Abstract
Klinefelter syndrome (KS) is a chromosomal condition (47, XXY) that may help us to unravel gene–brain behavior pathways to psychopathology. The phenotype includes social cognitive impairments and increased risk for autism traits. We used functional MRI to study neural mechanisms underlying social information processing. Eighteen nonclinical controls and thirteen men with XXY were scanned during judgments of faces with regard to trustworthiness and age. While judging faces as untrustworthy in comparison to trustworthy, men with XXY displayed less activation than controls in (i) the amygdala, which plays a key role in screening information for socio-emotional significance, (ii) the insula, which plays a role in subjective emotional experience, as well as (iii) the fusiform gyrus and (iv) the superior temporal sulcus, which are both involved in the perceptual processing of faces and which were also less involved during age judgments in men with XXY. This is the first study showing that KS can be associated with reduced involvement of the neural network subserving social cognition. Studying KS may increase our understanding of the genetic and hormonal basis of neural dysfunctions contributing to abnormalities in social cognition and behavior, which are considered core abnormalities in psychiatric disorders such as autism and schizophrenia.
Keywords: X chromosome, social cognition, autism, schizophrenia, amygdala, Klinefelter
INTRODUCTION
The study of de novo occurring genetic variations that are associated with neural, cognitive and behavioral abnormalities may increase our understanding of complex gene–brain behavior relations. In this regard, Klinefelter syndrome (KS), which is defined by the presence of an additional X chromosome in men, is of significant interest, especially considering that the X chromosome is enriched with genes involved in neural development and related cognitive and mental functioning. Even though behavioral outcome may be variable, group-wise analysis has indicated that on average, there is an increased vulnerability for difficulties in social competence in individuals with KS. Social competence refers to ‘the active and skilful coordination of multiple processes and resources available to the individual to meet social demands and achieve social goals in a particular type of social interaction and within a specific context’ (Iarocci et al., 2007). At a behavioral level the social difficulties in KS include social withdrawal, social anxiety, shyness, impulsivity, unassertiveness and emotion regulation problems (Ratcliffe, 1999; Boone et al., 2001; Geschwind and Dykens, 2004; van Rijn et al., 2006b, 2008; Visootsak and Graham, 2009). The severity of social difficulties is illustrated by the reported increased levels of autism traits such as problems in areas of social skills, communication, attention switching and imagination, and increased attention to details (van Rijn et al., 2008). Also, an increased risk for autism spectrum disorders (ASD) has been reported for individuals with XXY. Bruining et al. (2009) reported that 27% of the boys with KS in their sample (a mixed group of referred cases and prenatal follow-up) met criteria for ASD, and Bishop et al. (in press) found that 11% of the boys with KS in their sample (prenatal follow-up) had autism.
Social competence involves complex behaviors that heavily rely on an intact and efficient working of an intricate cognitive and neural system (Beauchamp and Anderson, 2010). It is generally thought that the social difficulties in KS are attributable to the language disablities observed in KS (Samango-Sprouse, 2001). A range of language deficits in the face of spared general intelligence is typically seen in individuals with KS (Geschwind et al., 2000; Boada et al., 2009). However, recent studies have indicated that deficits in social cognition and emotion processing may play an important role as well. These difficulties include impairments in decoding facial expressions of emotion, specifically anger (van Rijn et al., 2006b), as well as impairments in interpreting affective tone of voice, i.e. prosody (van Rijn et al., 2007). Abnormal experience of emotions in Klinefelter men has also been found (van Rijn et al., 2006b). In that study, Klinefelter men reported increased emotional arousal in response to emotion-inducing events and were more influenced by their emotions in a strategic decision making game. Difficulties in identification and expression of one’s own emotions were also found in this group, as indicated by the alexithymia measures. Taken together, Klinefelter men seem less accurate in perception of social–emotional cues, experience increased levels of emotional arousal, but are less able to identify and verbalize the emotions they experience, in comparison to the general population.
Findings of reductions in volume of brain areas important for social cognitive processing suggest that these social cognitive disturbances may have a direct neurobiological basis. Structural Magnetic Resonance Imaging (MRI) studies have pointed to volume reductions in the amygdala, insula, anterior cingulate and superior temporal gyrus in men with XXY (Patwardhan et al., 2002; Shen et al., 2004; DeLisi et al., 2005; Giedd et al., 2007; Steinman et al., 2009). However, it remains unclear whether and to what degree these neural networks are dysfunctioning during social information processing.
In this study, we investigated functioning of key brain regions involved in social information processing in adults with XXY. To our knowledge, this is the first study of functional brain mechanisms underlying social information processing in KS. Using functional Magnetic Resonance Imaging (fMRI) we measured activity in a neural network including the amygdala, fusiform gyrus, insula and superior temporal sulcus (STS) during trustworthiness evaluations of faces. We hypothesized atypical functioning of this network in men with KS. As a control condition, we also assessed brain activation when judging faces with regard to age, to examine whether functioning of this network is only disturbed when assessing social aspects of faces or also when assessing nonsocial, physical aspects of faces.
The observation of reduced engagement of neural networks underlying social cognition in men with the XXY karyotype would have several implications. First, it would suggest a direct neural basis for the social cognitive impairments that have been observed in men with KS. Second, it would support the notion of KS as a model for studying the role of the X chromosome, and androgens, in the development of brain areas subserving social cognition. Morover, the shared symptomatology in KS and ASD may fuel the generation of hypotheses with regard to genetic and hormonal mechanisms involved in the etiology of autism.
METHODS AND MATERIALS
Subjects
Thirteen men with XXY (mean age 39.0, s.d. 10.3) and 18 control men from the general population (mean age 32.2, s.d. 9.4) participated in the fMRI study. Controls were recruited using advertisements in local newspapers or were drawn from a database in our department. None of the control subjects had a history of psychiatric illness as confirmed with the Mini International Neuropsychiatric Interview plus (MINI) (Sheehan et al., 1998). Men with XXY were recruited with help of the Dutch Klinefelter Association, and were not preselected for psychological, behavioral or cognitive abnormalities. Symptoms preceding diagnosis in this sample were small testes, delayed puberty or infertility. Diagnosis of KS was confirmed by genetic analysis (i.e. karyotyping) using standard procedures. Of the 13 men with XXY, 11 were treated with testosterone supplements [mean age of treatment onset was 23.4 (s.d. 6.8), mean duration of testosterone treatment was 11.5 years (s.d. 6.3)]. Data on intellectual abilities as measured with the WAIS-III (Wechsler Adult Intelligence Scale) were available in our database for 11 of the 13 men with XXY (intellectual abilities were measured within one year of the fMRI session). Intelligence in men with XXY was within the normal range, with a mean score of 94.4 (s.d. 14.35) for general intelligence, a score of 96.3 (s.d. 14.9) for verbal intelligence and 93.7 (s.d. 12.0) for performance intelligence. There were no significant differences in age [t(1,29) = 3.5, P = 0.07] or years of education between the groups [Klinefelter group 14.9 (s.d. 2.1), control group 15.9 (s.d. 1.8), t(1,29) = 1.29, P = 0.18]. Exclusion criteria for both Klinefelter men and controls were IQ < 80, neurological conditions or history of head injury with loss of consciousness, recent history of substance abuse and intellectual disability. After complete description of the study (which was approved by the local ethical board) to the subjects, written informed consent was obtained according to the declaration of Helsinki.
Autism traits
The Autism-spectrum Quotient (AQ) (Baron-Cohen et al., 2001) is a self-administered questionnaire for adults that assesses the degree to which any individual adult of normal intelligence might have features of the core autistic phenotype. In a clinical population it has shown good test–retest reliability and good discriminative validity for Asperger syndrome at a cutoff score of 26 (Woodbury-Smith et al., 2005). In the general population, it has good discriminative validity for high functioning autism/Asperger syndrome using a cutoff score of 32 (Baron-Cohen et al., 2001). Scores on the AQ have shown to be normally distributed in the general population. Five subscales cover personality traits associated with the autistic spectrum; social skills, communication, imagination, attention to detail and attention switching.
Social judgment task
The psychological task used in the present study was an adapted version of a task used by Winston et al. (2002) and Baas et al. (2008). A scanning session lasted for 25 min and consisted of 16 task blocks with a duration of 45 s, 16 baseline blocks with a duration of 45 s and 16 instruction trials with a duration of 3 s. There were two types of task blocks and these types of task blocks were presented in random order for each subject. At the start of each task block, the word ‘age’ or ‘trustworthiness’ appeared on screen during an instruction trial to inform the subject of the task requirement. During eight task blocks, which were preceded by the word ‘age’, subjects had to decide whether the faces that were presented in the subsequent task block were older or younger than 30 years of age. In the other eight task blocks, which were preceded by the word ‘trustworthiness’, subjects had to judge whether the faces were trustworthy or untrustworthy. Task blocks consisted of 15 trials that were presented sequentially, and each trial consisted of a stimulus that was presented for 1 s followed by a fixation cross that was presented for 2 s. Face stimuli were presented once to each subject, randomized to the different task blocks and presented in random order across subjects. In total there were 120 face stimuli. Every task block was followed by a baseline block, during which a fixation-cross remained on screen. All stimuli, fixation crosses and instructions were presented on a gray (50% white) background. During scanning, decisions with regard to age and trustworthiness were indicated by button presses. In contrast to the study by Winston et al. (2002), we did not ask participants to also rate the faces with regard to trustworthiness after scanning, as these ratings may be different from during scanning due to repeated presentation and related increased familiarity. As our aim was to compare brain activation in men with KS to nonclinical controls during judgment of faces with regard to social aspects (trustworthiness) as well as during judgment of faces with regard to nonsocial aspects, i.e. age, data on judgments during scanning were sufficient.
Stimuli
Hundred and twenty frontal grayscale images of faces (60 men and 60 women) with a neutral emotional expression were used as stimuli. More than 80% of the photos were of Caucasian persons. These images were selected from a larger set of images on the basis of trustworthiness and emotional valence ratings given by 36 nonclinical subjects in a separate pilot study (9 men, 27 women; mean age 21.6, s.d. = 3.3). Of the images used in the present study, 75 were from the set that Adolphs et al. (1998) used in their study of social cognition in patients with bilateral amygdala damage. In order to obtain a sufficient number of images for our analysis, these images were supplemented with 45 images from the psychological image collection of the psychology department of Stirling University (PICS). The set of faces represented a balanced distribution of faces ranging from low- to high trustworthiness as judged in the pilot study. Note however, that for fMRI data analysis we used an event-related design in which the participants’ subjective trustworthiness ratings of faces, as obtained during scanning, were used.
Scans
Brain imaging data were acquired using a 1.5 T Philips ACS-NT scanner (Philips Medical Systems, Best, the Netherlands). For functional scans, 2D-EPI with BOLD (blood oxygenation level dependent) contrast was used, with the following parameters: echo time 40 ms; repetition time 2500 ms; flip angle 90 degrees; field of view 192 × 192 mm. Each volume comprised 33 axial scans with 2.2 mm slice thickness (and a gap of 0.8 mm). Thus, voxel size was 3 mm isotropic and volumes were continuously acquired every 2.5 s in an interleaved fashion (bottom slice first). In total, 600 volumes were acquired. In the scanner, the head was held in place with a strap and padding. Each run was preceded by six ‘dummy’ scans (which were not used in further analyses) to allow for T1 equilibration effects. Finally, a T1-weighted structural image was acquired.
Regions of interest
Regions of interest (ROI’s) were selected based on the findings in the fMRI study of Winston et al. (2002), showing that the amygdala, insula, fusiform gyrus and STS are key brain regions involved in judging trustworthiness from faces. Using the WFU Pickatlas tool for SPM (Maldjian et al., 2003), ROI’s for the fusiform area, insula and STS were formed based on the talairach atlas (in MNI space) and a ROI for the amygdala was formed based on the AAL atlas (in MNI space). Size of the ROI’s was 162 voxels for the amygdala, 1029 voxels for the fusiform gyrus, 1436 voxels for the insula and 3872 voxels for the STS.
fMRI analysis
Functional MRI data preprocessing and analysis was done using SPM2 (Wellcome Department of Imaging Neuroscience, London, England; www.fil.ion.ucl.ac.uk). First, all raw data were examined for motion and other imaging artifacts. After slice-timing correction, all functional scans were registered to the last volume of the last block and coregistered to the anatomical scan. Next, all functional images were registered to an MNI standard brain, to enable group-wise comparisons. All volumes were then smoothed with a 6 mm full-width, half-maximum isotropic Gaussian kernel. Time series were high-pass filtered with a cutoff of 128 s to remove low frequency signal changes.
In this event-related design, brain activity maps were obtained by analyzing the fMRI scans categorized according to individual (subjective) judgments of trustworthiness and age as indicated during scanning. For each subject, a statistical map (i.e. t-map) was obtained from a general linear model regression analysis using a factor matrix that contained a regressor modeling the onsets of faces that were judged to be untrustworthy, a regressor modeling the onsets of faces that were judged to be trustworthy and a regressor which modeled the onsets of faces that were judged during the age condition.
First, a whole brain analysis was performed (within group analyses for the groups separately) on activation related to trustworthiness decisions (all faces) vs baseline. In this analysis, a threshold of P = 0.001 (uncorrected) with an extend threshold of 10 voxels was set. Second, significant activation in each region of interest was determined in each voxel by applying a statistical treshold. The threshold corresponded to a P-value of 0.05, FWE (family-wise-error) corrected for multiple comparisons and resulted in the control group to a critical t-value of 3.70 for the amygdala, 4.80 for the fusiform gyrus, 5.22 for the insula and 5.73 for the STS. To enhance the interpretability of the within-group activation patterns, the thresholds that we applied in the XXY group were identical to those in the control group. Note however, that group differences in brain activation were explicitly tested in two-sample t-tests per voxel. Several averaged contrasts maps were created for both groups, namely: trustworthy minus baseline, untrustworthy minus baseline, untrustworthy minus trustworthy and age minus baseline. The averaged contrast maps for each group (men with XXY and controls) were subsequently entered in a two-sample t-test and differential task activation was defined as differential activation above a significance threshold of P < 0.001. As the two-sample t-test provides a measure of the difference in brain activation between the two groups, results from this test were crucial with regard to our research questions.
RESULTS
Autism traits
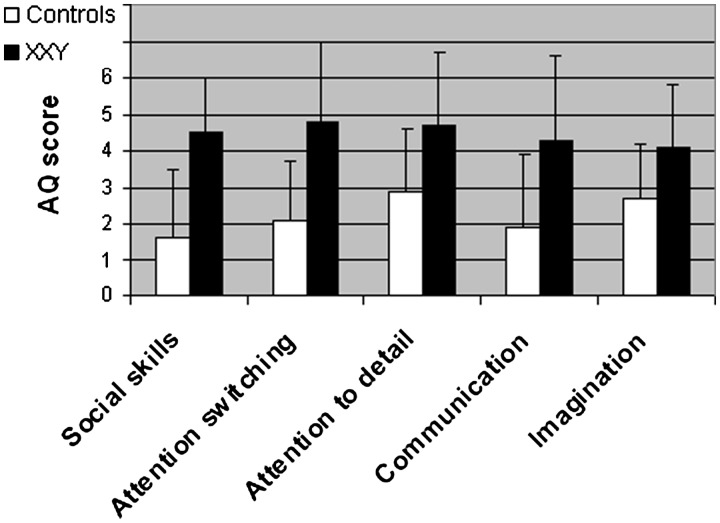
Data from three subjects in the control group were invalid due to incomplete questionnaires, and hence were left out the analyses involving the questionnaire. Mean total AQ score and all subscales separately were significantly higher in men with Klinefelter as compared to controls. Total score in the XXY group was 22.4 (s.d. 5.8) and in the control group 11.2 (s.d. 6.4), F(1,26) = 20.7, P < 0.001). As compared to men from the general population, men with XXY displayed more autism traits in all domains; social skills (F1,26) = 16.0, P < 0.001), attention switching (F1,26) = 13.7, P = 0.001, attention to detail (F1,26) = 6.2, P = 0.019), communication (F1,26) = 8.0, P = 0.009) and imagination [F(1,26) = 4.4, P = 0.045]. Effect sizes as expressed in Cohen’s d were 1.8 for total AQ score, 1.7 for social skills, 1.9 for attention switching, 1.0 for attention to detail, 1.1 for communication and 0.9 for imagination. See Figure 1 for mean scores and s.d.s on the five subscales of the AQ.
Fig. 1.
Levels of autism traits in men with XXY and men from the general population as measured with the AQ. Scores in all individual dimensions were significantly higher in men with XXY.
Behavioral performance
A repeated measures analysis with the factors ‘group’ (control, Klinefelter) and ‘judgement frequency’ (trustworthy, untrustworthy) indicated no significant main effect of group [F(1,29) = 0.45, P = 0.50] and no significant interactions between group and frequency of trustworthiness judgments [F(1,29) = 0.03, P = 0.87]. Within each group, no significant differences between the mean number of decisions of ‘trustworthy’ and ‘untrustworthy’ were observed, as indicated by post-hoc paired sample t-tests [t(1,12) = 1.21, P = 0.25 for men with XXY and t(1,17) = 1.70, P = 0.11 for controls].
Neural activations associated with judging trustworthiness of faces
Results of the within group whole-brain analyses (trustworthy and untrustworthy faces vs baseline) are presented in Table 1 for controls and Table 2 for men with XXY.
Table 1.
Areas with significant activation associated with trustworthiness judgments of faces in controls as indicated by a whole-brain analysis
| Regional peak activation | tmax value | zmax value | No. of voxels | x, y, z peak activation |
|---|---|---|---|---|
| Middle occipital gyrus | 8.99 | 5.39 | 528 | −48, −78, −12 |
| Lingual gyrus (region includes fusiform gyrus) | 8.47 | 5.23 | 1704 | −3, −75, 0 |
| Inferior frontal gyrus | 7.19 | 4.81 | 234 | 48, 6, 36 |
| Inferior frontal gyrus | 6.85 | 4.68 | 81 | 30, 30, −3 |
| Inferior frontal gyrus | 4.75 | 3.74 | 17 | 51, 36, −6 |
| Medial frontal gyrus | 6.88 | 4.69 | 90 | −6, 6, 48 |
| Insula | 6.53 | 4.56 | 61 | −33, 21, 0 |
| Cingulate gyrus | 6.53 | 4.56 | 18 | 9, 24, 30 |
| Caudate nucleus | 6.11 | 4.38 | 21 | −12, 9, 3 |
| Thalamus | 6.04 | 4.36 | 77 | 18, −24, 12 |
| Thalamus | 5.11 | 3.92 | 38 | −6, −18, 9 |
| Parahippocampal gyrus | 5.75 | 4.23 | 23 | −15, −3, 15 |
| Middle frontal gyrus | 5.27 | 4.00 | 26 | 36, 42, 27 |
| Middle frontal gyrus | 5.04 | 3.89 | 10 | 42, 42, 15 |
| Amygdala | 4.89 | 3.81 | 14 | 18, −6, −18 |
| Cerebellum | 4.38 | 3.54 | 11 | 24, 9, −9 |
Table 2.
Areas with significant activation associated with trustworthiness judgments of faces in men with XXY as indicated by a whole-brain analysis
| Regional peak activation | tmax value | zmax value | No. of voxels | x, y, z peak activation |
|---|---|---|---|---|
| Cuneus (occipital) | 9.80 | 5.05 | 17 | 21, −93, 18 |
| Fusiform gyrus | 8.93 | 4.86 | 64 | 45, −57, −24 |
| Fusiform gyrus | 5.28 | 3.73 | 26 | 33, −75, −18 |
| Lingual gyrus | 6.21 | 4.08 | 63 | 12, −87, −3 |
| Cerebellum | 6.15 | 4.06 | 14 | −18, −81, −21 |
| Cerebellum | 4.96 | 3.59 | 16 | −42, −57, −27 |
| Middle frontal gyrus | 6.05 | 4.02 | 17 | −39, 45, 12 |
With regard to our ROI, within-group analyses indicated significant activation in the fusiform gyrus in both groups when faces were judged trustworthy compared to baseline. In the control group the t-value was above threshold in 68 voxels (tmax = 7.9; x, y, z = −39, −69, −21), whereas in the XXY group this was 17 voxels (tmax = 8.2; x, y, z = 45, −57, −24). Indeed, a subsequent two-sample t-test (between group analyses) indicated slightly, but significantly, more activation in the fusiform gyrus in controls as compared to men with XXY (two voxels, tmax = 3.6; x, y, z = 30, −60, −15). No significant activation in the amygdala, STS or insula was observed in both groups.
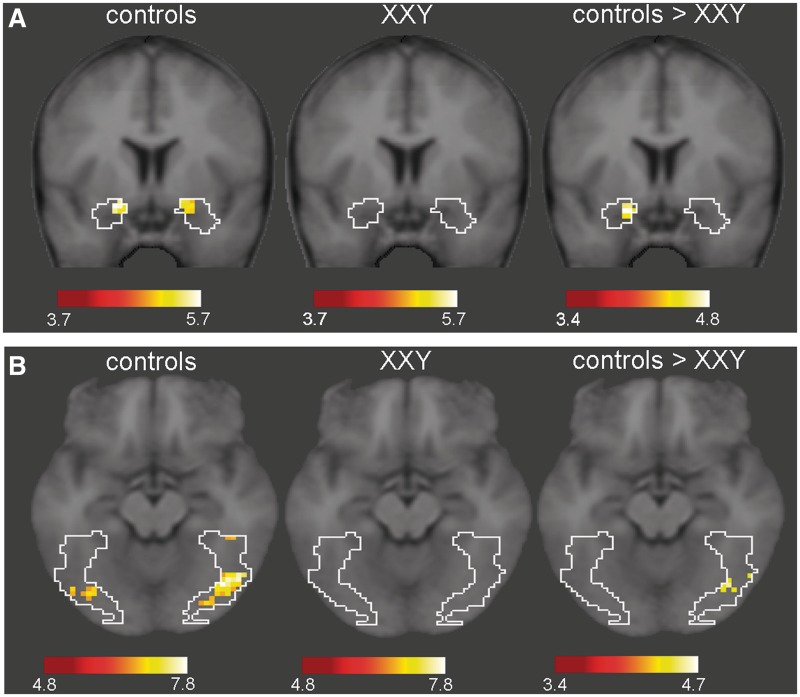
The crucial comparison was the difference in brain activation between the groups when faces were judged untrustworthy vs baseline (see Figure 2). Again, within group analyses of activation in our ROI’s indicated significant activation in the fusiform gyrus in the control group (62 voxels, tmax=7.8; x, y, z = 39, −69, −18) and the XXY group (one voxel, t = 4.9; x, y, z = 45, −57, −24). In addition, significant activation in the amygdala (bilaterally) was observed in the control group (16 voxels, tmax = 4.2; x, y, z = 21, −3, −18), but not in the XXY group. Between group analyses, i.e. a two-sample t-test, not only showed significantly more activation in the fusiform gyrus (17 voxels, tmax = 4.7; x, y, z = −30, −33, −24), including the fusiform face area (FFA, see Grill-Spector et al., 2004 for coordinates), but also in the amygdala (12 voxels, tmax = 4.77; x, y, z = −21, 3, −18) in the control group as compared to the XXY group. In order to assess whether group differences were specifically present for faces that convey threat and distrust, we examined group differences in brain activation when faces were judged untrustworthy as compared to trustworthy. Comparing the contrast maps untrustworthy minus trustworthy between the groups (using two-sample t-tests) revealed that activation specifically associated with distrust (untrustworthy minus trustworthy) significantly differed between the groups in the amygdala (four voxels, tmax = 3.2; P = 0.002), as well as in the insula (12 voxels, tmax = 2.9; P = 0.004), STS (39 voxels, tmax = 3.2, P = 0.002) and in the fusiform gyrus (11 voxels, tmax = 3.1, P = 0.002), which included part of the fusiform face area. Less activation in these area’s was observed in XXY men as compared to controls.
Fig. 2.
T-maps showing voxels in the amygdala (A) and the fusiform gyrus (B) with a t-value above threshold (P < 0.05, FWE corrected for multiple comparisons) during untrustworthy judgments of faces in the control group and XXY group.
Neural activations associated with judging age of faces
When judging the age of the presented faces, within group analyses showed significant activation (as compared to baseline) in the selected ROI’s in both groups. In controls, significant activation in the fusiform gyrus (143 voxels, tmax = 10.2; x, y, z = −39, −48, −24), insula (12 voxels, tmax = 6.6; x, y, z = −33, 18, 9) and amygdala (two voxels, tmax = 4.9; x, y, z = 21, −3, −12) was observed. In men with XXY, significant activation was found in the fusiform gyrus (30 voxels, tmax = 7.8; x, y, z = 42, −54, −21) and slight activation in the insula (one voxel, t = 6.1; x, y, z = −39, 12, 15) and amygdala (one voxel, t = 3.9; x, y, z = −24, −3, −27). Between group analyses, i.a. a two-sample t-test, showed that activation in the fusiform gyrus (11 voxels, tmax = 4.3; x, y, z = −30, −66, −15) and STS (12 voxels, tmax = 4.05; x, y, z = −45, −54, 12), but not in the amygdala and insula, was significantly stronger in in the control group than in the XXY group.
DISCUSSION
This fMRI study examined brain activation patterns during social evaluation of faces in men with an extra X chromosome (XXY chromosomal pattern) as compared to men from the general population. Based on previous studies using a comparable paradigm (Winston et al., 2002; Baas et al., 2008), we focused on four ROI: the amygdala, the fusiform gyrus, the insula and the STS. Our main interest was in brain activation during judgements of trustworthiness. As expected, men from the general population displayed significant activation in the amygdala and fusiform gyrus when judging faces as untrustworthy. These brain regions are key neural structures subserving the perception of social signals from faces. Compared to control men, men with XXY displayed decreased activation in the amygdala and fusiform gyrus during judging faces as untrustworthy. Less involvement of the amygdala and fusiform gyrus, together with reduced activation in the STS and insula, in men with XXY appeared to be specifically related to processing of threat and distrust (untrustworthy faces as compared to trustworthy faces). A second analysis was performed on brain activation during nonsocial (age) judgements. As expected, we observed activation in the amygdala, fusiform gyrus and insula in men from the general population when faces were evaluated with regard to age. During age-judgments less activation in the fusiform gyrus and STS was observed in men with XXY as compared to control men. Although speculative, our findings suggest that in men with XXY, the areas involved in perceptual processing, i.e. the fusiform gyrus and STS, may be less involved in evaluation of faces in general, as less activation was observed during judgments of both trustworthiness and age. The amygdala and insula may be specifically less involved when detecting threat from faces, rather than evaluations of faces in general, as less activation was only observed during trustworthiness judgments and specifically during untrustworthy as compared to trustworthy faces.
The amygdala plays a central role in the neural circuit subserving social cognition and is known for its engagement in screening information for emotional and social significance, especially threat-related information (Amaral, 2003; Phelps, 2006). As expressions on faces provide a crucial source of information needed for decoding social and emotional signals, the amygdala generally activates in response to facial expressions, especially to faces that indicate threat (Adolphs, 2001; Haxby et al., 2002; Phan et al., 2002). Reduced amygdala involvement in men with XXY was specifically related to processing of threat and distrus, as distrust related activity (untrustworthy minus trustworthy) was lower in men with XXY as compared to controls. This suggests that involvement of the amygdala, in men with Klinefelter is less when faces convey negative social signals such as threat. This would be in line with neuro-cognitive studies on KS showing that the identification of happy faces is intact, but that identification of angry faces is impaired (van Rijn et al., 2006b). Interestingly, reduced engagement of the amygdala in men with XXY as found in this study is in line with findings of volume reductions of this area that have been observed in structural MRI studies in KS (Patwardhan et al., 2002; Shen et al., 2004; Giedd et al., 2007; Steinman et al., 2009).
Interstingly, similar to findings in the amygdala there were other regions that showed lower distrust-related activation in men with XXY as compared to controls. This was the insula, which is involved in the subjective, physiological experience of emotion and the STS, which is involved in perceptual processing of faces. We can not exclude however, that reduced activation in these regions was in part due to less involvement of the amygdala signaling social and emotional significance.
One of the areas that receives input from the amygdala is the fusiform gyrus, a region in the occipital cortex that includes the ‘fusiform face area’ (FFA) (Puce et al., 1996). This area appears to be important for visual processing of the structural, static properties of faces, which are used to determine personal identity (Haxby et al., 2000; Adolphs, 2001). Morris et al. (1998) have shown that activation in the fusiform gyrus can be modulated by activation in the amygdala. They suggested that increasing threat-related social significance as processed in the amygdala may elicit re-allocation of attentional resources to allow detailed visual analysis of a socially significant stimulus. Involvement of the fusiform gyrus was less in men with XXY as compared to controls during processing of untrustworthy faces, and also during evaluation of faces with regard to age. Distrust related activity (untrustworthy minus trustworthy) was also lower in the XXY group than controls. This suggests that in the XXY group, the fusiform gyrus may be less involved in evaluation of faces in general.
Our observations of reduced engagement of a neural network for social cognition in men with XXY has several implications. First, abnormal engagement of a neural network subserving social information processing may underlie some of the social cognitive dysfunctions and impaired social adaptation that have been described in men with XXY (Geschwind and Dykens, 2004; Simm and Zacharin, 2006; van Rijn et al., 2006b, 2007). In line with other studies on vulnerability for ASD (Bruining et al., 2009; Bishop et al., in press) and in accordance with a previous report on increased levels of autism traits in a larger sample men with XXY in our department (of which the present is a subsample) (van Rijn et al., 2008), levels of autism traits were higher in the XXY group. Effect sizes were large, ranging from 0.9 (Imagination) to 1.7 (Social skills) for the various subscales of the AQ. Our present findings provide a specific neuro-anatomical basis for the social behavioral phenotype in KS.
Second, we can extrapolate from these findings that the X chromosome may play an important role, directly or indirectly through androgen effects, in the development of some of the brain areas subserving social cognition. Besides possible direct genetic effects on neural development in KS, hormonal effects should also be considered. KS is associated with hypogonadism and levels of testosterone are often abnormal. Although prenatal and infant levels of testosterone might be increased (Ratcliffe et al., 1994; but see Ross et al., 2005; Aksglaede et al., 2007), low testosterone levels are typically seen from puberty onwards (Salbenblatt et al., 1985; Aksglaede et al., 2008). It is thought that during development circulating gonadal hormones can modify brain structure and -function of target areas. Interestingly, as shown by animal studies, one of the major target areas of testosterone is the amygdala (Simerly et al., 1990). However, the relation between testosterone levels and neural development in KS is probably complex; timing of exposure, sensitivity to testosterone reflected in androgen receptor density and modulation by environmental factors such as androgen supplements are important determinants in the effects of testosterone (Craig et al., 2004). It will be interesting to explore the role of the X chromosome and tesosterone deficiencies in future studies.
Third, our observations may help in understanding pathways to psychopathology that extend beyond KS. Deviant social behavior and impairments in social cognitive functions are considered core abnormalities in neurodevelopmental disorders such as autism and schizophrenia (Fein et al., 1986; Corrigan and Penn, 2001; Abdi and Sharma, 2004). Neuroimaging studies have consistently shown abnormalities of the amygdala and fusiform gyrus in both autism and schizophrenia (Abdi and Sharma, 2004). Interestingly, these disorders are more prevalent in men. ASD are diagnosed approximately four times more often in boys than in girls (Volkmar et al., 1993). This fact has led others to propose that dysfunctional neural circuits underlying social cognitive impairments in autism may be related to genes on the X chromosome, that are differentially expressed in men and women (Skuse, 2000; Baron-Cohen et al., 2005). However, such dysfunctions may not be specific for autism spectrum pathology, but may also be related to schizophrenia spectrum pathology. Similarities between the autism and schizophrenia spectrum have also been observed with regard to the neurobiological underpinnings of impaired social cognition. When asking individuals to judge faces with regard to trustworthiness, i.e. attribute intentions to others, fMRI shows very similar patterns of brain activation in individuals with an autism-spectrum disorder and paranoid schizophrenia patients (Pinkham et al., 2008). Our studies on KS also suggest an overlap in social-cognitive dysfunctions associated with autistic and psychotic behavior. Men with KS display various social-cognitive deficits (van Rijn et al., 2006b) and do not only show increased levels of autistic traits but also schizotypal traits (van Rijn et al., 2006a) which is in line with a described increased risk for psychotic disorders (Bojesen et al., 2006; Boks et al., 2007).
A limitation of this study was that IQ scores were not available for all subjects. However, it has been shown that IQ scores are strongly correlated with the number of years of education individuals receive (Brody, 1992). Based on such findings, the general consensus is that IQ measures a characteristic of persons that also influences their educational experiences such as years of education, independent of socio-economic background (Brody, 1992), and that ‘number of years of education’ can be seen as a proxy of intellectual functioning. As the groups were carefully matched on this parameter (which was available for all subjects), we are confident that the groups were very comparable with regard to level of intellectual functioning. Another limitation is that the XXY group was too small to perform correlational analysis between percentage signal change in specific brain areas and scores on the autism questionnaire.
In future studies, it would be interesting to assess the role of hormonal imbalances (testosterone) in relation to anatomy and function of the amygdala. Also it would be interesting to more thoroughly examine how men with KS form complex social judgments. The present study showed that the frequency of untrustworthy judgments was equal in both group, but apparently different neural circuits are involved in making these judgments.
In sum, this study has revealed reduced engagement of the amygdala, fusiform gyrus, insula and STS during social information processing in men with XXY as compared to men from the general population. Compromised function of these areas may underlie some of the deficits in social cognition and vulnerability for autism that have been observed in XXY boys and men. In addition, as KS is defined by an X chromosomal abnormality and related androgen dysfunctions, our findings suggest a link between these developmental mechanisms and maturation of some of the neural regions supporting social cognition. In addition, our data suggest that KS may serve as a more general model for studying the genetic and hormonal basis of developmental abnormalities in social cognition. This may be particularly relevant since these deficits are considered core abnormalities in severe psychiatric disorders such as autism.
FUNDING
This work was supported by a VernieuwingsImpuls grant [016.026.027 to A.A.] and a Veni grant [016.095.060 to S.v.R.] from the Netherlands Organization for Scientific Research (NWO).
Conflict of Interest
None declared.
REFERENCES
- Abdi Z, Sharma T. Social cognition and its neural correlates in schizophrenia and autism. CNS Spectrums. 2004;9(5):335–43. doi: 10.1017/s1092852900009317. [DOI] [PubMed] [Google Scholar]
- Adolphs R. The neurobiology of social cognition. Current Opinion in Neurobiology. 2001;11(2):231–9. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393(6684):470–4. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Aksglaede L, Petersen JH, Main KM, Skakkebaek NE, Juul A. High normal testosterone levels in infants with non-mosaic Klinefelter’s syndrome. European Journal of Endocrinology. 2007;157(3):345–50. doi: 10.1530/EJE-07-0310. [DOI] [PubMed] [Google Scholar]
- Aksglaede L, Skakkebaek NE, Juul A. Abnormal sex chromosome constitution and longitudinal growth: serum levels of insulin-like growth factor (IGF)-I, IGF binding protein-3, luteinizing hormone, and testosterone in 109 males with 47,XXY, 47,XYY, or sex-determining region of the y chromosome (SRY)-Positive 46,XX Karyotypes. Journal of Clinical Endocrinology & Metabolism. 2008;93(1):169–76. doi: 10.1210/jc.2007-1426. [DOI] [PubMed] [Google Scholar]
- Amaral DG. The amygdala, social behavior, and danger detection. Annals of the New York Academy of Sciences. 2003;1000(1):337–47. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, Vink M, Ramsey NF, de Haan EHF, Kahn RS. Evidence of altered cortical and amygdala activation during social decision-making in schizophrenia. Neuroimage. 2008;40(2):719–27. doi: 10.1016/j.neuroimage.2007.12.039. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: implications for explaining autism. Science. 2005;310(5749):819–23. doi: 10.1126/science.1115455. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders. 2001;31(1):5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Anderson V. SOCIAL: an integrative framework for the development of social skills. Psychological Bulletin. 2010;136(1):39–64. doi: 10.1037/a0017768. [DOI] [PubMed] [Google Scholar]
- Bishop DV, Jacobs PA, Lachlan K, et al. Autism, language and communication in children with sex chromosome trisomies. Archives of Disease in Childhood. in press doi: 10.1136/adc.2009.179747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boada R, Janusz J, Hutaff-Lee C, Tartaglia N. The cognitive phenotype in Klinefelter syndrome: a review of the literature including genetic and hormonal factors. Developmental Disabilities Research Reviews. 2009;15(4):284–94. doi: 10.1002/ddrr.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojesen A, Juul S, Birkebaek NH, Gravholt CH. Morbidity in Klinefelter syndrome; a Danish register study based on hospital discharge diagnoses. The Journal of Clinical Endocrinology and Metabolism. 2006;91(4):1254–60. doi: 10.1210/jc.2005-0697. [DOI] [PubMed] [Google Scholar]
- Boks MPM, de Vette MHT, Sommer IE, et al. Psychiatric morbidity and X-chromosomal origin in a Klinefelter sample. Schizophrenia Research. 2007;93(1–3):399–402. doi: 10.1016/j.schres.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Boone KB, Swerdloff RS, Miller BL, et al. Neuropsychological profiles of adults with Klinefelter syndrome. Journal of the International Neuropsychological Society. 2001;7(4):446–56. doi: 10.1017/s1355617701744013. [DOI] [PubMed] [Google Scholar]
- Brody N. Intelligence. New York: Academic Press; 1992. [Google Scholar]
- Bruining H, Swaab H, Kas M, Van Engeland H. Psychiatric characteristics in a self-selected sample of boys with klinefelter syndrome. Pediatrics. 2009;123(5):e865–70. doi: 10.1542/peds.2008-1954. [DOI] [PubMed] [Google Scholar]
- Corrigan, P.W., Penn, D.L., editors. (2001). Social Cognition and Schizophrenia. Washington, DC: American Psychological Association.
- Craig IW, Harper E, Loat CS. The genetic basis for sex differences in human behaviour: role of the sex chromosomes. Annals of Human Genetics. 2004;68:269–84. doi: 10.1046/j.1529-8817.2004.00098.x. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Maurizio AM, Svetina C, et al. Klinefelter’s syndrome (XXY) as a genetic model for psychotic disorders. American Journal of Medical Genetics B Neuropsychiatric Genetics. 2005;135(1):15–23. doi: 10.1002/ajmg.b.30163. [DOI] [PubMed] [Google Scholar]
- Fein D, Pennington B, Markowitz P. Toward a neuropsychological model of infantile autism: are the social deficits primary? Journal of the American Academy of Child Psychiatry. 1986;25(2):198. doi: 10.1016/s0002-7138(09)60227-2. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Boone KB, Miller BL, Swerdloff RS. Neurobehavioral phenotype of Klinefelter syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2000;6(2):107–16. doi: 10.1002/1098-2779(2000)6:2<107::AID-MRDD4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Dykens E. Neurobehavioral and psychosocial issues in Klinefelter syndrome. Learning Disabilities Research & Practice. 2004;19(3):166–73. [Google Scholar]
- Giedd JN, Clasen LS, Wallace GL, et al. XXY (Klinefelter syndrome): a pediatric quantitative brain magnetic resonance imaging case-control study. Pediatrics. 2007;119(1):e232. doi: 10.1542/peds.2005-2969. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nature Neuroscience. 2004;7(5):555. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4(6):223. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biological Psychiatry. 2002;51(1):59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- Iarocci G, Yager J, Elfers T. What gene-environment interactions can tell us about social competence in typical and atypical populations. Brain and Cognition. 2007;65(1):112–27. doi: 10.1016/j.bandc.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Büchel C, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121(1):47. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Patwardhan AJ, Brown WE, Bender BG, Linden MG, Eliez S, Reiss AL. Reduced size of the amygdala in individuals with 47,XXY and 47,XXX karyotypes. American Journal of Medical Genetics. 2002;114(1):93–8. doi: 10.1002/ajmg.10154. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- PICS. Psychological Image Collection at Stirling (PICS) from the University of Stirling Psychology Department. http://pics.psych.stir.ac.uk/
- Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophrenia Research. 2008;99(1–3):164–75. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: a functional magnetic resonance imaging study. Journal of Neuroscience. 1996;16(16):5205. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe S. Long-term outcome in children of sex chromosome abnormalities. Archives of Disease in Childhood. 1999;80(2):192–5. doi: 10.1136/adc.80.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe SG, Read G, Pan H, Fear C, Lindenbaum R, Crossley J. Prenatal testosterone levels in XXY and XYY males. Hormone Research. 1994;42(3):106–09. doi: 10.1159/000184157. [DOI] [PubMed] [Google Scholar]
- Ross JL, Samango-Sprouse C, Lahlou N, Kowal K, Elder FF, Zinn A. Early androgen deficiency in infants and young boys with 47,XXY Klinefelter syndrome. Hormone Research. 2005;64(1):39. doi: 10.1159/000087313. [DOI] [PubMed] [Google Scholar]
- Salbenblatt JA, Bender BG, Puck MH. Pituitary-gonadal function in Klinefelter syndrome before and during puberty. Pediatric Research. 1985;19(1):82–6. doi: 10.1203/00006450-198501000-00022. [DOI] [PubMed] [Google Scholar]
- Samango-Sprouse C. Mental development in polysomy X Klinefelter syndrome (47,XXY; 48,XXXY): effects of incomplete X inactivation. Seminars in Reproductive Medicine. 2001;19(2):193–202. doi: 10.1055/s-2001-15400. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl. 20):22–33; quiz 34–57. [PubMed] [Google Scholar]
- Shen D, Liu D, Liu H, Clasen L, Giedd J, Davatzikos C. Automated morphometric study of brain variation in XXY males. Neuroimage. 2004;23(2):648–53. doi: 10.1016/j.neuroimage.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen-receptor messenger RNA-containing cells in the rat-brain - an insitu hybridization study. Journal of Comparative Neurology. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simm PJ, Zacharin MR. The psychosocial impact of Klinefelter syndrome - a 10 year review. Journal of Pediatric Endocrinology and Metabolism. 2006;19(4):499–505. [PubMed] [Google Scholar]
- Skuse DH. Imprinting, the X-chromosome, and the male brain: explaining sex differences in the liability to autism. Pediatric Research. 2000;47(1):9–16. doi: 10.1203/00006450-200001000-00006. [DOI] [PubMed] [Google Scholar]
- Steinman K, Ross J, Lai S, Reiss A, Hoeft F. Structural and functional neuroimaging in Klinefelter (47,XXY) syndrome: a review of the literature and preliminary results from a functional magnetic resonance imaging study of language. Developmental Disabilities Research Reviews. 2009;15(4):295–308. doi: 10.1002/ddrr.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn S, Aleman A, Swaab H, Kahn R. Klinefelter’s syndrome (karyotype 47,XXY) and schizophrenia-spectrum pathology. British Journal of Psychiatry. 2006a;189(5):459–61. doi: 10.1192/bjp.bp.105.008961. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Swaab H, Aleman A, Kahn RS. X Chromosomal effects on social cognitive processing and emotion regulation: a study with Klinefelter men (47,XXY) Schizophrenia Research. 2006b;84(2–3):194–203. doi: 10.1016/j.schres.2006.02.020. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Aleman A, Swaab H, Krijn T, Vingerhoets G, Kahn R. What it is said versus how it is said: comprehension of affective prosody in men with Klinefelter (47,XXY) syndrome. Journal of the International Neuropsychological Society (JINS) 2007;13(6):1065–70. doi: 10.1017/S1355617707071044. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Swaab H, Aleman A, Kahn RS. Social behavior and autism traits in a sex chromosomal disorder: Klinefelter (47XXY) syndrome. Journal of Autism and Developmental Disorders. 2008;38(9):1634–41. doi: 10.1007/s10803-008-0542-1. [DOI] [PubMed] [Google Scholar]
- Visootsak J, Graham JM., Jr Social function in multiple X and Y chromosome disorders: XXY, XYY, XXYY, XXXY. Developmental Disabilities Research Reviews. 2009;15(4):328–32. doi: 10.1002/ddrr.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR, Szatmari P, Sparrow SS. Sex differences in pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1993;V23(4):579–91. doi: 10.1007/BF01046103. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O’Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience. 2002;5(3):277–83. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Woodbury-Smith MR, Robinson J, Wheelwright S, Baron-Cohen S. Screening adults for asperger syndrome using the AQ: a preliminary study of its diagnostic validity in clinical practice. Journal of Autism and Developmental Disorders. 2005;V35(3):331–5. doi: 10.1007/s10803-005-3300-7. [DOI] [PubMed] [Google Scholar]