Abstract
In vivo, termination of transcription at the attenuator site of the tryptophan (trp) operon of E. coli is influenced by the protein termination factor rho. In vitro, termination does not depend on rho factor, and is very efficient in a purified system consisting only of RNA polymerase, the DNA template, nucleoside triphosphates, and buffer. The extent of termination in this system is unaffected over a wide range of salt and nucleoside triphosphate concentration. However, there is a 10-fold stimulation of trp leader mRNA synthesis if rho factor is present during the transcription reaction. This stimulation occurs only at low molar ratios of polymerase to template, and can be blocked by rifampicin. It is thus most likely due to the recycling of RNA polymerase molecules that have been released from the attenuator site by rho factor. In fact, transcription of the trp leader region in vitro results in the fomration of a stable termination complex which can be observed on sucrose gradients or by binding to nitrocellulose filters. These data indicate that a major function of rho at the trp attenuator is to release completed transcripts from a pre-formed termination complex, rather than to cause the cessation of elongation.
Full text
PDF


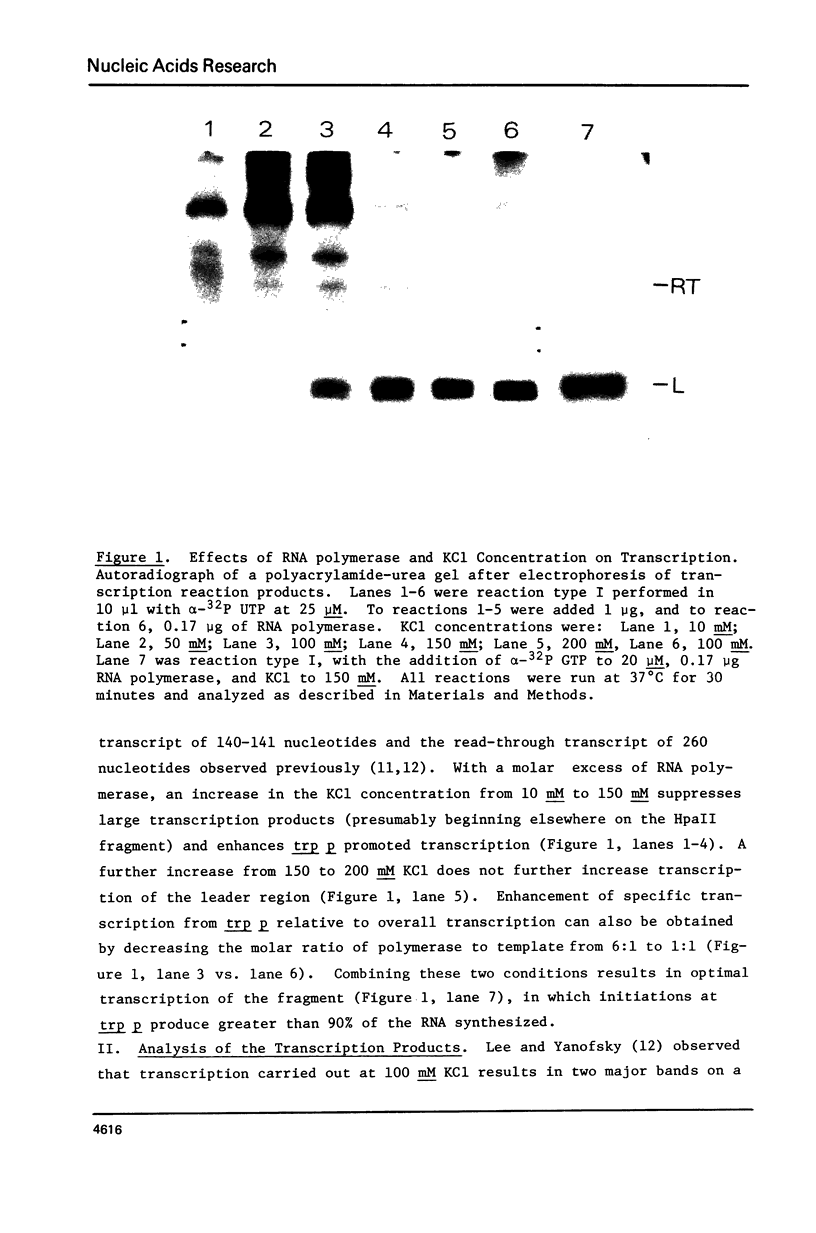







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P. Bacterial mutants in which the gene N function of bacteriophage lambda is blocked have an altered RNA polymerase. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2977–2981. doi: 10.1073/pnas.68.12.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysen A., Pironio M. Relationship between the N function of bacteriophage lambda and host RNA polymerase. J Mol Biol. 1972 Mar 28;65(2):259–272. doi: 10.1016/0022-2836(72)90281-1. [DOI] [PubMed] [Google Scholar]
- Grimberg J. I., Daniel V. In vitro transcription of E. coli tRNA genes. Nucleic Acids Res. 1977 Nov;4(11):3743–3752. doi: 10.1093/nar/4.11.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. P., Beckwith J. Mutant RNA polymerase of Escherichia coli terminates transcription in strains making defective rho factor. Proc Natl Acad Sci U S A. 1978 Jan;75(1):294–297. doi: 10.1073/pnas.75.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. P., Mitchell D. H., Beckwith J. Transcription termination at the end of the tryptophan operon of Escherichia coli. J Mol Biol. 1977 May 25;112(3):423–436. doi: 10.1016/s0022-2836(77)80190-3. [DOI] [PubMed] [Google Scholar]
- Hinkle D. C., Chamberlin M. J. Studies of the binding of Escherichia coli RNA polymerase to DNA. I. The role of sigma subunit in site selection. J Mol Biol. 1972 Sep 28;70(2):157–185. doi: 10.1016/0022-2836(72)90531-1. [DOI] [PubMed] [Google Scholar]
- Howard B. H., de Crombrugghe B. ATPase activity required for termination of transcription by the Escherichia coli protein factor rho. J Biol Chem. 1976 Apr 25;251(8):2520–2524. [PubMed] [Google Scholar]
- Howard B. H., de Crombrugghe B., Rosenberg M. Transcription in vitro of bacteriophage lambda 4S RNA: studies on termination and rho protein. Nucleic Acids Res. 1977 Apr;4(4):827–842. doi: 10.1093/nar/4.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer M., Neff N., Chamberlin M. J. Transcriptional termination at the end of the early region of bacteriophages T3 and T7 is not affected by polarity suppressors. J Virol. 1977 May;22(2):548–552. doi: 10.1128/jvi.22.2.548-552.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn L. J., Yanofsky C. Polarity suppressors defective in transcription termination at the attenuator of the tryptophan operon of Escherichia coli have altered rho factor. J Mol Biol. 1976 Sep 15;106(2):231–241. doi: 10.1016/0022-2836(76)90082-6. [DOI] [PubMed] [Google Scholar]
- Lee F., Squires C. L., Squires C., Yanofsky C. Termination of transcription in vitro in the Escherichia coli tryptophan operon leader region. J Mol Biol. 1976 May 15;103(2):383–393. doi: 10.1016/0022-2836(76)90318-1. [DOI] [PubMed] [Google Scholar]
- Lee F., Yanofsky C. Transcription termination at the trp operon attenuators of Escherichia coli and Salmonella typhimurium: RNA secondary structure and regulation of termination. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4365–4369. doi: 10.1073/pnas.74.10.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery C., Richardson J. P. Characterization of the nucleoside triphosphate phosphohydrolase (ATPase) activity of RNA synthesis termination factor p. II. Influence of synthetic RNA homopolymers and random copolymers on the reaction. J Biol Chem. 1977 Feb 25;252(4):1381–1385. [PubMed] [Google Scholar]
- Maizels N. M. The nucleotide sequence of the lactose messenger ribonucleic acid transcribed from the UV5 promoter mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3585–3589. doi: 10.1073/pnas.70.12.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Weissman S., deCrombrugghe B. Termination of transcription in bacteriophage lambda. Heterogeneous, 3'-terminal oligo-adenylate additions and the effects of rho factor. J Biol Chem. 1975 Jun 25;250(12):4755–4764. [PubMed] [Google Scholar]
- Yanofsky C. Mutations affecting tRNATrp and its charging and their effect on regulation of transcription termination at the attenuator of the tryptophan operon. J Mol Biol. 1977 Jul 15;113(4):663–677. doi: 10.1016/0022-2836(77)90229-7. [DOI] [PubMed] [Google Scholar]