Summary
Secretion is a fundamental cell process underlying different physiological and pathological events. In cells from the human immune system such as eosinophils, secretion of mediators generally occurs by means of piecemeal degranulation, an unconventional secretory pathway characterized by vesicular transport of small packets of materials from the cytoplasmic secretory granules to the cell surface. During piecemeal degranulation in eosinophils, a distinct transport vesicle system, which includes large, pleiomorphic vesiculotubular carriers is mobilized and enables regulated release of granule-stored proteins such as cytokines and major basic protein. Piecemeal degranulation underlies distinct functions of eosinophils as effector and immunoregulatory cells. This review focuses on the structural and functional advances that have been made over the last years concerning the intracellular trafficking and secretion of eosinophil proteins by piecemeal degranulation during inflammatory responses.
Keywords: Human eosinophils, Vesicle-mediated secretion, Inflammation, Piecemeal degranulation, Electron microscopy, Electron tomography
Introduction
Secretion is an essential biological activity of all eukaryotic cells by which they release specific products in the extracellular space during physiological and pathological events. Most eukaryotic proteins are secreted through the conventional endoplasmic reticulum (ER)-Golgi secretory pathway. The classical picture of the cell secretory pathway includes protein synthesis within the ER, transport of cargo inwards towards the Golgi apparatus and then through the Golgi and trans-Golgi network (TGN) en route to the plasma membrane, all carried by transport vesicles (Watson and Stephens, 2005). In cells from the human immune system such as eosinophils, basophils, neutrophils and mast cells, additional secretory vesicle traffic is active. This secretory pathway is characterized by vesicular transport of small packets of materials from the cytoplasmic secretory granules to the cell surface (Dvorak et al., 1991, 1992; Beil et al., 1993). Termed piecemeal degranulation (PMD) because of a “piece by piece” release of secretory granule contents, this secretory process is now recognized as a central secretion mode during inflammatory responses. In contrast to classical granule exocytosis which involves granule fusion with the plasma membrane and release of the total granule content, piecemeal degranulation enables release of specific granule-stored proteins.
Since the early description in basophils, PMD has been documented in diverse inflammatory cells and in a variety of experimental models and diseases (Dvorak, 1992b, 2005a). In human eosinophils, PMD is the most frequently encountered secretory process in cells from subjects with a range of inflammatory and allergic disorders (Dvorak et al., 1980; Karawajczyk et al., 2000; Erjefalt et al., 2001; Ahlstrom-Emanuelsson et al., 2004) and is responsible for the regulated release of cytokines and other proteins during eosinophil responses. We have been studying structural mechanisms underlying PMD in human eosinophils during different situations. This review focuses on the structural and functional advances that have been made over the last years concerning the intracellular trafficking and secretion of eosinophil proteins by PMD during inflammatory responses.
Eosinophil as a secretory cell
Eosinophils are leukocytes of the innate immune system with a wide range of functions. They are typically associated with host defense against helminthic pathogens and with inflammatory and allergic diseases such as asthma. Moreover, emerging evidence indicate that eosinophils are essential cells for tissue remodeling, repair and immunoregulation. It is now becoming apparent that eosinophils modulate acute phase and innate inflammatory responses as well as acquired immunity associated with both TH1 and TH2 immune responses (reviewed in Gleich, 2000; Adamko et al., 2005; Rothenberg and Hogan, 2006; Foley et al., 2007; Jacobsen et al., 2007; Trivedi and Lloyd, 2007).
In response to varied stimuli, including cross-linking of different subclasses of immunoglobulin receptors, interferon–γ (INF-γ), and the chemokines, eotaxin (CCL11), and RANTES (CCL5), eosinophils are recruited from the circulation into inflammatory foci, where they modulate immune responses through the extracellular release of granule-derived products (reviewed in Gleich, 2000; Munitz and Levi-Schaffer, 2004; Adamko et al., 2005; Rothenberg and Hogan, 2006; Rosenberg et al., 2007). The inflammatory action of eosinophils is therefore effected by degranulation mechanisms. In addition to PMD, two other mechanisms can be involved in eosinophil secretion: i) classical exocytosis by which granules release their entire contents following granule fusion with the plasma membrane, including compound exocytosis, which also involves intracellular granule-granule fusion before extracellular release; and ii) cytolysis, which consists of extracellular deposition of intact granules upon lysis of the cell (reviewed in Erjefalt and Persson, 2000; Moqbel and Coughlin, 2006; Weller et al., 2009). Granule exocytosis is rarely documented during inflammatory responses while cytolysis and PMD have been reported more frequently during human diseases. For example, in a recent study conducted in patients with nasal polyposis, while 30.7% of eosinophils were inactive, 41.7% exhibited PMD and 27.5% showed cytolysis (Armengot et al., 2009).
Classical, acute effector eosinophil responses involve secretion of four distinct cationic proteins: major basic protein (MBP) (Gleich et al., 1973; Lewis et al., 1978); eosinophil cationic protein (ECP) (Egesten et al., 1986); eosinophil-derived neurotoxin (EDN) (Peters et al., 1986); and eosinophil peroxidase (EPO) (Egesten et al., 1986). Secretory responses of human eosinophils also involve release of numerous cytokines with multiple biologic activities including transforming growth factor-alpha (TGF-α) (Wong et al., 1990), granulocyte macrophage colony-stimulating factor (GM-CSF) (Broide et al., 1992; Levi-Schaffer et al., 1995; Desreumaux et al., 1998); tumor necrosis factor-alpha (TNF-α) (Beil et al., 1993); INF-γ (Spencer et al., 2009); IL-2 (Levi-Schaffer et al., 1996); IL-3 (Fujisawa et al., 1994; Desreumaux et al., 1998); IL-4 (Moqbel et al., 1995; Moller et al., 1996b); IL-5 (Dubucquoi et al., 1994, Moller et al., 1996a, Desreumaux et al., 1998); IL-6 (Lacy et al., 1998); IL-10 (Spencer et al., 2009); IL-12 (Spencer et al., 2009); IL-13 (Woerly et al., 2002); IL-16 (Lim et al., 1996), regulated on activation, normal, T cell expressed, and secreted (RANTES/CCL5) (Ying et al., 1996; Lacy et al., 1999); eotaxin (CCL11) (Nakajima et al., 1998); vascular endothelial growth factor (VEGF) (Horiuchi and Weller, 1997), stem cell factor (SCF) (Hartman et al., 2001); epithelial cell-derived neutrophil activating peptide (ENA-78/CXCL5) (Persson et al., 2003); growth-related oncogene-alpha (GRO-α) (Persson-Dajotoy et al., 2003) and nerve growth factor (NGF) (Toyoda et al., 2003).
Different from other immune cells, such as most lymphocytes which must synthesize proteins prior to secretion; both cationic proteins and cytokines are stored as preformed pools within eosinophil secretory granules. Notably, secretion of the preformed eosinophil cytokines is a rapid and stimulus-specific event (Bandeira-Melo et al., 2003; Spencer et al., 2009). Using different stimuli to represent Th1, Th2 and inflammatory and regulatory microenvironments, we observed differential secretion of cytokines. For example, despite preformed, intragranular stores of IL-12 and IL-4, eosinophils responded to Th1 and proinflammatory cytokine stimuli dose-dependently with secretion of IL-4 but not IL-12. On the other hand, INF-γ was secreted in response to Th1, Th2 and inflammatory stimuli. These findings provide insights into the functions of human eosinophils in mediating inflammatory processes and initiation of specific immunity (Spencer et al., 2009).
Ultrastructural views of piecemeal degranulation in activated eosinophils
Structural changes in secretory granules
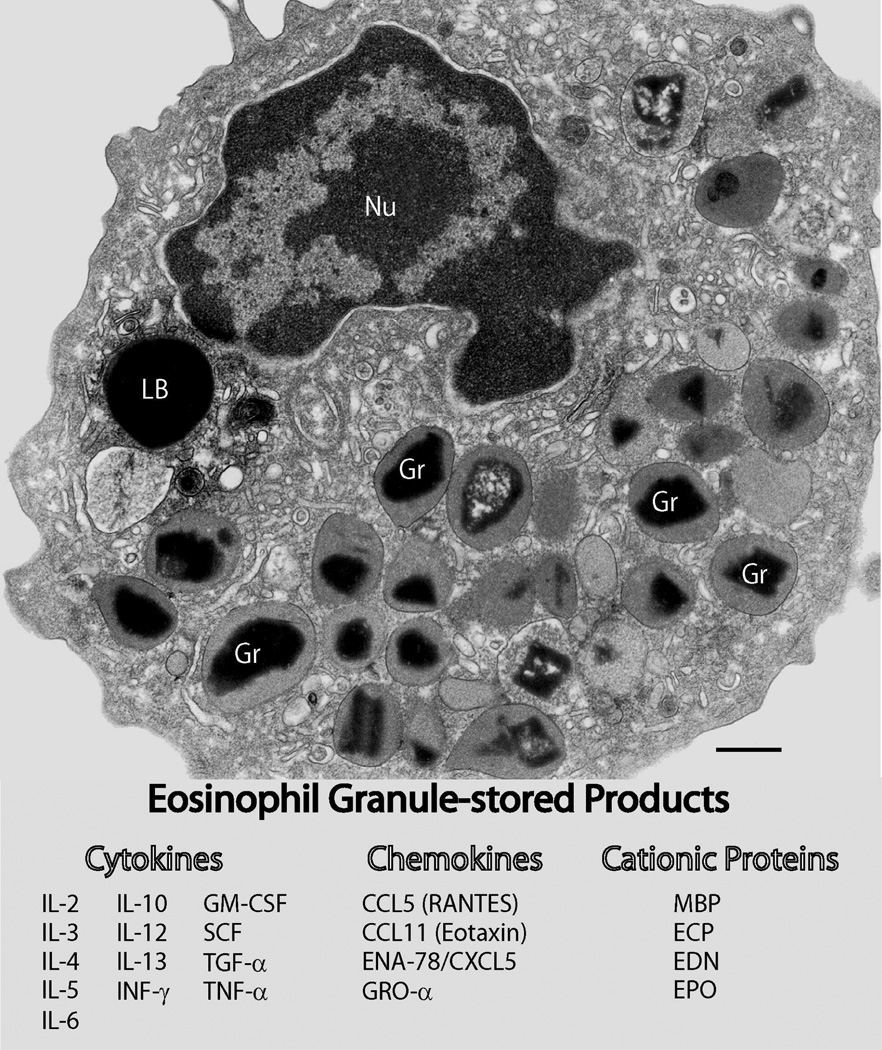
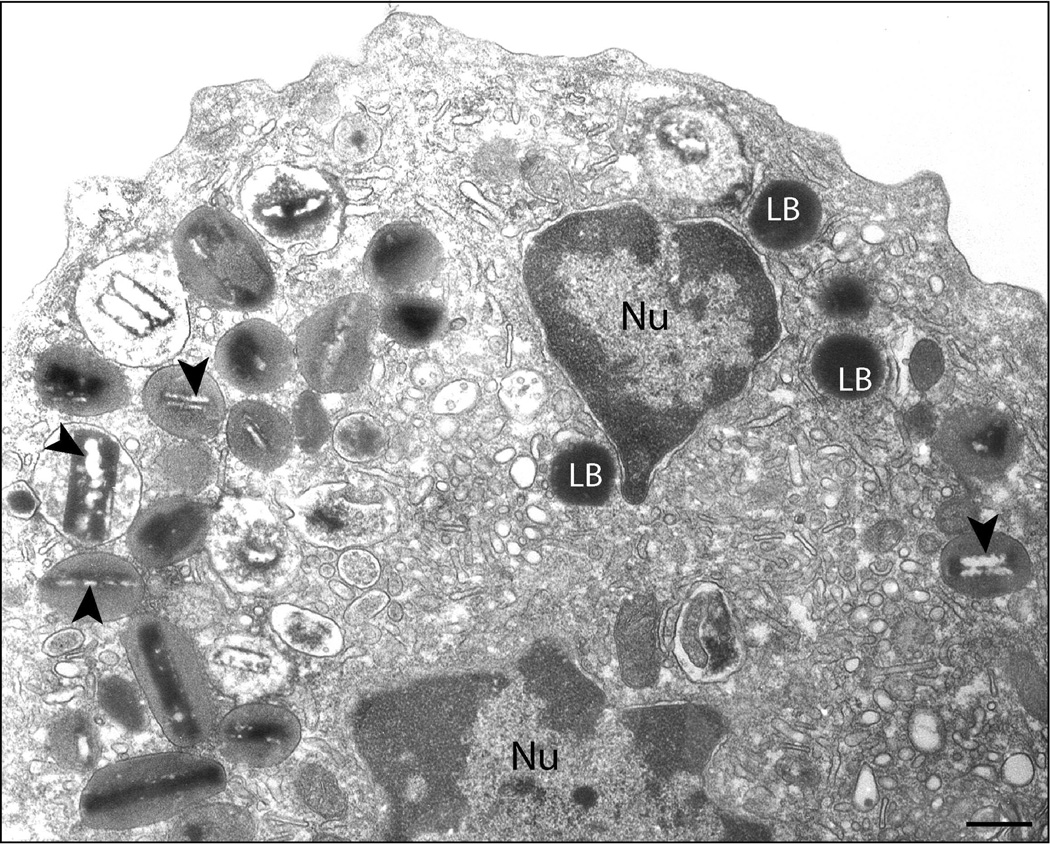
Human eosinophils are characterized by a major population of secretory specific granules (Kita et al., 1998; Lacy and Moqbel, 2000; Munitz and Levi-Schaffer, 2004), also termed secondary or crystalline granules. These granules exhibit a distinctive morphology with a central crystalline core compartment and an outer matrix surrounded by a delimiting trilaminar membrane (Fig. 1). Because of their unique morphology, this granule population defines the eosinophil lineage in multiple species (Dvorak and Weller, 2000).
Fig. 1.
Multifunctional granule-stored products within human eosinophils. Ultrastructural image of a human eosinophil shows the cytoplasm packed with specific granules containing an internal often electron-dense crystalline core. Cores are surrounded by an electron-lucent matrix. In response to a variety of stimuli, eosinophils secrete cytotoxic cationic proteins and an array of cytokines and chemokines. Figure 1 list: eosinophil proteins documented within specific granules. Bar, 480 nm. Gr, specific granules; Nu, nucleus; LB, lipid body. CCL5 (RANTES), regulated on activation, normal T cell expressed and secreted (Lacy et al., 1999); CCL11 (eotaxin) (Nakajima et al., 1998); ECP, eosinophil cationic protein (Egesten et al., 1986); EDN, eosinophil-derived neurotoxin (Peters et al., 1986); ENA-78/CXCL5, epithelial cell-derived neutrophil activating peptide (Persson et al., 2003); EPO, eosinophil peroxidase (Egesten et al., 1986); GM-CSF, granulocyte macrophage colony-stimulating factor (Levi-Schaffer et al., 1995, Desreumaux et al., 1998); GRO-α, growth-related oncogene-alpha (Persson-Dajotoy et al., 2003); IL-2 (Levi-Schaffer et al., 1996); IL-3 (Desreumaux et al., 1998); IL-4 (Moqbel et al., 1995; Moller et al., 1996b); IL-5 (Moller et al., 1996a; Desreumaux et al., 1998); IL-6 (Lacy et al., 1998); IL-10 (Spencer et al., 2009); IL-12 (Spencer et al., 2009); IL-13 (Woerly et al., 2002); INF-γ, interferon gamma (Spencer et al., 2009); MBP, major basic protein (Gleich et al., 1973; Lewis et al., 1978; Melo et al., 2009); NGF, nerve growth factor (Toyoda et al., 2003); SCF, stem cell factor (Hartman et al., 2001); TGF-α; transforming growth factor-alpha (Egesten et al., 1996); TNF-α, tumor necrosis factor-alpha (Beil et al., 1993). Reprinted from (Melo et al., 2008b) with permission.
Piecemeal deranulation is defined by the ultrastructural identification of emptying secretory granules. During PMD secretion, secretory granules undergo a progressive emptying of their contents, a phenomenon identified by transmission electron microscopy (TEM) by the presence of lucent areas within their internal structure, reduced electron density, disassembled contents or membrane empty chambers (Erjefalt et al., 2001; Dvorak, 2005b; Melo et al., 2005a; 2005c (Figs. 2, 3). Structural alterations within secretory granules associated with PMD are described in a diversity of human inflammatory and allergic disorders including asthma (Karawajczyk et al., 2000); nasal polyposis (Erjefalt et al., 2001; Armengot et al., 2009); allergic rhinitis (Erjefalt et al., 2001; Ahlstrom-Emanuelsson et al., 2004); ulcerative colitis (Erjefalt et al., 2001); Crohn’s disease (Erjefalt et al., 2001); atopic dermatitis (Cheng et al., 1997); gastric carcinoma (Caruso et al., 2005); shigellosis (Raqib et al., 2003) and cholera (Qadri et al., 2004). In this form of secretion, human eosinophils secrete granule matrix and/or core contents, but retain their granule containers. The result in electron-microscopic images is a cell filled with partially empty, and/or fully empty specific granules (reviewed in Dvorak and Weller, 2000) (Figs. 2, 3).
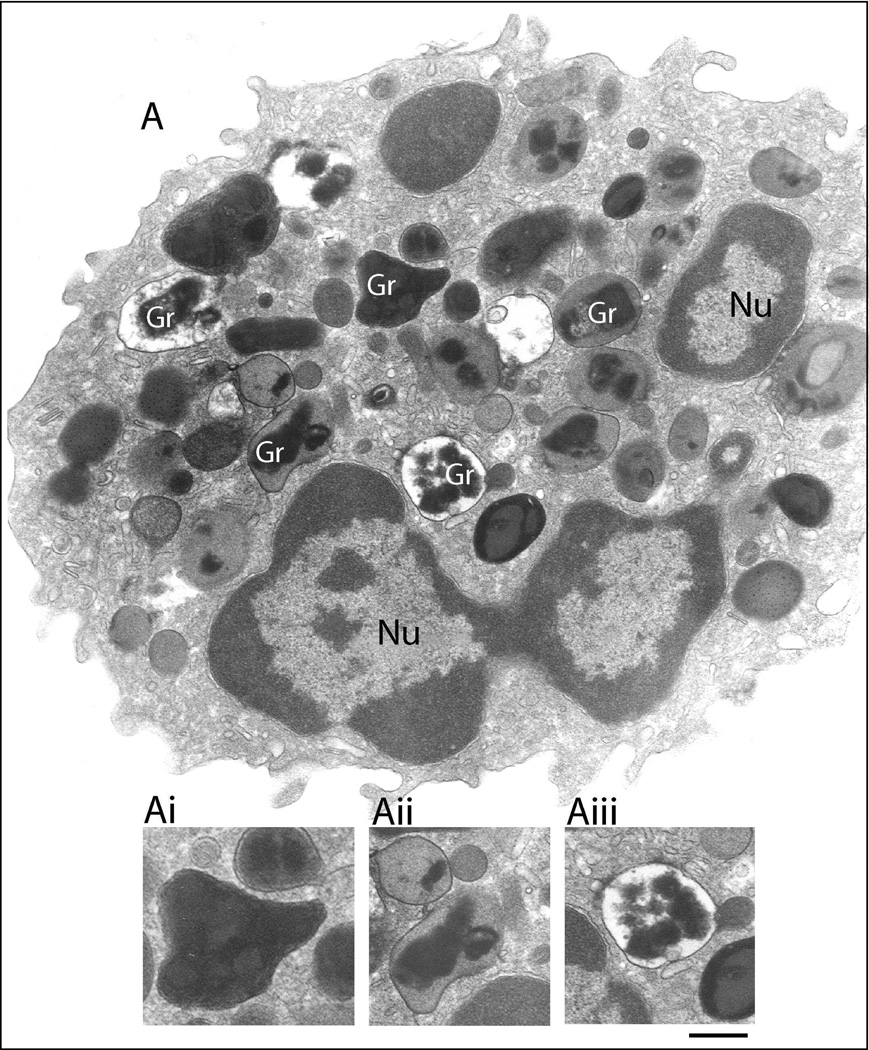
Fig. 2.
Ultrastructure of an eotaxin-activated human eosinophil showing piecemeal degranulation (PMD). After stimulation with eotaxin, specific granules (Gr) exhibit different degrees of emptying of their contents and show morphological diversity indicative of PMD. Granules can appear as irregular structures with protrusions from their surfaces (Ai); show disassembled matrices and cores or residual cores (Aii) or lucent areas in their cores, matrices or both (Aiii). Cells were incubated with eotaxin for 1h, immediately fixed and prepared for transmission electron microscopy as before (Melo et al., 2005b). Nu, nucleus. Scale bar: 1.0 µm.
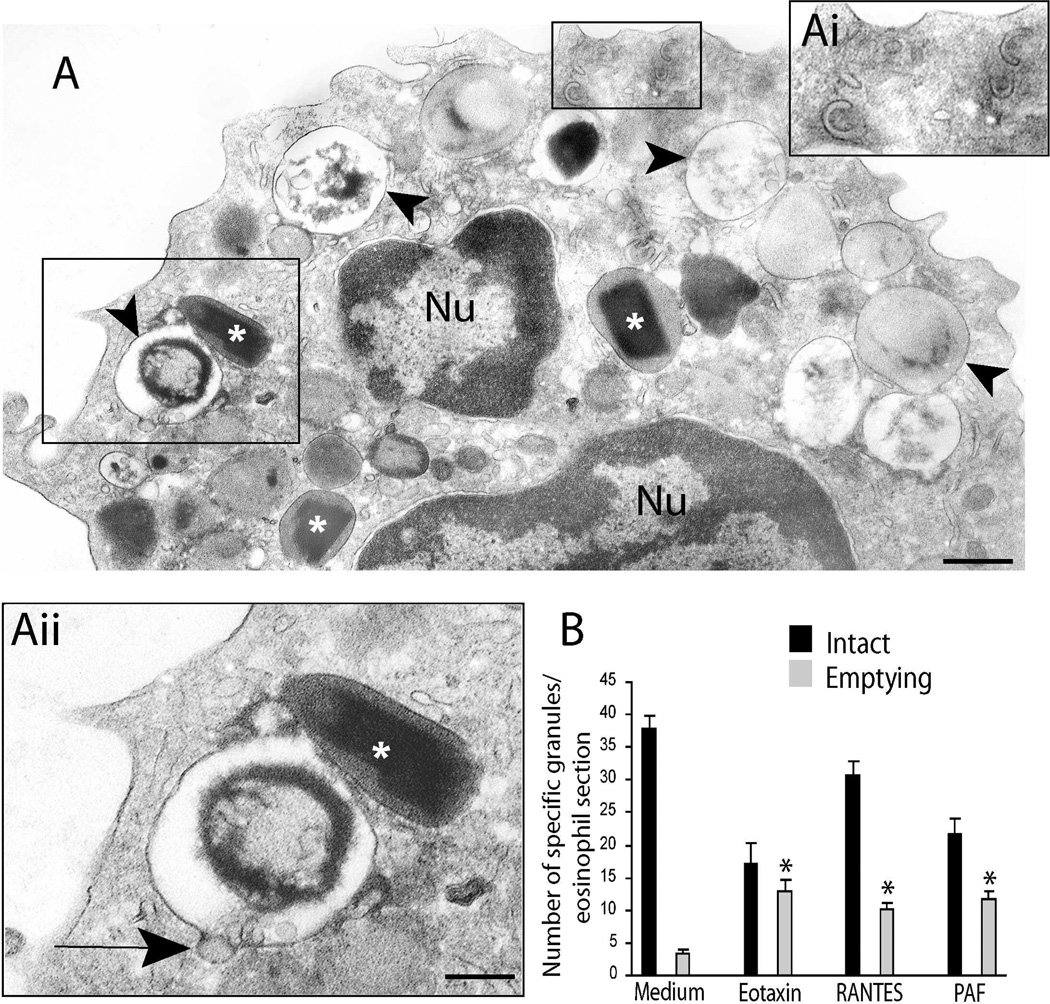
Fig. 3.
A. Piecemeal degranulation (PMD) in a platelet activating factor (PAF)-stimulated human eosinophil. Stimulation with PAF induced granule losses characteristic of PMD. Granules showing reduced electron density, with residual cores or membrane empty chambers are clearly observed (arrowheads). Intact, non-emptying granules with typical morphology (*) are seen close to emptying granules. Boxed areas in A are shown in high magnification in Ai and Aii. Eosinophil Sombrero vesicles with characteristic morphology are seen in Ai while Aii shows a vesicle profile (arrow) budding from a secretory granule. B. Significant increases in numbers of emptying granules occurred after stimulation with eosinophil agonists (*: P<0.05). Eosinophils were isolated by negative selection from healthy donors, stimulated, immediately fixed and prepared for transmission electron microscopy. Counts were derived from 3 experiments with a total of 3,945 granules counted in 95 electron micrographs randomly taken and showing the entire cell profile and nucleus. Reprinted from (Melo et al., 2005b) with permission. Nu, nucleus. Scale bar: A, 600 nm; Ai, Aii, 300 nm.
The number of emptying granules within human eosinophils increases when the cells are activated, both in vivo and in vitro in different conditions (Karawajczyk et al., 2000; Erjefalt et al., 2001; Ahlstrom-Emanuelsson et al., 2004; Melo et al., 2005b). In addition, eosinophil specific granules in the process of secreting their contents can show larger size than resting granules in the same cell, a phenomenon likely due to the changes that occur within granules in response to degranulating stimuli (Figs. 3A, 4C).
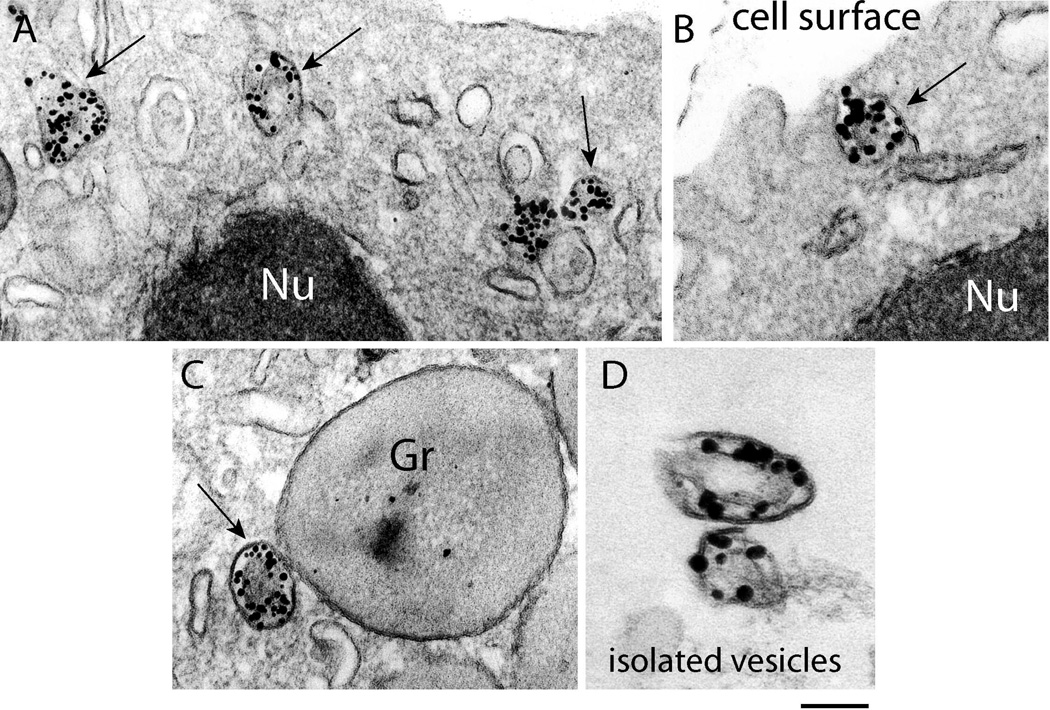
Fig. 4.
Large tubular carriers actively transport major basic protein (MBP). A–C. Eosinophil sombrero vesicles - EoSVs - (arrows) are observed in the cytoplasm by transmission electron microscopy (TEM) after immunonanogold labeling for MBP. Vesicles are seen beneath the plasma membrane in the cytoplasm (A), fused with the plasma membrane (B) and attached to an enlarged partially empty granule (C), typically indicative of PMD. D. EoSVs, isolated by subcellular fractionation, are densely labeled for MBP. Note that MBP is preferentially localized within the vesicle lumen. Eosinophils were stimuated by eotaxin as described in Material and Methods. Gr, specific granules; Nu, nucleus. Reprinted from (Melo et al., 2009) with permission. Scale bars: A, 400 nm; B, 230 nm; C, 250 nm; D, 200 nm.
We have been using inflammatory stimuli, such as the classical eosinophil agonists, eotaxin (CCL11), RANTES (CCL5) or platelet activating factor (PAF), and different technical approaches, especially electron microscopy to study PMD in human eosinophils (Figs. 2, 3). These stimuli trigger PMD, and pretreatment with brefeldin-A, a potential inhibitor of vesicular transport (Nebenfuhr et al., 2002), inhibits agonist-induced granule emptying (Melo et al., 2005b). Early aspects of stimulus-induced eosinophil PMD, when most granules did not yet show signs indicative of content losses, can be observed after 30 min of stimulation. At this time, granules develop into irregular structures with progressive protrusions from their surfaces, preferentially present on intact granules that had an ill-defined core and matrix (Melo et al., 2005b).
After 1h of stimulation, specific granules show dramatic changes in ultrastructure compared to those in unstimulated cells. In unstimulated eosinophils, granules are seen as round or elliptical structures with their classical morphology and full of contents (Fig. 1). Upon stimulation, granule contents exhibit clear losses classically associated with PMD (Figs. 2, 3) (Melo et al., 2005b).
Of interest, not all eosinophil specific granules are uniformly, coordinately, and simultaneously responsive to stimuli (Fig. 2). Studies using classical eosinophil agonists showed that whereas only 8% of granules in unactivated eosinophils had granules undergoing piecemeal degranulation, 43% (eotaxin), 25% (RANTES) and 34% (PAF) showed emptying granules (Melo et al., 2005b). Moreover, the responses of eosinophils were not uniformly distributed. For example, in scoring the numbers of granules that exhibited loss of granule contents indicative of piecemeal degranulation, in unstimulated cells 70% of eosinophils had <10% of granules with losses whereas eotaxin elicited a marked heterogeneity of granule emptying responses within eosinophils such that >15% of eosinophils had >90% of their granules exhibiting content losses (Melo et al., 2005b). In vivo, the numbers of emptying eosinophil granules in nasal biopsies from patients with seasonal allergic rhinitis obtained before and after the pollen season was also quantitated (Ahlstrom-Emanuelsson et al., 2004). Among the pre-seasonal tissue eosinophils, an average of 37±2.7% (mean ± SD) of the granules were altered as a result of piecemeal degranulation, while the extent of piecemeal degranulation was increased to 87±1.8% in association with seasonal pollen exposure (Ahlstrom-Emanuelsson et al., 2004). Moreover, eosinophils showing signs of severe-to-complete loss of granule content were exclusively observed during the pollen season and the degree of eosinophil degranulation was correlated with levels of ECP in lavage fluids obtained at histamine challenge (Ahlstrom-Emanuelsson et al., 2004).
Therefore, morphological changes in emptying granules reflect the activation state of granules. As observed in eosinophils and other cells undergoing PMD, granules can show different stages of emptying (from loosening of the matrix and core to more advanced stages up to complete emptying). Of note, in contrast to classical regulated secretion characterized by extrusion of entire granules, PMD sustains a pool of intact secretory granules. The presence of intact secretory granules intermingled with granules undergoing depletion of their contents has been described in different types of secretory cells, including other cells from the immune system such as mast cells and basophils and seems to be a general feature of piecemeal degranulation (reviewed in Crivellato et al., 2003). In eosinophils, this may contribute to the special capability of these cells to rapidly release their products under different or repetitive stimuli (Melo et al., 2005b).
Secretory vesicular trafficking from eosinophil specific granules
As noted, a large number of studies have documented the occurrence of PMD in different cell types based on the ultrastructural identification of emptying secretory granules (Dvorak, 1998; Crivellato et al., 2003; Melo et al., 2005b). By conventional TEM, also frequently reported is the presence of vesicles as well as tubules attached to, surrounding or budding from secretory granules within activated human eosinophils (reviewed in Melo et al., 2008b).
Using a cytochemical method for EPO detection, an early study has documented the presence of EPO-containing, membrane-bound vesicles attached to specific granules and plasma membrane and also free in the intervening cytoplasm in eosinophils arising in growth factor-supplemental cultures of human cord blood mononuclear cells. These EPO-loaded vesicles were identified as the vesicular system involved in the transport of products from granules to the cell surface (Dvorak et al., 1994).
Although numerous products are known to be localized within the eosinophil secretory granules for decades (Fig. 1), the ultrastructural immunolocalization of granule-stored proteins at transport vesicles was consistently documented only during the last 5 years (Melo et al., 2005c, 2009; Spencer et al., 2006).
By using a pre-embedding immunolabeling approach which is performed before standard EM processing, we have identified consistent secretory vesicle traffic of granule-stored proteins from eosinophil specific granules to cell membrane (Melo et al., 2005c, 2009). Pre-embedding immunoEM optimizes antigen preservation and is more sensitive to detect small molecules than post-embedding labeling that is done after conventional EM processing. Moreover, to reach antigens at membrane microdomains such as vesicles, we used Fab-fragments linked to very small (1.4 nm) gold particles as secondary antibodies. This strategy has enabled the identification of vesicular trafficking of typical granule-stored proteins such as IL-4 and MBP (Fig. 4) (Melo et al., 2005c, 2008a, 2009), recognized for a long time only within cores of eosinophil granules (Moqbel et al., 1995, Moller et al., 1996b). This vesicle-mediated secretion from secretory granules has likely been previously underestimated because of technical issues – inadequate preservation of vesicles and/or inability of antibodies access to them.
Until recently, it was believed that the transport of secretory proteins between the eosinophil cytoplasmic granules and cell membrane was carried out only by small round vesicles (Dvorak et al., 1991; Dvorak, 1992a; Beil et al., 1993). Interestingly, our EM studies have recently brought conclusive evidence for the participation of morphologically distinct, large membrane-bound tubular compartments, referred to as Eosinophil Sombrero Vesicles (EoSVs), in the eosinophil secretory route (Spencer et al., 2006; Melo et al., 2008b, 2009).
In activated human eosinophils, EoSVs undergo a remarkable formation and redistribution. When eosinophils are stimulated with classical eosinophil agonists, such as eotaxin, there is an increase of the total number of cytoplasmic EoSVs (Melo et al., 2005c). In addition, EoSVs are more frequently observed surrounding and/or in contact with secretory granules (Melo et al., 2005c). By quantitative TEM, it was demonstrated that activation induces a significant increase in the numbers of granule-attached EoSVs. Interestingly, the majority of these EoSVs (90%) are associated with granules showing ultrastructural changes typical of PMD (reviewed in Melo et al., 2008b).
Both small round vesicles and EoSVs compartments are positively immunolabeled for typical granule products (Melo et al., 2005b,c). Eosinophil peroxidase (EPO)-loaded vesicles and tubules were initially detected within eosinophils that developed from human-cord blood mononuclear cell cultures supplemented with interleukin-5 (IL-5) (Dvorak et al., 1994). Accordingly, mobilization of MBP into large tubular vesicles was demonstrated more recently by immunonanogold electron microscopy when eosinophils were stimulated with eotaxin. Vesicles containing MBP were identified within and extending from granules as well as around emptying granules and underneath the plasma membrane (Fig. 4A–C) (Melo et al., 2009). Eosinophil sombrero vesicles within intact eosinophils (Fig. 4A–C) or isolated from these cells by subcellular fractionation (Fig. 4D) were extensively labeled for MBP, which was clearly localized within the vesicle lumina (Fig. 4). MBP-loaded vesicles had an effective interaction with the secretory granules and seemed to be, at least in part, structurally linked to them (Fig. 4C). This interaction may be important for vesicular replenishment of MBP from granules. As noted, the MBP-positive vesicular system was associated with a secretory pathway transporting MBP from eosinophil specific granules and not with a synthetic route from the trans-Golgi network, which was rarely labeled for MBP (Melo et al., 2009).
In a recent study, we demonstrated that the total numbers of EoSVs are significantly increased within eosinophils from a patient with hypereosinophilic syndrome (HES) (Melo et al., 2009). Eosinophils from HES individuals are typically activated (Ackerman and Bochner, 2007), compared to cells from normal donors. The identification of increased number of EoSVs in HES eosinophils is important because they would explain the reason for the loss of electron dense cores (enriched in crystallized MBP) observed in tissue eosinophils from a range of disorders, such as Crohn’s disease, eosinophilic gastroenteritis and HES (Fig. 5) (Dvorak, 1980; Torpier et al., 1988; Dvorak et al., 1990). In fact, deposition of MBP can be demonstrated in affected tissues of patients with HES (Tai et al., 1987) and vesicular trafficking is likely involved in this secretory mechanism.
Fig. 5.
Ultrastructure of an eosinophil from a patient with hypereosinophilia. Secretory granules and a large number of tubular vesicles are observed in the cytoplasm. Arrows indicate content losses from granules electron dense cores. Nu, nucleus; LB, lipid body. Scale bar: 800 nm.
Vesicular trafficking of IL-4, a hallmark, granule-stored cytokine was identified in human eosinophils using different approaches (Melo et al., 2005c). This study clearly demonstrated a consistent and preferential labeling for IL-4 on vesicle membranes and not on their internal content as observed for MBP-labeled vesicles. Labeling for TGF-α, another granule-stored cytokine, was also documented at transport vesicles membranes in a previous EM study (Egesten et al., 1996). These findings showing preferential cytokine labeling at vesicle membranes instead of vesicle lumina provide more evidence for the occurrence of distinct cellular mechanisms involved in the mobilization of specific proteins from eosinophil granules. A functional implication of a membrane-bound vesicular transport of cytokines is that it adds support to the occurrence of selective release of products from eosinophils as previously indicated (Lacy et al., 1999; Bandeira-Melo et al., 2001). Interestingly, pools of IL-4 and MBP-loaded vesicles were also identified in unstimulated eosinophils. This may contribute to the rapid protein mobilization and release following cell activation (Melo et al., 2005c) and may underline the eosinophil role as a regulator of local immune and inflammatory responses (reviewed in Jacobsen et al., 2007; Adamko et al., 2005; Akuthota et al, 2008). Major basic protein, for example, in addition to being a recognized molecule for defense against parasites, seems to be involved in the regulation of cytokine responses (Specht et al., 2006).
Altogether, these studies provide new insights into the intracellular mechanisms mediating secretion of eosinophil granule-derived proteins. This is important to understand the pathological basis of allergic and other eosinophil-associated inflammatory diseases.
Electron tomography of secretory granules and vesicles
Studies using electron tomography have been redefining our understanding of the organization of several organelles and membrane systems and leading to many new insights into the functional organization of varied cells (Trucco et al., 2004; Melo et al., 2005b; Chen et al., 2008; Staehelin and Kang, 2008).
Novel insights into the structural mechanisms underlying PMD were identified using electron tomography. We used fully automated dual-axis electron tomography to study the 3D architecture of mobilized eosinophil secretory granules and EoSVs in high resolution. By tracking 4nm-thick serial digital sections of human eosinophils, we found that eosinophil specific granules are not merely storage stations, but are elaborate and compartmentalized organelles with internal, membranous vesiculotubular domains which undergo dynamic changes in their structure and contents in response to stimuli (Melo et al., 2005b) (Fig. 6). Intragranular membranous sub-compartments are imaged in 3D models as an aggregate of flattened tubular networks and tubules with interconnections in some planes and structural connections between the intragranular membranous network and the granule limiting membrane (Fig. 6). Moreover, the tomographic reconstructions revealed that intragranular vesiculotubular compartments can sequester and relocate granule products (Melo et al., 2005b) (Fig. 6). These findings have the important functional implication that proteins may be specifically sorted and segregated within granule sub-compartments before reaching the outer granule membrane in order to be delivered to the cell surface through vesicular compartments. These events of sequestration and relocation of granule products are important by enabling the differential secretion of granule products, as previously demonstrated by us (Bandeira-Melo et al., 2001; Spencer et al., 2006) and other groups (Lacy et al., 1999).
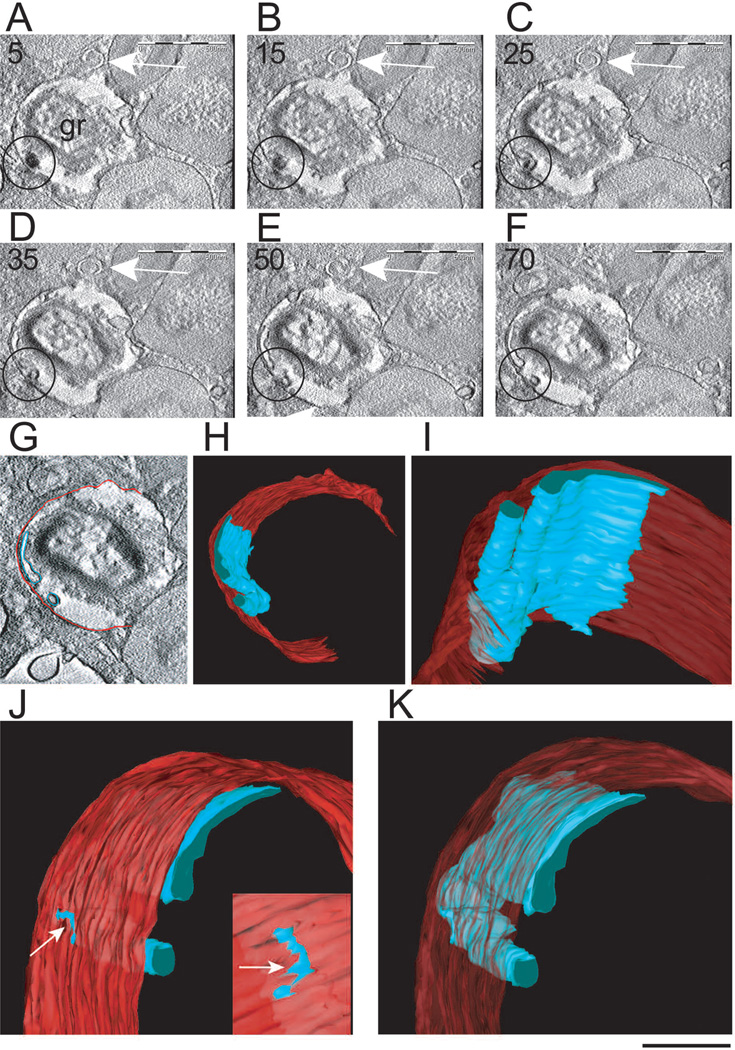
Fig. 6.
Tomographic slices and 3D models from an emptying specific granule. A–F. The tomographic volume shows intragranular sub-compartments in conjunction with mobilized content. Circles indicate the same sub-compartment surrounding part of the electron dense content that is relocated to the granule outer membrane. Note in C and D that the electron density of this membrane changes at the site of contact with the membranous intragranular sub-compartment. The arrows point to a forming Eosinophil Sombrero Vesicle (EoSV). Seventy and five serial single virtual slices as in F were extracted from the tomogram, outer granule membrane was partially traced in red and intragranular vesiculotubular structures were outlined in blue as in G so as to generate 3D models. H–K. 3D models of the same granule show intragranular membrane domains (blue) organized as a flattened tubular network and tubules. In J and K, the model has been rotated to provide another view. An area of continuity between the intragranular membranous network and the limiting granule membrane is indicated in J (arrows). The slices (~ 4 nm of thickness) were extracted from 3D reconstructions of a 400 nm eosinophil section analyzed by automated electron tomography at 200 Kv. The numbers on the upper left corner indicate the slice number through the tomographic volume. gr, granule. Cells were stimulated with eotaxin as in Figure 2 and processed for transmission electron microscopy. Reprinted from (Melo et al., 2005b) with permission. Also see Movie 1 and Movie 2 in supplementary material at www.traffic.dk/videos/6_10.asp. Scale bars: A–F, 500 nm; G, 450 nm; H, 400 nm; I, 180 nm; J, K, 150 nm.
The presence within granules of membranes was confirmed by immunonanogold labeling for CD63 (Melo et al., 2005b), a tetraspanin membrane protein previously implicated in eosinophil granule secretion (Mahmudi-Azer et al., 2002). Internal CD63-positive membranes have been recognized in some other lysosome-related organelles such as platelet alpha granules (Heijnen et al., 1998) and MHC class II compartments in dendritic cells (Barois et al., 2002). However, the origin of the CD63-bearing membranes within eosinophil granules has not been ascertained. It is likely over time these extensive intragranular membranous compartments are refreshed from endocytic recycling, from granule membranes and/or from biosynthetic pathways, but this remains to be delineated.
Electron tomography has also provided new insights into the intriguing structure of EoSVs. Three-dimensional reconstructions and models generated from digital serial sections revealed that individual EoSVs are curved tubular structures with cross-sectional diameters of approximately 150–300 nm. Along the length of EoSVs, both continuous, fully connected, cylindrical and circumferential domains and incompletely connected and only partially circumferential curved domains were identified (reviewed in Melo et al., 2008b). These two domains explain both the “C” shaped morphology of these vesicles and the presence of elongated tubular profiles very close to typical EoSV, as frequently seen in 2D cross-sectional images of eosinophils. Electron tomography revealed therefore that EoSVs present substantial membrane surfaces and are larger and more pleiomorphic than the small, spherical vesicles (~50 nm in diameter) classically involved in intracellular transport (Melo et al., 2005c, 2008b). In fact, the findings using electron tomography highlight EoSVs as a dynamic system with a remarkable ability to change their shape and to interact with secretory granules (Melo et al., 2005c, 2008b). In addition, tracking of vesicle formation using 4 nm-thickness digital sections by electron tomography revealed that EoSVs can indeed emerge from mobilized granules through a tubulation process (Melo et al., 2005c). Remarkably, the use of Brefeldin A dramatically suppressed EoSV numbers, perhaps by its previous demonstrated direct action within eosinophil granules to collapse the intragranular membranotubular networks with formation of lipid-rich deposits (Melo et al., 2005b) and inhibit tubulation needed for EoSV formation (Melo et al., 2005c). Electron tomography also showed that small round vesicles bud from eosinophil specific granules. These findings provide direct evidence for the origin of vesicular compartments from granules undergoing release of their products by PMD.
Receptor-mediated secretion of cytokines from human eosinophils
Functional and structural events within eosinophil granules and transport vesicles are crucial for the regulated release of cytokines and other proteins during eosinophil responses to allergic and inflammatory diseases. Upon cell stimulation, specific cytokines are selectively mobilized, from among over two dozen other preformed, granule-stored proteins, into secretory vesicles.
A major advance in the understanding of piecemeal degranulation in eosinophils was the demonstration that the differential secretion of cytokines is mediated through their cognate receptors which are highly expressed in eosinophil secretory granules and vesicles (Moqbel and Coughlin, 2006; Spencer et al., 2006).
Our EM observations of a strong association of IL-4 with membranes of secretory vesicles, suggesting participation of a docking molecule, led us to analyze IL-4 receptor expression throughout eotaxin-induced piecemeal degranulation of IL-4 (Spencer et al., 2006). In addition to nominal surface expression, we have detected intracellular stores of each component of functional type I and II IL-4 receptors. Both IL-4 and IL-4 receptor alpha chain colocalize in eosinophil granules; and after eotaxin-stimulation, IL-4 receptor alpha chain, bearing bound IL-4, is mobilized into secretory vesicles (Spencer et al., 2006). Importantly, the signal transducing accessory chain of the IL-4 receptor complex (γc chain) did not exhibit eotaxin-induced mobilization. Thus, trafficking of IL-4Rα-chaperoned IL-4 within tubular carriers may be accomplished without initiating an IL-4R-mediated signaling cascade (Spencer et al., 2006).
Eosinophils also contain substantial intracellular quantities of other granule- and vesicle-associated cytokine receptors, including for IL-6α, and IL-13. Intracellular stores of CCR3 were also expressed within human eosinophils, and its intracellular detection increased upon stimulus-induced release of RANTES, a known CCR3 ligand (Spencer et al., 2006).
Receptor chain involvement in cytokine secretion may provide a crucial link to unlocking regulatory mechanisms governing specificity of rapid, stimulus-induced release of preformed immunomodulatory proteins from human innate immune cells. Receptor-mediated trafficking of cytokines, a mechanism ideally suited to the large surface area inherent in tubular carriers, is thus a likely mechanism governing both the selectivity and rapid transit of cytokines for secretion.
Once loaded, granule-derived vesicles dock at appropriate cell membrane locales, and release their specific cargo. Electron microscopic visualization of vesicles transporting granule-stored proteins, such as MBP, was clearly demonstrated at the cell membrane.
The molecular mechanisms involved in the docking/fusion of these specific eosinophil transport carriers at the cell membrane remain to be fully elucidated. It was demonstrated that SNARE complexes, comprised of v (vesicle) and t (target)-SNARES, are involved in the process of eosinophil secretion (reviewed in Moqbel and Coughlin, 2006). SNARE (SNAP receptors) complexes formation is a critical event preceding membrane fusion and mediator release in a variety of cells (Wickner and Schekman, 2008). Specifically, eosinophil secretory vesicles, but not granules, express the v-SNARE VAMP-2 (vesicle associated membrane protein 2), which co-localized with RANTES throughout IFN-γ-induced piecemeal degranulation of RANTES (Lacy et al., 2001), and likely mediates specific membrane docking through interaction with plasma membrane t-SNARES, SNAP-23 and syntaxin-4 (Logan et al., 2002, 2003).
Concluding remarks
Activated eosinophils within tissues modulate immune responses and elicit effector functions through secretion of cytokine, lipid and cationic protein mediators. An understanding of the intrinsic complexity of the eosinophil secretory pathway is beginning to emerge.
Our studies have identified vesicular trafficking in activated human eosinophils that directs transport of granule-stored proteins from secretory granules to the cell surface. This secretory process, termed PMD, is central to eosinophil inflammatory responses.
It is recognized that eosinophils have a remarkable capacity to select cytokines and proteins to be secreted from their cytoplasmic granules through PMD in response to varied stimuli (Melo et al., 2005b; Moqbel and Coughlin, 2006). How a specific cytokine is chaperoned through the secretory pathway in eosinophils? The differential secretion of cytokines is mediated through intracellular receptors, including IL-4, IL-6, and IL-13 receptors as well as CCR3, identified at secretory granules and vesicles (Spencer et al., 2006). Receptor-mediated differential secretion of proteins, characterized in human eosinophils, may be a more general mechanism, occurring in other leukocytes.
Large tubular carriers provide an additional mechanism to rapidly transport material between membranes in different secretory pathways and are also responsible for moving the bulk of the secretory traffic between distant compartments (Luini et al., 2005; Simpson et al., 2006). In eosinophils, large carriers termed EoSVs may be fundamental for the diversity of proteins that need to be rapidly transported from within these cells (Melo et al., 2008b). During secretion, these specialized large tubular carriers in conjunction with small vesicles are actively formed and, in parallel, specific granules undergo highly dynamic changes related to the progressive release of their contents. Studies using electron tomography unveiled the three dimensional structure of EoSVs which exhibit distinct morphology, including substantial membrane surface, important for membrane-bound intracellular transport (Melo et al., 2008b).
Our studies have demonstrated vesicular trafficking of MBP and cytokines, such as IL-4, from secretory granules in activated eosinophils. Interestingly, rapid release of these granule-stored proteins may involve the presence of small storage/transient sites (vesicular pools) in the cytoplasm as identified in unstimulated eosinophils (Melo et al., 2005c, 2009). These extragranular sites appear to be relevant for the rapid release of small concentrations of proteins under cell activation without immediate disarrangement of the intricate crystalline cores within eosinophil specific granules. This is important for the eosinophil roles as an effector or immunoregulatory cell.
Knowledge of the secretory trafficking events within eosinophils underlying secretion from granule-stored products requires further mechanistic studies. Different aspects concerning the regulation of this vesicle-mediated traffic remain to be defined. One main question is whether granule-stored proteins are synthesized in a ER-independent way. A recent work has provided evidence for DNA and RNA synthesis in eosinophil secretory granules (Behzad et al., 2009). Structural studies from our group have previously demonstrated that these granules are highly elaborate organelles with internal membranes (Melo et al., 2005b). Altogether, these findings indicate new roles for eosinophil secretory granules as organelles with potential ability to synthesize and sort their battery of proteins.
The recognition of PMD as a secretory process to release granule-stored proteins is important to understand not only normal leukocyte functions but also the pathological basis of allergic and other eosinophil-associated inflammatory diseases. The understanding of all events and mechanisms governing differential sorting, packing and secretion of granule-stored mediators may be also fundamental to the goal of specifically blocking eosinophil secretion as a therapeutic strategy in the management of serious immune and inflammatory conditions (Moqbel and Coughlin, 2006).
Acknowledgements
The work of the authors is supported by National Institutes of Health, USA (grants AI020241, AI051645, AI022571), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil, grant CBB-APQ-01294-08). We thank Ann M. Dvorak, Rita Monahan-Earley, Tracey Sciuto, Ellen Morgan (Electron Microscopy Unit, Dept. of Pathology, BIDMC, Harvard Medical School) and Wim Voorhout of FEI Company (Eindhoven, The Netherlands) for helpful discussions and previous electron microscopy assistance.
References
- Ackerman SJ, Bochner BS. Mechanisms of eosinophilia in the pathogenesis of hypereosinophilic disorders. Immunol. Allergy Clin. North Am. 2007;27:357–375. doi: 10.1016/j.iac.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamko DJ, Odemuyiwa SO, Vethanayagam D, Moqbel R. The rise of the phoenix: The expanding role of the eosinophil in health and disease. Allergy. 2005;60:13–22. doi: 10.1111/j.1398-9995.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- Ahlstrom-Emanuelsson CA, Greiff L, Andersson M, Persson CG, Erjefalt JS. Eosinophil degranulation status in allergic rhinitis: Observations before and during seasonal allergen exposure. Eur. Respir. J. 2004;24:750–757. doi: 10.1183/09031936.04.00133603. [DOI] [PubMed] [Google Scholar]
- Armengot M, Garin L, Carda C. Eosinophil degranulation patterns in nasal polyposis: An ultrastructural study. Am. J. Rhinol. Allergy. 2009;23:466–470. doi: 10.2500/ajra.2009.23.3357. [DOI] [PubMed] [Google Scholar]
- Bandeira-Melo C, Sugiyama K, Woods LJ, Weller PF. Cutting edge: Eotaxin elicits rapid vesicular transport-mediated release of preformed IL-4 from human eosinophils. J. Immunol. 2001;166:4813–4817. doi: 10.4049/jimmunol.166.8.4813. [DOI] [PubMed] [Google Scholar]
- Bandeira-Melo C, Perez SAC, Melo RCN, Ghiran I, Weller PF. Elicell assay for the detection of released cytokines from eosinophils. J. Immunol. Methods. 2003;276:227–237. doi: 10.1016/s0022-1759(03)00076-0. [DOI] [PubMed] [Google Scholar]
- Barois N, de Saint-Vis B, Lebecque S, Geuze HJ, Kleijmeer MJ. MHC class II compartments in human dendritic cells undergo profound structural changes upon activation. Traffic. 2002;3:894–905. doi: 10.1034/j.1600-0854.2002.31205.x. [DOI] [PubMed] [Google Scholar]
- Behzad AR, Walker DC, Abraham T, McDonough J, Mahmudi-Azer S, Chu F, Shaheen F, Hogg JC, Pare PD. Localization of DNA and RNA in eosinophil secretory granules. Int. Arch. Allergy Immunol. 2009;152:12–27. doi: 10.1159/000260079. [DOI] [PubMed] [Google Scholar]
- Beil WJ, Weller PF, Tzizik DM, Galli SJ, Dvorak AM. Ultrastructural immunogold localization of tumor necrosis factor-alpha to the matrix compartment of eosinophil secondary granules in patients with idiopathic hypereosinophilic syndrome. J. Histochem. Cytochem. 1993;41:1611–1615. doi: 10.1177/41.11.8409368. [DOI] [PubMed] [Google Scholar]
- Broide DH, Paine MM, Firestein GS. Eosinophils express interleukin 5 and granulocyte macrophage-colony-stimulating factor mRNA at sites of allergic inflammation in asthmatics. J. Clin. Invest. 1992;90:1414–1424. doi: 10.1172/JCI116008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso RA, Ieni A, Fedele F, Zuccala V, Riccardo M, Parisi E, Parisi A. Degranulation patterns of eosinophils in advanced gastric carcinoma: An electron microscopic study. Ultrastruct. Pathol. 2005;29:29–36. doi: 10.1080/019131290882303. [DOI] [PubMed] [Google Scholar]
- Chen X, Winters CA, Reese TS. Life inside a thin section: Tomography. J. Neurosci. 2008;28:9321–9327. doi: 10.1523/JNEUROSCI.2992-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JF, Ott NL, Peterson EA, George TJ, Hukee MJ, Gleich GJ, Leiferman KM. Dermal eosinophils in atopic dermatitis undergo cytolytic degeneration. J. Allergy Clin. Immunol. 1997;99:683–692. doi: 10.1016/s0091-6749(97)70031-9. [DOI] [PubMed] [Google Scholar]
- Crivellato E, Nico B, Mallardi F, Beltrami CA, Ribatti D. Piecemeal degranulation as a general secretory mechanism? Anat. Rec. 2003;274A:778–784. doi: 10.1002/ar.a.10095. [DOI] [PubMed] [Google Scholar]
- Desreumaux P, Delaporte E, Colombel JF, Capron M, Cortot A, Janin A. Similar IL-5, IL-3, and GM-CSF syntheses by eosinophils in the jejunal mucosa of patients with celiac disease and dermatitis herpetiformis. Clin. Immunol. Immunopathol. 1998;88:14–21. doi: 10.1006/clin.1997.4494. [DOI] [PubMed] [Google Scholar]
- Dubucquoi S, Desreumaux P, Janin A, Klein O, Goldman M, Tavernier J, Capron A, Capron M. Interleukin 5 synthesis by eosinophils: Association with granules and immunoglobulin-dependent secretion. J. Exp. Med. 1994;179:703–708. doi: 10.1084/jem.179.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak AM. Ultrastructural evidence for release of major basic protein-containing crystalline cores of eosinophil granules in vivo: Cytotoxic potential in Crohn's disease. J. Immunol. 1980;125:460–462. [PubMed] [Google Scholar]
- Dvorak AM. Human mast cells. Ultrastructural observations of in situ, ex vivo, and in vitro studies, sources, and systems. In: Kaliner MAM, editor. The mast cell in health and disease. 1992a. pp. 1–90. [Google Scholar]
- Dvorak AM. Basophils and mast cells: Piecemeal degranulation in situ and ex vivo: A possible mechanism for cytokine-induced function in disease. In: Coffey RG, editor. Granulocyte responses to cytokines - basic and clinical research. New York: Marcel Dekker; 1992b. pp. 169–271. [PubMed] [Google Scholar]
- Dvorak AM. A role for vesicles in human basophil secretion. Cell Tissue Res. 1998;293:1–22. doi: 10.1007/s004410051093. [DOI] [PubMed] [Google Scholar]
- Dvorak AM. Ultrastructure of mast cells and basophils. Chemical Immunology Series. S. Karger. Basel. 2005a:1–250. [Google Scholar]
- Dvorak AM. Piecemeal degranulation of basophils and mast cells is effected by vesicular transport of stored secretory granule contents. Chem. Immunol. Allergy. 2005b;85:135–184. doi: 10.1159/000086516. [DOI] [PubMed] [Google Scholar]
- Dvorak AM, Weller PF. Ultrastructural analysis of human eosinophils. In: Marone G, editor. Human eosinophils: Biological and chemical aspects. Basel. Karger; 2000. pp. 1–28. [DOI] [PubMed] [Google Scholar]
- Dvorak AM, Monahan RA, Osage JE, Dickersin GR. Crohn's disease: Transmission electron microscopic studies. II. Immunologic inflammatory response. Alterations of mast cells, basophils, eosinophils, and the microvasculature. Hum. Pathol. 1980;11:606–619. doi: 10.1016/s0046-8177(80)80072-4. [DOI] [PubMed] [Google Scholar]
- Dvorak AM, Weller PF, Monahan-Earley RA, Letourneau L, Ackerman SJ. Ultrastructural localization of charcot-leyden crystal protein (lysophospholipase) and peroxidase in macrophages, eosinophils, and extracellular matrix of the skin in the hypereosinophilic syndrome. Lab. Invest. 1990;62:590–607. [PubMed] [Google Scholar]
- Dvorak AM, Furitsu T, Letourneau L, Ishizaka T, Ackerman SJ. Mature eosinophils stimulated to develop in human cord blood mononuclear cell cultures supplemented with recombinant human interleukin-5. I. Piecemeal degranulation of specific granules and distribution of charcot-leyden crystal protein. Am. J. Pathol. 1991;138:69–82. [PMC free article] [PubMed] [Google Scholar]
- Dvorak AM, Ackerman SJ, Furitsu T, Estrella P, Letourneau L, Ishizaka T. Mature eosinophils stimulated to develop in human-cord blood mononuclear cell cultures supplemented with recombinant human interleukin-5. II. Vesicular transport of specific granule matrix peroxidase, a mechanism for effecting piecemeal degranulation. Am. J. Pathol. 1992;140:795–807. [PMC free article] [PubMed] [Google Scholar]
- Dvorak AM, Estrella P, Ishizaka T. Vesicular transport of peroxidase in human eosinophilic myelocytes. Clin. Exp. Allergy. 1994;24:10–18. doi: 10.1111/j.1365-2222.1994.tb00910.x. [DOI] [PubMed] [Google Scholar]
- Egesten A, Alumets J, von Mecklenburg C, Palmegren M, Olsson I. Localization of eosinophil cationic protein, major basic protein, and eosinophil peroxidase in human eosinophils by immunoelectron microscopic technique. J. Histochem. Cytochem. 1986;34:1399–1403. doi: 10.1177/34.11.3772075. [DOI] [PubMed] [Google Scholar]
- Egesten A, Calafat J, Knol EF, Janssen H, Walz TM. Subcellular localization of transforming growth factor-alpha in human eosinophil granulocytes. Blood. 1996;87:3910–3918. [PubMed] [Google Scholar]
- Erjefalt JS, Persson CG. New aspects of degranulation and fates of airway mucosal eosinophils. Am. J. Respir. Crit. Care Med. 2000;161:2074–2085. doi: 10.1164/ajrccm.161.6.9906085. [DOI] [PubMed] [Google Scholar]
- Erjefalt JS, Greiff L, Andersson M, Adelroth E, Jeffery PK, Persson CG. Degranulation patterns of eosinophil granulocytes as determinants of eosinophil driven disease. Thorax. 2001;56:341–344. doi: 10.1136/thorax.56.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley SC, Prefontaine D, Hamid Q. Images in allergy and immunology: Role of eosinophils in airway remodeling. J. Allergy Clin. Immunol. 2007;119:1563–1566. doi: 10.1016/j.jaci.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Fujisawa T, Fukuda S, Atsuta J, Ichimi R, Kamiya H, Sakurai M. Interferon-gamma induces interleukin-3 release from peripheral blood eosinophils. Int. Arch. Allergy Immunol. 1994;104(Suppl 1):41–43. doi: 10.1159/000236748. [DOI] [PubMed] [Google Scholar]
- Gleich GJ. Mechanisms of eosinophil-associated inflammation. J. Allergy Clin. Immunol. 2000;105:651–663. doi: 10.1067/mai.2000.105712. [DOI] [PubMed] [Google Scholar]
- Gleich GJ, Loegering DA, Maldonado JE. Identification of a major basic protein in guinea pig eosinophil granules. J. Exp. Med. 1973;137:1459–1471. doi: 10.1084/jem.137.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman M, Piliponsky AM, Temkin V, Levi-Schaffer F. Human peripheral blood eosinophils express stem cell factor. Blood. 2001;97:1086–1091. doi: 10.1182/blood.v97.4.1086. [DOI] [PubMed] [Google Scholar]
- Heijnen HF, Debili N, Vainchencker W, Breton-Gorius J, Geuze HJ, Sixma JJ. Multivesicular bodies are an intermediate stage in the formation of platelet alpha-granules. Blood. 1998;91:2313–2325. [PubMed] [Google Scholar]
- Horiuchi T, Weller PF. Expression of vascular endothelial growth factor by human eosinophils: Upregulation by granulocyte macrophage colony-stimulating factor and interleukin-5. Am. J. Respir. Cell. Mol. Biol. 1997;17:70–77. doi: 10.1165/ajrcmb.17.1.2796. [DOI] [PubMed] [Google Scholar]
- Jacobsen EA, Taranova AG, Lee NA, Lee JJ. Eosinophils: Singularly destructive effector cells or purveyors of immunoregulation? J. Allergy Clin. Immunol. 2007;119:1313–1320. doi: 10.1016/j.jaci.2007.03.043. [DOI] [PubMed] [Google Scholar]
- Karawajczyk M, Seveus L, Garcia R, Bjornsson E, Peterson CG, Roomans GM, Venge P. Piecemeal degranulation of peripheral blood eosinophils: A study of allergic subjects during and out of the pollen season. Am. J. Respir. Cell. Mol. Biol. 2000;23:521–529. doi: 10.1165/ajrcmb.23.4.4025. [DOI] [PubMed] [Google Scholar]
- Kita H, Adolphson C, Gleich G. Biology of eosinophils. In: Adkinson NF, Ellis EF, Middleton E, Reed CE, Yunginger JW, editors. Allergy: Principles and practice. 5 ed. Mosby: St. Louis; 1998. pp. 242–260. [Google Scholar]
- Lacy P, Moqbel R. Eosinophil cytokines. Chem. Immunol. 2000;76:134–155. doi: 10.1159/000058782. [DOI] [PubMed] [Google Scholar]
- Lacy P, Levi-Schaffer F, Mahmudi-Azer S, Bablitz B, Hagen SC, Velazquez J, Kay AB, Moqbel R. Intracellular localization of interleukin-6 in eosinophils from atopic asthmatics and effects of interferon gamma. Blood. 1998;91:2508–2516. [PubMed] [Google Scholar]
- Lacy P, Mahmudi-Azer S, Bablitz B, Hagen SC, Velazquez JR, Man SF, Moqbel R. Rapid mobilization of intracellularly stored RANTES in response to interferon-gamma in human eosinophils. Blood. 1999;94:23–32. [PubMed] [Google Scholar]
- Lacy P, Logan MR, Bablitz B, Moqbel R. Fusion protein vesicle-associated membrane protein 2 is implicated in IFN-gamma-induced piecemeal degranulation in human eosinophils from atopic individuals. J. Allergy Clin. Immunol. 2001;107:671–678. doi: 10.1067/mai.2001.113562. [DOI] [PubMed] [Google Scholar]
- Levi-Schaffer F, Lacy P, Severs NJ, Newman TM, North J, Gomperts B, Kay AB, Moqbel R. Association of granulocyte-macrophage colony-stimulating factor with the crystalloid granules of human eosinophils. Blood. 1995;85:2579–2586. [PubMed] [Google Scholar]
- Levi-Schaffer F, Barkans J, Newman TM, Ying S, Wakelin M, Hohenstein R, Barak V, Lacy P, Kay AB, Moqbel R. Identification of interleukin-2 in human peripheral blood eosinophils. Immunology. 1996;87:155–161. [PMC free article] [PubMed] [Google Scholar]
- Lewis DM, Lewis JC, Loegering DA, Gleich GJ. Localization of the guinea pig eosinophil major basic protein to the core of the granule. J. Cell Biol. 1978;77:702–713. doi: 10.1083/jcb.77.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KG, Wan HC, Bozza PT, Resnick MB, Wong DT, Cruikshank WW, Kornfeld H, Center DM, Weller PF. Human eosinophils elaborate the lymphocyte chemoattractants. IL-16 (lymphocyte chemoattractant factor) and RANTES. J. Immunol. 1996;156:2566–2570. [PubMed] [Google Scholar]
- Logan MR, Odemuyiwa SO, Moqbel R. Understanding exocytosis in immune and inflammatory cells: The molecular basis of mediator secretion. J. Allergy Clin. Immunol. 2003;111:923–932. quiz 933. [PubMed] [Google Scholar]
- Logan MR, Lacy P, Bablitz B, Moqbel R. Expression of eosinophil target snares as potential cognate receptors for vesicle-associated membrane protein-2 in exocytosis. J. Allergy Clin. Immunol. 2002;109:299–306. doi: 10.1067/mai.2002.121453. [DOI] [PubMed] [Google Scholar]
- Luini A, Ragnini-Wilson A, Polishchuck RS, De Matteis MA. Large pleiomorphic traffic intermediates in the secretory pathway. Curr. Opin. Cell Biol. 2005;17:353–361. doi: 10.1016/j.ceb.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Mahmudi-Azer S, Downey GP, Moqbel R. Translocation of the tetraspanin CD63 in association with human eosinophil mediator release. Blood. 2002;99:4039–4047. doi: 10.1182/blood.v99.11.4039. [DOI] [PubMed] [Google Scholar]
- Melo RCN, Weller PF, Dvorak AM. Activated human eosinophils. Int. Arch. Allergy Immunol. 2005a;138:347–349. doi: 10.1159/000089189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo RCN, Perez SAC, Spencer LA, Dvorak AM, Weller PF. Intragranular vesiculotubular compartments are involved in piecemeal degranulation by activated human eosinophils. Traffic. 2005b;6:866–879. doi: 10.1111/j.1600-0854.2005.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo RCN, Spencer LA, Perez SAC, Ghiran I, Dvorak AM, Weller PF. Human eosinophils secrete preformed, granulestored interleukin-4 (IL-4) through distinct vesicular compartments. Traffic. 2005c;6:1047–1057. doi: 10.1111/j.1600-0854.2005.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo RCN, Dvorak AM, Weller PF. Electron tomography and immunonanogold electron microscopy for investigating intracellular trafficking and secretion in human eosinophils. J. Cell. Mol. Med. 2008a;12:1416–1419. doi: 10.1111/j.1582-4934.2008.00346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo RCN, Spencer LA, Dvorak AM, Weller PF. Mechanisms of eosinophil secretion: Large vesiculotubular carriers mediate transport and release of granule-derived cytokines and other proteins. J. Leukoc. Biol. 2008b;83:229–236. doi: 10.1189/jlb.0707503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo RCN, Spencer LA, Perez SA, Neves JS, Bafford SP, Morgan ES, Dvorak AM, Weller PF. Vesicle-mediated secretion of human eosinophil granule-derived major basic protein. Lab. Invest. 2009;89:769–781. doi: 10.1038/labinvest.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller GM, de Jong TA, Overbeek SE, van der Kwast TH, Postma DS, Hoogsteden HC. Ultrastructural immunogold localization of interleukin 5 to the crystalloid core compartment of eosinophil secondary granules in patients with atopic asthma. J. Histochem. Cytochem. 1996a;44:67–69. doi: 10.1177/44.1.8543784. [DOI] [PubMed] [Google Scholar]
- Moller GM, de Jong TA, van der Kwast TH, Overbeek SE, Wierenga-Wolf AF, Thepen T, Hoogsteden HC. Immunolocalization of interleukin-4 in eosinophils in the bronchial mucosa of atopic asthmatics. Am. J. Respir. Cell. Mol. Biol. 1996b;14:439–443. doi: 10.1165/ajrcmb.14.5.8624248. [DOI] [PubMed] [Google Scholar]
- Moqbel R, Coughlin JJ. Differential secretion of cytokines. Sci. STKE. 2006;338:pe26. doi: 10.1126/stke.3382006pe26. (2006) [DOI] [PubMed] [Google Scholar]
- Moqbel R, Ying S, Barkans J, Newman TM, Kimmitt P, Wakelin M, Taborda-Barata L, Meng Q, Corrigan CJ, Durham SR. Identification of messenger RNA for IL-4 in human eosinophils with granule localization and release of the translated product. J. Immunol. 1995;155:4939–4947. [PubMed] [Google Scholar]
- Munitz A, Levi-Schaffer F. Eosinophils: 'new' roles for 'old' cells. Allergy. 2004;59:268–275. doi: 10.1111/j.1398-9995.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Yamada H, Iikura M, Miyamasu M, Izumi S, Shida H, Ohta K, Imai T, Yoshie O, Mochizuki M, Schroder JM, Morita Y, Yamamoto K, Hirai K. Intracellular localization and release of eotaxin from normal eosinophils. FEBS Lett. 1998;434:226–230. doi: 10.1016/s0014-5793(98)00863-1. [DOI] [PubMed] [Google Scholar]
- Nebenfuhr A, Ritzenthaler C, Robinson DG. Brefeldin A: Deciphering an enigmatic inhibitor of secretion. Plant Physiol. 2002;130:1102–1108. doi: 10.1104/pp.011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson-Dajotoy T, Andersson P, Bjartell A, Calafat J, Egesten A. Expression and production of the CXC chemokine growth-related oncogene-alpha by human eosinophils. J. Immunol. 2003;170:5309–5316. doi: 10.4049/jimmunol.170.10.5309. [DOI] [PubMed] [Google Scholar]
- Persson T, Monsef N, Andersson P, Bjartell A, Malm J, Calafat J, Egesten A. Expression of the neutrophil-activating CXC chemokine ENA-78/CXCL5 by human eosinophils. Clin. Exp. Allergy. 2003;33:531–537. doi: 10.1046/j.1365-2222.2003.01609.x. [DOI] [PubMed] [Google Scholar]
- Peters MS, Rodriguez M, Gleich GJ. Localization of human eosinophil granule major basic protein, eosinophil cationic protein, and eosinophil-derived neurotoxin by immunoelectron microscopy. Lab. Invest. 1986;54:656–662. [PubMed] [Google Scholar]
- Qadri F, Bhuiyan TR, Dutta KK, Raqib R, Alam MS, Alam NH, Svennerholm AM, Mathan MM. Acute dehydrating disease caused by vibrio cholerae serogroups 01 and 0139 induce increases in innate cells and inflammatory mediators at the mucosal surface of the gut. Gut. 2004;53:62–69. doi: 10.1136/gut.53.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raqib R, Moly PK, Sarker P, Qadri F, Alam NH, Mathan M, Andersson J. Persistence of mucosal mast cells and eosinophils in shigella-infected children. Infect. Immun. 2003;71:2684–2692. doi: 10.1128/IAI.71.5.2684-2692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin. Immunol. 2007;119:1303–1310. doi: 10.1016/j.jaci.2007.03.048. quiz 1311-1302. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, Hogan SP. The eosinophil. Annu. Rev. Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- Simpson JC, Nilsson T, Pepperkok R. Biogenesis of tubular ER-to-golgi transport intermediates. Mol. Biol. Cell. 2006;17:723–737. doi: 10.1091/mbc.E05-06-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht S, Saeftel M, Arndt M, Endl E, Dubben B, Lee NA, Lee JJ, Hoerauf A. Lack of eosinophil peroxidase or major basic protein impairs defense against murine filarial infection. Infect. Immun. 2006;74:5236–5243. doi: 10.1128/IAI.00329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer LA, Melo RCN, Perez SA, Bafford SP, Dvorak AM, Weller PF. Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc. Natl. Acad. Sci. USA. 2006;103:3333–3338. doi: 10.1073/pnas.0508946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer LA, Szela CT, Perez SA, Kirchhoffer CL, Neves JS, Radke AL, Weller PF. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J. Leukoc. Biol. 2009;85:117–123. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin LA, Kang BH. Nanoscale architecture of endoplasmic reticulum export sites and of Golgi membranes as determined by electron tomography. Plant Physiol. 2008;147:1454–1468. doi: 10.1104/pp.108.120618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai PC, Ackerman SJ, Spry CJ, Dunnette S, Olsen EG, Gleich GJ. Deposits of eosinophil granule proteins in cardiac tissues of patients with eosinophilic endomyocardial disease. Lancet. 1987;1:643–647. doi: 10.1016/s0140-6736(87)90412-0. [DOI] [PubMed] [Google Scholar]
- Torpier G, Colombel JF, Mathieu-Chandelier C, Capron M, Dessaint JP, Cortot A, Paris JC, Capron A. Eosinophilic gastroenteritis: Ultrastructural evidence for a selective release of eosinophil major basic protein. Clin. Exp. Immunol. 1988;74:404–408. [PMC free article] [PubMed] [Google Scholar]
- Toyoda M, Nakamura M, Makino T, Morohashi M. Localization and content of nerve growth factor in peripheral blood eosinophils of atopic dermatitis patients. Clin. Exp. Allergy. 2003;33:950–955. doi: 10.1046/j.1365-2222.2003.01719.x. [DOI] [PubMed] [Google Scholar]
- Trivedi SG, Lloyd CM. Eosinophils in the pathogenesis of allergic airways disease. Cell. Mol. Life Sci. 2007;64:1269–1289. doi: 10.1007/s00018-007-6527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco A, Polishchuk RS, Martella O, Di Pentima A, Fusella A, Di Giandomenico D, San Pietro E, Beznoussenko GV, Polishchuk EV, Baldassarre M, Buccione R, Geerts WJ, Koster AJ, Burger KN, Mironov AA, Luini A. Secretory traffic triggers the formation of tubular continuities across Golgi sub-compartments. Nat. Cell Biol. 2004;6:1071–1081. doi: 10.1038/ncb1180. [DOI] [PubMed] [Google Scholar]
- Watson P, Stephens DJ. ER-to-Golgi transport: Form and formation of vesicular and tubular carriers. Biochim. Biophys. Acta. 2005;1744:304–315. doi: 10.1016/j.bbamcr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Weller PF, Neves JS, Perez SA, Melo RCN, Spencer LA, Dvorak AM. Mechanisms of human eosinophil secretion. Cytokine. 2009;48:40. [Google Scholar]
- Wickner W, Schekman R. Membrane fusion. Nat. Struct. Mol. Biol. 2008;15:658–664. doi: 10.1038/nsmb.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woerly G, Lacy P, Younes AB, Roger N, Loiseau S, Moqbel R, Capron M. Human eosinophils express and release IL-13 following CD28-dependent activation. J. Leukoc. Biol. 2002;72:769–779. [PubMed] [Google Scholar]
- Wong DT, Weller PF, Galli SJ, Elovic A, Rand TH, Gallagher GT, Chiang T, Chou MY, Matossian K, McBride J. Human eosinophils express transforming growth factor alpha. J. Exp. Med. 1990;172:673–681. doi: 10.1084/jem.172.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying S, Meng Q, Taborda-Barata L, Corrigan CJ, Barkans J, Assoufi B, Moqbel R, Durham SR, Kay AB. Human eosinophils express messenger RNA encoding RANTES and store and release biologically active RANTES protein. Eur. J. Immunol. 1996;26:70–76. doi: 10.1002/eji.1830260111. [DOI] [PubMed] [Google Scholar]