ADAM17 functions as a molecular rheostat to control neutrophil influx at sites of infection by regulating the surface density of L-selectin.
Keywords: inflammation, metalloprotease, TACE
Abstract
Neutrophil infiltration and bacterial clearance occur earlier in conditional knockout mice with leukocytes lacking the metalloprotease ADAM17 than in control mice. We investigated cell-intrinsic changes in neutrophils lacking ADAM17 and alterations in the inflammatory environment in conditional ADAM17 knockout mice to determine how the sheddase exerts its effects on neutrophil recruitment. In vivo analyses comparing control and ADAM17-deficient neutrophils revealed that the latter cells accumulated at increased levels in the inflamed mesenteric microvasculature and in the peritoneal cavity following bacterial challenge, indicating changes in their adhesive properties. Consistent with this, bacterial infection caused a marked down-regulation of L-selectin, an adhesion protein and substrate of ADAM17, from the surface of circulating neutrophils in control mice but not in conditional ADAM17 knockout mice. Neutrophils from gene-targeted mice with leukocytes expressing a noncleavable form of L-selectin also displayed a competitive advantage in the presence of control neutrophils when infiltrating a site of infection. Taken together, our findings reveal that impaired L-selectin shedding is a key mechanism underlying early neutrophil recruitment in conditional ADAM17 knockout mice during bacterial infection. Disrupting only the shedding of L-selectin, however, did not increase bacterial clearance, indicating that additional substrates also contribute to the detrimental role of ADAM17 during severe infection.
Introduction
A proteolytic process referred to as ectodomain shedding regulates the cell surface density of various adhesion molecules on leukocytes, as well as the release of soluble proinflammatory factors [1]. ADAM17 is a membrane-associated metalloprotease that plays a primary role in ectodomain shedding by leukocytes [2]. Of interest is that chimeric mice and conditional ADAM17 knockout mice that lack ADAM17 in their leukocytes exhibited a change in the temporal recruitment pattern of neutrophils into sites of inflammation, which occurred more rapidly than in control mice, but was not prolonged [3–5]. Moreover, conditional ADAM17 knockout mice demonstrated enhanced bacterial clearance and an increased survival rate during Escherichia coli-mediated peritonitis, indicating that excessive ADAM17 activity in leukocytes has a detrimental effect on the host response.
Neutrophil migration from the blood into sites of bacterial infection occurs in a stepwise manner, which is orchestrated by members of several families of adhesion molecules (e.g., selectins, integrins, and the Ig superfamily) [6]. Our study examined how ADAM17 exerts its effects on neutrophil recruitment during bacterial infection. Our findings reveal that ADAM17 can be induced in circulating neutrophils during infection, and this decreases the surface density of the adhesion protein L-selectin. We examined neutrophils expressing a noncleavable form of L-selectin to directly determine the effects of L-selectin shedding on neutrophil migration. Our findings implicate L-selectin shedding as a key mechanism underlying early neutrophil infiltration into an infectious locus in conditional ADAM17 knockout mice. However, in contrast to targeting ADAM17, increased bacterial clearance was not observed in mice with leukocytes expressing noncleavable L-selectin, indicating that additional substrates contribute to the detrimental role of ADAM17 during infection.
MATERIALS AND METHODS
Gene-targeted mice
The Animal Care and Use Committee of the University of Minnesota approved the experimental procedures involving animals. Conditional ADAM17 knockout mice (Adam17flox/ΔZn/Vav-Cre) lacking functional ADAM17 in all leukocytes and L(E) mice have been described previously [3, 4, 7]. L-selectin in the latter mice contains a membrane-proximal region from mouse E-selectin, which prevents its shedding upon leukocyte activation in vitro and in vivo [7]. All mice were of mixed genetic background (C57BL/6J;129), and control mice for all experiments consisted of sex-matched littermates.
E. coli-mediated peritonitis
The Institutional Biosafety Committee of the University of Minnesota approved the experimental procedures involving bacteria. E. coli 0111:B4 was injected into mice in a Biosafety Level 2 laboratory at a sublethal dose (5×107 CFU), as described previously [3].
Leukocyte staining and counts
The fluorophore-conjugated mAb anti-L-selectin/CD62L (MEL-14), anti-Mac-1/CD11b (M1/70), Ly-6G (RB6-8C5), and anti-LFA-1/CD11a (M17/4) were purchased from eBioscience (San Diego, CA, USA). Anti-TLR4 (MTS510) was purchased from BioLegend (San Diego, CA, USA). Isotype-matched negative control antibodies were purchased from the respective companies. Bone marrow, peripheral blood, and peritoneal lavage leukocytes were treated with the indicated antibodies, and their staining levels, as well as leukocyte differentials, were determined by flow cytometry using a FACSCanto instrument (BD Biosciences, San Jose, CA, USA), as described previously [3].
ELISA
Quantitative ELISA was performed as described previously [3, 4, 8]. For nonquantitative ELISA, a RayBio Mouse Cytokine Antibody Array G Series 3 glass slide (RayBiotech, Norcross, GA, USA) was used for determining the relative levels of 62 analytes, which included KC, MIP-2, and LIX, as per the manufacturer's instructions.
In vivo assays
Fluorescent intravital microscopy and image analysis of neutrophil accumulation in the peritoneal microcirculation were performed, as described previously [9, 10]. Bone marrow neutrophils were isolated from conditional ADAM17 knockout and control mice, as described previously [3, 11, 12], and were labeled immediately with the fluorophore CFSE, purchased from Invitrogen (Carlsbad, CA, USA) at 5 μM for 15 min at 37°C. After washing, CFSE-labeled neutrophils lacking or expressing ADAM17 (1×107 cells) were infused via the left carotid artery into separate C57BL/6J mice, 2 h after they were administered E. coli 0111:B4 LPS i.p. (100 μg; Sigma-Aldrich, St. Louis, MO, USA). Interaction of fluorescently labeled neutrophils with the postcapillary vascular endothelium of the mesentery was made visible by fluorescent epi-illumination and all images were recorded using StreamPix digital video recording software (NorPix, Montreal, Quebec, Canada), which was followed by off-line analysis. After a brief period of stabilization, fluorescent neutrophils interacting with the microvascular endothelium at a slower rate than the main bloodstream in a vessel segment were enumerated for a specific duration of time (≤30 min) and expressed as the number of accumulated cells/250 μm/min. Following intravital microscopy, the mice were bled via facial vein to determine the proportion of fluorescent neutrophils in the blood.
A competitive leukocyte migration assay was performed, as described previously, with some modifications [13]. Bone marrow neutrophils isolated from conditional ADAM17 knockout and control mice were separately labeled with CFSE or orange-fluorescent tetramethylrhodamine (CMTMR; Invitrogen) at 5 μM for 15 min at 37°C. A 1:1 mixture of CFSE- and CMTMR-labeled neutrophils (2×107 total cells) was infused via tail vein injection into C57BL/6J mice, 2 h after they were administered 5 × 107 E. coli i.p. After an additional 2 h, peritoneal and blood samples were collected, and the fluorescent cells in these samples were analyzed by flow cytometry.
In vitro neutrophil chemotaxis assay
Bone marrow neutrophils were isolated from C57BL/6J mice and labeled with CFSE, as described above. CFSE-labeled neutrophils were washed with PBS, counted, and resuspended in PBS to a final concentration of 1 × 106/ml. Control and conditional ADAM17 knockout mice, challenged 2 h earlier by i.p. injection of 5 × 107 E. coli, were lavaged with 1 ml PBS, which was then subjected to centrifugation (10 min at 2040 g at 4°C) and sterile filtration (0.22 μm pore size). Chemotaxis was analyzed using a disposable, 96-well microchemotaxis transwell plate (Corning, Corning, NY, USA). Peritoneal lavage samples were loaded in duplicate into wells of the transwell plate. A serial dilution of fluorescence-labeled neutrophils was also performed (ranging from 1500 to 5×104) and loaded directly into wells of the plate to provide a standard curve for the determination of migrated cells and to account for any interassay variability in cell labeling. The lid was installed, and 100 μl CFSE-labeled neutrophils were aliquoted into the lid wells (with filter containing 5 μm-diameter pores), and the chamber was incubated for 2 h at 37°C and 5% CO2. After incubation, nonmigrated cells were removed from the top of the filter, the chamber containing the filter apparatus was centrifuged (400 g for 5 min), and migrated cells in the bottom of the chamber were quantified by measuring fluorescence with a plate reader.
Statistical analysis
Differences between groups were analyzed using a conventional two-tailed Student's t test. Reported P values were considered significant at P < 0.05.
RESULTS AND DISCUSSION
Circulating neutrophils lacking ADAM17 demonstrate enhanced adhesive properties during bacterial infection
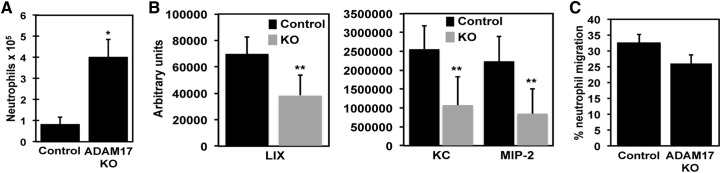
Homozygous deletion of the Adam17 gene in mice is lethal [14], whereas conditional ADAM17 knockout mice lacking ADAM17 expression in leukocytes do not exhibit obvious developmental abnormalities or changes in circulating leukocyte levels or their proportions [3, 4]. We have reported, however, that neutrophils in these mice infiltrate a site of bacterial infection much earlier than neutrophils in control mice [3]. This may be the result of alterations in the cell-intrinsic properties of neutrophils lacking ADAM17 and/or changes in the local inflammatory environment in conditional ADAM17 knockout. The latter process seemed less likely to account for the enhanced recruitment of neutrophils considering that the production of several proinflammatory cytokines was greatly decreased in conditional ADAM17 knockout mice following bacterial infection [3]. Proinflammatory cytokines, such as TNF-α, have been reported to induce the expression of chemokines that promote neutrophil recruitment [15, 16], and we observed that various neutrophil chemokines in the infectious locus were reduced in conditional ADAM17 knockout mice as well. For instance, as early as 2 h after i.p. E. coli injection, peritoneal neutrophil counts were considerably higher in conditional ADAM17 knockout mice than in control mice (Fig. 1A), yet peritoneal levels of CXCL1 (KC), CXCL2 (MIP-2), and CXCL5 (LIX) were lower in these mice (Fig. 1B). As these chemokines do not account for all of the neutrophil chemoattractants at a site of infection, we also compared the overall chemotactic activity of the inflammatory milieu from conditional ADAM17 knockout and control mice following bacterial challenge. WT neutrophils were fluorescently labeled and placed in the upper compartment of an in vitro chemotaxis chamber, and peritoneal lavage fluid from control or conditional ADAM17 knockout mice, collected 2 h after E. coli challenge, was placed in the lower compartment. Neutrophil migration was quantified using a fluorescence plate reader. We found that the peritoneal lavage fluid from conditional ADAM17 knockout mice did not exhibit greater chemotactic activity than the peritoneal lavage fluid from control mice (Fig. 1C). These findings thus suggest that accelerated neutrophil recruitment in conditional ADAM17 knockout mice was not a result of augmented chemoattraction by the inflammatory milieu.
Figure 1. Chemotactic activity of peritoneal lavage from control and conditional ADAM17 knockout (KO) mice following bacterial challenge.
(A and B) Two hours after i.p. injection of E. coli (5×107 CFU) into control and conditional ADAM17 knockout mice, peritoneal neutrophil (A) and chemokine (B) levels were determined. Control and conditional ADAM17 knockout mice had few, if any, peritoneal neutrophils prior to infection [3]. (C) The collected peritoneal lavage fluid was also analyzed for its chemotactic activity using neutrophils from C57BL/6J WT mice. All results are expressed as the mean (±sd) of at least four mice in each group. *P < 0.001 versus control; **P < 0.05 versus control.
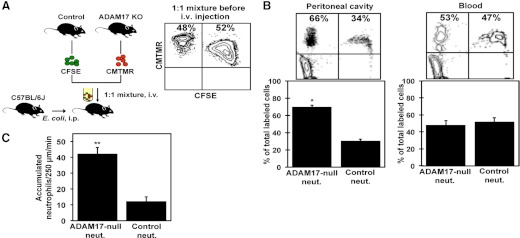
Next, we directly compared the adhesive properties of neutrophils expressing or lacking ADAM17. This was accomplished by adoptive transfer of the neutrophil populations into WT mice following bacterial challenge. This approach eliminated differences in the inflammatory environment between control and conditional ADAM17 knockout mice. Enriched neutrophils from control and conditional ADAM17 knockout mice were separately labeled with the fluorescent dyes CFSE and CMTMR. The labeling schemes were alternated to ensure that any observed differences in neutrophil recruitment were not a result of the fluorophore. Flow cytometric analysis of the labeled cells also revealed that the fluorophores did not differentially alter the surface expression levels of various adhesion molecules (data not shown). WT mice were i.p.-injected with E. coli and then i.v.-infused with a 1:1 ratio of fluorescent neutrophils expressing or lacking ADAM17. Two hours after leukocyte injection, peritoneal lavage and blood samples were collected, and the fluorescent cells in these samples were analyzed by flow cytometry (Fig. 2A). The ratio of fluorescent neutrophils, expressing or lacking ADAM17 in the blood, was found to be similar, demonstrating that there was no disparity in the clearance of the labeled cell populations (Fig. 2B). However, for the fluorescent neutrophils that infiltrated the peritoneal cavity, the percentage of these cells lacking ADAM17 was, on average, 2.3-fold higher than the percentage of neutrophils expressing ADAM17 (Fig. 2B), revealing a competitive advantage by ADAM17-deficient neutrophils when infiltrating a site of infection.
Figure 2. Peritoneal cavity and mesenteric microvasculature accumulation by neutrophils expressing or lacking ADAM17.
(A) Neutrophils from control and conditional ADAM17 knockout mice were labeled with distinct fluorophores (CFSE and CMTMR), mixed 1:1, and infused i.v. into C57BL/6J recipient mice, i.p.-injected with E. coli (5×107 CFU), 2 h previously, as illustrated. (B) Peripheral blood and peritoneal lavage fluid were harvested 2 h later, and the proportion of total fluorescent cells in each sample, which represents neutrophils expressing (Control neut.) or lacking ADAM17 (ADAM17-null neut.), was determined by flow cytometry. The contour plots show representative data from four separate experiments. The indicated percentages represent the proportion of total cells in the top two quadrants. Results shown in the bar graph are expressed as the mean (±sd) of four mice in each group. *P < 0.01 versus control. (C) Neutrophils from control and conditional ADAM17 knockout mice were fluorescently labeled (CFSE), and equivalent cell numbers were i.v.-injected into separate C57BL/6J mice, 2 h after i.p. injection of LPS. Neutrophil interactions with the vascular endothelium of postcapillary venules were analyzed by fluorescent intravital microscopy of the exteriorized mesentery. Fluorescent neutrophils attached to the microvascular endothelium (rolling or firmly adhered) are expressed as the number of accumulated cells/250 μm/min. Results are expressed as the mean (±sd) of at least four mice in each group. **P < 0.001 versus control.
With the use of intravital microscopy, we also examined whether ADAM17 expression affected neutrophil accumulation along the endothelium of the mesenteric microvasculature. Neutrophils expressing and lacking ADAM17 were labeled with CFSE, and equal numbers of each population were injected into separate WT mice, previously administered E. coli LPS i.p. LPS was used for consistent induction of inflammation and stimulation of microvasculature endothelium between mice. We found that the rate of accumulation along the vascular endothelium for circulating neutrophils lacking ADAM17 was 3.5-fold higher than for control neutrophils (Fig. 2C); however, the levels of fluorescent cells in the blood of mice receiving neutrophils that expressed or lacked ADAM17, were similar (data not shown). The above findings are thus consistent with cell-intrinsic changes in blood neutrophils lacking ADAM17 that affect their adhesive properties during bacterial infection.
ADAM17 activation in circulating neutrophils during bacterial infection
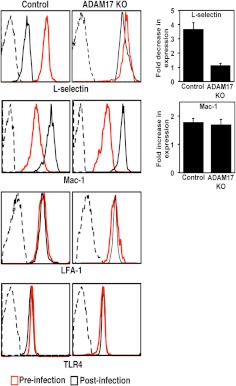
The adhesion protein L-selectin is constitutively expressed at high levels on the surface of circulating neutrophils and has a specialized function in facilitating neutrophil attachment to the vascular endothelium [6, 17, 18]. L-selectin is also a well-described ADAM17 substrate [4, 11, 14]. L-selectin underwent a considerable down-regulation in surface expression on circulating neutrophils in control mice but not in conditional ADAM17 knockout mice following E. coli challenge (Fig. 3). In contrast, the adhesion protein LFA-1 was expressed at equivalent levels on neutrophils from control and conditional ADAM17 knockout mice before and after bacterial challenge (Fig. 3). This was the case for TLR4 as well (Fig. 3), which is required for neutrophil activation by LPS from E. coli. Consistent with this, circulating neutrophils from both groups of mice demonstrated an equivalent up-regulation in expression of the cell activation marker Mac-1 (Fig. 3), indicating that these cells were similarly activated during infection. These results demonstrate for the first time that host and bacterial products in the blood during infection can induce ADAM17 activity in circulating neutrophils, causing a reduction in the surface density of L-selectin.
Figure 3. ADAM17 activation in circulating neutrophils during bacterial infection.
The relative surface expression levels of L-selectin, LFA-1, Mac-1, and TLR4 were determined by flow cytometry on peripheral blood neutrophils from control and conditional ADAM17 knockout mice, 2 h after E. coli challenge (5×107 CFU). For all histogram plots, isotype-matched negative control antibody staining is indicated by dashed lines. y-axis, Cell number; x-axis, Log 10 fluorescence. Data are representative of four mice in each group. The bar graphs show the average fold decrease in L-selectin surface expression and average fold increase in Mac-1 surface expression for neutrophils from control or conditional ADAM17 knockout mice following E. coli challenge. Results are expressed as the mean (±sd) of four mice in each group.
Effects of directly targeting L-selectin shedding or ADAM17 on the host response
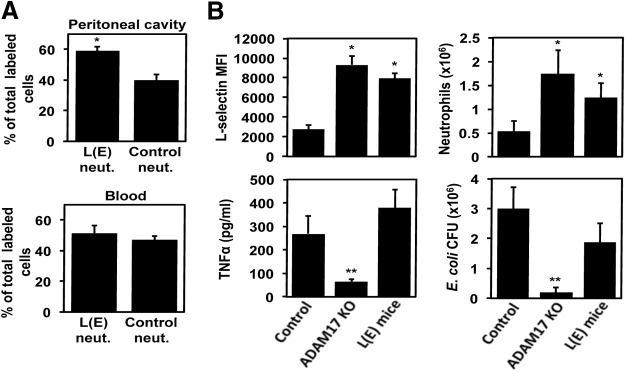
ADAM17 cleaves several proteins on the surface of leukocytes involved in their migration [1], and thus, we sought to determine the effects of specifically blocking the shedding of L-selectin on neutrophil infiltration into a site of bacterial infection. Venturi et al. [7] have generated mice, referred to as L(E), in which their leukocytes express a mutated version of L-selectin that does not undergo cleavage. In Fig. 4A, we show that neutrophils expressing noncleavable L-selectin demonstrated a competitive advantage in the presence of control neutrophils when infiltrating the peritoneal cavity following E. coli challenge, as did neutrophils lacking ADAM17 (Fig. 2B). Tang et al. [5] provide complementary results in a recent study using Adam17−/− bone marrow radiation chimeric mice by showing enhanced neutrophil accumulation during sterile inflammation, which was blocked by an L-selectin function-blocking antibody. Taken together, these findings suggest that the maintenance of L-selectin surface expression accounts for the enhanced infiltration of neutrophils seen in conditional ADAM17 knockout mice.
Figure 4. Selective inhibition of L-selectin shedding increases neutrophil influx into the infectious locus.
(A) Neutrophils from L(E) and control mice were examined in a competitive leukocyte migration assay, as described in Fig. 2A and B. Data are the mean (±sd) of four mice. *P < 0.05 versus control. (B) Conditional ADAM17 knockout, L(E), and control mice were injected i.p. with E. coli (5×107 CFU). Four hours later, L-selectin surface expression levels on peripheral blood neutrophils were determined by flow cytometry [mean fluorescence intensity (MFI)], plasma levels of TNF-α were determined by ELISA, and neutrophil and bacteria levels in the peritoneal cavity were quantified. Results are expressed as the mean (±sd) of four to eight mice in each group. *P < 0.05 versus control; **P < 0.001 versus control.
At this time, the impact of L-selectin shedding on the host response to bacterial challenge in comparison with conditional ADAM17 knockout mice has not been investigated. Similar to conditional ADAM17 knockout mice, circulating neutrophils in L(E) mice retained high levels of surface L-selectin during E. coli-mediated peritonitis (Fig. 4B); however, the shedding of other ADAM17 substrates, such as TNF-α, was not diminished in L(E) mice (Fig. 4B). The lack of L-selectin shedding by neutrophils in L(E) mice corresponded with their enhanced infiltration into the infectious locus (Fig. 4B). Of interest, however, is that peritoneal E. coli levels were reduced significantly in conditional ADAM17 knockout mice but only trended lower in L(E) mice when compared with control mice (Fig. 4B). These findings suggest that ADAM17 substrates, in addition to L-selectin, exert broad effects on the early host response during bacterial infection.
In conclusion, ADAM17, in neutrophils, appears to function as a molecular rheostat to control their infiltration at sites of inflammation by regulating the surface density of L-selectin. However, ADAM17 overactivation in circulating neutrophils during severe infection promotes excessive L-selectin shedding, which, in turn, impairs neutrophil migration into the infectious locus. It is well described that a marked defect in neutrophil recruitment occurs during sepsis and its more severe form, septic shock [19]. Considering our findings, it is tempting to speculate that this may involve overactivation of ADAM17 in blood neutrophils. The magnitude of ADAM17 activation likely increases its detrimental activity, considering its broad putative substrates. We found that targeting ADAM17, but not L-selectin shedding alone, enhanced bacterial clearance. An important aspect of severe infection is wide-spread inflammation, which can promote immunosuppression and end organ damage [19, 20]. A key cytokine and ADAM17 substrate involved in these events is TNF-α [21], and its systemic levels were much higher in L(E) mice than conditional ADAM17 knockout mice (Fig. 4B), which may have attenuated bacterial clearance. Hence, therapeutic interventions targeting ADAM17 might provide the dual benefit of enhancing the host response and diminishing widespread inflammation.
ACKNOWLEDGMENTS
The study was supported in part or in full by grants AI083521 and AI082291 (B.W.) and AI35796 and HL108000 (P.S.) from the U.S. National Institutes of Health. We thank Drs. Douglas Steeber (University of Wisconsin-Milwaukee) and Thomas Tedder (Duke University) for providing the L(E) mice.
Footnotes
- ADAM17
- a disintegrin and metalloprotease 17
- KC
- keratinocyte-derived chemokine
- LIX
- lipopolysaccharide-induced CXC chemokine
- Mac-1
- macrophage antigen 1
AUTHORSHIP
C.L., M.R.H., and Y.W. performed the experimental work and contributed to the interpretation and preparation of the manuscript. P.S. and B.W. designed the experiments and contributed to interpretation and preparation of the manuscript.
REFERENCES
- 1. Garton K. J., Gough P. J., Raines E. W. (2006) Emerging roles for ectodomain shedding in the regulation of inflammatory responses. J. Leukoc. Biol. 79, 1105–1116 [DOI] [PubMed] [Google Scholar]
- 2. Reiss K., Saftig P. (2009) The “a disintegrin and metalloprotease” (ADAM) family of sheddases: physiological and cellular functions. Semin. Cell. Dev. Biol. 20, 126–137 [DOI] [PubMed] [Google Scholar]
- 3. Long C., Wang Y., Herrera A. H., Horiuchi K., Walcheck B. (2010) In vivo role of leukocyte ADAM17 in the inflammatory and host responses during E. coli-mediated peritonitis. J. Leukoc. Biol. 87, 1097–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arndt P. G., Strahan B., Wang Y., Long C., Horiuchi K., Walcheck B. (2011) Leukocyte ADAM17 regulates acute pulmonary inflammation. PLoS ONE 6, e19938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang J., Zarbock A., Gomez I., Wilson C. L., Lefort C. T., Stadtmann A., Bell B., Huang L. C., Ley K., Raines E. W. (2011) Adam17-dependent shedding limits early neutrophil influx but does not alter early monocyte recruitment to inflammatory sites. Blood 118, 786–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ley K., Laudanna C., Cybulsky M. I., Nourshargh S. (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 [DOI] [PubMed] [Google Scholar]
- 7. Venturi G. M., Tu L., Kadono T., Khan A. I., Fujimoto Y., Oshel P., Bock C. B., Miller A. S., Albrecht R. M., Kubes P., Steeber D. A., Tedder T. F. (2003) Leukocyte migration is regulated by L-selectin endoproteolytic release. Immunity 19, 713–724 [DOI] [PubMed] [Google Scholar]
- 8. Bell J. H., Herrera A. H., Li Y., Walcheck B. (2007) Role of ADAM17 in the ectodomain shedding of TNF-α and its receptors by neutrophils and macrophages. J. Leukoc. Biol. 82, 173–176 [DOI] [PubMed] [Google Scholar]
- 9. Broide D. H., Humber D., Sullivan S., Sriramarao P. (1998) Inhibition of eosinophil rolling and recruitment in P-selectin- and intracellular adhesion molecule-1-deficient mice. Blood 91, 2847–2856 [PubMed] [Google Scholar]
- 10. Zuberi R. I., Ge X. N., Jiang S., Bahaie N. S., Kang B. N., Hosseinkhani R. M., Frenzel E. M., Fuster M. M., Esko J. D., Rao S. P., Sriramarao P. (2009) Deficiency of endothelial heparan sulfates attenuates allergic airway inflammation. J. Immunol. 183, 3971–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Y., Brazzell J., Herrera A., Walcheck B. (2006) ADAM17 deficiency by mature neutrophils has differential effects on L-selectin shedding. Blood 108, 2275–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y., Zhang A. C., Ni Z., Herrera A., Walcheck B. (2010) ADAM17 activity and other mechanisms of soluble L-selectin production during death receptor-induced leukocyte apoptosis. J. Immunol. 184, 4447–4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bahaie N. S., Kang B. N., Frenzel E. M., Hosseinkhani M. R., Ge X. N., Greenberg Y., Ha S. G., Demetriou M., Rao S. P., Sriramarao P. (2011) N-glycans differentially regulate eosinophil and neutrophil recruitment during allergic airway inflammation. J. Biol. Chem. 286, 38231–38241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peschon J. J., Slack J. L., Reddy P., Stocking K. L., Sunnarborg S. W., Lee D. C., Russell W. E., Castner B. J., Johnson R. S., Fitzner J. N., Boyce R. W., Nelson N., Kozlosky C. J., Wolfson M. F., Rauch C. T., Cerretti D. P., Paxton R. J., March C. J., Black R. A. (1998) An essential role for ectodomain shedding in mammalian development. Science 282, 1281–1284 [DOI] [PubMed] [Google Scholar]
- 15. Calkins C. M., Heimbach J. K., Bensard D. D., Song Y., Raeburn C. D., Meng X., McIntyre R. C., Jr., (2001) TNF receptor I mediates chemokine production and neutrophil accumulation in the lung following systemic lipopolysaccharide. J. Surg. Res. 101, 232–237 [DOI] [PubMed] [Google Scholar]
- 16. Elizur A., Adair-Kirk T. L., Kelley D. G., Griffin G. L., Demello D. E., Senior R. M. (2008) Tumor necrosis factor-α from macrophages enhances LPS-induced clara cell expression of keratinocyte-derived chemokine. Am. J. Respir. Cell Mol. Biol. 38, 8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walcheck B., Moore K. L., McEver R. P., Kishimoto T. K. (1996) Neutrophil-neutrophil interactions under hydrodynamic shear stress involve L-selectin and PSGL-1. A mechanism that amplifies initial leukocyte accumulation on P-selectin in vitro. J. Clin. Invest. 98, 1081–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. St Hill C. A., Alexander S. R., Walcheck B. (2003) Indirect capture augments leukocyte accumulation on P-selectin in flowing whole blood. J. Leukoc. Biol. 73, 464–471 [DOI] [PubMed] [Google Scholar]
- 19. Alves-Filho J. C., Spiller F., Cunha F. Q. (2010) Neutrophil paralysis in sepsis. Shock 34 (Suppl. 1), 15–21 [DOI] [PubMed] [Google Scholar]
- 20. Boomer J. S., To K., Chang K. C., Takasu O., Osborne D. F., Walton A. H., Bricker T. L., Jarman S. D., II, Kreisel D., Krupnick A. S., Srivastava A., Swanson P. E., Green J. M., Hotchkiss R. S. (2011) Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306, 2594–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gordon A. C., Lagan A. L., Aganna E., Cheung L., Peters C. J., McDermott M. F., Millo J. L., Welsh K. I., Holloway P., Hitman G. A., Piper R. D., Garrard C. S., Hinds C. J. (2004) TNF and TNFR polymorphisms in severe sepsis and septic shock: a prospective multicentre study. Genes Immun. 5, 631–640 [DOI] [PubMed] [Google Scholar]