable health outcomes? Does the core gut microbiome depend on the age of the infant (i.e., should we have milestones of colonization to aim for), and should this change depending on other factors such as ethnicity? Notably, breastmilk has been shown to be the optimum nutrition source for infants, thus investigating the composition of the breastfed infant’s gut microbiota at different time points may provide targets to aim for. If we know the benefits of breastfeeding should we try to prolong these effects and mimic them in the weaning period? While this review has outlined the significant advances in our understanding of the acquisition and development of the infant gut microbiota, it is evident that large knowledge gaps still exist. Most notably we still struggle to define what constitutes a beneficial or normal gut microbiota. In the future, additional research of the long-term impacts on health arising from an altered infant gut microbiota and, of the duration of the effects of different factors (such as breastfeeding or antibiotic use) on these populations is needed. To date, high-throughput sequencing technologies have been relatively under-utilized with respect to investigations of the infant gut microbiota, despite their widespread use in other fields of microbial ecology, or even the gut microbiota of adults. Where these technologies have been utilized, in many cases the results correlate closely with those from culture-based approaches, thus showing the ability to corroborate and supplement the culture-based results with those generated using newer technologies. Additionally, it is evident the significantly more comprehensive insights that can be achieved by using the new technologies available. Accordingly, it is anticipated that our movement into the metagenomic era will provide us with detailed insights into the gut microbiota of infants, and the factors, which influence this microbiota, in the very near future. The value of such research has the potential to be immense.
Acknowledgments
F.F. is in receipt of an Irish Research Council for Science Engineering and Technology EMBARK scholarship and a Teagasc Walsh fellowship. Research in the R.P.R., G.F., C.S. and P.D.C. labs is supported by the Science Foundation of Ireland (SFI) funded Centre for Science, Engineering and Technology, the Alimentary Pharmabiotic Centre (APC) and P.D.C. is also supported by a SFI PI award “Obesibiotics” (11/PI/1137).
Glossary
Abbreviations:
- GIT
gastrointestinal tract
- GALT
gut-associated lymphoid tissue
- FISH
fluorescent in situ hybridization
- TGGE
temperature gradient gel electrophoresis
- DGGE
denaturing gradient gel electrophoresis
- qPCR
quantitative PCR
- WHO
World Health Organization
- NEC
necrotizing enterocolitis
- LAB
lactic acid bacteria
- VLBW
very low birth weight
- ESPGHAN
European Society for Paediatric Gastroenterology Hepatology and Nutrition
- FAO
Food and Agricultural Organization
- EFSA
European Food Safety Authority
- FOS
fructooligosaccharides
- GOS
galactooligosaccharides
- HMOS
human milk oligosaccharides
- FCM
flow cytometry
- RCT
Randomized control trial
- SCFA
Short chain fatty acid
- MAC
microflora associated characteristics
- AAD
antibiotic associated diarrhoea
- CDAC
Clostridium difficile associated diarrhoea
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/20169
References
- 1.Adlerberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta Paediatr. 2009;98:229–38. doi: 10.1111/j.1651-2227.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- 2.Vaishampayan PA, Kuehl JV, Froula JL, Morgan JL, Ochman H, Francino MP. Comparative metagenomics and population dynamics of the gut microbiota in mother and infant. Genome Biol Evol. 2010;2:53–66. doi: 10.1093/gbe/evp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fagerås M, Tomičić S, Voor T, Björkstén B, Jenmalm MC. Slow salivary secretory IgA maturation may relate to low microbial pressure and allergic symptoms in sensitized children. Pediatr Res. 2011;70:572–7. doi: 10.1203/PDR.0b013e318232169e. [DOI] [PubMed] [Google Scholar]
- 5.Conroy ME, Shi HN, Walker WA. The long-term health effects of neonatal microbial flora. Curr Opin Allergy Clin Immunol. 2009;9:197–201. doi: 10.1097/ACI.0b013e32832b3f1d. [DOI] [PubMed] [Google Scholar]
- 6.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–93. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–9. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 8.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman AL, Kallstrom G, Faith JJ, Reyes A, Moore A, Dantas G, et al. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc Natl Acad Sci U S A. 2011;108:6252–7. doi: 10.1073/pnas.1102938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tjellström B, Stenhammar L, Högberg L, Fälth-Magnusson K, Magnusson KE, Midtvedt T, et al. Gut microflora associated characteristics in first-degree relatives of children with celiac disease. Scand J Gastroenterol. 2007;42:1204–8. doi: 10.1080/00365520701320687. [DOI] [PubMed] [Google Scholar]
- 11.Cardona ME. Effect of probiotics on five biochemical microflora-associated characteristics, in vitro and in vivo. Food & Nutrition Research. 2002;46:73–9. [Google Scholar]
- 12.Böttcher MF, Nordin EK, Sandin A, Midtvedt T, Björkstén B. Microflora-associated characteristics in faeces from allergic and nonallergic infants. Clin Exp Allergy. 2000;30:1590–6. doi: 10.1046/j.1365-2222.2000.00982.x. [DOI] [PubMed] [Google Scholar]
- 13.Bennet R, Eriksson M, Nord CE. The fecal microflora of 1-3-month-old infants during treatment with eight oral antibiotics. Infection. 2002;30:158–60. doi: 10.1007/s15010-002-2140-z. [DOI] [PubMed] [Google Scholar]
- 14.Hascoët JM, Hubert C, Rochat F, Legagneur H, Gaga S, Emady-Azar S, et al. Effect of formula composition on the development of infant gut microbiota. J Pediatr Gastroenterol Nutr. 2011;52:756–62. doi: 10.1097/MPG.0b013e3182105850. [DOI] [PubMed] [Google Scholar]
- 15.Fallani M, Amarri S, Uusijarvi A, Adam R, Khanna S, Aguilera M, et al. INFABIO team Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology. 2011;157:1385–92. doi: 10.1099/mic.0.042143-0. [DOI] [PubMed] [Google Scholar]
- 16.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–6. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Midtvedt T, Bjørneklett A, Carlstedt-Duke B, Gustafsson BE, Høverstad T, Lingaas E, et al. The influence of antibiotics upon microflora-associated characteristics in man and mammals. Prog Clin Biol Res. 1985;181:241–4. [PubMed] [Google Scholar]
- 18.Tjellström B, Stenhammar L, Högberg L, Fälth-Magnusson K, Magnusson KE, Midtvedt T, et al. Gut microflora associated characteristics in children with celiac disease. Am J Gastroenterol. 2005;100:2784–8. doi: 10.1111/j.1572-0241.2005.00313.x. [DOI] [PubMed] [Google Scholar]
- 19.Bezirtzoglou E, Stavropoulou E. Immunology and probiotic impact of the newborn and young children intestinal microflora. Anaerobe. 2011;17:369–74. doi: 10.1016/j.anaerobe.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Thompson-Chagoyan OC, Fallani M, Maldonado J, Vieites JM, Khanna S, Edwards C, et al. Faecal microbiota and short-chain fatty acid levels in faeces from infants with cow’s milk protein allergy. Int Arch Allergy Immunol. 2011;156:325–32. doi: 10.1159/000323893. [DOI] [PubMed] [Google Scholar]
- 21.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 22.O’Toole PW, Claesson MJ. Gut microbiota: Changes throughout the lifespan from infancy to elderly. Int Dairy J. 2010;20:281–91. doi: 10.1016/j.idairyj.2009.11.010. [DOI] [Google Scholar]
- 23.Malinen E, Kassinen A, Rinttilä T, Palva A. Comparison of real-time PCR with SYBR Green I or 5′-nuclease assays and dot-blot hybridization with rDNA-targeted oligonucleotide probes in quantification of selected faecal bacteria. Microbiology. 2003;149:269–77. doi: 10.1099/mic.0.25975-0. [DOI] [PubMed] [Google Scholar]
- 24.Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes (Lond) 2008;32:1720–4. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- 25.Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205–11. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Overturf K. Quantitative PCR. Molecular Research in Aquaculture 2009; 39-61. [Google Scholar]
- 27.Zwielehner J, Liszt K, Handschur M, Lassl C, Lapin A, Haslberger AG. Combined PCR-DGGE fingerprinting and quantitative-PCR indicates shifts in fecal population sizes and diversity of Bacteroides, bifidobacteria and Clostridium cluster IV in institutionalized elderly. Exp Gerontol. 2009;44:440–6. doi: 10.1016/j.exger.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Collado MC, Isolauri E, Laitinen K, Salminen S. Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: a prospective follow-up study initiated in early pregnancy. Am J Clin Nutr. 2010;92:1023–30. doi: 10.3945/ajcn.2010.29877. [DOI] [PubMed] [Google Scholar]
- 29.Brown PO, Botstein D. Exploring the new world of the genome with DNA microarrays. Nat Genet. 1999;21(Suppl):33–7. doi: 10.1038/4462. [DOI] [PubMed] [Google Scholar]
- 30.Frank DN, Pace NR. Gastrointestinal microbiology enters the metagenomics era. Curr Opin Gastroenterol. 2008;24:4–10. doi: 10.1097/MOG.0b013e3282f2b0e8. [DOI] [PubMed] [Google Scholar]
- 31.Mardis ER. Next-generation DNA sequencing methods. Annu Rev Genomics Hum Genet. 2008;9:387–402. doi: 10.1146/annurev.genom.9.081307.164359. [DOI] [PubMed] [Google Scholar]
- 32.Strausberg RL, Levy S, Rogers YH. Emerging DNA sequencing technologies for human genomic medicine. Drug Discov Today. 2008;13:569–77. doi: 10.1016/j.drudis.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 33.Mardis ER. The impact of next-generation sequencing technology on genetics. Trends Genet. 2008;24:133–41. doi: 10.1016/j.tig.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Weber JL, Myers EW. Human whole-genome shotgun sequencing. Genome Res. 1997;7:401–9. doi: 10.1101/gr.7.5.401. [DOI] [PubMed] [Google Scholar]
- 35.Hattori M, Taylor TD. The human intestinal microbiome: a new frontier of human biology. DNA Res. 2009;16:1–12. doi: 10.1093/dnares/dsn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurokawa K, Itoh T, Kuwahara T, Oshima K, Toh H, Toyoda A, et al. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 2007;14:169–81. doi: 10.1093/dnares/dsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. The sequence of the human genome. Science. 2001;291:1304–51. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 38.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4586–91. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schadt EE, Turner S, Kasarskis A. A window into third-generation sequencing. Hum Mol Genet. 2010;19(R2):R227–40. doi: 10.1093/hmg/ddq416. [DOI] [PubMed] [Google Scholar]
- 42.Shendure J, Porreca GJ, Reppas NB, Lin X, McCutcheon JP, Rosenbaum AM, et al. Accurate multiplex polony sequencing of an evolved bacterial genome. Science. 2005;309:1728–32. doi: 10.1126/science.1117389. [DOI] [PubMed] [Google Scholar]
- 43.Levene MJ, Korlach J, Turner SW, Foquet M, Craighead HG, Webb WW. Zero-mode waveguides for single-molecule analysis at high concentrations. Science. 2003;299:682–6. doi: 10.1126/science.1079700. [DOI] [PubMed] [Google Scholar]
- 44.Kasianowicz JJ, Brandin E, Branton D, Deamer DW. Characterization of individual polynucleotide molecules using a membrane channel. Proc Natl Acad Sci U S A. 1996;93:13770–3. doi: 10.1073/pnas.93.24.13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clarke J, Wu HC, Jayasinghe L, Patel A, Reid S, Bayley H. Continuous base identification for single-molecule nanopore DNA sequencing. Nat Nanotechnol. 2009;4:265–70. doi: 10.1038/nnano.2009.12. [DOI] [PubMed] [Google Scholar]
- 46.Roesch LFW, Lorca GL, Casella G, Giongo A, Naranjo A, Pionzio AM, et al. Culture-independent identification of gut bacteria correlated with the onset of diabetes in a rat model. ISME J. 2009;3:536–48. doi: 10.1038/ismej.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gophna U, Sommerfeld K, Gophna S, Doolittle WF, Veldhuyzen van Zanten SJO. Differences between tissue-associated intestinal microfloras of patients with Crohn’s disease and ulcerative colitis. J Clin Microbiol. 2006;44:4136–41. doi: 10.1128/JCM.01004-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 49.Maitra A, Cohen Y, Gillespie SED, Mambo E, Fukushima N, Hoque MO, et al. The Human MitoChip: a high-throughput sequencing microarray for mitochondrial mutation detection. Genome Res. 2004;14:812–9. doi: 10.1101/gr.2228504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas RK, Baker AC, Debiasi RM, Winckler W, Laframboise T, Lin WM, et al. High-throughput oncogene mutation profiling in human cancer. Nat Genet. 2007;39:347–51. doi: 10.1038/ng1975. [DOI] [PubMed] [Google Scholar]
- 51.Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010;26:5–11. doi: 10.1097/MOG.0b013e328333d751. [DOI] [PubMed] [Google Scholar]
- 52.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grönlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr. 1999;28:19–25. doi: 10.1097/00005176-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 55.Edwards CA, Parrett AM. Intestinal flora during the first months of life: new perspectives. Br J Nutr. 2002;88:11–8. doi: 10.1079/BJN2002625. [DOI] [PubMed] [Google Scholar]
- 56.Salminen S, Gibson GR, McCartney AL, Isolauri E. Influence of mode of delivery on gut microbiota composition in seven year old children. Gut. 2004;53:1388–9. doi: 10.1136/gut.2004.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stark PL, Lee A. The microbial ecology of the large bowel of breast-fed and formula-fed infants during the first year of life. J Med Microbiol. 1982;15:189–203. doi: 10.1099/00222615-15-2-189. [DOI] [PubMed] [Google Scholar]
- 58.Mshvildadze M, Neu J. The infant intestinal microbiome: friend or foe? Early Hum Dev. 2010;86(Suppl 1):67–71. doi: 10.1016/j.earlhumdev.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–21. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 61.Fitzpatrick F, Oza A, Gilleece A, O’Byrne AM, Drudy D, C. difficile subcommittee of the Health Protection Surveillance Centre Laboratory diagnosis of Clostridium difficile-associated disease in the Republic of Ireland: a survey of Irish microbiology laboratories. J Hosp Infect. 2008;68:315–21. doi: 10.1016/j.jhin.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 62.Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J., Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16:459–77. doi: 10.1086/648363. [DOI] [PubMed] [Google Scholar]
- 63.Thomas C, Stevenson M, Riley TV. Antibiotics and hospital-acquired Clostridium difficile-associated diarrhoea: a systematic review. J Antimicrob Chemother. 2003;51:1339–50. doi: 10.1093/jac/dkg254. [DOI] [PubMed] [Google Scholar]
- 64.van Nimwegen FA, Penders J, Stobberingh EE, Postma DS, Koppelman GH, Kerkhof M, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol. 2011;128:948–55, e1-3. doi: 10.1016/j.jaci.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 65.Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E, Retetangos C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev. 2010;86(Suppl 1):13–5. doi: 10.1016/j.earlhumdev.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 66.Adlerberth I, Strachan DP, Matricardi PM, Ahrné S, Orfei L, Åberg N, et al. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J Allergy Clin Immunol. 2007;120:343–50. doi: 10.1016/j.jaci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 67.Huurre A, Kalliomäki M, Rautava S, Rinne M, Salminen S, Isolauri E. Mode of delivery - effects on gut microbiota and humoral immunity. Neonatology. 2008;93:236–40. doi: 10.1159/000111102. [DOI] [PubMed] [Google Scholar]
- 68.Leahy SC, Higgins DG, Fitzgerald GF, van Sinderen D. Getting better with bifidobacteria. J Appl Microbiol. 2005;98:1303–15. doi: 10.1111/j.1365-2672.2005.02600.x. [DOI] [PubMed] [Google Scholar]
- 69.Picard C, Fioramonti J, Francois A, Robinson T, Neant F, Matuchansky C. Review article: bifidobacteria as probiotic agents -- physiological effects and clinical benefits. Aliment Pharmacol Ther. 2005;22:495–512. doi: 10.1111/j.1365-2036.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- 70.Liepke C, Adermann K, Raida M, Mägert HJ, Forssmann WG, Zucht HD. Human milk provides peptides highly stimulating the growth of bifidobacteria. Eur J Biochem. 2002;269:712–8. doi: 10.1046/j.0014-2956.2001.02712.x. [DOI] [PubMed] [Google Scholar]
- 71.EFSA Opinion of the Scientific Committee on a request from EFSA on the introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA. The EFSA Journal. 2007;587:1–16. [Google Scholar]
- 72.Goldani HAS, Bettiol H, Barbieri MA, Silva AAM, Agranonik M, Morais MB, et al. Cesarean delivery is associated with an increased risk of obesity in adulthood in a Brazilian birth cohort study. Am J Clin Nutr. 2011;93:1344–7. doi: 10.3945/ajcn.110.010033. [DOI] [PubMed] [Google Scholar]
- 73.Neu J, Rushing J. Cesarean versus vaginal delivery: long-term infant outcomes and the hygiene hypothesis. Clin Perinatol. 2011;38:321–31. doi: 10.1016/j.clp.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.WHO. Global strategy on infant and young child feeding. Available online at (www.who.int/nutrition/publications/infantfeeding) 2003.
- 75.Kristiansen AL, Lande B, Øverby NC, Andersen LF. Factors associated with exclusive breast-feeding and breast-feeding in Norway. Public Health Nutr. 2010;13:2087–96. doi: 10.1017/S1368980010002156. [DOI] [PubMed] [Google Scholar]
- 76.Tarrant RC, Younger KM, Sheridan-Pereira M, White MJ, Kearney JM. The prevalence and determinants of breast-feeding initiation and duration in a sample of women in Ireland. Public Health Nutr. 2010;13:760–70. doi: 10.1017/S1368980009991522. [DOI] [PubMed] [Google Scholar]
- 77.Survey NI. Breastfeeding Among US Children Born 1999–2007. CDC National Immunization Survey 2007.
- 78.Allen J, Hector D. Benefits of breastfeeding. N S W Public Health Bull. 2005;16:42–6. doi: 10.1071/NB05011. [DOI] [PubMed] [Google Scholar]
- 79.Fox PF, Kelly AL. Indigenous enzymes in milk: Overview and historical aspects—Part 2. Int Dairy J. 2006;16:517–32. doi: 10.1016/j.idairyj.2005.09.017. [DOI] [Google Scholar]
- 80.Manning TS, Gibson GR. Microbial-gut interactions in health and disease. Prebiotics. Best Pract Res Clin Gastroenterol. 2004;18:287–98. doi: 10.1016/j.bpg.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 81.Bezirtzoglou E, Tsiotsias A, Welling GW. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH) Anaerobe. 2011;17:478–82. doi: 10.1016/j.anaerobe.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 82.Fallani M, Young D, Scott J, Norin E, Amarri S, Adam R, et al. and Other Members of the INFABIO Team Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J Pediatr Gastroenterol Nutr. 2010;51:77–84. doi: 10.1097/MPG.0b013e3181d1b11e. [DOI] [PubMed] [Google Scholar]
- 83.Le Huërou-Luron I, Blat S, Boudry G. Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr Res Rev. 2010;23:23–36. doi: 10.1017/S0954422410000065. [DOI] [PubMed] [Google Scholar]
- 84.Roger LC, Costabile A, Holland DT, Hoyles L, McCartney AL. Examination of faecal Bifidobacterium populations in breast- and formula-fed infants during the first 18 months of life. Microbiology. 2010;156:3329–41. doi: 10.1099/mic.0.043224-0. [DOI] [PubMed] [Google Scholar]
- 85.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368:1271–83. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 86.Martín R, Langa S, Reviriego C, Jimínez E, Marín ML, Xaus J, et al. Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr. 2003;143:754–8. doi: 10.1016/j.jpeds.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 87.Martín R, Heilig GHJ, Zoetendal EG, Smidt H, Rodríguez JM. Diversity of the Lactobacillus group in breast milk and vagina of healthy women and potential role in the colonization of the infant gut. J Appl Microbiol. 2007;103:2638–44. doi: 10.1111/j.1365-2672.2007.03497.x. [DOI] [PubMed] [Google Scholar]
- 88.Sinkiewicz G, Ljunggren L. Occurrence of Lactobacillus reuteri in human breast milk. Microb Ecol Health Dis. 2008;20:122–6. doi: 10.1080/08910600802341007. [DOI] [Google Scholar]
- 89.Solís G, de Los Reyes-Gavilan CG, Fernández N, Margolles A, Gueimonde M. Establishment and development of lactic acid bacteria and bifidobacteria microbiota in breast-milk and the infant gut. Anaerobe. 2010;16:307–10. doi: 10.1016/j.anaerobe.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 90.Mohan P, Abrams SA. Oral lactoferrin for the treatment of sepsis and necrotizing enterocolitis in neonates. Cochrane Database Syst Rev. 2009:CD007138. doi: 10.1002/14651858.CD007138.pub2. [DOI] [PubMed] [Google Scholar]
- 91.Agostoni C, Decsi T, Fewtrell M, Goulet O, Kolacek S, Koletzko B, et al. ESPGHAN Committee on Nutrition Complementary feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2008;46:99–110. doi: 10.1097/01.mpg.0000304464.60788.bd. [DOI] [PubMed] [Google Scholar]
- 92.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4578–85. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McKeever TM, Lewis SA, Smith C, Hubbard R. The importance of prenatal exposures on the development of allergic disease: a birth cohort study using the West Midlands General Practice Database. Am J Respir Crit Care Med. 2002;166:827–32. doi: 10.1164/rccm.200202-158OC. [DOI] [PubMed] [Google Scholar]
- 95.Strachan DP. Family size, infection and atopy: the first decade of the ‘hygiene hypothesis’. Thorax. 2000;55:2–10. doi: 10.1136/thorax.55.suppl_1.S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.WHO. WHO obesity factsheet. Available online at (http://www.who.int/mediacentre/factsheets/fs311/en/index.html) 2011.
- 97.Lawlor DA, Smith GD, O’Callaghan M, Alati R, Mamun AA, Williams GM, et al. Epidemiologic evidence for the fetal overnutrition hypothesis: findings from the mater-university study of pregnancy and its outcomes. Am J Epidemiol. 2007;165:418–24. doi: 10.1093/aje/kwk030. [DOI] [PubMed] [Google Scholar]
- 98.Kalliomäki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–8. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 99.Gueimonde M, Sakata S, Kalliomäki M, Isolauri E, Benno Y, Salminen S. Effect of maternal consumption of lactobacillus GG on transfer and establishment of fecal bifidobacterial microbiota in neonates. J Pediatr Gastroenterol Nutr. 2006;42:166–70. doi: 10.1097/01.mpg.0000189346.25172.fd. [DOI] [PubMed] [Google Scholar]
- 100.Mangin I, Suau A, Gotteland M, Brunser O, Pochart P. Amoxicillin treatment modifies the composition of Bifidobacterium species in infant intestinal microbiota. Anaerobe. 2010;16:433–8. doi: 10.1016/j.anaerobe.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 101.Parker RB. Probiotics, the other half of the antibiotic story. Animal Nutrition and Health. 1974;29:4–8. [Google Scholar]
- 102.Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66:365–78. doi: 10.1111/j.1365-2672.1989.tb05105.x. [DOI] [PubMed] [Google Scholar]
- 103.Salminen S, Ouwehand A, Benno Y, Lee YK. Probiotics: how should they be defined? Trends Food Sci Technol. 1999;10:107–10. doi: 10.1016/S0924-2244(99)00027-8. [DOI] [Google Scholar]
- 104.Lilly DM, Stillwell RH. Probiotics: growth-promoting factors produced by microorganisms. Science. 1965;147:747–8. doi: 10.1126/science.147.3659.747. [DOI] [PubMed] [Google Scholar]
- 105.FAO/WHO. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Foods Including Powder Milk with Live Lactic Acid Bacteria. Basel, Switzerland: World Health Organization 2001. [Google Scholar]
- 106.Parracho H, McCartney AL, Gibson GR. Probiotics and prebiotics in infant nutrition. Proc Nutr Soc. 2007;66:405–11. doi: 10.1017/S0029665107005678. [DOI] [PubMed] [Google Scholar]
- 107.Holzapfel WH, Schillinger U. Introduction to pre-and probiotics. Food Res Int. 2002;35:109–16. doi: 10.1016/S0963-9969(01)00171-5. [DOI] [Google Scholar]
- 108.EFSA. EFSA on Dietetic Products, Nutrition and Allergies (NDA); Scientific Opinion on on the scientific requirements for health claims related to gut and immune function. Available online at (www.efsa.europa.eu/efsajournal.html) 2010.
- 109.Aureli P, Capurso L, Castellazzi AM, Clerici M, Giovannini M, Morelli L, et al. Probiotics and health: an evidence-based review. Pharmacol Res. 2011;63:366–76. doi: 10.1016/j.phrs.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 110.Preidis GA, Versalovic J. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era. Gastroenterology. 2009;136:2015–31. doi: 10.1053/j.gastro.2009.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rastall RA, Gibson GR, Gill HS, Guarner F, Klaenhammer TR, Pot B, et al. Modulation of the microbial ecology of the human colon by probiotics, prebiotics and synbiotics to enhance human health: an overview of enabling science and potential applications. FEMS Microbiol Ecol. 2005;52:145–52. doi: 10.1016/j.femsec.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 112.Wallace TC, Guarner F, Madsen K, Cabana MD, Gibson G, Hentges E, et al. Human gut microbiota and its relationship to health and disease. Nutr Rev. 2011;69:392–403. doi: 10.1111/j.1753-4887.2011.00402.x. [DOI] [PubMed] [Google Scholar]
- 113.Kitajima H, Sumida Y, Tanaka R, Yuki N, Takayama H, Fujimura M. Early administration of Bifidobacterium breve to preterm infants: randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 1997;76:F101–7. doi: 10.1136/fn.76.2.F101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grześkowiak Ł, Grönlund MM, Beckmann C, Salminen S, von Berg A, Isolauri E. The impact of perinatal probiotic intervention on gut microbiota: double-blind placebo-controlled trials in Finland and Germany. Anaerobe. 2012;18:7–13. doi: 10.1016/j.anaerobe.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 115.Gerrard JW, Geddes CA, Reggin PL, Gerrard CD, Horne S. Serum IgE levels in white and metis communities in Saskatchewan. Ann Allergy. 1976;37:91–100. [PubMed] [Google Scholar]
- 116.Sepp E, Julge K, Vasar M, Naaber P, Björksten B, Mikelsaar M. Intestinal microflora of Estonian and Swedish infants. Acta Paediatr. 1997;86:956–61. doi: 10.1111/j.1651-2227.1997.tb15178.x. [DOI] [PubMed] [Google Scholar]
- 117.Bourlioux P, Koletzko B, Guarner F, Braesco V. The intestine and its microflora are partners for the protection of the host: report on the Danone Symposium “The Intelligent Intestine,” held in Paris, June 14, 2002. Am J Clin Nutr. 2003;78:675–83. doi: 10.1093/ajcn/78.4.675. [DOI] [PubMed] [Google Scholar]
- 118.Saavedra JM. Use of probiotics in pediatrics: rationale, mechanisms of action, and practical aspects. Nutr Clin Pract. 2007;22:351–65. doi: 10.1177/0115426507022003351. [DOI] [PubMed] [Google Scholar]
- 119.Johansson MA, Sjögren YM, Persson JO, Nilsson C, Sverremark-Ekström E. Early colonization with a group of Lactobacilli decreases the risk for allergy at five years of age despite allergic heredity. PLoS One. 2011;6:e23031. doi: 10.1371/journal.pone.0023031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–20. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 121.Rautava S, Kalliomäki M, Isolauri E. Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J Allergy Clin Immunol. 2002;109:119–21. doi: 10.1067/mai.2002.120273. [DOI] [PubMed] [Google Scholar]
- 122.Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:192–8. doi: 10.1016/j.jaci.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 123.Viljanen M, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, et al. Probiotics in the treatment of atopic eczema/dermatitis syndrome in infants: a double-blind placebo-controlled trial. Allergy. 2005;60:494–500. doi: 10.1111/j.1398-9995.2004.00514.x. [DOI] [PubMed] [Google Scholar]
- 124.Prescott SL, Björkstén B. Probiotics for the prevention or treatment of allergic diseases. J Allergy Clin Immunol. 2007;120:255–62. doi: 10.1016/j.jaci.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 125.Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, Murrell DF, Tang ML. Probiotics for the treatment of eczema: a systematic review. Clin Exp Allergy. 2009;39:1117–27. doi: 10.1111/j.1365-2222.2009.03305.x. [DOI] [PubMed] [Google Scholar]
- 126.Kalliomäki M, Antoine JM, Herz U, Rijkers GT, Wells JM, Mercenier A. Guidance for substantiating the evidence for beneficial effects of probiotics: prevention and management of allergic diseases by probiotics. J Nutr. 2010;140:713S–21S. doi: 10.3945/jn.109.113761. [DOI] [PubMed] [Google Scholar]
- 127.Wang M, Karlsson C, Olsson C, Adlerberth I, Wold AE, Strachan DP, et al. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J Allergy Clin Immunol. 2008;121:129–34. doi: 10.1016/j.jaci.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 128.Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156:3216–23. doi: 10.1099/mic.0.040618-0. [DOI] [PubMed] [Google Scholar]
- 129.Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007;1:56–66. doi: 10.1038/ismej.2007.3. [DOI] [PubMed] [Google Scholar]
- 130.Claud EC, Walker WA. Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. FASEB J. 2001;15:1398–403. doi: 10.1096/fj.00-0833hyp. [DOI] [PubMed] [Google Scholar]
- 131.Deshpande G, Rao S, Patole S. Probiotics for prevention of necrotising enterocolitis in preterm neonates with very low birthweight: a systematic review of randomised controlled trials. Lancet. 2007;369:1614–20. doi: 10.1016/S0140-6736(07)60748-X. [DOI] [PubMed] [Google Scholar]
- 132.Braga TD, da Silva GAP, de Lira PIC, de Carvalho Lima M. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. Am J Clin Nutr. 2011;93:81–6. doi: 10.3945/ajcn.2010.29799. [DOI] [PubMed] [Google Scholar]
- 133.Caplan MS, Lickerman M, Adler L, Dietsch GN, Yu A. The role of recombinant platelet-activating factor acetylhydrolase in a neonatal rat model of necrotizing enterocolitis. Pediatr Res. 1997;42:779–83. doi: 10.1203/00006450-199712000-00010. [DOI] [PubMed] [Google Scholar]
- 134.Caplan MS, Miller-Catchpole R, Kaup S, Russell T, Lickerman M, Amer M, et al. Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Gastroenterology. 1999;117:577–83. doi: 10.1016/S0016-5085(99)70450-6. [DOI] [PubMed] [Google Scholar]
- 135.Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, Yeh TF, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2005;115:1–4. doi: 10.1542/peds.2004-1463. [DOI] [PubMed] [Google Scholar]
- 136.Hoyos AB. Reduced incidence of necrotizing enterocolitis associated with enteral administration of Lactobacillus acidophilus and Bifidobacterium infantis to neonates in an intensive care unit. Int J Infect Dis. 1999;3:197–202. doi: 10.1016/S1201-9712(99)90024-3. [DOI] [PubMed] [Google Scholar]
- 137.Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr. 2005;147:192–6. doi: 10.1016/j.jpeds.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 138.Alfaleh K, Anabrees J, Bassler D, Al-Kharfi T. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2011;CD005496 doi: 10.1002/14651858.CD005496.pub3. [DOI] [PubMed] [Google Scholar]
- 139.Barclay AR, Stenson B, Simpson JH, Weaver LT, Wilson DC. Probiotics for necrotizing enterocolitis: a systematic review. J Pediatr Gastroenterol Nutr. 2007;45:569–76. doi: 10.1097/MPG.0b013e3181344694. [DOI] [PubMed] [Google Scholar]
- 140.Deshpande G, Rao S, Patole S, Bulsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics. 2010;125:921–30. doi: 10.1542/peds.2009-1301. [DOI] [PubMed] [Google Scholar]
- 141.Gooch WM, 3rd, Blair E, Puopolo A, Paster RZ, Schwartz RH, Miller HC, et al. Effectiveness of five days of therapy with cefuroxime axetil suspension for treatment of acute otitis media. Pediatr Infect Dis J. 1996;15:157–64. doi: 10.1097/00006454-199602000-00013. [DOI] [PubMed] [Google Scholar]
- 142.Hoberman A, Paradise JL, Burch DJ, Valinski WA, Hedrick JA, Aronovitz GH, et al. Equivalent efficacy and reduced occurrence of diarrhea from a new formulation of amoxicillin/clavulanate potassium (Augmentin) for treatment of acute otitis media in children. Pediatr Infect Dis J. 1997;16:463–70. doi: 10.1097/00006454-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 143.Cremonini F, Di Caro S, Nista EC, Bartolozzi F, Capelli G, Gasbarrini G, et al. Meta-analysis: the effect of probiotic administration on antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2002;16:1461–7. doi: 10.1046/j.1365-2036.2002.01318.x. [DOI] [PubMed] [Google Scholar]
- 144.D’Souza AL, Rajkumar C, Cooke J, Bulpitt CJ. Probiotics in prevention of antibiotic associated diarrhoea: meta-analysis. BMJ. 2002;324:1361–7. doi: 10.1136/bmj.324.7350.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tankanow RM, Ross MB, Ertel IJ, Dickinson DG, McCormick LS, Garfinkel JF. A double-blind, placebo-controlled study of the efficacy of Lactinex in the prophylaxis of amoxicillin-induced diarrhea. DICP. 1990;24:382–4. doi: 10.1177/106002809002400408. [DOI] [PubMed] [Google Scholar]
- 146.Agostoni C, Axelsson I, Braegger C, Goulet O, Koletzko B, Michaelsen KF, et al. ESPGHAN Committee on Nutrition Probiotic bacteria in dietetic products for infants: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2004;38:365–74. doi: 10.1097/00005176-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 147.Segarra-Newnham M. Probiotics for Clostridium difficile-associated diarrhea: focus on Lactobacillus rhamnosus GG and Saccharomyces boulardii. Ann Pharmacother. 2007;41:1212–21. doi: 10.1345/aph.1K110. [DOI] [PubMed] [Google Scholar]
- 148.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–12. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 149.Veereman-Wauters G. Application of prebiotics in infant foods. Br J Nutr. 2005;93(Suppl 1):S57–60. doi: 10.1079/BJN20041354. [DOI] [PubMed] [Google Scholar]
- 150.Playne MJ, Crittenden R. Commercially available oligosaccharides. Bulletin-FIL-IDF (Belgium); International Dairy Federation 1996. [Google Scholar]
- 151.Wang X, Gibson GR. Effects of the in vitro fermentation of oligofructose and inulin by bacteria growing in the human large intestine. J Appl Bacteriol. 1993;75:373–80. doi: 10.1111/j.1365-2672.1993.tb02790.x. [DOI] [PubMed] [Google Scholar]
- 152.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104:1–63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 153.Reddy BS. Possible mechanisms by which pro- and prebiotics influence colon carcinogenesis and tumor growth. J Nutr. 1999;129(Suppl):1478S–82S. doi: 10.1093/jn/129.7.1478S. [DOI] [PubMed] [Google Scholar]
- 154.Arslanoglu S, Moro GE, Boehm G. Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J Nutr. 2007;137:2420–4. doi: 10.1093/jn/137.11.2420. [DOI] [PubMed] [Google Scholar]
- 155.Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–75. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- 156.Macfarlane S, Macfarlane GT, Cummings JH. Review article: prebiotics in the gastrointestinal tract. Aliment Pharmacol Ther. 2006;24:701–14. doi: 10.1111/j.1365-2036.2006.03042.x. [DOI] [PubMed] [Google Scholar]
- 157.Agostoni C, Axelsson I, Goulet O, Koletzko B, Michaelsen KF, Puntis JWL, et al. ESPGHAN Committee on Nutrition Prebiotic oligosaccharides in dietetic products for infants: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2004;39:465–73. doi: 10.1097/00005176-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 158.Ben XM, Zhou XY, Zhao WH, Yu WL, Pan W, Zhang WL, et al. Supplementation of milk formula with galacto-oligosaccharides improves intestinal micro-flora and fermentation in term infants. Chin Med J (Engl) 2004;117:927–31. [PubMed] [Google Scholar]
- 159.Waligora-Dupriet AJ, Campeotto F, Nicolis I, Bonet A, Soulaines P, Dupont C, et al. Effect of oligofructose supplementation on gut microflora and well-being in young children attending a day care centre. Int J Food Microbiol. 2007;113:108–13. doi: 10.1016/j.ijfoodmicro.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 160.Boehm G, Moro G. Structural and functional aspects of prebiotics used in infant nutrition. J Nutr. 2008;138:1818S–28S. doi: 10.1093/jn/138.9.1818S. [DOI] [PubMed] [Google Scholar]
- 161.Howie PW, Forsyth JS, Ogston SA, Clark A, Florey CD. Protective effect of breast feeding against infection. BMJ. 1990;300:11–6. doi: 10.1136/bmj.300.6716.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Boehm G, Stahl B, Jelinek J, Knol J, Miniello V, Moro GE. Prebiotic carbohydrates in human milk and formulas. Acta Paediatr Suppl. 2005;94:18–21. doi: 10.1080/08035320510043493. [DOI] [PubMed] [Google Scholar]
- 163.Haarman M, Knol J. Quantitative real-time PCR assays to identify and quantify fecal Bifidobacterium species in infants receiving a prebiotic infant formula. Appl Environ Microbiol. 2005;71:2318–24. doi: 10.1128/AEM.71.5.2318-2324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rinne MM, Gueimonde M, Kalliomäki M, Hoppu U, Salminen SJ, Isolauri E. Similar bifidogenic effects of prebiotic-supplemented partially hydrolyzed infant formula and breastfeeding on infant gut microbiota. FEMS Immunol Med Microbiol. 2005;43:59–65. doi: 10.1016/j.femsim.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 165.Salvini F, Riva E, Salvatici E, Boehm G, Jelinek J, Banderali G, et al. A specific prebiotic mixture added to starting infant formula has long-lasting bifidogenic effects. J Nutr. 2011;141:1335–9. doi: 10.3945/jn.110.136747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Finegold SM. Interaction of antimicrobial therapy and intestinal flora. Am J Clin Nutr. 1970;23:1466–71. doi: 10.1093/ajcn/23.11.1466. [DOI] [PubMed] [Google Scholar]
- 167.Sakata H, Yoshioka H, Fujita K. Development of the intestinal flora in very low birth weight infants compared to normal full-term newborns. Eur J Pediatr. 1985;144:186–90. doi: 10.1007/BF00451911. [DOI] [PubMed] [Google Scholar]
- 168.Tanaka S, Kobayashi T, Songjinda P, Tateyama A, Tsubouchi M, Kiyohara C, et al. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol Med Microbiol. 2009;56:80–7. doi: 10.1111/j.1574-695X.2009.00553.x. [DOI] [PubMed] [Google Scholar]
- 169.Favier CF, de Vos WM, Akkermans ADL. Development of bacterial and bifidobacterial communities in feces of newborn babies. Anaerobe. 2003;9:219–29. doi: 10.1016/j.anaerobe.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 170.Hussey S, Wall R, Gruffman E, O’Sullivan L, Ryan CA, Murphy B, et al. Parenteral antibiotics reduce bifidobacteria colonization and diversity in neonates. Int J Microbiol. 2011;2011:130574. doi: 10.1155/2011/130574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ajslev TA, Andersen CS, Gamborg M, Sørensen TI, Jess T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes (Lond) 2011;35:522–9. doi: 10.1038/ijo.2011.27. [DOI] [PubMed] [Google Scholar]
- 172.Jedrychowski W, Gałaś A, Whyatt R, Perera F. The prenatal use of antibiotics and the development of allergic disease in one year old infants. A preliminary study. Int J Occup Med Environ Health. 2006;19:70–6. doi: 10.2478/v10001-006-0010-0. [DOI] [PubMed] [Google Scholar]
- 173.Foliaki S, Pearce N, Björkstén B, Mallol J, Montefort S, von Mutius E, International Study of Asthma and Allergies in Childhood Phase III Study Group Antibiotic use in infancy and symptoms of asthma, rhinoconjunctivitis, and eczema in children 6 and 7 years old: International Study of Asthma and Allergies in Childhood Phase III. J Allergy Clin Immunol. 2009;124:982–9. doi: 10.1016/j.jaci.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 174.Kozyrskyj AL, Ernst P, Becker AB. Increased risk of childhood asthma from antibiotic use in early life. Chest. 2007;131:1753–9. doi: 10.1378/chest.06-3008. [DOI] [PubMed] [Google Scholar]
- 175.Marra F, Marra CA, Richardson K, Lynd LD, Kozyrskyj A, Patrick DM, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. 2009;123:1003–10. doi: 10.1542/peds.2008-1146. [DOI] [PubMed] [Google Scholar]
- 176.Bedford Russell AR, Murch SH. Could peripartum antibiotics have delayed health consequences for the infant? BJOG. 2006;113:758–65. doi: 10.1111/j.1471-0528.2006.00952.x. [DOI] [PubMed] [Google Scholar]
- 177.Penders J, Kummeling I, Thijs C. Infant antibiotic use and wheeze and asthma risk: a systematic review and meta-analysis. Eur Respir J. 2011;38:295–302. doi: 10.1183/09031936.00105010. [DOI] [PubMed] [Google Scholar]
- 178.Celedón JC, Fuhlbrigge A, Rifas-Shiman S, Weiss ST, Finkelstein JA. Antibiotic use in the first year of life and asthma in early childhood. Clin Exp Allergy. 2004;34:1011–6. doi: 10.1111/j.1365-2222.2004.01994.x. [DOI] [PubMed] [Google Scholar]
- 179.Kummeling I, Stelma FF, Dagnelie PC, Snijders BEP, Penders J, Huber M, et al. Early life exposure to antibiotics and the subsequent development of eczema, wheeze and allergic sensitization in the first 2 years of life: the KOALA Birth Cohort Study. Pediatrics. 2007;119:225–31. doi: 10.1542/peds.2006-0896. [DOI] [PubMed] [Google Scholar]
- 180.Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012;129:434–40, 440, e1-2. doi: 10.1016/j.jaci.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 181.Johnston B, Supina A, Ospina M, Vohra S. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2007:2007. doi: 10.1002/14651858.CD004827.pub2. [DOI] [PubMed] [Google Scholar]