2011 SLB Bonazinga Award Winner: Perspective on the impact of genome-scale technologies on our understanding of macrophage biology, and the evolution of innate immune cell function.
Keywords: networks, clusters, lipopolysaccharide, dendritic cell, CSF-1, chromatin
Abstract
Monocytes and macrophages differentiate from progenitor cells under the influence of colony-stimulating factors. Genome-scale data have enabled the identification of the set of genes that distinguishes macrophages from other cell types and the ways in which thousands of genes are regulated in response to pathogen challenge. Although there has been a focus on a small subset of lineage-enriched transcription factors, such as PU.1, more than one-half of the transcription factors in the genome can be expressed in macrophage lineage cells under some state of activation, and they interact in a complex network. The network architecture is conserved across species, but many of the target genes evolve rapidly and differ between mouse and human. The data and publication deluge related to macrophage biology require the development of new analytical tools and ways of presenting information in an accessible form. The website www.macrophages.com is a community website that partly fills this niche.
MACROPHAGE BIOLOGY—A HISTORICAL PERSPECTIVE
It is an extraordinary honor to receive the Bonazinga Award from the Society of Leukocyte Biology. The list of previous awardees on the Society website reads like a “who's who” of the most influential people I have known in my field, including two of my direct mentors, Siamon Gordon and Josh Fidler. In this plenary perspective, I do not intend to try and review the field of macrophage biology. I will start where I started in the field—with the identification of markers of mononuclear phagocytes. I will progress through a discussion as to why I consider the use of such markers misleading, because they are not correlated with underlying functions, and macrophages are infinitely heterogeneous. And, I will end, where I am now—with the data deluge and progress toward understanding transcriptional networks.
THE CELLS OF THE MONONUCLEAR PHAGOCYTE SYSTEM
The MPS was defined as a family of cells of the innate immune system derived from hematopoietic progenitor cells under the influence of specific growth factors. Differentiated cells of the MPS, monocytes, and macrophages, are major effectors of innate immunity, engulfing and killing pathogens. They are also needed for tissue repair and resolution of inflammation. Their biology and differentiation have been reviewed by a number of authors [1–4]. The original definition of the MPS considered an essentially linear sequence, from pluripotent progenitors through committed myeloid progenitors shared with granulocytes to promonocytes and blood monocytes and thence, to tissue macrophages (reviewed in ref. [5]). The number of monocyte-derived macrophages in tissues was considered to increase by recruitment in response to a wide range of inflammatory challenges, and the function of these recruited macrophages varied depending on the nature of the stimulus.
Challenges to the unified concept of a MPS have been discussed elsewhere [3]. The clear separation from polymorphonuclear cells implied by the term mononuclear is no longer clear. We and others have shown that at least in vitro, inflammatory granulocytes (polymorphs) can transdifferentiate into macrophages [6]. The most recent challenge to the MPS concept relates to whether some populations of tissue macrophages, notably the microglia of the brain [7] and the epidermal macrophages (Langerhans cells) of the skin [8], [9] do not derive from blood monocytes in the steady-state. Instead, they may be seeded from yolk sac or other primitive progenitors during embryonic development and are thereafter renewed by local proliferation. The former study on the origin of microglia used a lineage tracer, in which an inducible cre-recombinase was activated by administration of the inducer to the mother before the onset of definitive hematopoiesis in the embryo. Thereafter, cells that expressed the recombinase were tagged permanently with a fluorescent reporter gene. The conclusions of that lineage tracer experiment are very difficult to reconcile with clear evidence that blood-derived monocytes do infiltrate the brain as well as the retina in late gestation and postnatally, engulf dying neurons, and transmogrify into classical microglia [10, 11]. The derivation of microglia from yolk sac progenitors was documented well before the recent lineage trace experiments [12, 13]. Within the yolk sac, proliferating phagocytic cells arise without any apparent monocyte-like stage. The tracer data provide support for the idea that these cells can be retained in adult animals and that there is relatively little infiltration of the brain from phagocytes generated from definitive progenitors formed before 10.5 days after coitus. It also supports the view that definitive progenitors arise independently of those formed in the yolk sac. However, it does not eliminate a role for definitive progenitors “born” later in development, in the aorta-gonad-mesonephros, and blood monocytes derived from them [14]. A more recent study examined mice that lacked the transcription factor, c-myb [15]. C-myb is expressed in pluripotent hematopoietic progenitors, but in the knockout mice, yolk sac-derived macrophages developed normally. In an adult mouse, induced deletion of the c-myb gene in a conditional knockout selectively depleted blood monocytes but did not ablate tissue macrophages. This study also used an inducible lineage trace to demonstrate that at least some cells generated in the yolk sac gave rise to descendents that are retained in the adult mouse.
A previous Bonazinga awardee, Richard Stanley, made a major contribution to the field of macrophage biology through the discovery of Macrophage colony-stimulating factor (CSF-1) and subsequent identification with Charles Sherr of the CSF-1R (reviewed in ref. [16]). More recently, a second growth factor, IL-34, was identified that can also interact with the CSF-1R. The two-ligands, one-receptor system is conserved through evolution [17]. How this could be maintained while both ligands diverge through evolution became evident with the recent solution of the crystal structure of the complex of IL-34 with the receptor [18, 19]. The majority of the tissue mononuclear phagocyte populations, including the brain microglia [7, 20], depends on their migration, survival, and (re)population upon signaling through the CSF-1R, with distinct roles for its two ligands, CSF-1 and IL-34. The CSF-1-deficient osteopetrotic mouse and toothless rat both have gross deficiencies of tissue macrophages and many pleiotropic consequences of that deficiency [21]. In the brain, at least it appears that the two ligands may exert region-specific functions on microglia [22]. My group characterized the transcription control elements of the mouse csf1r locus and used that information to generate transgenic MacGreen mice, in which all of the tissue macrophages express an EGFP reporter gene [23], a tool that illustrates the very large numbers of these cells within tissues.
ANTIGEN PRESENTATION AND RELATIONSHIP BETWEEN MACROPHAGES AND DCs
The activation of T lymphocytes requires the presentation of the antigen on MHC molecules on the surface of an APC. In the early 1970s, Steinman and Cohn [24] identified cells with unusual morphology that had very high levels of class II MHC and appeared to be especially adapted to present antigen. They were termed DCs, and as defined originally, the DC was a nonphagocytic cell that was particularly active at stimulation in the allogeneic MLR. The literature in this area has been very confused by the loose use of the term “DC” to be synonymous with APC [3, 4, 25, 26]. What I consider to be a myth emerged: that DCs are a separate cell lineage uniquely able to present antigen to naïve T cells [26]. DCs were identified originally in lymphoid tissues but were isolated subsequently from peripheral organs, such as the gut lamina propria [27], again clearly distinguished from active phagocytes/macrophages. The literature became confused when it was discovered that monocytes could differentiate into DCs [28] and that APC activity could be elicited in active phagocytes grown from bone marrow or peripheral blood monocytes in the hematopoietic growth factor, GM-CSF [29]. It was further confused when the DC came to be defined in the mouse as any cell that expressed the surface marker CD11c [30]. What is now generally accepted is that the nonphagocytic DC identified by Steinman and Cohn [24] and the tissue macrophage shared a common, committed progenitor in the bone marrow [4], and they share responsiveness to CSF-1. The Steinman-Cohn DC, unlike the macrophage, retains expression of the alternative growth factor receptor Flt3, which is also expressed upon pluripotent hematopoietic progenitor cells. Consequently, Steinman-Cohn DCs are expanded substantially and selectively in response to Flt3 ligand [4], where CSF-1 expands macrophages and Steinman-Cohn DC populations [31]. Steinman-Cohn DCs most probably seed T cell areas in response to Flt3 ligand, produced constitutively by T lymphocytes. Indirect evidence, based on a lysozyme M-cre-mediated lineage tracer, suggests that Steinman-Cohn DCs have never passed through a mature monocyte intermediate [32]. A CDP has been proposed to arise from a common MDP [33], but the proposed CDP has more in common with the common myeloid progenitor, including expression of Flt3 (see data on www.biogps.org). So, I am more inclined to the view that if it is a real entity, the CDP lies upstream rather than downstream of the MDP. The confusion in the literature evaporates if we accept that classical, monocyte-derived macrophages can also acquire antigen-presenting function for naïve T cells [25, 26], and the only clear functional and differentiation dichotomy is between phagocytic (macrophages) and non (or much less)-phagocytic APCs (Steinman-Cohn DC). Some of the transcriptional data in support of this view will be considered below. A substantial proportion of the actively phagocytic, resident tissue macrophages associated with epithelia/mucosal surfaces expresses class II MHC and are able to stimulate naïve T cells, sometimes more directed toward tolerance/suppression [34].
TRANSCRIPTIONAL REGULATION IN THE MONONUCLEAR PHAGOCYTE SYSTEM
Studies of transcriptional regulation in cells of the mononuclear phagocyte system began in the early 1980s. Such studies were constrained significantly in the first instance by the fact that macrophages recognize and respond to bacterial DNA. Classical promoter transfection studies were difficult to achieve, because primary macrophages undergo apoptosis in response to DNA introduced into the cytoplasm [35]. Only recently, the receptor responsible for the response, AIM2, was identified by a number of groups. including my own [36]. This constraint was overcome by the use of a mouse macrophage cell line, RAW264, which is resistant to apoptosis probably because of its transformation with the v-abl oncogene. However, this cell line, in common with primary mouse macrophages, is still activated by bacterial DNA through the activation of the TLR9 receptor [37]. So, the act of transfection in mouse macrophages intrinsically alters gene expression. Interestingly, this is a mouse-specific phenomenon; human macrophages do not express TLR9 [38].
The understanding of macrophage differentiation and transcriptional regulation gained major impetus with the cloning of the transcription factor PU.1 (or Spi-1 or sfpi1). A member of the Ets transcription factor family and named for its binding to purine-rich sequence motifs, PU.1 was found to be restricted in its expression to macrophages and B lymphocytes [39]. PU.1 was identified as a regulator of the Ig transcription, in partnership with another factor, subsequently identified as IRF4, in a manner that was dependent on phosphorylation [40, 41]. In B cells, its function is partly redundant with the closely related transcription factor Spi-B, which is absent from macrophages [42, 43]. Ross et al. [44] showed shortly after the original cloning of PU.1 that the level of PU.1 mRNA is substantially higher in macrophages than B cells. The level of nuclear PU.1 protein detected by electrophoretic mobility shift assay was also a great deal higher in macrophages, and macrophage nuclei contained reproducible, high levels of PU.1 proteolytic cleavage products that retained DNA binding activity. Overexpression of PU.1 in B cells was able to suppress Ig enhancer activity. Later studies emphasized the view that high expression of PU.1 was essential to macrophage lineage commitment and macrophage-specific gene expression [45].
PU.1 has two apparent functions in macrophage transcriptional regulation. First, a specific subset of promoters that is active in macrophages, exemplified by that of the csf1r gene, lacks a TATA box or a CpG island and instead, contains repeats of a purine-rich motif that binds PU.1. Purine-rich motifs alone can generate transcription initiation in macrophages, but PU.1 must act in concert with another member of the Ets transcription factor family, such as Ets2, to achieve maximal transcription [46]. To initiate transcription on such promoters and to specify the transcription start site, PU.1 cooperates with Ewing sarcoma protein [47]. Genome-scale chromatin immunoprecipitation analysis to identify PU.1 binding sites and the dynamic regulation of PU.1 binding has revealed that the major function of this transcription factor is to generate open chromatin around enhancers that are constitutively or potentially activated in cells of the macrophage lineage and that can subsequently be occupied by other transcription factors [48, 49]. The role of PU.1 in chromatin remodeling has been reviewed by Lawrence and Natoli [50].
With the major focus on PU.1 in the literature, one could be forgiven for concluding that PU.1 is the only factor needed to consider understanding macrophage differentiation. However, there are many open questions. First, the phenotype of the PU.1 knockout, which certainly reduces greatly the numbers of all mature cells of the mononuclear phagocyte lineage, macrophages, DCs, and osteoclasts, as well as other myeloid cells [51], depends on mouse genetic background [52]. On at least some genetic backgrounds, PU.1 is not detected on yolk-sac-derived phagocytes in the developing mouse embryo, and these cells still express Csf1r in a PU.1−/− mouse [12]. Secondly, the PU.1 locus itself has a purine-rich TATA-less promoter, and at some point in myeloid lineage commitment, PU.1 must itself be transcriptionally activated. Recent studies have characterized functional enhancers in the PU.1 locus and defined a positive feed-forward amplification by PU.1 and roles for other factors, CEBPa and runt-related transcription factor 1 [53]. As noted above, PU.1 collaborates with other Ets factors on the Csf1r promoter. Some time ago, we screened CSF-1-stimulated bone marrow-derived macrophages for expression of the 29 members of the Ets family (unpublished results). The data are largely confirmed within the large dataset available on www.biogps.org. In summary, PU.1 (Spi-1), Ets2, ETV3, ETV5, Spi-C, Fli1, GA-binding proptein α, ELF1, ELF2, ELF4, Ets repressor factor, Elk1, and ELK3 were all detected at substantial levels in macrophages. The BioGPS data set also contains well-validated hematopoietic stem cells, and within these cells, Fli1, ETV6, and ERG appear as candidates for regulators of purine-rich promoters. Fli1 and ERG were able to transactivate the csf1r promoter in transient transfections of RAW264 cells (unpublished results). Hoogenkamp et al. [54] reported that Fli1 can occupy PU.1 sites and partly compensate for the absence of PU.1 in myeloid differentiation. The transcription factor Ets2 is a key part of the macrophage differentiation cascade, being a direct target of the CSF-1R signaling pathway that leads to phosphorylation of the pointed domain [55, 56]. So, the purine element recognition network is really rather more complex than just PU.1, and as we will discuss below, PU.1/purine-rich elements do not act in isolation.
MACROPHAGE ACTIVATION
Monocytes are recruited from the blood or expanded locally in response to numerous challenges, including normal turnover during development, infections, wounds, and stresses and tumors. The phenotype of the cells recruited adapts to the demands of the situation with distinct patterns of gene regulation and expression. Monocytes in peripheral blood have been subdivided into subsets based on certain surface markers [57]. This is probably much more of a continuum of maturation with time in response to CSF-1 [58], and the less-mature cells (ly6C+ in mouse, CD14 hi/CD16 lo in human) are more likely to be recruited in inflammation. A number of groups have advocated subclassification of the activation states seen in recruited macrophages, broadly into M1 and M2, or classically activated and alternatively activated, respectively [1, 59, 60]. The M1 and M2 nomenclature links the state of activation of the macrophages to the activation of Th1 and Th2 lymphocytes, which in turn, links them to the actions of IFN-γ (originally known as macrophage-activating factor [61]) and IL-4. Broadly speaking, classical activation or M1 phenotypes are associated with the defense against bacterial pathogens and the acquisition of microbicidal activity. Classically activated macrophages also express high levels of class II MHC and present antigen to T lymphocytes. Some therefore chose to call them Tip-DC [62], although as noted in a recent review [25], no one has ever separately identified Tip-DC and M1 macrophages in the same location. M2, or alternatively activated, macrophages appear most prevalent in parasite infections and tumors and are commonly defined through expression of known targets of IL-4 signaling [50]. Interestingly, they may derive in part from local proliferation of resident macrophages rather than monocyte recruitment [63].
The classifications of macrophages invite subtle subclassification [64]. The activation state varies depending on the precise stimulus and also varies with time during the inflammatory process, as one proceeds from acute to chronic phase or resolution. Others have confused matters further by considering cells grown in GM-CSF to be M1-like and those grown in CSF-1 to be M2-like [50]. Different activation states may be associated with the actions of distinct transcription factors, notably, members of the IRF transcription factor family; STAT1 and IRF5 collaborate to induce M1 polarization, whereas STAT6 and IRF4 interact to polarize toward M2 [50].
The M1/M2 nomenclature is really somewhat indefensible. There is really not a binary divide. Other inducible transcription factors (peroxisome proliferator-activated receptor-γ, TFEC, CEBPb, CREB) appear to be recruited in different tissues, in tumors, and in tissue repair [50]. The most sustainable view in my opinion sees the spectrum of macrophage phenotypes much like the spectrum of colors on a color wheel [65]. One might identify pure “red”, “yellow”, and “blue” macrophages in extreme situations, but every combination and shade is possible, and they can be interconverted. At the single-cell level, there is also evidence of stochastic drivers of transcriptional regulation that ensure that every single macrophage has a unique gene-expression profile [66]. That may actually ensure that each cell provides a unique challenge to a pathogen. It also ensures that the number of “subsets” that can be defined is infinite—a function of the number of markers. Not surprisingly, as there is a powerful evolutionary selection by pathogens, there is also considerable variation in the gene-expression profiles, especially of regulated genes, between mouse strains [67] and especially between species. When one comes down to detail, the gene-expression profiles of “activated” mouse and human macrophages are very different [68].
DYNAMIC NETWORKS IN MACROPHAGE DIFFERENTIATION AND ACTIVATION
It is not the purpose of the review to document every transcription factor that is active in macrophages and the way that they interact. The very brief consideration of the biology of PU.1 serves to illustrate the fact that to paraphrase John Donne, “No transcription factor is an island, entire of itself, every one is a piece of a continent”. Each transcription factor must itself be regulated at the transcriptional (and post-translational) level. The large majority also engages in numerous protein–protein interactions to generate novel complexes [69]. In the case of PU.1, aside from the many related Ets factors that can bind to similar DNA motifs, there are numerous other interaction partners. A current search of the interaction database iHOP (www.ihop-net.org) reveals at least 42 other transcription factors that can interact directly with PU.1, and that list is expanding.
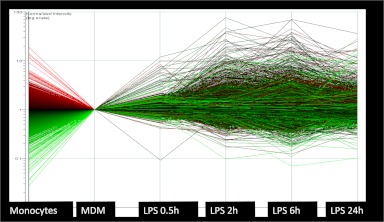
To understand how transcriptional regulation is achieved in macrophages or any other system, it is worthwhile to start to look at the entire system. Looking at one gene at a time can be rather like gazing at the individual brush strokes of an impressionist painting. System-wide views of transcriptional networks have been greatly expedited by the advent of genome-scale technologies. Figure 1 shows a microarray profile of human monocyte-derived macrophages responding to the TLR4 agonist LPS. It illustrates a number of features that are common to many dynamic gene regulation responses.
Figure 1. Dynamic regulation of gene expression in macrophages.
This figure shows unpublished data that formed the basis for initial identification of LPS-inducible genes in human monocytes [68]. It is shown solely to illustrate the pattern seen in a global analysis of macrophage biology. In this experiment, human blood monocytes were first differentiated in CSF-1 for 7 days to generate MDMs and then stimulated for the times indicated with a maximal dose of bacterial LPS. Gene expression for the whole time course was normalized to one in the MDM; curves for genes that were down-regulated during differentiation are colored red, and those that are up-regulated are colored green. The notable features are the very large dynamic range and extent of regulation and the apparent balance between activation and repression. Note also that there is no consistent pattern for the genes coded red and green in their subsequent response to LPS.
There is a sequential cascade of gene regulation. In this case, the early response genes, which include a number of classical inflammatory cytokines, such as TNF-α, have been shown to be subject to regulation primarily at the level of transcription elongation from poised RNA polymerase II complexes [70, 71]. Later, response genes are regulated by autocrine factors, including TNF, and regulated transcription factors.
The numbers and magnitudes of regulation/expression of induced genes are balanced by transcriptional repression of other genes. In a sense, this is obvious, as the total amount of mRNA/cell does not change radically, but the overall mRNA abundance profile is maintained. In fact, analysis of very large numbers of CAGE tag libraries (genome-scale 5′-RACE) [72, 73] demonstrated that individual transcript abundance follows a power law relationship with an exponent that is relatively constant even between cell types [74]. Relatively few genes in any cell are very highly expressed at any time. So, high-level gene induction must be balanced by repression. This is not simply a matter of attrition. Among the repressed genes are numerous transcriptional repressors that would otherwise block the response to LPS and genes involved in other pathways.
Many of the early response genes, both induced and repressed, return to the prestimulation level with time; the response is in some measure self-limiting, even in the continued presence of the agonist. Among the LPS-inducible genes are numerous additional feedback regulators, which we have referred to as “inflammation suppressor genes” [75].
In broad terms, we might think of the control of macrophage activation like an automatic vehicle, in which forward progress requires the release of the brake as well as the application of the accelerator and in which the accelerator links back to the reapplication of the brake.
A number of groups have analyzed the transcriptional cascade of macrophage activation by LPS [48, 50, 76, 77]. The signaling pathway from the receptor TLR4, leading to transcriptional activation, has been described in considerable detail; the major focus has been on the transcription factor complex NF-κB and on the IRFs, which are induced, in part, by autocrine IFN-β [78]. Motif over-representation among LPS-inducible promoters led to identification of the transcription factor ATF3 as a key inducible feedback regulator of the response to LPS [76]. They also identified the stress response factors NRF1 and NRF2 as candidate regulators, based on the presence of binding site motifs in active promoters [77]. However, most importantly, the data revealed that some two-thirds of annotated transcription factors can be expressed in primary macrophages, and if one assesses their potential interactions, the system has the characteristics of a scale-free or small-world network [77]. A relatively small number of expressed transcription factors are very highly connected to others, whereas others occupy peripheral niches within the network with relatively few direct connections. Nevertheless, every transcription factor in the network is connected in some way to every other factor via multiple paths. So, the system, in this case, macrophage activation, will respond in some way to perturbation of any of the components of the network. No component can be truly redundant. A more recent study by Amit et al. [79] on the response of macrophages to LPS confirmed this prediction by systematically perturbing candidate regulators using lentiviral tranduction of short hairpin RNAs. This study also identified a number of regulators, including circadian clock genes, which could not be inferred from motif analysis. The study of regulatory networks of human and mouse macrophages responding to LPS was expedited by the availability in both species of CAGE data that precisely defined transcription start sites so that we were able to look at DNA sequence motif over-representation in the promoters of LPS-inducible genes in both species, including the numerous genes that were induced in one species and not the other [68]. The list of motifs over-represented in the promoters and their relative over-representation was identical between the two species, suggesting that in both species, the promoters sample a common transcriptional milieu. In essence, the network architecture is conserved. However, a pairwise comparison of individual promoters revealed that there was very little conservation of individual elements between the species, although we did not specify direct alignment. This is consistent with a model in which gain and loss of individual motifs, including the TATA box, are significant drivers of evolution, and few individual motifs/binding sites have indispensible functions. Those data also support the model in which each transcription factor binding site contributes independently to the probability of transcription [80].
Genome-scale technologies were used in a tour de force study of a model system of macrophage differentiation, using the induced differentiation of the THP-1 human cell line in response to phorbol ester [81]. The study combined the use of CAGE to identify all of the active promoters and their dynamic regulation across time, quantitative RT-PCR of all predicted transcription factors, and knockdown of a substantial set of candidate regulators. Of a curated list of ∼1300 candidate transcription factors, 610 were detected by CAGE and microarray at some time-point during differentiation, ∼100 distinguished the immature from the mature cells, and another 100 were regulated transiently during the state transition. So, as with the activation of macrophages by LPS, this system involved the dynamic regulation of numerous transcription factors in a sequential cascade. Although PU.1 occupied a central position in the network, it was clearly not the only driver, and it was also evident that subsets of macrophage-expressed genes required different combinations of transcriptional regulators. Again, in common with the response of macrophages to LPS, there were also many genes down-regulated during the differentiation of THP-1 cells, including many transcription factors. To assess the importance of individual factors and especially their down-regulation, 52 of the factors were knocked down using small interfering RNA. Knockdowns of myb, Homeobox A9, CEBPG, growth factor-independent 1 transcription repressor, CEBPA, FLI1, and mixed lineage leukemia translocated to 3 were each sufficient of themselves to cause partial differentiation of the THP-1 cells. Additional double knockdowns indicated that this was not a result of the factors operating in a common pathway; each had an independent function. Several of these factors have well-documented repressive roles. The function of myb, in particular, reflects its down-regulation from high levels in progenitor cells (see www.biogps.org) and its ability to repress macrophage differentiation and to directly repress expression of the csf1r promoter [82]. Myb is most likely also a direct repressor of PU.1 expression, as PU.1 was rapidly induced upon myb knockdown [81].
CLUSTER ANALYSIS OF LARGE MICROARRAY DATASETS IDENTIFIES MACROPHAGE-SPECIFIC GENES AND CHARACTERIZES TRANSCRIPTION STATES
The advent of microarray technologies has made it possible to characterize populations of cells based on shared expression of large sets of genes rather than individual markers. This has had a particular impact on macrophage biology. The Symatlas initiative, which assembled high-quality microarray data sets from a large variety of different cell types [83] pioneered a number of similar initiatives, some of which have been more focused on cells of the immune system (e.g., www.immgen.org). The mouse component of Symatlas, now available on www.biogps.org, includes a very wide range of leukocyte populations, including macrophages in various states of activation, microglia, and purified Steinman-Cohn DCs from lymphoid tissues (including CD8-positive and -negative and pDCs). The purified Steinman-Cohn DCs display the expected expression of known genes, including the putative marker CD11c, which is also expressed in inflammatory macrophages and NK cells. In an initial application of this powerful data set, we identified the set of GPCRs that are expressed in macrophages [84].
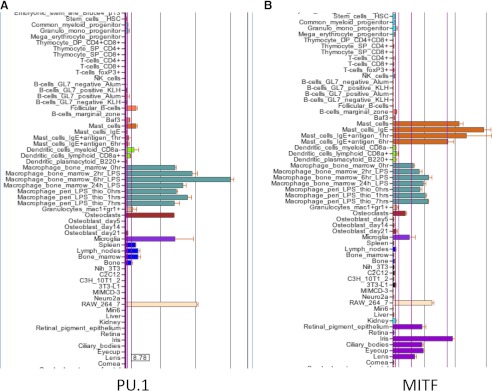
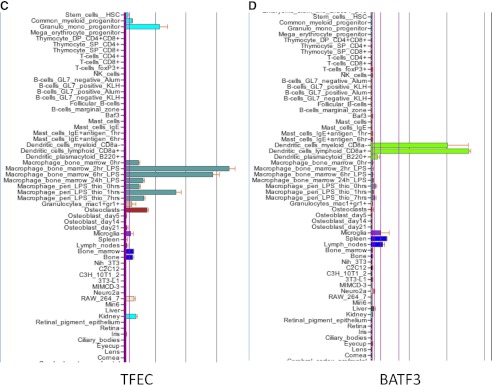
We subsequently used the network clustering tool, BioLayout3D to group transcripts based on their correlated coexpression in the primary cells from this very large dataset [85]. Among the largest clusters was a set of genes that is shared by all of the phagocytes (including osteoclasts) and almost absent from the nonphagocytic Steinman-Cohn DCs, as well as most of the other cell types represented. This cluster was strongly, internally validated by the presence of the large majority of known genes associated with lysosomes and included the transcription factors, such as PU.1 and CEBPα and -β, which likely contribute to transcriptional control. We analyzed the promoters of these phagocyte-restricted genes, and in common with a study from humans of the promoter of known lysosomal proteins [86], we identified, in addition to purine-rich motifs, the recognition motifs for basic helix-loop-helix transcription factors of the microphthalmia transcription factor family—MITF, TFEB, TFEC, and TFE3. We had previously shown that all four MITF family members are expressed in macrophages, that TFEC is a macrophage-specific transcription factor and a PU.1 target gene [87], that MITF interacts physically and genetically with PU.1 [52], and that MITF can transactivate the promoter of the acid phosphatase 5 lysosomal enzyme [52]. But MITF does not appear as one of the transcription factors in the phagocyte gene cluster. Figure 2 shows screen shots of the BioGPS profiles for PU.1, MITF, and TFEC. The profiles show why MITF does not appear to be phagocyte-restricted. Although MITF is expressed at considerably higher levels in all of the phagocytes than it is in any of the DC populations, it is also expressed in mast cells and pigmented cells (retinal pigment epithelium). In both of these sites, MITF is actually transcribed from a separate promoter [73], so one mRNA isoform is, in fact, phagocyte-restricted. TFE3, a binding partner for MITF, is also differentially overexpressed in the macrophages relative to the Steinman-Cohn DCs on BioGPS, and CEBPa and CEBPb also distinguish the macrophages from the DCs on the BioGPS profiles (not shown). So, one might conclude that PU.1, the MITF family, and CEBP families are sufficient to distinguish the phagocytes from their nonphagocytic cousins, the Steinman-Cohn DCs.
Figure 2. Expression of key myeloid transcription factors.
The images are screen shots of part of the display on the website www.biogps.org for the transcription factors indicated. Note that PU.1 is most highly expressed in the macrophages, osteoclasts, isolated microglia, and the macrophage line RAW264 and much lower in granulocytes, B cells, and DCs. MITF is shared by macrophages, mast cells, and retinal pigment epithelium. The MITF-related transcription factor TFEC is also macrophage-restricted, except for a previously unreported expression in myeloid progenitors. BATF3 is expressed at high levels only in the myeloid DCs.
Steinman-Cohn DCs are not distinguished from macrophages solely by what they do not express. Belz and Nutt [88] have reviewed transcriptional control in the development of Steinman-Cohn DCs, and the BioGPS data support/predict some of the biology. The transcriptional repressor BATF3, which binds to the transcription factor c-jun, is very strongly DC-restricted (Fig. 2). The binding partner of BATF3, c-jun, is required for macrophage viability [89]. Many of its other dimerization partners and other members of the bZIP family (ATF2, ATF3, ATF4, junB, and fra2) are also highly expressed in macrophages and absent from the DCs on the BioGPS data (not shown). Other members of the b-ZIP family, c-maf and mafB, bind to related motifs (an extended AP1 site) and are also very strongly expressed in macrophages relative to Steinman-Cohn DCs, and these factors also have been implicated in macrophage differentiation [51]. So, the BATF3 factor probably acts, in part, to oppose phagocyte/macrophage gene expression. Of the other factors highlighted by Belz and Nutt [88], IRF8 and transcription factor 4, are indeed overexpressed selectively by pDCs but do not clearly distinguish these cells from macrophages (not shown). Among the STAT family, STAT1, -2, -4, -5A/B, and -6, all are substantially higher in macrophages than Steinman-Cohn DCs, and among the IRFs, IRF1, -5, and -7, all are substantially higher in macrophages. Of course, all of these factors regulate each other and other factors. NRF1 and -2 also are both much higher in macrophages than Steinman-Cohn DCs, and these two factors can also dimerize with Maf transcription factors [90]. The take-home message is that each cellular state is metastable, involving a complex interacting network of factors, none of which can really be viewed as a master regulator. Essentially, similar conclusions have come from an exhaustive analysis of 38 human hematopoietic cell populations [91].
WHAT DOES TRANSCRIPTIONAL CLUSTERING TELL US ABOUT LINEAGE MARKERS?
The other important fact that emerges from the clustering analysis is that very few of the candidate surface markers that are commonly used to purify cell types are actually contained within a cluster. As noted above, CD11c is clearly not a DC marker [92]. When surface proteins have a clear function, or a common key regulator, they tend to be associated with cell lineage-specific or functional clusters. Hence, siglecH is, as expected from its function, present in a pDC-specific cluster [85]. The macrophage marker, F4/80 (EGF module-containing mucin-like receptor 1), is in a very small cluster with c-maf and MafB, both of which are required for expression of the gene [93] but separate from the phagocyte cluster, as F4/80 is absent from osteoclasts (and from several tissue macrophage populations) and present in myeloid DCs. Class II MHC genes, required for antigen presentation, do not correlate with any other surface marker but do correlate with genes required for antigen processing and with the known regulator, class II MHC transactivator. There is actually no Steinman-Cohn DC-specific cluster. These cells are largely defined by the fact that they share some myeloid markers with macrophages, but they are not phagocytes. They certainly differ from macrophages in expressing high levels of Flt3, but this marker is expressed equally in hematopoietic progenitor cells. The conclusion one must reach is that the knowledge of the presence of a surface marker on a cell has no predictive value in terms of the likely expression of other genes in the same cell, including other surface markers. The lack of clear correlation means that the number of subpopulations of mononuclear phagocytes that can be defined using multiple markers is an exponential function of the number of markers examined. In the end, each macrophage is unique.
The current microarray platforms are now sufficiently reproducible so that one can actually integrate datasets from independent laboratories, and it is not necessary to do it all in one laboratory. We recently performed a clustering meta-analysis that integrated the BioGPS data with numerous quality datasets downloaded from the National Center for Biotechnology Information Gene Expression Omnibus repository. These profiles can be used to determine the relatedness of different populations of cells based on their transcription profiles. The analysis confirmed the clear separation of Steinman-Cohn DCs from all independent macrophage profiles but grouped the so-called DCs generated by cultivation in GM-CSF very clearly with the macrophages [94]. The datasets used in this analysis included macrophage populations polarized with different stimuli, but the clustering failed to identify any set of genes equivalent to the proposed markers of the M1 (classical) or M2 (alternative) activation, suggesting that this classification is also something of an illusion. As with DCs becoming synonymous with APCs, the “alternatively activated” macrophage in the mouse has come to be conflated with IL-4-stimulated macrophage. For example, a very interesting, recent study documents the effect of “alternatively activated macrophages” in adaptive thermogenesis, when they are actually studying IL-4-inducible target genes [95]. Interestingly, the macrophage-specific transcription factor TFEC appears to have a specific function in the inducible expression of a subset of IL-4-response genes [96].
DATABASES, WEBSITES, AND THE FUTURE
The escalating amount of data on mononuclear phagocyte biology coming from genome-scale technologies now taxes the capacity of any individual to access all of the useful information about the regulation of his or her favorite gene. Table 1 summarizes some of the more useful portals for finding macrophage-related data. BioGPS is one example of a new era of more user-friendly portals. We have established the website www.macrophages.com as a community website for sharing access to macrophage-related genomic and other information [97], including the massive promoter-related datasets arising from the FANTOM projects, in which mouse and human macrophage systems were analyzed heavily. The latest phase of this project FANTOM5 will provide a comprehensive overview of the human promoterome and includes human mononuclear phagocytes analyzed in hundreds of different states. This website (www.macrophages.com) also provides macrophage-related pathway annotation data and links to the growing InnateDB (www.innateDB.org), which curates molecular interactions among macrophage-expressed proteins. As noted above, alongside this information, there is already significant baseline data on the chromatin state of the genome in mature macrophages, and these data will continue to expand with ChIP-Seq for more factors in more states. There is a need to integrate this kind of data into a common viewer, based on the convenient University of California Santa Cruz genome browser format (UCSC Genome Bioinformatics). The website also contains a curated compendium of major reviews on macrophage biology and transcriptional regulation, many of which provide much more comprehensive coverage of subtopics of this brief oversight.
Table 1. Useful Sources of Data Related to Macrophage Biology.
| Website | Description |
|---|---|
| www.macrophages.com | Transcriptomic data linked to FANTOM projects. Microarray datasets. Curated database of reviews on macrophage biology. Images and movies. |
| www.lsbm.org | Database of microarray datasets on human tissues and cells, including monocytes and monocyte-derived macrophages. |
| www.biogps.org | Database of microarray datasets from mouse, human, and pig, including macrophages in various states of activation. Correlated expression function enables identification of genes with similar expression. |
| fantom.gsc.riken.jp | Databases containing transcriptomic analysis from the FANTOM projects, includes identification of active promoters in mouse and human macrophages in a wide range of conditions (FANTOM3 and [hyphen]5) and time course analysis of differentiation of THP-1 cells (FANTOM4). |
| www.immgen.org | Immunological Genome Project contains a compendium of microarray expression profiles in the mouse, including myeloid cells in many different states of activation. |
| www.innatedb.org | Database of the genes, proteins, experimentally verified interactions, and signaling pathways involved in the innate immune response of humans, mice, and bovines to microbial infection. |
| db.systemsbiology.net/IIDB | Database of predicted transcription factor binding sites and microarray coexpression clusters of mouse macrophages responding to TLR ligands. |
| www.ihop-net.org | A gene network prediction database based on coincidence of gene names in the literature. Summary overview gives tables of coincidence. |
| www.ncbi.nlm.nih.gov/geoprofiles | A large database of published microarray expression profiles. A search on gene name and macrophage provides access to several hundred datasets. |
| www.macgate.qfab.org | Database containing data from a comparative analysis of the response of mouse and human macrophages to LPS. |
| www.emouseatlas.org | Edinburgh mouse atlas. Localization of genes in developing embryo by in situ hybridization enables phenotyping of fetal macrophages. |
So much macrophage biology has been carried out on the mouse, with limited crossover to human systems. Comparative analyses have revealed some degree of orthology in gene-expression profiles among the major monocyte subsets in the two species [98] and substantial discordance among the sets of inducible genes [68]. With completed genomes, we are seeing comparable datasets available for other species, including the domestic pig [99] that will underpin more rigorous studies of the evolution of innate immunity and also the recognition that there is very substantial genetic variation within species that underlies disease susceptibility loci (a subject too large to review herein). We are in the era of the data deluge.
ACKNOWLEDGMENTS
The Roslin Institute is supported by Institute Strategic Programme grants from the Biotechnology and Biological Sciences Research Council. I am grateful for the support and input of the current and past members of my laboratory (especially Matt Sweet, Kate Schroder, and Kate Irvine) and from Roslin colleagues (especially Tom Freeman and Kim Summers). This review is based on a plenary talk presented at the Society for Leukocyte Biology Meeting in Kansas City in 2011. It is therefore a personal perspective, focused predominantly on work from my group and from consortia with whom I have worked. That focus is not intended to detract from the work of numerous respected colleagues in the field of macrophage biology. A curated database of major reviews in the area of macrophage biology is available on www.macrophages.com.
Footnotes
- ATF
- activating transcription factor
- BATF
- basic leucine zipper transcription factor
- bZIP
- basic leucine zipper domain
- CAGE
- Cap analysis gene expression
- CDP
- common DC progenitor
- CEBPG/A/b
- C/EBP γ/α/β
- ELF
- E74-like factor
- ELK
- E26-like transcription factor
- Fli1
- friend leukemia integration 1 transcription factor
- Flt3
- fms-like tyrosine kinase 3
- IRF
- IFN regulatory factor
- MDP
- macrophage-DC progenitor
- MITF
- microphthalmia-associated transcription factor
- MPS
- mononuclear phagocyte system
- NRF
- nuclear respiratory factor
- pDC
- plasmacytoid DC
- TFEC/-B/-3
- transcription factor EC/EB/E3
- Tip-DC
- TNF, iNOS-producing DC
REFERENCES
- 1. Gordon S., Taylor P. R. (2005) Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 [DOI] [PubMed] [Google Scholar]
- 2. Hume D. A. (2006) The mononuclear phagocyte system. Curr. Opin. Immunol. 18, 49–53 [DOI] [PubMed] [Google Scholar]
- 3. Hume D. A. (2008) Differentiation and heterogeneity in the mononuclear phagocyte system. Mucosal Immunol. 1, 432–441 [DOI] [PubMed] [Google Scholar]
- 4. Geissmann F., Manz M. G., Jung S., Sieweke M. H., Merad M., Ley K. (2010) Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hume D. A., Ross I. L., Himes S. R., Sasmono R. T., Wells C. A., Ravasi T. (2002) The mononuclear phagocyte system revisited. J. Leukoc. Biol. 72, 621–627 [PubMed] [Google Scholar]
- 6. Sasmono R. T., Ehrnsperger A., Cronau S. L., Ravasi T., Kandane R., Hickey M. J., Cook A. D., Himes S. R., Hamilton J. A., Hume D. A. (2007) Mouse neutrophilic granulocytes express mRNA encoding the macrophage colony-stimulating factor receptor (CSF-1R) as well as many other macrophage-specific transcripts and can transdifferentiate into macrophages in vitro in response to CSF-1. J. Leukoc. Biol. 82, 111–123 [DOI] [PubMed] [Google Scholar]
- 7. Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M. F., Conway S. J., Ng L. G., Stanley E. R., Samokhvalov I. M., Merad M. (2012) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chorro L., Sarde A., Li M., Woollard K. J., Chambon P., Malissen B., Kissenpfennig A., Barbaroux J. B., Groves R., Geissmann F. (2009) Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J. Exp. Med. 206, 3089–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoeffel G., Wang Y., Greter M., See P., Teo P., Malleret B., Leboeuf M., Low D., Oller G., Almeida F., Choy S. H., Grisotto M., Renia L., Conway S. J., Stanley E. R., Chan J. K., Ng L. G., Samokhvalov I. M., Merad M., Ginhoux F. (2012) Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 209, 1167–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perry V. H., Hume D. A., Gordon S. (1985) Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience 15, 313–326 [DOI] [PubMed] [Google Scholar]
- 11. Ransohoff R. M., Perry V. H. (2009) Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 27, 119–145 [DOI] [PubMed] [Google Scholar]
- 12. Lichanska A. M., Browne C. M., Henkel G. W., Murphy K. M., Ostrowski M. C., McKercher S. R., Maki R. A., Hume D. A. (1999) Differentiation of the mononuclear phagocyte system during mouse embryogenesis: the role of transcription factor PU.1. Blood 94, 127–138 [PubMed] [Google Scholar]
- 13. Lichanska A. M., Hume D. A. (2000) Origins and functions of phagocytes in the embryo. Exp. Hematol. 28, 601–611 [DOI] [PubMed] [Google Scholar]
- 14. Rybtsov S., Sobiesiak M., Taoudi S., Souilhol C., Senserrich J., Liakhovitskaia A., Ivanovs A., Frampton J., Zhao S., Medvinsky A. (2011) Hierarchical organization and early hematopoietic specification of the developing HSC lineage in the AGM region. J. Exp. Med. 208, 1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schulz C., Gomez E., Perdiguero, Chorro L., Szabo-Rogers H., Cagnard N., Kierdorf K., Prinz M., Wu B., Jacobsen S. E., Pollard J. W., Frampton J. K. J., Liu, Geissmann F. (2012) A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 [DOI] [PubMed] [Google Scholar]
- 16. Chitu V., Stanley E. R. (2006) Colony-stimulating factor-1 in immunity and inflammation. Curr. Opin. Immunol. 18, 39–48 [DOI] [PubMed] [Google Scholar]
- 17. Garceau V., Smith J., Paton I. R., Davey M., Fares M. A., Sester D. P., Burt D. W., Hume D. A. (2010) Pivotal Advance: Avian colony-stimulating factor 1 (CSF-1), interleukin-34 (IL-34), and CSF-1 receptor genes and gene products. J. Leukoc. Biol. 87, 753–764 [DOI] [PubMed] [Google Scholar]
- 18. Ma X., Lin W. Y., Chen Y., Stawicki S., Mukhyala K., Wu Y., Martin F., Bazan J. F., Starovasnik M. A. (2012) Structural basis for the dual recognition of helical cytokines IL-34 and CSF-1 by CSF-1R. Structure 20, 676–687 [DOI] [PubMed] [Google Scholar]
- 19. Liu H., Leo C., Chen X., Wong B. R., Williams L. T., Lin H., He X. (2012) The mechanism of shared but distinct CSF-1R signaling by the non-homologous cytokines IL-34 and CSF-1. Biochim. Biophys. Acta 1824, 938–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Erblich B., Zhu L., Etgen A. M., Dobrenis K., Pollard J. W. (2012) Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS One 6, e26317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pollard J. W. (2009) Trophic macrophages in development and disease. Nat. Rev. Immunol. 9, 259–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nandi S., Gokhan S., Dai X. M., Wei S., Enikolopov G., Lin H., Mehler M. F., Richard Stanley E. (2012) The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev. Biol. 367, 100–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sasmono R. T., Oceandy D. J. W., Pollard, Tong W., Pavli P., Wainwright B. J., Ostrowski M. C., Himes S. R., Hume D. A. (2003) A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood 101, 1155–1163 [DOI] [PubMed] [Google Scholar]
- 24. Steinman R. M., Cohn Z. A. (1973) Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 137, 1142–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Geissmann F., Gordon S., Hume D. A., Mowat A. M., Randolph G. J. (2010) Unravelling mononuclear phagocyte heterogeneity. Nat. Rev. Immunol. 10, 453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hume D. A. (2008) Macrophages as APC and the dendritic cell myth. J. Immunol. 181, 5829–5835 [DOI] [PubMed] [Google Scholar]
- 27. Pavli P., Woodhams C. E., Doe W. F., Hume D. A. (1990) Isolation and characterization of antigen-presenting dendritic cells from the mouse intestinal lamina propria. Immunology 70, 40–47 [PMC free article] [PubMed] [Google Scholar]
- 28. Randolph G. J., Beaulieu S., Lebecque S., Steinman R. M., Muller W. A. (1998) Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science 282, 480–483 [DOI] [PubMed] [Google Scholar]
- 29. Inaba K., Inaba M., Romani N., Aya H., Deguchi M., Ikehara S., Muramatsu S., Steinman R. M. (1992) Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176, 1693–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jung S., Unutmaz D., Wong P., Sano G., De, los Santos K., Sparwasser T., Wu S., Vuthoori S., Ko K., Zavala F., Pamer E. G., Littman D. R., Lang R. A. (2002) In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17, 211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. MacDonald K. P., Rowe V., Bofinger H. M., Thomas R., Sasmono T., Hume D. A., Hill G. R. (2005) The colony-stimulating factor 1 receptor is expressed on dendritic cells during differentiation and regulates their expansion. J. Immunol. 175, 1399–1405 [DOI] [PubMed] [Google Scholar]
- 32. Jakubzick C., Bogunovic M., Bonito A. J., Kuan E. L., Merad M., Randolph G. J. (2008) Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J. Exp. Med. 205, 2839–2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Auffray C., Fogg D. K., Narni-Mancinelli E., Senechal B., Trouillet C., Saederup N., Leemput J., Bigot K., Campisi L., Abitbol M., Molina T., Charo I., Hume D. A., Cumano A., Lauvau G., Geissmann F. (2009) CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J. Exp. Med. 206, 595–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Denning T. L., Wang Y. C., Patel S. R., Williams I. R., Pulendran B. (2007) Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 8, 1086–1094 [DOI] [PubMed] [Google Scholar]
- 35. Stacey K. J., Ross I. L., Hume D. A. (1993) Electroporation and DNA-dependent cell death in murine macrophages. Immunol. Cell. Biol. 71, 75–85 [DOI] [PubMed] [Google Scholar]
- 36. Roberts T. L., Idris A., Dunn J. A., Kelly G. M., Burnton C. M., Hodgson S., Hardy L. L., Garceau V., Sweet M. J., Ross I. L., Hume D. A., Stacey K. J. (2009) HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323, 1057–1060 [DOI] [PubMed] [Google Scholar]
- 37. Stacey K. J., Sweet M. J., Hume D. A. (1996) Macrophages ingest and are activated by bacterial DNA. J. Immunol. 157, 2116–2122 [PubMed] [Google Scholar]
- 38. Schroder K., Lichtinger M., Irvine K. M., Brion K., Trieu A., Ross I. L., Ravasi T., Stacey K. J., Rehli M., Hume D. A., Sweet M. J. (2007) PU.1 and ICSBP control constitutive and IFN-γ-regulated Tlr9 gene expression in mouse macrophages. J. Leukoc. Biol. 81, 1577–1590 [DOI] [PubMed] [Google Scholar]
- 39. Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A. (1990) The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell 61, 113–124 [DOI] [PubMed] [Google Scholar]
- 40. Pongubala J. M., Van Beveren C., Nagulapalli S., Klemsz M. J., McKercher S. R., Maki R. A., Atchison M. L. (1993) Effect of PU.1 phosphorylation on interaction with NF-EM5 and transcriptional activation. Science 259, 1622–1625 [DOI] [PubMed] [Google Scholar]
- 41. Pongubala J. M., Nagulapalli S., Klemsz M. J., McKercher S. R., Maki R. A., Atchison M. L. (1992) PU.1 recruits a second nuclear factor to a site important for immunoglobulin κ 3′ enhancer activity. Mol. Cell. Biol. 12, 368–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sokalski K. M., Li S. K., Welch I., Cadieux-Pitre H. A., Gruca M. R., DeKoter R. P. (2011) Deletion of genes encoding PU.1 and Spi-B in B cells impairs differentiation and induces pre-B cell acute lymphoblastic leukemia. Blood 118, 2801–2808 [DOI] [PubMed] [Google Scholar]
- 43. DeKoter R. P., Geadah M., Khoosal S., Xu L. S., Thillainadesan G., Torchia J., Chin S. S., Garrett-Sinha L. A. (2011) Regulation of follicular B cell differentiation by the related E26 transformation-specific transcription factors PU.1, Spi-B, and Spi-C. J. Immunol. 185, 7374–7384 [DOI] [PubMed] [Google Scholar]
- 44. Ross I. L., Dunn T. L., Yue X., Roy S., Barnett C. J., Hume D. A. (1994) Comparison of the expression and function of the transcription factor PU.1 (Spi-1 proto-oncogene) between murine macrophages and B lymphocytes. Oncogene 9, 121–132 [PubMed] [Google Scholar]
- 45. DeKoter R. P., Singh H. (2000) Regulation of B lymphocyte and macrophage development by graded expression of PU.1. Science 288, 1439–1441 [DOI] [PubMed] [Google Scholar]
- 46. Ross I. L., Yue X., Ostrowski M. C., Hume D. A. (1998) Interaction between PU.1 and another Ets family transcription factor promotes macrophage-specific basal transcription initiation. J. Biol. Chem. 273, 6662–6669 [DOI] [PubMed] [Google Scholar]
- 47. Hume D. A., Sasmono T., Himes S. R., Sharma S. M., Bronisz A., Constantin M., Ostrowski M. C., Ross I. L. (2008) The Ewing sarcoma protein (EWS) binds directly to the proximal elements of the macrophage-specific promoter of the CSF-1 receptor (csf1r) gene. J. Immunol. 180, 6733–6742 [DOI] [PubMed] [Google Scholar]
- 48. Ghisletti S., Barozzi I., Mietton F., Polletti S., De Santa F., Venturini E., Gregory L., Lonie L., Chew A., Wei C. L., Ragoussis J., Natoli G. (2010) Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity 32, 317–328 [DOI] [PubMed] [Google Scholar]
- 49. Heinz S., Benner C., Spann N., Bertolino E., Lin Y. C., Laslo P., Cheng J. X., Murre C., Singh H., Glass C. K. (2010) Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell. 38, 576–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lawrence T., Natoli G. (2011) Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 11, 750–761 [DOI] [PubMed] [Google Scholar]
- 51. Friedman A. D. (2007) Transcriptional control of granulocyte and monocyte development. Oncogene 26, 6816–6828 [DOI] [PubMed] [Google Scholar]
- 52. Luchin A., Suchting S., Merson T., Rosol R. J., Hume D. A., Cassady A. I., Ostrowski M. C. (2001) Genetic and physical interactions between microphthalmia transcription factor and PU.1 are necessary for osteoclast gene expression and differentiation. J. Biol. Chem. 276, 36703–36710 [DOI] [PubMed] [Google Scholar]
- 53. Leddin M., Perrod C., Hoogenkamp M., Ghani S., Assi S., Heinz S., Wilson N. K., Follows G., Schonheit J., Vockentanz L., Mosammam A. M., Chen W., Tenen D. G., Westhead D. R., Gottgens B., Bonifer C., Rosenbauer F. (2011) Two distinct auto-regulatory loops operate at the PU.1 locus in B cells and myeloid cells. Blood 117, 2827–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hoogenkamp M., Krysinska H., Ingram R., Huang G., Barlow R., Clarke D., Ebralidze A., Zhang P., Tagoh H., Cockerill P. N., Tenen D. G., Bonifer C. (2007) The Pu.1 locus is differentially regulated at the level of chromatin structure and noncoding transcription by alternate mechanisms at distinct developmental stages of hematopoiesis. Mol. Cell. Biol. 27, 7425–7438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fowles L. F., Martin M. L., Nelsen L., Stacey K. J., Redd D., Clark Y. M., Nagamine Y., McMahon M., Hume D. A., Ostrowski M. C. (1998) Persistent activation of mitogen-activated protein kinases p42 and p44 and ets-2 phosphorylation in response to colony-stimulating factor 1/c-fms signaling. Mol. Cell. Biol. 18, 5148–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fowles L. F., Stacey K. J., Marks D., Hamilton J. A., Hume D. A. (2000) Regulation of urokinase plasminogen activator gene transcription in the RAW264 murine macrophage cell line by macrophage colony-stimulating factor (CSF-1) is dependent upon the level of cell-surface receptor. Biochem. J. 347, 313–320 [PMC free article] [PubMed] [Google Scholar]
- 57. Geissmann F., Jung S., Littman D. R. (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 [DOI] [PubMed] [Google Scholar]
- 58. Macdonald K. P., Palmer J. S., Cronau S., Seppanen E., Olver S., Raffelt N. C., Kuns R., Pettit A. R., Clouston A., Wainwright B., Branstetter D., Smith J., Paxton R. J., Cerretti D. P., Bonham L., Hill G. R., Hume D. A. (2010) An antibody against the colony-stimulating factor 1 receptor (CSF1R) depletes the resident subset of monocytes and tissue and tumor-associated macrophages but does not inhibit inflammation. Blood 116, 3955–3963 [DOI] [PubMed] [Google Scholar]
- 59. Gordon S., Martinez F. O. (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32, 593–604 [DOI] [PubMed] [Google Scholar]
- 60. Taylor P. R., Gordon S. (2003) Monocyte heterogeneity and innate immunity. Immunity 19, 2–4 [DOI] [PubMed] [Google Scholar]
- 61. Schroder K., Hertzog P. J., Ravasi T., Hume D. A. (2004) Interferon-γ: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75, 163–189 [DOI] [PubMed] [Google Scholar]
- 62. Serbina N. V., Salazar-Mather T. P., Biron C. A., Kuziel W. A., Pamer E. G. (2003) TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19, 59–70 [DOI] [PubMed] [Google Scholar]
- 63. Jenkins S. J., Ruckerl D., Cook P. C., Jones L. H., Finkelman F. D., van Rooijen N., MacDonald A. S., Allen J. E. (2011) Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332, 1284–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Martinez F. O., Sica A., Mantovani A., Locati M. (2008) Macrophage activation and polarization. Front. Biosci. 13, 453–461 [DOI] [PubMed] [Google Scholar]
- 65. Mosser D. M., Edwards J. P. (2008) Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ravasi T., Wells C., Forest A., Underhill D. M., Wainwright B. J., Aderem A., Grimmond S., Hume D. A. (2002) Generation of diversity in the innate immune system: macrophage heterogeneity arises from gene-autonomous transcriptional probability of individual inducible genes. J. Immunol. 168, 44–50 [DOI] [PubMed] [Google Scholar]
- 67. Wells C. A., Ravasi T., Faulkner G. J., Carninci P., Okazaki Y., Hayashizaki Y., Sweet M., Wainwright B. J., Hume D. A. (2003) Genetic control of the innate immune response. BMC Immunol. 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schroder K., Irvine K. M., Taylor M. S., Bokil N. J., Cao K-A. L., Masterman K-A., Labzin L. I., Semple C. A., Kapetanovic R., Fairbairn L.., et al. (2012) Conservation and divergence in Toll-like receptor 4-regulated gene expression in primary human versus mouse macrophages. Proc. Natl. Acad. Sci. USA 109, E944–E953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ravasi T., Suzuki H., Cannistraci C. V., Katayama S., Bajic V. B., Tan K., Akalin A., Schmeier S., Kanamori-Katayama M., Bertin N.., et al. (2009) An atlas of combinatorial transcriptional regulation in mouse and man. Cell 140, 744–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Medzhitov R., Horng T. (2009) Transcriptional control of the inflammatory response. Nat. Rev. Immunol. 9, 692–703 [DOI] [PubMed] [Google Scholar]
- 71. Hargreaves D. C., Horng T., Medzhitov R. (2009) Control of inducible gene expression by signal-dependent transcriptional elongation. Cell 138, 129–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Carninci P., Kasukawa T., Katayama S., Gough J., Frith M. C., Maeda N., Oyama R., Ravasi T., Lenhard B., Wells C.., et al. (2005) The transcriptional landscape of the mammalian genome. Science 309, 1559–1563 [DOI] [PubMed] [Google Scholar]
- 73. Carninci P., Sandelin A., Lenhard B., Katayama S., Shimokawa K., Ponjavic J., Semple C. A., Taylor M. S., Engstrom P. G., Frith M. C.., et al. (2006) Genome-wide analysis of mammalian promoter architecture and evolution. Nat. Genet. 38, 626–635 [DOI] [PubMed] [Google Scholar]
- 74. Balwierz P. J., Carninci P., Daub C. O., Kawai J., Hayashizaki Y., Van Belle W., Beisel C., van Nimwegen E. (2009) Methods for analyzing deep sequencing expression data: constructing the human and mouse promoterome with deepCAGE data. Genome Biol. 10, R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wells C. A., Ravasi T., Hume D. A. (2005) Inflammation suppressor genes: please switch out all the lights. J. Leukoc. Biol. 78, 9–13 [DOI] [PubMed] [Google Scholar]
- 76. Gilchrist M., Thorsson V., Li B., Rust A. G., Korb M., Roach J. C., Kennedy K., Hai T., Bolouri H., Aderem A. (2006) Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature 441, 173–178 [DOI] [PubMed] [Google Scholar]
- 77. Nilsson R., Bajic V. B., Suzuki H., di Bernardo D., Bjorkegren J., Katayama S., Reid J. F., Sweet M. J., Gariboldi M., Carninci P., Hayashizaki Y., Hume D. A., Tegner J., Ravasi T. (2006) Transcriptional network dynamics in macrophage activation. Genomics 88, 133–142 [DOI] [PubMed] [Google Scholar]
- 78. Takeuchi O., Akira S. (2010) Pattern recognition receptors and inflammation. Cell 140, 805–820 [DOI] [PubMed] [Google Scholar]
- 79. Amit I., Garber M., Chevrier N., Leite A. P., Donner Y., Eisenhaure T., Guttman M., Grenier J. K., Li W., Zuk O.., et al. (2009) Unbiased reconstruction of a mammalian transcriptional network mediating pathogen responses. Science 326, 257–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hume D. A. (2000) Probability in transcriptional regulation and its implications for leukocyte differentiation and inducible gene expression. Blood 96, 2323–2328 [PubMed] [Google Scholar]
- 81. Suzuki H., Forrest A. R., van Nimwegen E., Daub C. O., Balwierz P. J., Irvine K. M., Lassmann T., Ravasi T., Hasegawa Y., de Hoon M. J.., et al. (2009) The transcriptional network that controls growth arrest and differentiation in a human myeloid leukemia cell line. Nat. Genet. 41, 553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Reddy M. A., Yang B. S., Yue X., Barnett C. J., Ross I. L., Sweet M. J., Hume D. A., Ostrowski M. C. (1994) Opposing actions of c-ets/PU.1 and c-myb protooncogene products in regulating the macrophage-specific promoters of the human and mouse colony-stimulating factor-1 receptor (c-fms) genes. J. Exp. Med. 180, 2309–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Su A. I., Wiltshire T., Batalov S., Lapp H., Ching K. A., Block D., Zhang J., Soden R., Hayakawa M., Kreiman G., Cooke M. P., Walker J. R., Hogenesch J. B. (2004) A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. USA 101, 6062–6067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lattin J., Zidar D. A., Schroder K., Kellie S., Hume D. A., Sweet M. J. (2007) G-protein-coupled receptor expression, function, and signaling in macrophages. J. Leukoc. Biol. 82, 16–32 [DOI] [PubMed] [Google Scholar]
- 85. Hume D. A., Summers K. M., Raza S., Baillie J. K., Freeman T. C. (2009) Functional clustering and lineage markers: insights into cellular differentiation and gene function from large-scale microarray studies of purified primary cell populations. Genomics 95, 328–338 [DOI] [PubMed] [Google Scholar]
- 86. Settembre C., Di Malta C., Polito V. A., Garcia M., Arencibia, Vetrini F., Erdin S., Erdin S. U., Huynh T., Medina D., Colella P., Sardiello M., Rubinsztein D. C., Ballabio A. (2011) TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rehli M., Lichanska A., Cassady A. I., Ostrowski M. C., Hume D. A. (1999) TFEC is a macrophage-restricted member of the microphthalmia-TFE subfamily of basic helix-loop-helix leucine zipper transcription factors. J. Immunol. 162, 1559–1565 [PubMed] [Google Scholar]
- 88. Belz G. T., Nutt S. L. (2010) Transcriptional programming of the dendritic cell network. Nat. Rev. Immunol. 12, 101–113 [DOI] [PubMed] [Google Scholar]
- 89. Himes S. R., Sester D. P., Ravasi T., Cronau S. L., Sasmono T., Hume D. A. (2006) The JNK are important for development and survival of macrophages. J. Immunol. 176, 2219–2228 [DOI] [PubMed] [Google Scholar]
- 90. Biswas M., Chan J. Y. (2010) Role of Nrf1 in antioxidant response element-mediated gene expression and beyond. Toxicol. Appl. Pharmacol. 244, 16–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Novershtern N., Subramanian A., Lawton L. N., Mak R. H., Haining W. N., McConkey M. E., Habib N., Yosef N., Chang C. Y., Shay T.., et al. (2011) Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell 144, 296–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hume D. A. (2010) Applications of myeloid-specific promoters in transgenic mice support in vivo imaging and functional genomics but do not support the concept of distinct macrophage and dendritic cell lineages or roles in immunity. J. Leukoc. Biol. 89, 525–538 [DOI] [PubMed] [Google Scholar]
- 93. Moriguchi T., Hamada M., Morito N., Terunuma T., Hasegawa K., Zhang C., Yokomizo T., Esaki R., Kuroda E., Yoh K., Kudo T., Nagata M., Greaves D. R., Engel J. D., Yamamoto M., Takahashi S. (2006) MafB is essential for renal development and F4/80 expression in macrophages. Mol. Cell. Biol. 26, 5715–5727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mabbott N., Kenneth A., Baillie J., Hume D. A., Freeman T. C. (2011) Meta-analysis of lineage-specific gene expression signatures in mouse leukocyte populations. Immunobiology 215, 724–736 [DOI] [PubMed] [Google Scholar]
- 95. Nguyen K. D., Qiu Y., Cui X., Goh Y. P., Mwangi J., David T., Mukundan L., Brombacher F., Locksley R. M., Chawla A. (2011) Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480, 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rehli M., Sulzbacher S., Pape S., Ravasi T., Wells C. A., Heinz S., Sollner L., El Chartouni C., Krause S., Steingrimsson E., Hume D. A., Andreesen R. (2005) Transcription factor Tfec contributes to the IL-4-inducible expression of a small group of genes in mouse macrophages including the granulocyte colony-stimulating factor receptor. J. Immunol. 174, 7111–7122 [DOI] [PubMed] [Google Scholar]
- 97. Robert C., Lu X., Law A., Freeman T. C., Hume D. A. (2011) Macrophages.com: an on-line community resource for innate immunity research. Immunobiology 216, 1203–1211 [DOI] [PubMed] [Google Scholar]
- 98. Ingersoll M. A., Spanbroek R., Lottaz C., Gautier E. L., Frankenberger M., Hoffmann R., Lang R., Haniffa M., Collin M., Tacke F., Habenicht A. J., Ziegler-Heitbrock L., Randolph G. J. (2010) Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 115, e10–e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kapetanovic R., Fairbairn L., Beraldi D., Sester D. P., Archibald A. L., Tuggle C. K., Hume D. A. (2012) Pig bone marrow-derived macrophages resemble human macrophages in their response to bacterial lipopolysaccharide. J. Immunol. 188, 3382–3394 [DOI] [PubMed] [Google Scholar]