Abstract
Smith-Lemli-Opitz syndrome (SLOS) is a rare multiple congenital anomaly neurodevelopmental syndrome of impaired cholesterol synthesis. Growth restriction and developmental delay are very common clinical manifestations of SLOS. The degree, etiology, and consequences of growth restriction in SLOS remain an area of limited knowledge to the scientific community. There have been no studies describing the growth parameters and providing reference growth charts for individuals with SLOS. Our longitudinal data from 78 patients between the ages of 0.1 and 16 years with SLOS shows a growth restriction of about 2 standard deviations below the Centers for Disease Control (CDC) norms for age. This study represents comprehensive anthropometric data from the largest cohort available, and proposes growth charts for widespread use in the management and study of individuals with SLOS.
Keywords: Smith-Lemli-Opitz Syndrome, SLOS, growth, height, weight, head circumference, anthropometry
INTRODUCTION
Smith-Lemli-Opitz syndrome (SLOS) is a multiple congenital anomaly, autosomal recessive neurodevelopmental disorder caused by mutations in 7-dehydrocholesterol reductase (DHCR7) resulting in low levels of cholesterol and excess sterol precursors including 7- dehydrocholesterol (7-DHC) [Irons et al., 1993; Tint et al., 1994; Wassif et al., 1998; Fitzky et al., 1998; Waterham et al., 1998; Porter, 2000]. There is an estimated incidence of 1 in 10,000 to 60,000 live births, with a carrier frequency of about 0.8% to 2% [Tsukahara et al., 1998; Nowaczyk et al., 2001; Battaile et al., 2001; Wright et al., 2003]. Cholesterol is a biologic substrate for the formation of many important compounds such as myelin, cell membranes, bile acids, and steroid hormones. The phenotypic spectrum is broad and includes typical facial features (ptosis, small nose with anteverted nares, micrognathia), acral dysgenesis (syndactyly, polydactyly), maxillofacial anomalies (cleft palate, bifid uvula, sublingual cysts), neurodevelopmental disability (intellectual disability, autistic traits, cerebral palsy, special sensory impairment, cataracts, hypotonia, microcephaly), gastrointestinal dysfunction (hepatic insufficiency, Hirschsprung disease, pancreatitis), cardiac (atrial and ventricular septal defects, cyanotic heart disease) and genital-renal malformations (ambiguous genitalia, hypospadias, renal malformations) [Ryan, et al., 1998; Kelley and Hennekam, 2000; Porter, 2000; Porter, 2003].
The most common finding in the neonatal period is intolerance to standard oral feeding resulting in growth failure, which has been attributed to poor sucking and swallowing, and an apparent lack of feeding drive [Ryan, et al., 1998; Kelley and Hennekam, 2000]. Thus, gastrostomy tube insertion and supportive care is often required for adequate caloric intake during infancy. Growth parameters in individuals with SLOS are often lower than those of their age-matched peers, but data have not been published in a robust and clinically useful format. Furthermore, growth charts for individuals with SLOS have not yet been developed. The establishment of references to detect deviation from the expected pattern is important for maintenance of health for children of all ages.
Data used to develop these charts are derived from measurements taken during a longitudinal study of the natural history of SLOS conducted at the National Institutes of Health in Bethesda, Maryland. The cohort represents the largest and most comprehensive anthropometric database of SLOS available to date. The goal of this project is to describe growth parameters and create growth charts for SLOS that conform to the current Centers for Disease Control (CDC) structure for typically developing individuals. This manuscript is the first to provide growth curves for this disorder. The application of these charts will support therapeutic research initiatives, and provide outcome measures relevant to the health of individuals with SLOS.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board (IRB) of the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Maryland and the Kennedy Krieger Institute in Baltimore, Maryland. This study satisfied the ethical requirements of the Hamilton Health Sciences/Faculty of Health at McMaster University Research Ethics Board. Over the course of 13 years (1998–2011) a National Institutes of Health (NIH) longitudinal natural history study of individuals with SLOS has established a robust database of anthropometric measures. Of the 78 patients included in this report, 72 patients were seen at the NIH and 6 were seen at the McMaster University Medical Centre in Ontario, Canada. The ethnic distribution of the SLOS cohort was 74 Caucasian (94.9%), 2 Hispanic (2.6%), 1 Asian (1.3%), and 1 African American (1.3%). Data for weight, height, BMI, and HC were collected for subjects at each visit separated by a mean of 11 months. Each measurement was counted as one data point. Data related to weight was obtained using SR Instruments, Inc. model SR555 and Scale-Tronic, Inc. model 5002 scales for children, and Scaletronix models 4800 and 4802 for infants. Height was collected using SECA Stadiometer model 242 and Holtain Ltd. (U.K.) Stadiometer model NE210. The formula used to calculate body mass index is as follows: BMI = weight in kilograms/height in meters-squared. Occipito-frontal circumference (OFC) of the head was measured using a non-stretchable plastic measurement tape. All diagnoses of SLOS were made by clinical and biochemical or molecular means, and confirmed by a clinical geneticist (F.D.P. and M.N.) at the time of initial medical evaluation. Informed consent for diagnostic and anthropometric measures was obtained for all the individuals at the time of enrollment in the study.
Data were initially prepared using the statistical package Stata, and then exported to the package R for growth curve estimation [StataCorp, 2009; R Development Core Team, 2010]. We constructed growth curves for weight, height, BMI, and OFC from birth to 3 years, and birth to 16 years, each with 5th, 50th, and 95th centiles for age. Data from male and female individuals was combined because the total number of patients in the sample is small, resulting in limited data points. Normative data for growth parameters were obtained from tables published online by the 2000 CDC/NCHS growth curves [Kuczmarski et al., 2000; Kuczmarski et al., 2002]. The CDC data demonstrate no significant differences in growth rates due to race or ethnicity for nutritionally healthy typically developing children. The SLOS curves created were plotted on cross-sectional charts for normal growth based on data from the CDC. Normative data for BMI was limited to ages 2 to 16 years, and from birth to 3 years for OFC. Weight and height normative data were available from birth to 16 years. Therefore, comparisons were not performed beyond the scope of CDC published age groups for respective growth parameters.
In order to estimate the smoothed percentile curve for each of the four anthropomorphic measures, empirical percentiles were computed across the age range under study. A one month window (one month around each time point of interest) for the period of birth to 36 months, and a 3 month window for the period of 36 months to 192 months (16 years), was used for each estimate [Stasinopoulos, 2005]. The differing window choices were applied based on the relatively high density of data points in the former time frame compared to the latter time frame. The resulting percentile estimates were then smoothed across the age range using cubic penalized smoothing lines with the package gamlss in R statistical computing [Stasinopoulos et al., 2008; R Development Core Team, 2010]. Reference data for weight, height, BMI, and head circumference for average stature children from birth through 16 years was plotted along with the smoothed curve estimates for SLOS children using the statistical package R [R Development Core Team, 2010].
RESULTS
A total of 78 individuals (43 males, 35 females) were included in the anthropometry database representing a total of 1,854 observations. Sample size data and demographic characteristics of the SLOS population are presented in Table I. Each subject had a similar number of observations for each growth parameter included in the database. The total number of data for analysis of weight was 493, height 477, BMI 477, and OFC 407. The number of subjects (n) and measurements were n=43 and 139 measurements for weight, n=43 and 136 measurements for height, n=43 and 136 measurements for BMI, and n=55 and 224 measurements for OFC. Both subjectively and statistically there was no difference in the average weight, height, BMI and OFC between male and female individuals with SLOS in the period of 0-16 years. Further investigation showed no qualitative differences in the average differences adjusted for age, or differing age trajectories for males and females.
TABLE 1.
Sample & demographic data of SLOS subjects for whom growth curves were derived.
| Males | Females | Combined | |
|---|---|---|---|
| Weight | |||
| Number of subjects | 43 | 35 | 78 |
| Total number of measurements | 245 | 248 | 493 |
| Height | |||
| Number of subjects | 43 | 35 | 78 |
| Total number of measurements | 239 | 238 | 477 |
| Body Mass Index (BMI) | |||
| Number of subjects | 43 | 35 | 78 |
| Total number of measurements | 239 | 238 | 477 |
| Head Circumference (HC/OFC) | |||
| Number of subjects | 42 | 35 | 77 |
| Total number of measurements | 205 | 202 | 407 |
| Age, years (mean, SD) | 6.2 (SD=4.2) | 6.3 (SD=4.2) | 6.2 (SD=4.2) |
| SLOS severity scale score (mean, SD) | 14.5 (SD=11.1) | 23.1(SD=12.9) | 18.5 (SD=12.8) |
| Cholesterol, total serum (mg/dL) (mean, SD) at the time of growth measurement |
103.6 (SD=40.4) |
82.4 (SD=39.2) |
92.5 (SD=41.6) |
| 7-Dehydrocholesterol, serum (mg/dL) (mean, SD) at the time of growth measurement |
6.4 (SD=5.2) | 10.6 (SD=7.1) | 8.5 (SD=6.6) |
| Number of observations receiving gastric/jejunal-tube feeding, and no supplemental cholesterol (%) |
5/245 (2%) | 6/248 (2.4%) | 11/493 (2.2%) |
| Number of observations receiving gastric/jejunal-tube feeding, plus supplemental cholesterol (%) |
60/245 (24%) | 142/248 (57%) | 199/493 (41%) |
| Number of observations receiving supplemental oral cholesterol (%) |
160/245 (66%) | 87/248 (35%) | 246/493 (50%) |
| Number of observations without supplemental oral cholesterol(%) |
20/245 (8.2%) | 13/248 (5.3%) | 33/493 (6.7%) |
Note: SLOS severity scale scores are categorized as mild (0–12), moderate (13–25), and severe (26–45). Normal ranges for 7-DHC: Birth to 10 years, 0.5 – 1.5 mg/dL; 10 years to adult, 0.7 – 1.9 mg/dL. Normal ranges for total cholesterol in children were derived from NHANES data:
Growth Parameters
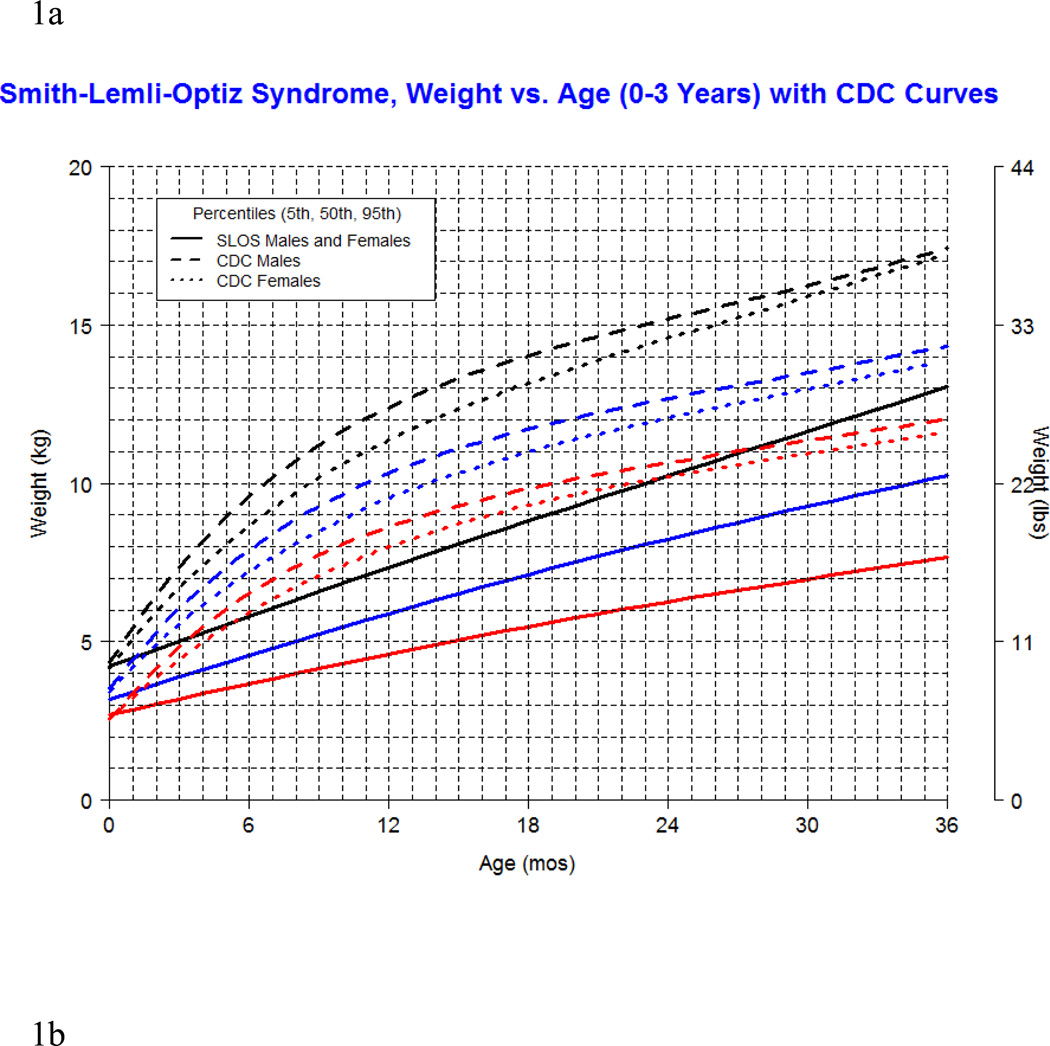
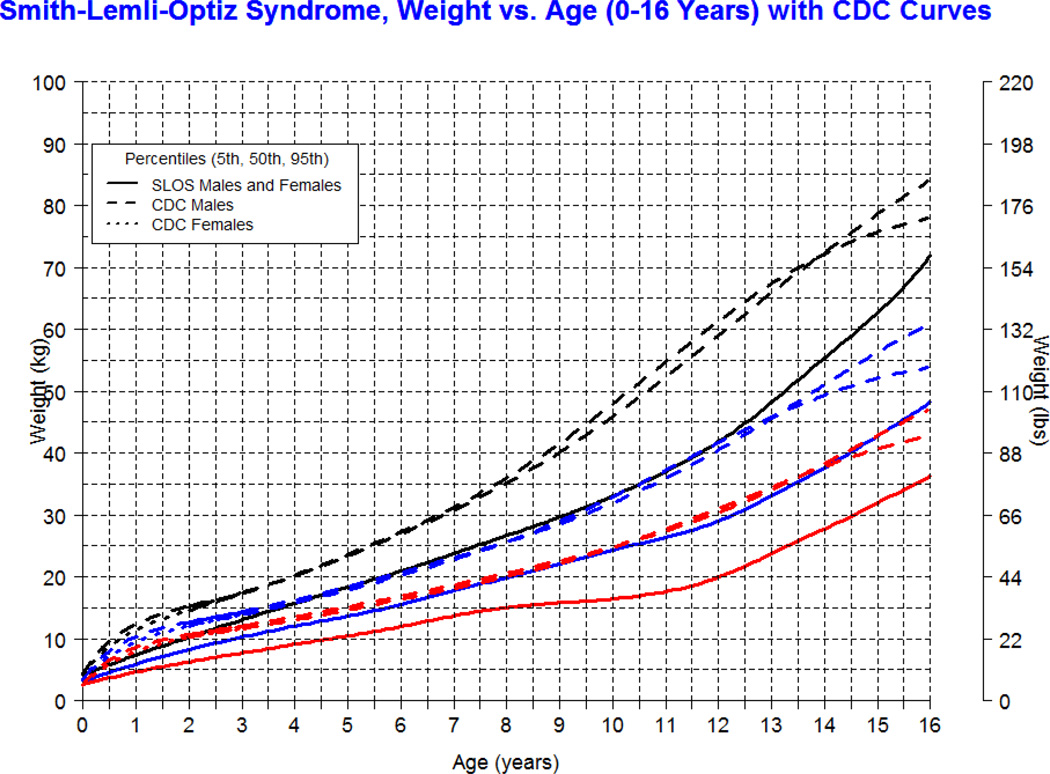
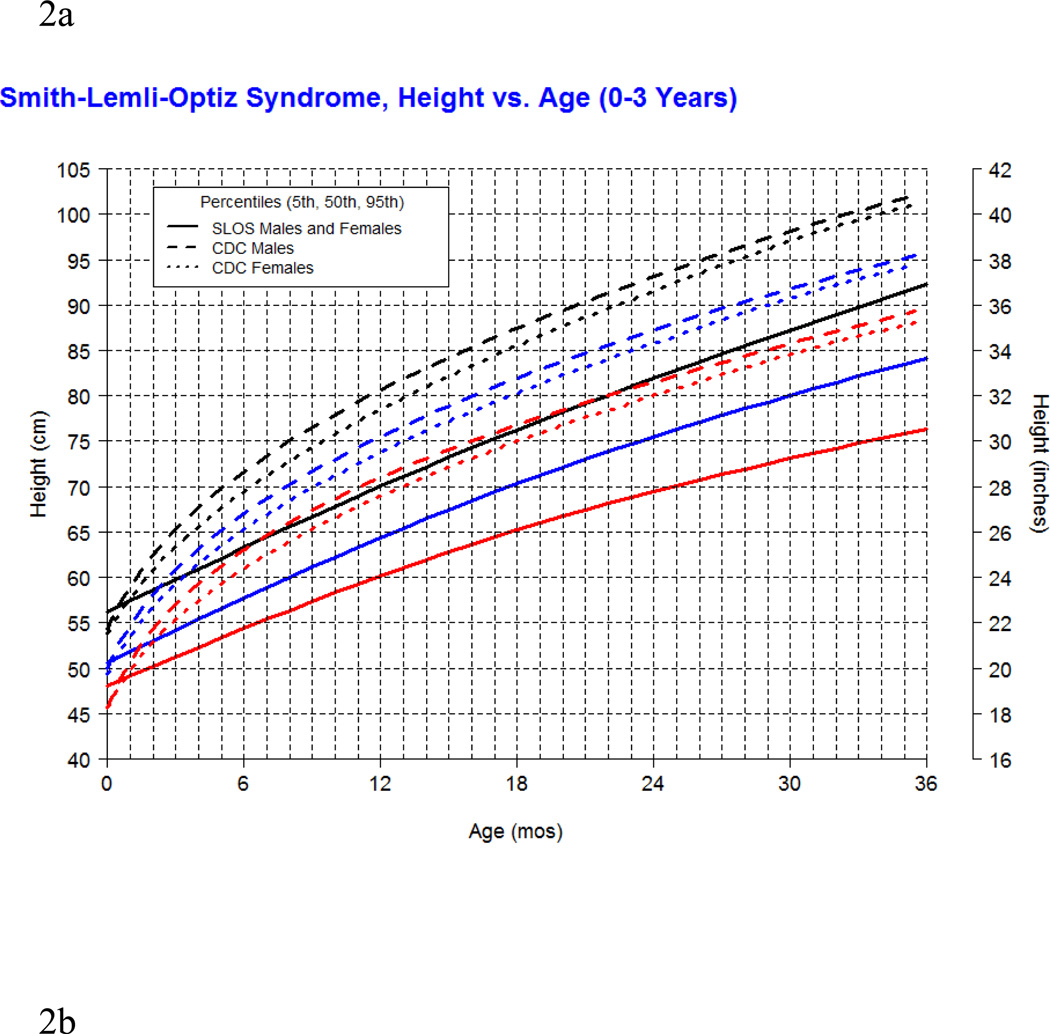
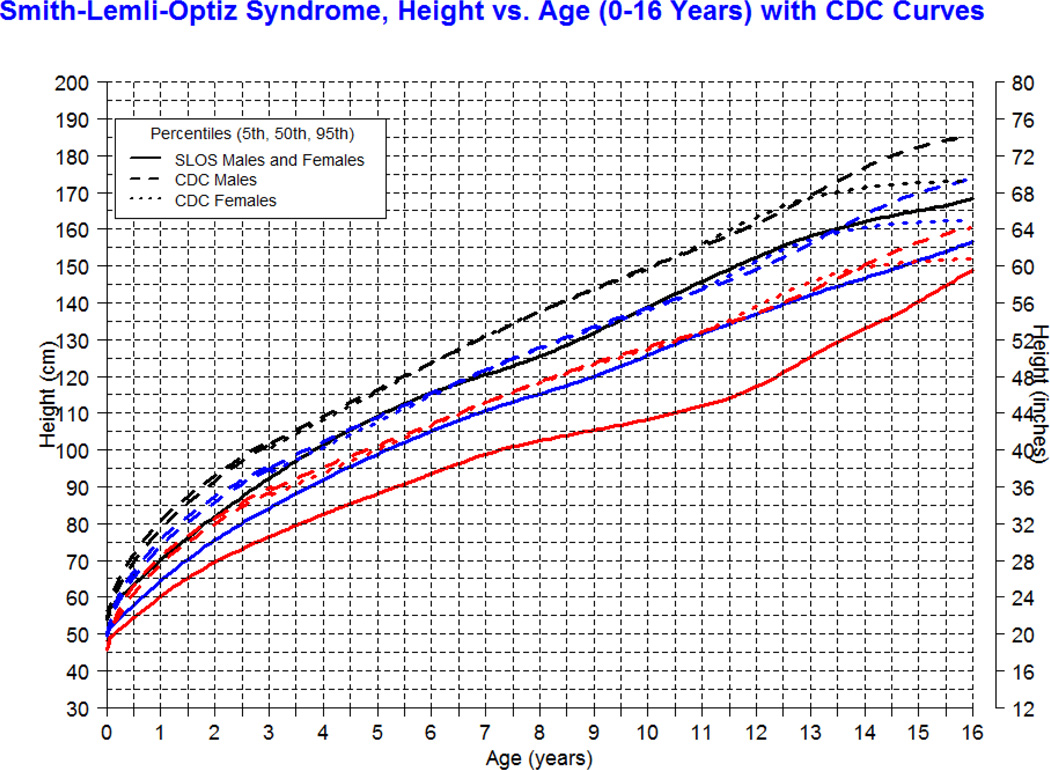
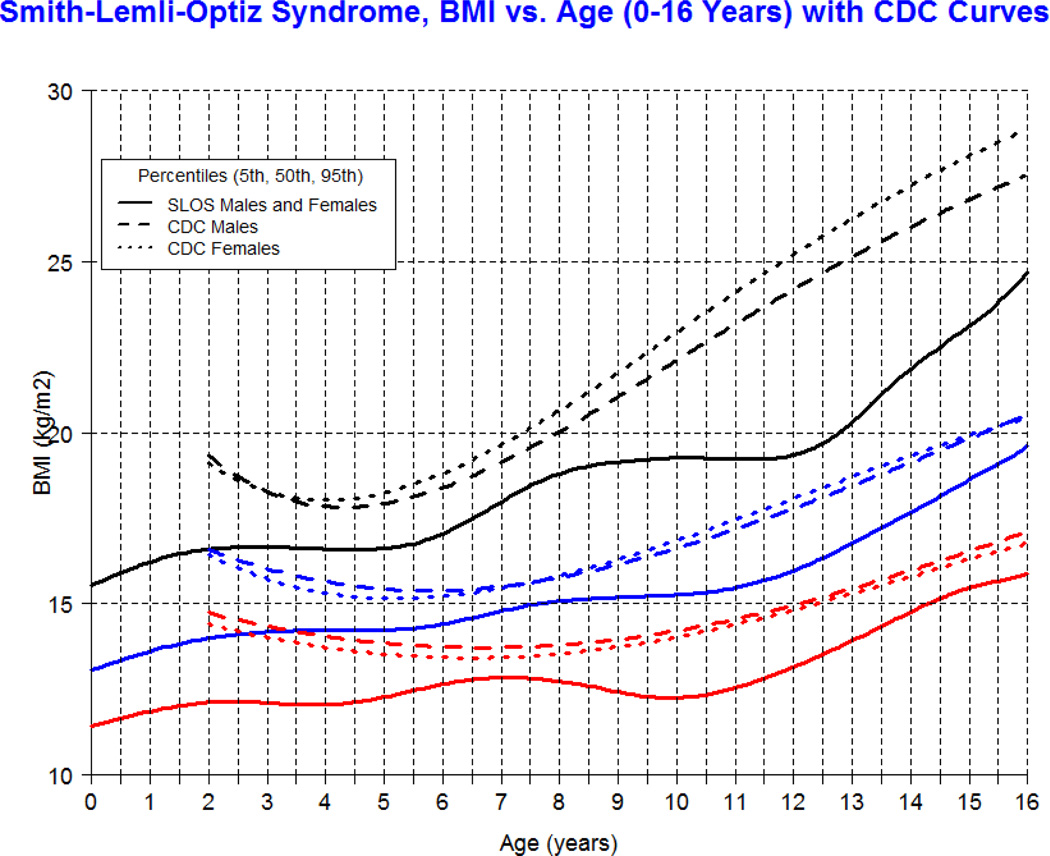
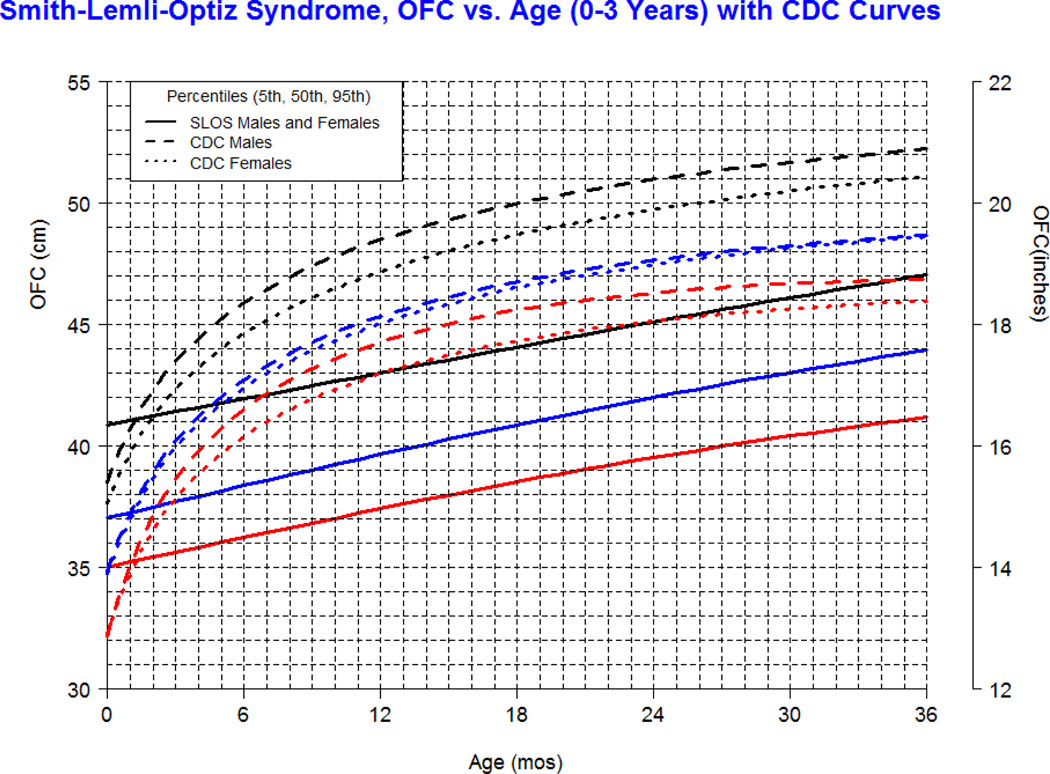
Our longitudinal data from 78 patients between the ages of 0.1 and 16 years with SLOS and 1,854 observations shows growth retardation of about 2 standard deviations below the Centers for Disease Control (CDC) norms for age. The mean weight of SLOS boys was 18.6 kg (n=245, SD=13.7) and mean weight of SLOS girls was 16.6 kg (n=248, SD=10.0) with no statistical difference between gender groups (p=0.41). Compared to typically developing individuals, the mean weight in SLOS falls about 2 standard deviations below the curve for agematched boys and girls (Fig 1). The mean height of SLOS boys was 104.2 cm (n=239, SD=28.2) and mean height of SLOS girls was 99.7 cm (n=238, SD=26.4) with no statistical difference between gender groups (p=0.44). Compared to typically developing individuals, the mean height in SLOS falls about 2 standard deviations below the curve for age-matched boys and girls (Fig 2). The mean BMI of SLOS boys was 15.0 kg/m2 (n=239, SD=2.7) and mean BMI of SLOS girls was 14.8 kg/m2 (n=238, SD=2.3) with no statistical difference between gender groups (p=0.71). Compared to typically developing individuals, the mean BMI in SLOS is about 2 standard deviations below the curve for age matched boys and girls (Fig 3). The mean OFC of SLOS boys was 46.8 cm (n=205, SD=4.8) and mean OFC of SLOS girls was 45.4 cm (n=202, SD=4.2) with no statistical difference between gender groups (p=0.15). Compared to typically developing individuals, the mean OFC in SLOS falls about 2 standard deviations below the curve for agematched boys and girls (Fig 4). Smith-Lemli-Opitz syndrome standard curves for growth parameters of weight, height, BMI and OFC are presented in Supplementary Data 1 through 8 (see Supporting Information online).
Figure 1.
Figure 2.
Figure 3.
Figure 4.
DISCUSSION
The charts presented in this manuscript are novel and proposed as tools to improve the care of individuals with SLOS, especially in areas of growth and nutrition. The large sampling of this rare disease provides a valuable representation of longitudinal data in North America, and spans the spectrum of disease severity. Our data show that overall growth in SLOS is retarded by about 2 standard deviations below the typically developing age-matched population in the United States of America. The shape of the SLOS growth curve appears relatively linear with advancing age, as opposed to the normal population which shows exponential growth early in childhood followed by a plateau in growth with advancing age. This abnormal growth pattern has not been described in SLOS and requires further study.
The pathoetiology of growth failure in SLOS has not been extensively studied. Possible etiologies include central nervous system dysfunction, hormonal or neurosteroid regulation, toxic metabolic effects, and peripheral organ involvement. Currently, there is no effective treatment to improve developmental progress and growth failure in SLOS. The majority of published studies report early accelerated growth and some improvements in development with administration of dietary supplemental cholesterol [Elias et al., 1997; Irons et al., 1997; Nwokoro and Mulvihill, 1997]. Other studies show that dietary cholesterol supplementation does not hold significant treatment benefit for growth or developmental progress [Starck et al., 2002; Sikora et al., 2004]. Studies on treatment with simvastatin report variable results for improved growth and development [Jira et al., 2000; Haas et al., 2007]. Although dietary supplemental cholesterol is widely used among patients, the benefits of the present therapeutic options remain inconclusive. While there has been progress in the elucidation of possible pathophysiologic mechanisms responsible for the broad phenotypic spectrum observed in SLOS, there exists a paucity of biomarkers for measurement of disease outcome. Markers of disease severity and outcome are increasingly important as targeted therapeutics for this genetic disorder appear to be a promising area of scientific research. Growth curves will serve as a fundamental physiologic outcome measure for future and retrospective studies of therapeutic efficacy.
The statistical modeling techniques in this manuscript are also novel and have been applied to the study of achondroplasia [Hoover-Fong et al., 2007]. A limitation of our study includes the relatively small number of patients available for analyses when compared to the CDC database. With even smaller numbers for each gender, separate curves for males and females with SLOS were difficult to report. There was a paucity of data available for SLOS patients beyond the age of 16 years, leaving descriptions of growth in SLOS adults an area of future study. However, growth measurements are typically performed by the health practitioner between the ages presented in this manuscript, making the charts relevant for clinical use. While an increase in number of patients may have resulted in smoother growth curves, the cohort in this manuscript represents the largest database of patients with this rare disorder. Therefore, these robust results have a good potential to significantly improve clinical practice. Additional limitations of this study include supplemental nutrition in a large portion of patients at time of measurement resulting in what may be interpreted as imprecise measurement of the natural growth history, i.e. in the absence of nutritional therapy. Because supplemental nutrition was the standard of care for patients diagnosed with growth failure, this potential confounding factor could not be avoided.
With exception to the extremes of growth, there is limited evidence to suggest that failure to attain optimal growth, in mild to moderate degrees, leads to damaging consequences for the individual. Therefore, further studies should be performed to define optimal growth and development in SLOS. It is widely believed that children should be given the opportunity to reach their full growth potential when community resources are available. In the case of genetic syndromes with poor somatic growth as a feature, many patients are subjected to ongoing dietary manipulation and supplementation to “optimize” growth. In most cases, the primary goals of nutritional therapy are to ensure the individual reaches growth curve parameters of normal children. These attempts often result in familial stress around mealtime and feelings of guilt if unsuccessful in meeting expectations for ideal weight gain. Occasionally, obesity with no or minimal linear and head growth is the result of inappropriate supplementation. It is hoped that with the publication of growth charts for SLOS these complications will be avoided. Detection of growth failure may assist the health practitioner in establishing goals for evaluation, therapeutic intervention and management of the patient. With the publication of growth parameters for SLOS, prospective studies analyzing the influence of growth on neurodevelopmental and systemic consequences will be greatly facilitated.
Supplementary Material
ACKNOWLEDGMENTS
This work is supported by the Intramural research program of the Eunice Kennedy Shriver National Institute of Health, and the Institute for Clinical and Translational Research at Johns Hopkins University.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- Battaile KP, Battaile BC, Merkens LS, Maslen CL, Steiner RD. Carrier frequency of the common mutation IVS8-1G>C in DHCR7 and estimate of the expected incidence of Smith-Lemli-Opitz syndrome. Mol Genet Metab. 2001;72:67–71. doi: 10.1006/mgme.2000.3103. [DOI] [PubMed] [Google Scholar]
- Elias ER, Irons MB, Hurley AD, Tint GS, Salen G. Clinical effects of cholesterol supplementation in six patients with the Smith-Lemli-Opitz syndrome (SLOS) Am J Med Genet. 1997;68:305–310. doi: 10.1002/(sici)1096-8628(19970131)68:3<305::aid-ajmg11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Fitzky BU, Witsch-Baumgartner M, Erdel M, Lee JN, Paik YK, Glossmann H, Utermann G, Moebius FF. Mutations in the Delta7-sterol reductase gene in patients with the Smith-Lemli-Opitz syndrome. Proc Natl Acad Sci USA. 1998;95:8181–8186. doi: 10.1073/pnas.95.14.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D, Garbade SF, Vohwinkel C, Muschol N, Trefz FK, Penzien JM, Zschocke J, Hoffmann GF, Burgard P. Effects of cholesterol and simvastatin treatment in patients with Smith-Lemli-Opitz syndrome (SLOS) J Inherit Metab Dis. 2007;30:375–387. doi: 10.1007/s10545-007-0537-7. [DOI] [PubMed] [Google Scholar]
- Hoover-Fong JE, McGready J, Schulze KJ, Barnes H, Scott CI. Weight for age charts for children with achondroplasia. Am J Med Genet Part A. 2007;143A:2227–2235. doi: 10.1002/ajmg.a.31873. [DOI] [PubMed] [Google Scholar]
- Irons M, Elias ER, Salen G, Tint GS, Batta AK. Defective cholesterol biosynthesis in Smith-Lemli-Opitz syndrome. Lancet. 1993;341(8857):1414. doi: 10.1016/0140-6736(93)90983-n. [DOI] [PubMed] [Google Scholar]
- Irons M, Elias ER, Abuelo D, Bull MJ, Greene CL, Johnson VP, Keppen L, Schanen C, Tint GS, Salen G. Treatment of Smith-Lemli-Opitz syndrome: results of a multicenter trial. Am J Med Genet. 1997;68:311–314. [PubMed] [Google Scholar]
- Jira PE, Wevers RA, de Jong J, Rubio-Gozalbo E, Janssen-Zijlstra FS, van Heyst AF, Sengers RC, Smeitink JA. Simvastatin. A new therapeutic approach for Smith-Lemli-Opitz syndrome. J Lipid Res. 2000;41:1339–1346. [PubMed] [Google Scholar]
- Kelley RI. A new face for an old syndrome. Am J Med Genet. 1997;68:251–256. doi: 10.1002/(sici)1096-8628(19970131)68:3<251::aid-ajmg1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Kelley RI, Hennekam RC. The Smith-Lemli-Opitz syndrome. J Med Genet. 2000;37:321–335. doi: 10.1136/jmg.37.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegel KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegel KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC Growth Charts for the United States: Methods and development. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- Nowaczyk MJ, Nakamura LM, Eng B, Porter FD, Waye JS. Frequency and ethnic distribution of the common DHCR7 mutation in Smith-Lemli-Opitz syndrome. Am J Med Genet. 2001;102:383–386. doi: 10.1002/ajmg.1441. [DOI] [PubMed] [Google Scholar]
- Nwokoro NA, Mulvihill JJ. Cholesterol and bile acid replacement therapy in children and adults with Smith-Lemli-Opitz (SLO/RSH) syndrome. Am J Med Genet. 1997;68:315–321. doi: 10.1002/(sici)1096-8628(19970131)68:3<315::aid-ajmg13>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Porter FD. RSH/Smith-Lemli-Opitz syndrome: a multiple congenital anomaly/mental retardation syndrome due to an inborn error of cholesterol biosynthesis. Mol Genet Metab. 2000;71:163–174. doi: 10.1006/mgme.2000.3069. [DOI] [PubMed] [Google Scholar]
- Porter FD. Human malformation syndromes due to inborn errors of cholesterol synthesis. Curr Opin Pediatr. 2003;15:607–613. doi: 10.1097/00008480-200312000-00011. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2010. ISBN 3-900051-07-0, URL http://www.R-project.org/ [Google Scholar]
- Ryan AK, Bartlett K, Clayton P, Eaton S, Mills L, Donnai D, Winter RM, Burn J. Smith-Lemli-Opitz syndrome: a variable clinical and biochemical phenotype. J Med Genet. 1998;35:558–565. doi: 10.1136/jmg.35.7.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora DM, Ruggiero M, Petit-Kekel K, Merkens LS, Connor WE, Steiner RD. Cholesterol supplementation does not improve developmental progress in Smith-Lemli-Opitz syndrome. J Pediatr. 2004;144:783–791. doi: 10.1016/j.jpeds.2004.02.036. [DOI] [PubMed] [Google Scholar]
- Starck L, Lövgren-Sandblom A, Björkhem I. Cholesterol treatment forever? The first Scandinavian trial of cholesterol supplementation in the cholesterol-synthesis defect Smith-Lemli-Opitz syndrome. J Intern Med. 2002;252:314–321. doi: 10.1046/j.1365-2796.2002.01037.x. [DOI] [PubMed] [Google Scholar]
- Stasinopoulos DM. Generalized additive models for location, scale and shape, (with discussion) Appl Statist. 2005;54(3):507–554. [Google Scholar]
- Stasinopoulos DM, Rigby B, Akantziliotou C. Instructions on how to use the gamlss package in R. (Second Edition) 2008 ( http://gamlss.org/images/stories/papers/gamlss-manual.pdf, last accessed 11/29/201).
- StataCorp. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- Tint GS, Irons M, Elias ER, Batta AK, Frieden R, Chen TS, Salen G. Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N Engl J Med. 1994;330:107–113. doi: 10.1056/NEJM199401133300205. [DOI] [PubMed] [Google Scholar]
- Tsukahara M, Fujisawa K, Yamamoto K, Hasui M, Saito C, Yamamaka T, Honda A, Honda M, Tint GS, Salen G. Smith-Lemli-Opitz syndrome in Japan. Am J Med Genet. 1998;75:118–119. [PubMed] [Google Scholar]
- Wassif CA, Maslen C, Kachilele-Linjewile S, Lin D, Linck LM, Connor WE, Steiner RD, Porter FD. Mutations in the human sterol delta7-reductase gene at 11q12-13 cause Smith-Lemli-Opitz syndrome. Am J Hum Genet. 1998;63:55–62. doi: 10.1086/301936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham HR, Wijburg FA, Hennekam RC, Vreken P, Poll-The BT, Dorland L, Duran M, Jira PE, Smeitink JA, Wevers RA, Wanders RJ. Smith-Lemli-Opitz syndrome is caused by mutations in the 7-dehydrocholesterol reductase gene. Am J Hum Genet. 1998;63:329–338. doi: 10.1086/301982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright BS, Nwokoro NA, Wassif CA, Porter FD, Waye JS, Eng B, Nowaczyk MJ. Carrier frequency of the RSH/Smith-Lemli-Opitz IVS8-1G>C mutation in African Americans. Am J Med Genet A. 2003;120:139–141. doi: 10.1002/ajmg.a.10207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.