Abstract
Autophagy represents an evolutionarily conserved catabolic mechanism that promotes cell survival by releasing energy substrates via degradation of cellular constituents and by eliminating defective organelles under conditions of stress, such as starvation and hypoxia. The link between enhanced autophagy and nutrient deprivation has been well established. For example, chronic myocardial ischemia, a condition of insufficient oxygen and nutrition, activates autophagy to degrade and recycle damaged cellular structures, thereby ameliorating cardiomyocyte injury.
Keywords: MTOR, autophagy, energy metabolism, mitochondrial biogenesis, apoptosis, metabolic syndrome
Obesity afflicts one-third of adults in the United States, and induces the metabolic syndrome (MetS) in approximately half of the severely obese young population. The MetS, which involves obesity and insulin resistance (IR) as major components, is associated with increased cardiovascular diseases, which have been attributed partially to inefficient energy metabolism. However, the myocardial autophagic activity in this state of over-nutrition is poorly understood.
A recent study from our group showed that myocardial autophagy is inhibited in the obesity-instigated MetS, potentially as a result of impaired energy metabolism and subsequent myocardial injury. In that study, we took advantage of a unique MetS-prone swine model to demonstrate the stepwise emergence of obesity and subsequent IR, and the novel imaging technology Blood-Oxygen-Level-Dependent (BOLD)-magnetic resonance imaging (MRI) to detect myocardial oxygenation in vivo, which was subsequently confirmed ex vivo. Although myocardial perfusion increased in MetS, we found paradoxical hypoxia by BOLD-MRI. As myocardial oxygen consumption remained unchanged in MetS, we speculated that decreased myocardial oxygenation after development of IR might be secondary to impaired glucose utilization. Accordingly, SIRT1/sirtuin 1 was downregulated in MetS, in association with decreased expression of PPARGC1A/peroxisome proliferator-activated receptor-gamma-coactivator-1-α, a master regulator of energy metabolism. Furthermore, activity of ATP synthase was inhibited in MetS, suggesting reduced ATP formation.
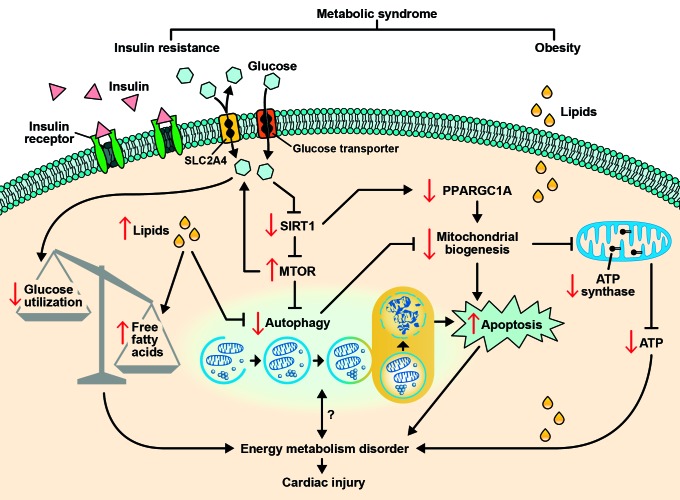
Considering the critical role of enhanced autophagy in response to insufficient energy, we then studied the expression of mechanistic target of rapamycin (MTOR), an important autophagy inhibitor. Surprisingly, MTOR in fact increased in MetS, accompanied by decreased conversion of LC3-I to LC3-II and expression of ULK1 and BECN1, indicating compromised autophagy in MetS. Depressed autophagic activity may reduce the recycling of amino acids for fuel, and exacerbate the disorder of energy metabolism. It may also lead to accumulation of damaged mitochondria that eventuates in apoptosis (Fig. 1). Indeed, we found impaired mitochondrial biogenesis accompanied by elevated apoptosis in MetS.
Figure 1. Involvement of autophagy in cardiac injury observed in the metabolic syndrome. Insulin resistance may downregulate SIRT1, which may inhibit MTOR, a major inhibitor of autophagy. Downregulation of SIRT1 may also decrease the expression of peroxisome proliferator-activated receptor-gamma-coactivator 1-α (PPARGC1A). Inhibition of PPARGC1A and autophagy may blunt mitochondrial biogenesis, leading to elevated apoptosis and decreased synthesis of ATP. Furthermore, increased cardiomyocyte lipids may inhibit autophagy, increase free fatty acids, and exacerbate the imbalance of glucose utilization due to insulin resistance. Impaired glucose utilization, autophagy and ATP synthesis, as well as increased apoptosis, may track together and cause energy metabolism disorder and subsequently cardiac injury. Nevertheless, the interaction between the level of autophagic activity and energy metabolism deserves further investigation.
To ascertain that impaired energy metabolism in association with compromised autophagy in MetS heart is mainly due to the development of IR rather than merely more severe or prolonged obesity, we used several different swine models with selectively induced IR and comparable duration of obesity, and found that similar mechanisms were activated. Alas, our study design did not establish a definite correlation between impaired energy metabolism and downregulated autophagy in MetS, yet these clinically relevant large models of diet-induced obesity with or without IR recapitulate the complexity of obesity-induced metabolic disorders, and thus represent a great translation power clinically.
Although we demonstrated in obesity-initiated MetS the coexistence of impaired energy metabolism and inhibited autophagy, partially responsible for myocardial injury, the cause and effect relationship and the temporal order may be difficult to determine. However, it is not unlikely that compromised autophagy may fail to compensate for early and mild energy insufficiency, which may be worsened by impaired mitochondrial biogenesis and the accumulation of defective mitochondria secondary to inhibited autophagy. Although we did not directly pursue the underlying cause for compromised myocardial autophagy in MetS, we speculate that the development of IR may play an important role. In fact, IR is associated with downregulation of SIRT1, which can activate autophagy by inhibition of MTOR (Fig. 1). Furthermore, upregulation of MTOR may in turn aggravate IR in the myocardium. Indeed, MTOR pathway overactivation contributes to impaired insulin signaling in the liver and skeletal muscle of obese rats. This vicious cycle may further lower the efficiency of glucose utilization, forcing the heart to produce larger proportion of energy from free fatty acids rather than glucose metabolism. This deleterious shift may lead to myocardial adiposity and subsequent injury.
Notably, increased autophagy activity has been implicated in other components of MetS, like hypertension. Autophagy is activated in myocardial hypertrophy secondary to hypertension, possibly as an adaptive process to supplement proteins and to alter the content of numerous sarcomeric components for hypertrophic remodeling. Of interest, the left ventricular remodeling observed in our study was conversely accompanied by inhibited autophagy, indicating that the underlying etiology of myocardial hypertrophy determines the level of autophagic activity. Further studies are needed to evaluate the dynamic changes of autophagic activity when obesity and hypertension coexist and to reveal the mechanisms underlying transition from adaptive to maladaptive autophagy.
Furthermore, it would be of great interest to explore autophagy activation in the heart upon coexistence of obesity and myocardial ischemia, two metabolically counteracting conditions. A recent study from Sadoshima’s group shows that autophagy is inhibited in MetS with prolonged myocardial ischemia, and responsible for a larger myocardial infarct size. However, autophagic activity was assessed in MetS mice with acute ischemia achieved by left descending coronary artery ligation. Further studies are needed to advance our understanding with respect to the autophagic activity in chronic coronary artery stenosis, as well as its potential as a therapeutic target in this important cardiovascular disease.
As a growing population worldwide has transitioned from famine to overnutrition, leading to increased prevalence of cardiovascular diseases, it is the right time to extend our understanding of cardiac autophagy, a conserved catabolism process, in epidemic obesity-initiated MetS.
Acknowledgments
This study was partly supported by NIH grants numbers DK73608, HL77131 and HL085307.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/20285