Abstract
The stroma in human carcinomas consists of extracellular matrix and various types of non-carcinoma cells, mainly leukocytes, endothelial cells, fibroblasts, myofibroblasts and bone marrow-derived progenitors. The tumor-associated stroma actively supports tumor growth by stimulating neo-angiogenesis, as well as proliferation and invasion of apposed carcinoma cells. It has long been accepted that alterations within carcinoma cells mediate metastasis in a cell-autonomous fashion. Recent studies have, however, suggested an additional notion that cancer cells instigate local and systemic changes in the tumor microenvironment and contribute to niche formation for metastasis. Research, aiming to establish the roles of the tumor-associated stroma in facilitating the spread of carcinoma cells into distant organs, has provided an abundance of data and greater knowledge of the biology of metastatic carcinoma cells and associated stromal cells. This has stimulated further advances in the development of novel therapeutic approaches targeting tumor metastasis.
Keywords: CAF, metastasis, multi-process of metastasis, tumor microenvironment, tumor-associated stroma
Introduction
Metastasis is a life-threatening disease that accounts for as much as 90% of cancer-related mortality.1-3 Carcinoma cells have often spread to distant organs at the time (or even before) patients present with cancer. Routine clinical examinations have produced significant progress in detecting metastasis but existing methods for screening cancer patients are incapable of detecting micro-metastasis and disseminated tumor cells (DTCs) in distant organs. Adjuvant chemotherapy and adjuvant radiotherapy are anticipated to prevent relapse and death. However, over periods of time ranging from years to decades, these metastatic cells residing in distant organs often relapse, corrupt the local microenvironment and acquire the ability to develop into macro-metastases. Metastatic nodules are known to be formed by carcinoma cells harboring increased numbers of epi/genetic alterations conferring aggressive and drug-resistant propensities.
The invasion-metastatic cascade consists of a series of distinct cellular events including (1) local invasion of cancer cells into surrounding tissue, (2) their entrance into the (micro)vasculature (intravasation), (3) survival and exit of circulating tumor cells (CTCs) from the bloodstream (extravasation), and (4) formation of micro and/or macroscopic metastases in distant organs (colonization).4,5 The ability of distinct carcinoma cells to metastasize into distant organs depends on their cellular origins and the epi/genetic alterations acquired and accumulated by these cells during the course of tumor progression.
In addition, more recently emerging evidence supports the notion that the tumor-associated stroma, consisting of endothelial cells, leukocytes, macrophages, myofibroblasts, bone marrow-derived progenitors and abundant extracellular matrix (ECM), significantly facilitates tumor metastasis.4-7 The molecular signaling underlying the complexity of heterogeneous stromal-tumor interactions that is relevant to tumor metastasis is the subject of intensive research. This review aims to highlight the role(s) of the tumor-associated stroma, in addition to tumor cell-autonomous alterations, at instigating and supporting progression of the multi-step processes of tumor metastasis.
Tumor Cell-Autonomous Alterations Influencing Metastasis
Evolution of metastasis
Genetic alterations harbored by carcinoma cells have long been considered to play major roles in promoting the invasion-metastasis cascade. Recent studies using whole genome sequencing and copy number analyses examined genetic alterations in detail in carcinomas, including those of the colon, pancreas, breast and prostate.8-13 For these studies, matched pairs of primary tumors and metastases were employed. Considerable sharing of somatic mutations identified in metastases with those found in the corresponding primary tumors was revealed. It was therefore concluded that metastases had originated from clonal evolution of small populations of primary carcinoma cells harboring additional alterations late in the genetic evolution of carcinomas. This conclusion supporting a linear progression model of carcinoma metastasis contradicts a parallel progression model. The latter proposes that carcinoma cells, which disseminate to distant organs early during tumor progression, may acquire genetic alterations independently of those present in primary tumor cells.14 This discrepancy may account for post-mortem samples derived from patients in the terminal stages of disease in most of the above studies. In such cases, the primary carcinoma cells that had accumulated numerous genetic alterations were likely to have spread into distant organs. In contrast, early metastases which account for small primary cancers at the time of their diagnosis (e.g., TNM classification; T1M1 and T2M1)15 are assumed to stem from carcinoma cells that were relatively less genetically altered. Metastatic cells disseminated from early-stage tumors may evolve independently within the local microenvironment of distant organs and therefore harbor alterations different from those present in primary tumors. Further analyses of samples derived from T1M1 and T2M1 cancer patients may help us to understand differences among the existing models of metastatic tumor evolution.
Experiments using mouse models of human tumors suggest that paracrine signaling instigated by the tumor-associated stroma provides carcinoma cells with pro-invasive and metastatic propensities during both early and late stages of tumorigenesis.16-18 However, detailed characterization of the contribution of the tumor-associated stroma to linear and parallel tumor progression models of metastasis remains to be addressed experimentally in future studies.
Cancer stem cells (CSCs) and epithelial mesenchymal transition (EMT)
The cells of origin for metastasis are also known to have major effects on the invasion-metastasis cascade. The concept of cancer stem cells (CSCs), whereby rare populations of carcinoma cells are capable of forming a tumor, derives from the well-established characteristics of normal tissue stem cells, including their self-renewal and multi-potency.19 Induction of the CSC state was repeatedly observed in various normal and carcinoma cells which underwent epithelial mesenchymal transition (EMT).20-22 The latter is a well-characterized process of cellular trans-differentiation through which epithelial cells acquire the mesenchymal phenotype.23-25 This trans-differentiation program is also reversible as judged by the cells undergoing mesenchymal-epithelial transition (MET). Of note, the bidrectional nature of the CSC phenotype (transition between CSC- and non-CSC-states) was often observed as associated with the reversible EMT trait and this encouraged revision of the definition of CSCs.26-28 In addition to pro-invasive and anoikis-resistant propensities in cells undergoing EMT, tumor-initiating ability was therefore highlighted as one of the EMT-associated phenotypes and as being important for the establishment of cancer metastasis.28 The link between EMT and CSCs has been further supported by a recent study employing a transgenic mouse model of pancreatic intraepithelial neoplasia (PanIN) and a cell lineage-specific labeling approach.29 Introduction of mutations in the genes encoding K-ras and p53 proteins along with a yellow fluorescent protein (YFP) marker specifically in pancreatic cells of the Pdx1-Cre transgenic mouse strain, allowed tracking of pancreatic epithelial cells during progression of PanIN toward pancreatic ductal adenocarcinomas (PDACs). Indeed, cells with the EMT phenotype emerged among YFP-positive (YFP+) carcinoma cells, as demonstrated by their decreased expression of E-cadherin, an epithelial marker, and increased expression of ZEB-1 and fibroblast-specific protein-1 (FSP-1), both of which are mesenchymal markers. To determine whether the carcinoma cells undergoing EMT also display the CSC phenotype, PanINs were dissociated into a single cell suspension and sorted for E-cad-negative YFP+ cells using flow cytometry. These cancer cells, when implanted orthotopically into recipient mice, showed a substantial increase in their tumor-initiating ability relative to the control cancer cells expressing the cell-surface E-cadherin. In contrast, cells extracted from PDACs were of similar size in primary tumors and liver metastases regardless of E-cadherin status. In this experimental setting, the EMT phenotype was associated with the CSC trait in PanINs but not in PDACs. This finding leads to speculation that induction of the CSC phenotype by EMT relies on a cell context-dependent process; in other words, the CSC trait in PDACs may be independent of EMT, but instigated by epi/genetic alterations harbored and accumulated during tumor progression.
Circulating tumor cells (CTCs) and metastasis
CTCs found in the bloodstream, and DTCs that have already spread and localized in distant organs, are believed to be precursors of metastatic nodules.30-32 Importantly, increased CTC numbers and the presence of DTCs in bone marrow predict a poor outcome in breast cancer patients, indicating that these cells can serve as an independent prognostic factor for this disease.33,34 Notably, particular genetic alterations harbored in CTCs, which are responsible for drug-resistance, such as those of the epidermal growth factor receptor (EGFR) gene, have been detected in non-small-cell lung cancer patients.35 This may serve as a new approach for monitoring the genetic changes which develop de novo in carcinoma cells during application of systemic therapy.
A newly emerging hypothesis suggests that CTCs that are capable of giving rise to distant metastases are enriched for CSCs, and as such show increased invasiveness, anoikis-resistance, tumor-initiating potential and the ability to avoid cellular dormancy during metastatic colonization.28,31,36,37 Indeed, it has been demonstrated that several CSC-enriched cancer cell populations show increased ability to form metastases when implanted intravenously into recipient mice.38-40 It is however unknown whether the emergence of spontaneous metastases observed in cancer patients is mediated by CTCs enriched for CSCs. Technical challenges of existing methods for handling and culturing CTCs make proving this assumption experimentally difficult. Low numbers of CTCs present in circulating blood, their short half-life and a paucity of functional markers available for their identification, render isolating CTCs very challenging. Interestingly, it has been shown that distinct populations of CTCs can be found in blood samples of cancer patients, including those circulating as single cells which show a mesenchymal or ameboid-like appearance and those found in the circulation in the form of epithelial sheets, indicative of collective movement of a group of epithelial cells.41,42 Moreover, CTCs appear to contain clusters of heterogeneous cell populations composed of platelets, leukocytes and mesenchymal cells.43-45
The half-life of CTCs in the circulation is short, i.e., measured in hours, which is partially related to their fast clearance from the circulation and/or apoptosis.46 To date, whether CTCs and DTCs extracted from either patients or mouse tumor models are capable of forming metastases when introduced into recipient mice has not been shown. These cells may require niche support to exert their potential to initiate and develop metastasis. Cells of primary tumors are known to be capable of inducing both local and systemic changes in the microenvironment that prime and facilitate their metastatic spread and promote colonization of distant organs.47,48 A variety of soluble factors secreted by tumor cells and supporting stromal cells contribute to increased vascular permeability and penetration of blood vessels by cancer cells, immune evasion, and vascular adhesion which allow cancer cells to intravasate, survive in the circulation and extravasate into sites of metastatic nodule formation.47,49,50 Support provided by a local, organ-specific metastatic niche is also thought to play a critical role in the abilities of DTCs to initiate and develop metastasis.38,47,51 Further improvements in the techniques allowing detailed characterization of CTCs with regard to their epi/genetic status and gene expression profiles is needed to advance our knowledge of the metastasis-forming ability and CSC phenotype of CTCs in future studies.
It has already been well documented in various studies that carcinoma cells and the surrounding stromal cells co-evolve with each other during the course of tumor progression.18,52,53 Signaling molecules produced by the tumor-promoting stroma, initially triggered by carcinoma cells, presumably cross-talk with cell-autonomous alterations in carcinoma cells, thereby further influencing metastasis. The roles of tumor-associated stroma and signaling pathways mediating stromal-tumor interactions in processes involved in tumor metastasis will be highlighted in the following section.
Metastasis-Promoting Signal from the Tumor-Associated Stroma
Immune cells
Various types of non-neoplastic stromal cells are frequently present within human primary carcinomas, including heterogeneous populations of immune cells, endothelial cells, fibroblasts, myofibroblasts and bone marrow-derived progenitors.54-57 Immune cells are represented by those of innate immunity, including macrophages, neutrophils, mast cells, myeloid-derived suppressor cells, dendritic cells and natural killer (NK) cells, and cells of adaptive immunity, such as T and B lymphocytes. The immune cells which infiltrate the tumor (excluding NK cells) produce tumor-promoting cytokines including tumor necrosis factor-α (TNF-α), IL-1β, IL-6 and IL-8, which increase NFκB and STAT3 signaling in nearby premalignant cells.49 This signaling not only stimulates tumorigenic progression, but also induces cytokine production by the carcinoma cells themselves. The newly established positive feedback loop allows further activation of immune cells and maintenance of their effects on cancer progression.
Tumor-associated macrophages (TAMs), which are mature myeloid cells, can be found within the tumor microenvironment in high numbers. TAMs, when educated by microenvironmental cues within the primary tumor, adopt the M2/trophic phenotype.58 M2 type TAMs produce paracrine factors promoting neo-angiogenesis, immunosuppression and local inflammation, all of which facilitate the invasion-metastasis cascade.59,60 For example, IL-4 and colony-stimulating factor-1 (CSF-1) cytokines produced by carcinoma cells and/or T-lymphocytes stimulate recruitment and activation of TAMs, which promote cancer cell invasion by producing epidermal growth factor (EGF) and cathepsin B and S proteinases (Fig. 1).61-63 TAMs also play essential roles in promoting colonization of DTCs in distant organs. Prior to their extravasation, carcinoma cells which enter the circulation need to overcome anchorage-independent growth conditions, i.e., survive sheer forces and resist anoikis. The subsequent colonization is thought to be a rate-limiting step of metastasis, as it has been estimated that less than 0.01% of CTCs which survive in the circulation, extravasate and give rise to micrometastases.6 It has been shown that TAMs, which were activated by the cancer cell-produced ECM proteoglycan versican and vascular cell adhesion molecule-1 (VCAM-1) via Toll-like receptor 2 (TLR2) and counterpart-receptor α4-integrins, respectively, stimulate pulmonary metastatic colonization through elevation of TNF-α and PI3K/Akt signaling in carcinoma cells (Fig. 2A).64,65
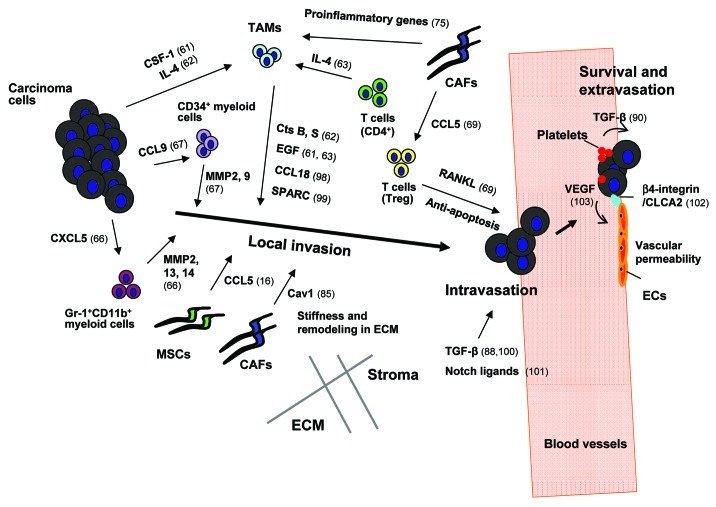
Figure 1. The roles of tumor-associated stroma in the induction of local invasion, intravasation, survival and extravasation of carcinoma cells. Breast cancer cells secrete colony-stimulating factor-1 (CSF-1), a hematopoietic growth factor recruiting tumor-associated macrophages (TAMs) into the primary tumor.61 Infiltrating perivascular TAMs secrete epidermal growth factor (EGF) which chemotactically attracts cancer cells toward the vasculature and thereby facilitates their intravasation.61 In addition, IL4 produced by breast cancer cells and CD4+ T lymphocytes boosts expression of EGF and cathepsin (Cts) B and S proteases by TAMs and further promotes TAM-instigated cancer cell invasion.62,63 In addition to the aforementioned factors TAM-derived CCL18 has also been shown to promote invasion of breast carcinoma cells via signaling through its cognate receptor, PITPNM3,98 whereas TAM-secreted protein acidic and rich in cysteine (SPARC) induces cancer cell migration by acting through αvβ5 integrin.99 CCR1+CD34+ immature myeloid cells respond to CCL9 chemokine secreted from colorectal carcinoma cells, and infiltrate the invasive front of the tumor epithelium, where they produce metalloproteinases (MMPs) 2 and 9.67 CXCL5, another chemokine produced by breast cancer cells, attracts a different population of immunosuppressive myeloid cells (Gr-1+CD11b+) toward the invasive front of tumor tissues where they produce MMP2, 13 and 14 facilitating invasion and metastasis of cancer cells.66 Similarly, CCL5 chemokine secreted by mesenchymal stem cells (MSCs) enhances invasion and metastasis of breast carcinoma cells by activating the CCR5 receptors on these cells.16 Caveolin-1 (Cav1) expressed on carcinoma-associated fibroblasts (CAFs) facilitates tumor invasion through the force-dependent architectural regulation of extracellular matrix (ECM), including its stiffening.85 The primary tumor microenvironment is a source of abundant TGF-β which induces transient activation of TGF-β signaling in breast carcinoma cells promoting their motility and intravasation.88 In addition, stromal-derived TGF-β stimulates expression of angiopoietin-like 4 (ANGPTL4) by breast cancer cells; ANGPTL4 primes cancer cells to dissociate cell-cell junctions between vascular endothelial cells and thereby increases the levels of their extravasation.100 Notch ligands, DLL4 and/or Jagged1 (Jag1), expressed by tumor-associated endothelial cells (EC), macrophages and fibroblasts have been shown to mediate Notch signaling-dependent invasion of colon cancer cells.101 Activation of NFκB signaling in CAFs mediated by IL-1β secreted from infiltrating immune cells results in the production of pro-inflammatory chemokines (e.g., CXCL1 and CXCL2) which chemotactically recruit TAMs in the primary tumor,75 whereas CCL5 secreted by CAFs signals via the CCR1 receptor expressed on regulatory T cells (T-reg).69 The recruited T-reg can express RANKL, a ligand for the RANK receptor on the surfaces of breast cancer cells.69 Signaling mediated upon activation of RANK resulting in IKKα and thus NFκB activation allows cancer cells to evade apoptosis and facilitates their extravasation. Platelet-derived TGF-β induces the TGF-β-Smad2/3 signaling in cancer cells, whereas direct physical contact between platelets and tumor cells results in activation of the NFκB pathway.90 Activation of both forms of signaling leads to induction of the EMT phenotype in CTCs that increases their extravasation.90 hCLCA2, a Ca2+-sensitive chloride channel protein which is expressed by ECs, is implicated in directing β4-integrin-dependent adhesion between these cells and breast carcinoma cells, allowing the latter to undergo extravasation effectively.102 Vascular endothelial growth factor (VEGF) secreted by tumor cells also aids tumor cell extravasation via induction of Src signaling in ECs and the resulting disruption of the integrity of the EC barrier/layer.103
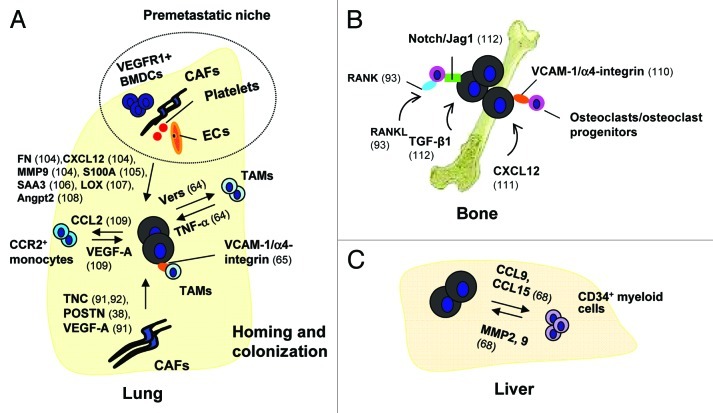
Figure 2. The role of tumor-associated stroma priming for infiltration and colonization by CTCs. (A) Contribution of tumor-associated stromal cells to development of pulmonary metastasis. The formation of a pre-metastatic niche, facilitating homing and colonization of CTCs specifically in the lung has been shown to be aided by signaling molecules secreted by cells from primary tumors [e.g., vascular endothelial growth factor A (VEGF-A)].47 It is believed that VEGFR1+ bone marrow-derived cells (BMDCs), endothelial cells, CAFs and platelets, expressed by the ECM, and signaling molecules, such as fibronectin (FN),104 CXCL12,104 MMP9,104 S100A,105 serum amyloid A (SAA) 3,106 lysyl oxidase (LOX)107 and angiopoietin 2 (Angpt2),108 are all involved in mediating the formation of a pre-metastatic niche. To further promote metastatic colonization, monocytes, TAMs and CAFs, regulated by cancer cells, continue to contribute to forming a specific metastatic niche. It has been shown that breast cancer cells forming pulmonary metastases secrete the CCL2 chemokine109 which mediates infiltration of lung tissue by CCR2 receptor-expressing monocytes. These cells, in turn, produce VEGF-A which stimulates extravasation and seeding of the lung by additional cancer cells. Pulmonary metastatic breast cancer cells secrete the ECM proteoglycan versican which allows recruitment and activation of TAMs via Toll-like receptor 2 (TLR2).64 Activated TAMs in turn produce TNF-α which stimulates pulmonary metastatic colonization by breast cancer cells. In addition, interaction of breast cancer cells expressing vascular cell adhesion molecule-1 (VCAM-1) with the α4-integrin receptor expressed by myeloid cells activates PI3K/Akt signaling in tumor cells and increases their survival.65 ECM proteins produced by CAFs, such as tenascin-C (TNC)91,92 and periostin (POSTN),38 are also implicated in metastatic niche formation in the lung and promotion of pulmonary colonization by breast cancer cells. Not only CAFs, but also S100A4+ fibroblasts, a cell population within CAFs, secrete VEGF-A which induces angiogenesis and thereby promotes metastatic spread and colonization by breast cancer cells.91 (B) The role of tumor-associated stroma in facilitating bone metastasis. Interaction between VCAM-1 expressed on breast carcinoma cells and α4-integrins expressed by osteoclast progenitors elevates local osteoclast activity, thereby aiding conversion of indolent micrometastasis to macrometastasis.110 Bone marrow mesenchymal cells promote survival of breast cancer cells via secretion of the CXCL12/SDF-1 chemokine and CXCL12-dependent activation of c-Src signaling.111 TGF-β released from its reservoir during ECM degradation accompanying bone destruction, induces Jag1 expression on breast carcinoma cells.112 Jag1-activated Notch signaling promotes carcinoma cell proliferation, but also activates osteoclast differentiation allowing further destruction of bone.112 RANKL produced by the bone microenvironment activates RANK receptors expressed on osteoclast progenitors and osteoclasts, leading to bone destruction and metastatic cancer cell colonization.93 (C) Involvement of tumor-associated stroma in liver metastasis. Colon cancer cells secrete CCL9 and CCL15 which recruit bone marrow-derived CCR1+CD34+ myeloid cells into the liver.68 These myeloid cells in turn produce MMP2 and 9 which both facilitate liver colonization by metastatic colon cancer cells.68
Immature myeloid cells, such as Gr-1+CD11b+myeloid-derived suppressor cells and CD34+Gr-1-bone-marrow-derived cells, infiltrate the invasive front of the tumor. These cells promote local micro-invasions of carcinoma cells mediated by CXCL5-CXCR2 and CCL9-CCR1 paracrine signaling (Fig. 1).66,67 CXCL5 and CCL9 chemokines secreted by carcinoma cells recruit myeloid cells upon activation of their receptors, CXCR2 and CCR1, respectively, which are expressed on these cells. The infiltrating myeloid cells, in turn, produce several metalloproteinases (MMPs) which promote ECM degradation, thereby allowing carcinoma cell invasion. In addition, it has been shown that CD34+Gr-1-bone marrow-derived myeloid cells, which were recruited into the liver, stimulated MMP-dependent colonization of this tissue by colon cancer cells (Fig. 2C).68 Tumor-infiltrating regulatory T cells (T-reg) are immunosuppressive lymphocytes that produce the cytokine RANKL (receptor activator of NFκB ligand), a ligand for the RANK receptor. Acting through RANK expressed on breast cancer cells, RANKL activates IKKα, which is essential for NFκB activation and induction of the anti-apoptotic effects of cancer cells and their efficient extravasation.69 Activation of RANK signaling also promotes EMT and CSC phenotypes in BRCA-1-deficient human mammary carcinoma cells that can promote tumorigenesis and metastasis.70
Mesenchymal cells
The presence of large numbers of stromal cells, and the high density and stiffness of ECM are characteristic features of the tumor stroma, which is often referred to as a “desmoplastic” stroma.55,71,72 Fibroblasts and myofibroblasts, collectively designated carcinoma-associated fibroblasts (CAFs), have been shown to substantially contribute to the development of desmoplastic stroma. Myofibroblasts are α-smooth muscle actin (α-SMA)-positive fibroblasts which are a hallmark of activated fibroblasts.56,71,73 Various cell types are thought to be a source for the emergence of tumor-promoting myofibroblasts. Among these there are resident normal fibroblasts, endothelial cells, pericytes, smooth muscle cells, preadipocytes and bone marrow-derived progenitors, such as fibrocytes and mesenchymal stem cells (MSCs).56,74 Our own work demonstrated that resident mammary fibroblasts evolve into CAF myofibroblasts via establishment of both TGF-β-Smad2/3 and CXCL12 (also called stromal cell-derived factor-1: SDF-1)-CXCR4 autocrine signaling pathways during tumor progression.52 Once acquired, this autocrine signaling allows these cells to stably maintain their myofibroblastic state and the associated tumor-promoting propensity, even in the absence of ongoing interaction with carcinoma cells. Another study also indicated that IL-1β secreted by immune cells activates NFκB signaling in locally resident stromal fibroblasts allowing their evolution into CAFs.75 Other signaling pathways including those of the phosphatase and tensin homolog (PTEN) and the Hedgehog (Hh) are also known to modulate activated, tumor-promoting phenotypes of CAFs.76-78 PTEN signaling in stromal fibroblasts suppresses myofibroblast differentiation in culture. Inhibition of such signaling in the tumor-associated stroma within a transgenic mouse model did, in fact, accelerate the initiation and progression of ErbB2-driven breast epithelial cancers.78 The observed tumor-promoting effect was presumably attributable to the increased desmoplastic stromal reaction, accompanied by numerous infiltrating macrophages, promoting the resulting tumor growth.78 It has also been shown that Hh ligands, released from carcinoma epithelial cells, induce the presumed Smo signaling into surrounding stroma in a paracrine fashion.79 In a colon tumor xenograft model, inhibition of Smo protein activity on tumor-associated stromal cells using a small molecular antagonist of the Hh pathway clearly attenuated primary tumor growth.79 Interestingly, another study also showed that systemic administration of a Smo inhibitor into a murine model of pancreatic cancer, which harbored mutant K-ras and p53 alleles, enhanced the efficiency of intratumoral delivery of the chemotherapeutic agent presumably due to altered composition of the desmoplastic stroma and attenuated stromal myofibroblast proliferation.77 Collectively, these studies demonstrated important roles of activation of the Hh-Smo pathway in the tumor-associated stroma that can increase primary tumor growth and suppress drug delivery.
CAFs interact with cancer cells and collaborate with other components of the stroma through their production and secretion of various growth factors, cytokines and chemokines. These signaling molecules effectively mediate neo-angiogenesis, as well as proliferation, survival, motility and invasion of cancer cells.56,80,81 For example, CAFs activated by infiltrating immune cells produce proinflammatory chemokines (e.g., CXCL1 and CXCL2) which mediate recruitment of TAMs into primary tumors,75 whereas the CCL5 chemokine secreted by CAFs recruits T-reg by signaling through the CCR1 receptor expressed on these cells.69 CCL5 secreted from mesenchymal stem cells (MSCs) (among CAFs’ precursors), also acts through the CCR5 receptor (another receptor against CCL5 besides CCR1) expressed on breast carcinoma cells, thereby enhancing invasion and metastasis.16 Moreover, CXCL12 and fibroblast growth factor 2 (FGF-2) released by CAFs stimulate neoangiogenesis by recruiting endothelial progenitor cells and vascular endothelial cells, respectively.82,83
Microenvironment-mediated tensile forces (e.g., matrix stiffness) promote breast cancer progression.84 CAFs, which are activated fibroblasts, can interact with ECM proteins to modulate intracellular adhesions, cell contractility and forces within the microenvironment. It has been shown that caveolin-1 (Cav-1), produced by CAFs, alters the alignment of ECM proteins and promotes stiffness of the tumor microenvironment in a force-dependent fashion via Rho GTPase activation, thereby stimulating tumor invasion and metastasis.85
Co-metastasis with host stromal cells
During the multi-step processes of tumor metastasis, intravasation of invasive carcinoma cells into the circulation is crucial for their spread to distant organs. As discussed earlier, some CTCs are present as clusters with stromal cell populations composed of platelets, leukocytes and mesenchymal cells, raising the possibility that host stromal cells may direct the spread of carcinoma cells. Collective cell migration is a principal of cancer cell migration.86 Leading cells are known to guide migrating cell groups that respond to intrinsic and extrinsic signals induced by interaction with their microenvironment. A study using time-lapse imaging showed that fibroblasts lead and direct collective invasion of co-cultured squamous cell carcinoma (SCC) cells in 3D collagen-Matrigel.87 The collective invasion of SCC cells retaining their epithelial characteristics depended on force- and protease-mediated matrix remodelling by fibroblasts. Another study also suggested that TGF-β, which is abundant in the host microenvironment of primary tumor activated canonical TGF-β-Smad2/3 signaling in carcinoma cells, thereby switching the cell motility from cohesive to the single cell mode.88 The fluorescence-based, TGF-β signaling-dependent reporter activity was examined in a rat mammary MTLn3E tumor xenograft model using intravital imaging. It was shown that each single cancer cell indicating active TGF-β signaling substantially intravasated and metastasized via the bloodstream.88 In contrast, cells without TGF-β signaling activation formed collections of cells which spread into lymph nodes. Taken together, these findings support the theory that tumor stroma-derived paracrine signaling contributes to modulating the motility and mode by which carcinoma cells invade and intravasate.
A recent study also proposed the notion that carcinoma cells co-metastasize with host stromal cells into distant organs. This was demonstrated in a study in which Lewis lung carcinoma cells (LLC1) expressing ds-Red fluorescent protein were implanted under the renal capsule graft in mice ubiquitously expressing GFP. Sampling of the blood from these mice when tumors reached approximately 10 mm in diameter showed that 80% of isolated ds-Red+ CTCs traveled as single cells, while some ds-Red+CTCs formed clusters containing viable GFP+ host stromal cells.44 In addition, mice having GFP+ cells specifically in their skin were generated by the parabiosis skin transplantation and ds-Red+GFP-LLC1 tumor cells were cutaneously implanted. These xenografts showed spontaneous lung metastasis formed by ds-Red+LLC1 cells that were accompanied by GFP+ cells, indicating their co-metastasis into the lungs. The GFP+ cells were represented by α-SMA+FSP-1+ myofibroblasts (75–80%) and F4/80+ macrophages (28%). Furthermore, human mammary CAFs were injected subcutaneously together with murine LLC1 cells into recipient mice and allowed to metastasize with carcinoma cells into the lung.44 To abolish support from CAFs for metastatic colonization, mice were systemically treated with diphtheria toxin after primary tumor resection.44 This toxin is described as being 1,000 times more potent at killing human cells than murine cells. As a result of diphtheria toxin treatment, the number of lung metastatic nodules was decreased relative to the control treatment. These observations demonstrated that CAFs co-metastasize with primary carcinoma cells and may thus be directly involved in promoting tumor metastasis. Similarly, another study showed pancreatic stellate cells orthotopically injected together with pancreatic carcinoma cells into recipient mice to be found in metastatic nodules of distant organs.89 However, it remains to be determined whether CAFs interact directly with carcinoma cells during their metastatic spread, or if CAFs interact with platelets and leukocytes,30,43 and what the signaling pathways mediating these heterogeneous aggregate formations are.
Cancer patients with thrombosis often have poor prognoses.50 It has been demonstrated that platelets prevent the elimination of CTCs by immune cells and stimulate adhesion of CTCs to vascular endothelial cells, thereby supporting their survival, extravasation and seeding into distant organs. A recent study identified molecular signaling by which platelets prime tumor cells for metastasis.90 It has been shown that interaction of CTCs with platelets leads to the induction of Smad2/3 and NFκB signaling pathways allowing circulating colon carcinoma cells to acquire an EMT phenotype facilitating their extravasation and subsequent colonization.90 This study supports the notion that the dynamic interactions between CTCs and heterogeneous stromal cell types in the bloodstream are important, if not prerequisite, for efficient formation of metastases.
Metastatic niche formation
Once CTCs lodge themselves in distant organs, metastatic colonization of these cells depends greatly on the support provided by the niche created by a unique composition of local stromal environmental factors. It has been shown that the CAF-produced ECM proteins tenascin C (TNC) and periostin (POSTN) provide breast cancer cells with tumor-initiating ability via activation of Notch and Wnt signaling. These signaling pathways boost the CSC phenotype of tumor cells and facilitate their pulmonary metastatic colonization (Fig. 2A).38,51,91,92 During metastatic colonization, TNC expression is also induced in S100A4+stromal fibroblasts, which constitute a heterogeneuos cell population within CAFs.91 Eradication of these fibroblasts in transgenic mice upon expression of viral thymidine kinase (tk) under the control of the S100A4 promoter (S100A4-tk mice) and ganciclovir treatment, resulted in decreased ability of orthotopically and intravenously implanted 4T1 breast carcinoma cells to develop into pulmonary metastases.91 Similar results were observed for TNC null mice into which 4T1 cells had been introduced intravenously. These results suggest that stromal-derived TNC plays essential role(s) during metastatic colonization. Notably, TNC is also highly expressed by lung metastatic breast carcinoma cell lines such as MDA-MB-231 and has been shown to be involved in activation of Notch and Wnt signaling, both of which allow these cells to maintain their CSC phenotype.92
As noted above, CAFs present within pulmonary metastasis produce the ECM protein POSTN, which has also been shown to be required for the maintenance and/or development of the CSC trait and metastasis-forming ability by carcinoma cells.38 This was illustrated by a study in which CD24+CD90+ CSC-enriched populations extracted from MMTV-PyMT breast carcinomas were introduced intravenously into recipient mice.38 Only these cells, and not CD24−CD90− carcinoma cells, were able to form lung metastases due to their increased ability to colonize lung tissue. Also, spontaneous lung metastasis was shown to be attenuated in MMTV-PyMT POSTN−/− mice as compared with the control POSTN+/+ genetic background. POSTN-deficient mammary carcinoma cells were extracted from the POSTN−/− mice and orthotopically implanted into wild-type POSTN+/+ recipients or POSTN−/− mice. A significant increase in lung metastasis and the CSC trait was observed only in POSTN+/+ mice, further supporting the key role of stromal POSTN in the development of metastatic nodules.
Human breast and prostate cancer cells preferentially metastasize to bone, wherein the local host microenvironment accelerates both bone destruction and tumor growth. Recent studies indicated crucial roles of RANKL, produced by the host microenvironment serving as a niche, in promoting bone metastasis (Fig. 2B).93 RANKL is produced by osteoblasts and other stromal cells and this cytokine binds to RANK receptors expressed mainly on osteoclast progenitors that stimulates these cells to differentiate into active osteoclasts, leading to excessive bone loss. Blocking the binding of RANKL to RANK using systemic administration of osteoprotegerin, which is a soluble decoy receptor for RANKL, clearly inhibited the development of bone metastasis of B16F10 melanoma cells expressing RANK, when introduced through intracardiac injection into recipient mice.93 In contrast, metastases of melanoma cells to other organs including the ovary, adrenal glands and brain were comparable between control and osteoprotegerin-treated groups.93 These findings therefore suggested that the host microenvironment-produced RANKL serves as a bone metastatic niche fostering colonization of disseminated carcinoma cells.
Conclusions and Perspectives
The importance of tumor-associated stroma in inducing and supporting the various stages of tumor progression and metastasis has become evident. Carcinoma cells possess the ability to instigate changes in the surrounding stroma in both primary and metastatic sites, which upon activation stimulate local invasion, dissemination and metastatic colonization of tumor cells. Various cell-autonomous alterations within carcinoma cells resulting in corruption of the stroma have been studied in organs including the lungs, bone and liver.7,38,49,69,94
Many reports suggest that increased numbers of CTCs in the bloodstream and the presence of DTCs in bone marrow are associated with poor prognoses in cancer patients. It remains to be determined what proportions of detected CTCs and DTCs are prone to apoptosis and what fractions of these cells are capable of forming metastases. Improving laboratory techniques, that would allow culturing and functional analyses of CTCs and DTCs extracted from cancer patients using both in vitro and in vivo experimental approaches, could aid in establishing the abilities of these cells to form metastases and facilitate determining the clinical outcomes of this disease.
CSC-niche formation and retarded immune surveillance, both of which support survival and growth of cancer cells within primary tumors and metastatic nodules, are also thought to be actively supported by the tumor-associated stroma. Exploration of the signaling which underpins the formation of the niche for CSCs suggests its dependence on complex interactions between tumor and stromal cells, and their functional cooperation with cell-autonomous alterations within carcinoma cells.
A better understanding of the interdependence of epithelial and stromal cells would lead to the development of novel pharmacological agents designed to specifically target these stromal-tumor interactions. A number of drugs anticipated to inhibit stromal-tumor interactions have indeed shown clinical benefits. For example, bisphosphonates blocking the osteoclast activity at bone metastatic sites disrupt the vicious cycle in bone remodeling and thus reduce the production of local growth factors in the bone microenvironment. Zoredronic acid has shown anticancer effects with a relative reduction of 36% in the risk of disease progression in adjuvant settings for endocrine therapy-treated breast cancer patients.95,96 Denosumab, a fully human RANKL monoclonal antibody, binds to the RANKL which is abundant in the bone microenvironment and prevents the interaction with RANK. This antibody thus blocked bone turnover and inhibited the development of metastatic bone lesions with greater efficacy than zoredronic acid in several large phase III studies.97 Furthermore, vismodegib, a Smo inhibitor, was approved for the treatment of basal-cell carcinoma by the FDA in 2012 and this drug is now in clinical trials for other tumor types. Hh-activated Smo signaling in tumor-associated stroma is anticipated to be inhibited by this drug. The development of therapeutic interventions targeting stromal-tumor interactions is crucial and combining novel approaches with conventional therapies should be considered for increasing the efficacy of these treatments.
Acknowledgments
We gratefully acknowledge Cancer Research UK Grant C147/A6058 (to A.O.), the Atopy Research Centre, Juntendo University School of Medicine, Tokyo, Japan for funding and Drs Ko Okumura and Sonoko Habu for supporting the research and equipment.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/20631
References
- 1.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 2.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 3.Weinberg RA. The Biology of Cancer. New York, USA: Garland Science, Taylor & Francis Group LLC, 2007. [Google Scholar]
- 4.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sethi N, Kang Y. Unravelling the complexity of metastasis - molecular understanding and targeted therapies. Nat Rev Cancer. 2011;11:735–48. doi: 10.1038/nrc3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–7. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–13. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones S, Chen WD, Parmigiani G, Diehl F, Beerenwinkel N, Antal T, et al. Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci U S A. 2008;105:4283–8. doi: 10.1073/pnas.0712345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navin N, Kendall J, Troge J, Andrews P, Rodgers L, McIndoo J, et al. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472:90–4. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu W, Laitinen S, Khan S, Vihinen M, Kowalski J, Yu G, et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med. 2009;15:559–65. doi: 10.1038/nm.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding L, Ellis MJ, Li S, Larson DE, Chen K, Wallis JW, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9:302–12. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- 15.Engel J, Eckel R, Kerr J, Schmidt M, Fürstenberger G, Richter R, et al. The process of metastasisation for breast cancer. Eur J Cancer. 2003;39:1794–806. doi: 10.1016/S0959-8049(03)00422-2. [DOI] [PubMed] [Google Scholar]
- 16.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 17.Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008;13:394–406. doi: 10.1016/j.ccr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–72. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 21.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 23.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–29. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–44. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 27.Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15:1010–2. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- 28.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 29.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–61. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. J Cell Biol. 2011;192:373–82. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pantel K, Alix-Panabières C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol. 2009;6:339–51. doi: 10.1038/nrclinonc.2009.44. [DOI] [PubMed] [Google Scholar]
- 32.Hou JM, Krebs M, Ward T, Sloane R, Priest L, Hughes A, et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol. 2011;178:989–96. doi: 10.1016/j.ajpath.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 34.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 35.Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366–77. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–9. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 37.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–46. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr HA, Delaloye JF, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–9. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 39.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Pang R, Law WL, Chu ACY, Poon JT, Lam CSC, Chow AKM, et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6:603–15. doi: 10.1016/j.stem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 42.Madsen CD, Sahai E. Cancer dissemination--lessons from leukocytes. Dev Cell. 2010;19:13–26. doi: 10.1016/j.devcel.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Im JH, Fu W, Wang H, Bhatia SK, Hammer DA, Kowalska MA, et al. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer Res. 2004;64:8613–9. doi: 10.1158/0008-5472.CAN-04-2078. [DOI] [PubMed] [Google Scholar]
- 44.Duda DG, Duyverman AM, Kohno M, Snuderl M, Steller EJ, Fukumura D, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A. 2010;107:21677–82. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stott SL, Hsu CH, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci U S A. 2010;107:18392–7. doi: 10.1073/pnas.1012539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Méhes G, Witt A, Kubista E, Ambros PF. Circulating breast cancer cells are frequently apoptotic. Am J Pathol. 2001;159:17–20. doi: 10.1016/S0002-9440(10)61667-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–93. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McAllister SS, Weinberg RA. Tumor-host interactions: a far-reaching relationship. J Clin Oncol. 2010;28:4022–8. doi: 10.1200/JCO.2010.28.4257. [DOI] [PubMed] [Google Scholar]
- 49.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–34. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oskarsson T, Massagué J. Extracellular matrix players in metastatic niches. EMBO J. 2012;31:254–6. doi: 10.1038/emboj.2011.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107:20009–14. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25:30–8. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 54.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–7. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 57.Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–49. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 58.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 59.Qian B-Z, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 61.Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–9. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 62.Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24:241–55. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–6. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Q, Zhang XH, Massagué J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 2011;20:538–49. doi: 10.1016/j.ccr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kitamura T, Kometani K, Hashida H, Matsunaga A, Miyoshi H, Hosogi H, et al. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat Genet. 2007;39:467–75. doi: 10.1038/ng1997. [DOI] [PubMed] [Google Scholar]
- 68.Kitamura T, Fujishita T, Loetscher P, Revesz L, Hashida H, Kizaka-Kondoh S, et al. Inactivation of chemokine (C-C motif) receptor 1 (CCR1) suppresses colon cancer liver metastasis by blocking accumulation of immature myeloid cells in a mouse model. Proc Natl Acad Sci U S A. 2010;107:13063–8. doi: 10.1073/pnas.1002372107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–53. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palafox M, Ferrer I, Pellegrini P, Vila S, Hernandez-Ortega S, Urruticoechea A, et al. RANK induces epithelial-mesenchymal transition and stemness in human mammary epithelial cells and promotes tumorigenesis and metastasis. Cancer Res. 2012;72:2879–88. doi: 10.1158/0008-5472.CAN-12-0044. [DOI] [PubMed] [Google Scholar]
- 71.Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999;250:273–83. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]
- 72.Shimoda M, Mellody KT, Orimo A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol. 2010;21:19–25. doi: 10.1016/j.semcdb.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pietras K, Östman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316:1324–31. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 74.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–72. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17:135–47. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 76.Theunissen JW, de Sauvage FJ. Paracrine Hedgehog signaling in cancer. Cancer Res. 2009;69:6007–10. doi: 10.1158/0008-5472.CAN-09-0756. [DOI] [PubMed] [Google Scholar]
- 77.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461:1084–91. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–10. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 80.Polanska UM, Mellody K, Orimo A. Tumor-Promoting Stromal Myofibroblasts in Human Carcinomas. The Tumor Microenvironment, 2010:325-49. [Google Scholar]
- 81.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–38. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 82.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 83.Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goetz JG, Minguet S, Navarro-Lérida I, Lazcano JJ, Samaniego R, Calvo E, et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146:148–63. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khalil AA, Friedl P. Determinants of leader cells in collective cell migration. Integr Biol (Camb) 2010;2:568–74. doi: 10.1039/c0ib00052c. [DOI] [PubMed] [Google Scholar]
- 87.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 88.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–96. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu Z, Vonlaufen A, Phillips PA, Fiala-Beer E, Zhang X, Yang L, et al. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol. 2010;177:2585–96. doi: 10.2353/ajpath.2010.090899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–90. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O’Connell JT, Sugimoto H, Cooke VG, MacDonald BA, Mehta AI, LeBleu VS, et al. VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc Natl Acad Sci U S A. 2011;108:16002–7. doi: 10.1073/pnas.1109493108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med. 2011;17:867–74. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440:692–6. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 94.Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 95.Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Pöstlberger S, Menzel C, et al. ABCSG-12 Trial Investigators Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–91. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 96.Onishi T, Hayashi N, Theriault RL, Hortobagyi GN, Ueno NT. Future directions of bone-targeted therapy for metastatic breast cancer. Nat Rev Clin Oncol. 2010;7:641–51. doi: 10.1038/nrclinonc.2010.134. [DOI] [PubMed] [Google Scholar]
- 97.Brown JE, Coleman RE. Denosumab in patients with cancer-a surgical strike against the osteoclast. Nat Rev Clin Oncol. 2012;9:110–8. doi: 10.1038/nrclinonc.2011.197. [DOI] [PubMed] [Google Scholar]
- 98.Chen J, Yao Y, Gong C, Yu F, Su S, Chen J, et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. 2011;19:541–55. doi: 10.1016/j.ccr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sangaletti S, Di Carlo E, Gariboldi S, Miotti S, Cappetti B, Parenza M, et al. Macrophage-derived SPARC bridges tumor cell-extracellular matrix interactions toward metastasis. Cancer Res. 2008;68:9050–9. doi: 10.1158/0008-5472.CAN-08-1327. [DOI] [PubMed] [Google Scholar]
- 100.Padua D, Zhang XHF, Wang QQ, Nadal C, Gerald WL, Gomis RR, et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sonoshita M, Aoki M, Fuwa H, Aoki K, Hosogi H, Sakai Y, et al. Suppression of colon cancer metastasis by Aes through inhibition of Notch signaling. Cancer Cell. 2011;19:125–37. doi: 10.1016/j.ccr.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 102.Abdel-Ghany M, Cheng HC, Elble RC, Pauli BU. The breast cancer beta 4 integrin and endothelial human CLCA2 mediate lung metastasis. J Biol Chem. 2001;276:25438–46. doi: 10.1074/jbc.M100478200. [DOI] [PubMed] [Google Scholar]
- 103.Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167:223–9. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–75. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 106.Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K, et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008;10:1349–55. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 107.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang Y, Song N, Ding Y, Yuan S, Li X, Cai H, et al. Pulmonary vascular destabilization in the premetastatic phase facilitates lung metastasis. Cancer Res. 2009;69:7529–37. doi: 10.1158/0008-5472.CAN-08-4382. [DOI] [PubMed] [Google Scholar]
- 109.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell. 2011;20:701–14. doi: 10.1016/j.ccr.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang XH, Wang Q, Gerald W, Hudis CA, Norton L, Smid M, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sethi N, Dai X, Winter CG, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell. 2011;19:192–205. doi: 10.1016/j.ccr.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]