Abstract
Objective
The error-related negativity (ERN) is a negative deflection in the event-related potential following an incorrect response, which is often increased in patients with obsessive-compulsive disorder (OCD). However, the relationship of the ERN to comorbid tic disorders has not been examined in patients with OCD. This study compared ERN amplitudes in patients with tic-related OCD, patients with non-tic-related OCD, and healthy controls.
Method
The ERN, correct response negativity, and error number were measured during an Eriksen flanker task to assess performance monitoring in 44 youth with a lifetime diagnosis of OCD and 44 matched healthy controls ranging in age from 10 to 19 years. Nine youth with OCD had a lifetime history of tics.
Results
ERN amplitudewas significantly increased in OCD patients compared to healthy controls. ERN amplitude was significantly larger in patients with non-tic-related OCD than either patients with tic-related OCD or controls. ERN amplitude had a significant negative correlation with age in healthy controls but not patients with OCD. Instead, in patients with non-tic-related OCD, ERN amplitude had a significant positive correlation with age at onset of OCD symptoms. ERN amplitude in patients was unrelated to OCD symptom severity, current diagnostic status, or treatment effects.
Conclusions
The results provide further evidence of increased error-related brain activity in pediatric OCD. The difference in the ERN between patients with tic-related and non-tic-related OCD provides preliminary evidence of a neurobiological difference between these two OCD subtypes. The results indicate the ERN is a trait-like measure that may serve as a biomarker for non-tic-related OCD.
Keywords: anxiety disorder, tic disorder, brain potential, performance monitoring, biomarker
Introduction
Obsessive-compulsive disorder (OCD) is a heterogeneous condition with lifetime prevalence estimates ranging from 1% to 3%.1 The National Comorbidity Survey Replication found a median age at onset of 19 years in OCD, with 21% of cases starting by age 10.1 About 10% to 40% of OCD cases diagnosed in childhood or adolescence have a lifetime history of a chronic tic disorder.2 It has been proposed that some forms of OCD are etiologically related to chronic tic disorders.2–5 Tic-related OCD is characterized by male predominance.2,3 The distinction between tic-related and non-tic-related OCD has been supported by studies of familial aggregation,4,5 treatment response,6–8 outcome,9 prolactin release,10 and prepulse inhibition.11
Functional brain imaging studies indicate the pathophysiology of OCD involves increased activity in cortico-striatal circuits connecting the anterior cingulate cortex (ACC) with other brain regions.12,13 Functional magnetic resonance imaging (MRI) studies of patients with OCD have found increased error-related brain activity localized to the ACC.14 The observation of increased error-related brain activity in patients with OCD is consistent with the hypothesis that OCD involves defects in an error-detection system, which may give rise to repeated doubts about actions and excessive worries about potential mistakes.15 It is unclear, however, whether increased error-related brain activity may contribute to the expression or suppression of OCD symptoms. Consistent with the latter possibility, improvement in OCD symptoms during intensive cognitive-behavioral therapy was associated in an imaging study with a significant increase in dorsal ACC metabolic activity.12
The error-related negativity (ERN)16,17 or error negativity (Ne)18 is a frontally maximal negative deflection in the response-locked event-related potential that peaks within 100 msec after an incorrect response, which can be evoked by errors committed outside of conscious awareness.19 Studies using fMRI,20 magnetoencephalography,21 and dipole source localization22 have suggested the ERN is generated mainly by the dorsal ACC. ERN amplitude generally increases with age, which may reflect ACC maturation.23 The ERN has been hypothesized to reflect error detection, response conflict, or reward prediction errors in which outcomes are worse than expected.17 The ERN shows substantial heritability, suggesting it may serve as an endophenotype in genetic studies of psychopathology.24
In studies using tasks eliciting response conflict, ERN amplitude has been increased in adult patients with OCD25–32 and young adults with self-reported obsessive-compulsive (OC) symptoms.33,34 Some of those studies also found that serotonergic antidepressants had no effect on the ERN in patients with OCD.29,31 ERN amplitude has also been increased in studies of pediatric patients with OCD34 and children with parent-reported OC symptoms.35 In the study of pediatric OCD with 18 patients and 18 controls, the ERN did not change as a function of the reduction in OCD symptoms with cognitive-behavioral therapy, indicating that increased ACC activity during response monitoring does not necessarily maintain OCD symptoms and that an increase in this brain potential may serve as a trait marker for OCD.34,36 Similarly, increased ERN amplitude has been found not only in patients with OCD but also in unaffected first-degree relatives of OCD probands, providing further evidence that overactive error monitoring may provide a biomarker for OCD that is independent of the presence of clinical symptoms.30
Enhanced ERN amplitude has been described in patients with Tourette’s disorder, although the majority of the patients in that study had significant obsessive-compulsive symptoms.37 An MRI study of siblings concordant for Tourette’s disorder found thinning in the cingulate cortex that was associated with higher OC symptom severity.38 Furthermore, a functional brain imaging study of patients with Tourette’s disorder found decreased metabolic activity in the ACC and dorsolateral prefrontal cortical regions that was associated with higher OC symptom severity.39 Hence, there may be substantial difference between patients with OCD and patients with Tourette’s disorder in ACC activity,12–14,38,39 suggesting that the ERN may be larger in patients with non-tic-related OCD than in patients with tic-related OCD.
Since the ERN has been examined to a limited extent in pediatric OCD34 and tic disorders,37 the following study was conducted with 44 youth with a lifetime diagnosis of OCD and 44 age-matched healthy controls using a flanker task that elicits response conflict. The primary aim was to determine that the ERN is larger in patients with OCD than controls and, more specifically, larger in patients with non-tic-related OCD than either patients with tic-related OCD or controls. The secondary aim was to examine the correlations of the ERN with age in all subjects and age at onset of OCD symptoms in patients.23
Method
Participants
Pediatric patients were recruited in the Department of Psychiatry at the University of Michigan and the surrounding community. Pediatric comparison subjects were recruited from the surrounding community. After complete description of the study, written informed consent was obtained from at least one parent of the participant and written informed assent from the participant. Participants were paid for their interviews and psychophysiological recordings.
All 44 patients had a lifetime diagnosis of OCD. Patients were excluded if they had a lifetime diagnosis of autistic disorder, Asperger’s disorder, schizophrenia, other psychotic disorder, bipolar I disorder, substance-related disorder, or anorexia nervosa, or a current diagnosis of major depressive disorder. All 44 comparison subjects had no history of an axis I disorder. Lifetime and current axis I diagnoses were made independently by two clinicians using all sources of information according to DSM-IV criteria. Patients and comparison subjects were excluded if they had a history of mental retardation, head injury with a sustained loss of consciousness, chronic neurological disorder such as a seizure disorder, or a score greater than 15 on the lifetime version of the Social Communication Questionnaire.40 All participants lived with at least one English-speaking biological parent who was willing to participate in research.
Consistent with prior studies of the ERN in OCD, patients were included in the study if they were taking a stable dose of a selective serotonin reuptake inhibitor but no other psychotropic medications. Medications being taken (and number of patients taking the medication) were the following: fluoxetine (11), sertraline (2), escitalopram (2), and citalopram (1). Prior studies have found serotonergic antidepressants have no effect on ERN amplitude.29,31
All 88 participants were interviewed with the Schedule for Schizophrenia and Affective Disorders for School-Aged Children-Present and Lifetime Version41 and Schedule for Obsessive-Compulsive and Other Behavioral Syndromes.42 The lifetime (maximum) and current severity of OCD was assessed in the patients with a modified version of the Children’s Yale-Brown Obsessive Compulsive Disorder Scale.43 The parent report scales completed for all participants consisted of the Child Behavior Checklist44 and Social Communication Questionnaire.40 The self-report scales completed by all participants consisted of the Multidimensional Anxiety Scale for Children45 and Children’s Depression Inventory.46
The average age of the patients with OCD was 13.8 years (range = 10–19), and the average age of the healthy controls was 13.9 years (range 10–18) (t86 = .09, p = .93). The group with OCD had 20 males and the comparison group had 22 males (χ21= 0.18, p = .67). The current and lifetime CY-BOCS scores in the patients with OCD ranged from 0 to 34 and 12 to 36, respectively. Although all patients had a lifetime diagnosis of OCD, 29 had a current diagnosis and 15 had a past diagnosis with minimal current OCD symptoms that no longer met criteria for diagnosis.
Nine patients with OCD had a lifetime history of tics, consisting of six with Tourette’s disorder, one with chronic motor tic disorder, one with transient tic disorder, and one with tic disorder not otherwise specified. All patients with tic-related OCD had a current OCD diagnosis. The tic-related group had six males and the non-tic-related group had 14 males (χ21= 2.05, p = .15). One patient with tic-related OCD had a history of attention-deficit hyperactivity disorder. There were no significant differences between the patients with tic-related and the patients with non-tic-related OCD in their history of depressive disorders (χ21= 0.15, p = .70) or non-OCD anxiety disorders (χ21= 2.46, p = .12). Table 1 summarizes other demographic and clinical data for the patients and controls.
TABLE 1.
Demographic and Clinical Data in Youth with Non-Tic-Related Obsessive-Compulsive Disorder (OCD), Youth with Tic-Related OCD, and Healthy Comparison Subjects
| Variable | Youth with Non- Tic-Related OCD (n = 35)
|
Youth with Tic- Related OCD (n = 9)
|
Healthy Control Subjects (n = 44)
|
Comparisons of Non-Tic- Related OCD, Tic-Related OCD, and Healthy Control Subjects
|
||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Test Statistic | p | |
| Age (years) | 13.9 | 2.3 | 13.6 | 3.5 | 13.9 | 2.3 | F2,85 = 0.04 | .96 |
| Child Behavior Checklist | ||||||||
| Total score | 31.1 | 20.5 | 44.6 | 23.4 | 6.9 | 5.4 | F2,85 = 36.9 | <.0001a,b |
| Internalizing score | 12.8 | 8.7 | 16.6 | 9.5 | 2.3 | 2.1 | F2,85 = 35.5 | <.0001c |
| Externalizing score | 5.7 | 6.5 | 7.6 | 7.8 | 1.7 | 1.9 | F2,85 = 9.0 | <.0003d,e |
| Obsessive-Compulsive Scale score | 5.7 | 3.8 | 8.8 | 3.6 | 0.6 | 0.8 | F2,85 = 53.0 | <.0001f,g |
| Multidimensional Anxiety Scale For Children | 47.0 | 19.9 | 43.2 | 21.8 | 26.9 | 13.5 | F2,85 = 15.1 | <.0001h,i |
| Children’s Depression Inventory | 10.0 | 7.2 | 9.3 | 5.7 | 2.6 | 2.7 | F2,85 = 6.19 | <.0001j,k |
| Age at Onset of Obsessive-Compulsive Symptoms (years) | 8.4 | 3.0 | 5.8 | 3.4 | F1,42 = 5.40 | .025 | ||
| Duration of Obsessive-Compulsive Symptoms (years) | 7.8 | 4.1 | 5.3 | 3.5 | F1,42 = 3.32 | .08 | ||
| Children’s Yale-Brown Obsessive | ||||||||
| Compulsive Scale - lifetime score | 27.4 | 6.3 | 29.9 | 4.7 | F1,42 = 1.17 | .29 | ||
| - current score | 15.2 | 8.9 | 21.1 | 5.8 | F1,42 = 3.60 | .06 | ||
Note:
Patients with non-tic-related OCD significantly different from patients with tic-related OCD, p = .02.
patients with non-tic-related OCD significantly different from healthy controls, and tic-related OCD patients significantly different from healthy controls, p < .0001.
Patients with non-tic-related OCD significantly different from healthy controls, and patients with tic-related OCD significantly different from healthy controls, p < .0001.
Patients with non-tic-related OCD significantly different from healthy controls, p = .002.
Patients with tic-related OCD significantly different from healthy controls, p = .0006.
Patients with non-tic-related OCD significantly different from healthy controls, and patients with tic-related OCD significantly different from healthy controls, p < .0001.
Patients with non-tic-related OCD significantly different from patients with tic-related OCD, p = .004.
Patients with non-tic-related OCD significantly different from healthy controls, p < .0001.
Patients with tic-related OCD significantly different from healthy controls, p =.01.
Patients with non-tic-related OCD significantly different from healthy controls, p < .0001.
Patients with tic-related OCD significantly different from healthy controls, p = .0007.
Task and Procedure
Participants performed a modified Eriksen flanker task in which arrows appeared on a personal computer display with congruent (e.g., →→→→→) and incongruent (e.g., →→←→→) conditions.47 They were instructed to respond to the central arrow target by pressing one of two buttons indicating the direction of the middle arrow (i.e., right versus left), while ignoring the adjacent arrows, and to respond as quickly and accurately as possible, while placing equal emphasis on speed and accuracy. The stimuli remained on the screen for 250 msec, with the interval between consecutive stimuli lasting 1500 msec.
Each participant was seated 0.65 meters directly in front of the computer monitor. Following a practice block of 32 trials, each subject completed 8 blocks of 64 trials for a total of 512 trials. Performance feedback was provided after every block to yield error rates of approximately 10%, ensuring an adequate number of trials for stable error-related waveforms.
Electrophysiological Recording, Data Reduction, and Analysis
The electroencephalography (EEG) was recorded from DC-104 Hz with 64 Ag/AgCl scalp electrodes, two mastoid electrodes, and two vertical and two horizontal electro-oculogram electrodes, using the BioSemi ActiveTwo system. Data were recorded referenced to a ground formed from a common mode sense active electrode and driven right leg passive electrode (see http://www.biosemi.com/faq/cms&drl.htm). Data were digitized at 512 Hz; following recording, the data were resampled at 256 Hz. Prior to eye movement correction, EEG data were screened using automated algorithms that rejected individual sweeps in which (a) the absolute voltage range for any individual electrode exceeded 500 μV, (b) a change greater than 50 μV was measured from one datapoint to the next, or (c) the data deviated by more than +25 or −100 dB in the 20–40 Hz frequency window (for detecting muscle artifacts). Data were also screened by visual inspection. Ocular movement artifacts were then corrected using the algorithm described by Gratton, Coles, and Donchin.48 Waveforms shown in figures were filtered with a nine-point Chebyshev II low-pass, zero-phase-shift digital filter (Matlab R2010a; Mathworks, Natick, MA), with a half-amplitude cutoff at approximately 12 Hz.
Behavioral measures included the number of correct and incorrect trials for each subject, as well as accuracy expressed as a percentage of valid trials. Average reaction times on error and correct trials were calculated separately. Reaction time and accuracy after errors were evaluated to determine if there were group differences in post-error behavioral adjustments. Reaction times were analyzed with group as a between-subject factor and response type as a within-subject factor. The mean number of errors per subject contributing to the analysis was 57.7 (SD = 24.5; range = 8–121).
The ERN was quantified using mean amplitude measures relative to a pre-response baseline −200 to −50 msec. The mean amplitude of the ERN was computed on incorrect response trials in a window from 0 to 80 msec following the incorrect response. The correct response negativity (CRN) consisted of the same measure computed on correct response trials. Amplitudes were calculated for the central frontal (FCz) and central (Cz) electrodes. Results are presented for both FCz and Cz, with findings generally stronger at Cz.
ERN amplitude was compared between groups using a repeated-measure analysis of covariance (ANCOVA) with error number included as a covariate. Analyses of clinical variables were conducted with analysis of variance and Student’s t-tests. Pearson correlation coefficients were used to examine associations of response-related amplitudes with age, behavioral measures, and clinical measures. All statistical tests were two-tailed with the alpha level set at 0.05.
Results
Behavioral Data in Obsessive-Compulsive Disorder Patients and Healthy Controls
Behavioral and event-related brain potential data for participants are presented in Table 2. Compared to the control group, there was a trend for the OCD group to make more errors (F1,86 = 3.74, p = .056), using the number of error trials that remained after data cleaning, with patients with non-tic-related OCD making more errors than healthy controls (F1,77 = 5.34, p = .024). No main effect of the OCD and control groups and no interaction between those groups and response type for reaction time reached significance. There was no significant difference between the OCD and control groups in post-error reaction time. In a comparison of patients with tic-related OCD, patients with non-tic-related OCD, and control subjects, there was no significant main effect for groups or significant interaction between those groups and response type for reaction time. There were no significant sex differences for error total, reaction time on correct or error trials, or post-error slowing.
TABLE 2.
Behavioral and Event-Related Brain Potential Data in Youth with Non-Tic-Related Obsessive-Compulsive Disorder (OCD), Youth with Tic-Related OCD, and Healthy Comparison Subjects
| Variable | Youth with Non- Tic-Related OCD (n = 35)
|
Youth with Tic- Related OCD (n = 9)
|
Healthy Control Subjects (n = 44)
|
Comparisons of Non-Tic- Related OCD, Tic-Related OCD, and Healthy Control Subjects
|
||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Test Statistic | p | |
| Behavioral Data | ||||||||
| Total number of errors | 65.3 | 24.1 | 52.2 | 22.3 | 52.7 | 24.1 | F2,85 = 2.96 | .06a |
| Correct reaction time (msec) | 464.0 | 103.5 | 524.8 | 112.6 | 504.0 | 136.8 | F2,85 = 1.44 | .24 |
| Error reaction time (msec) | 456.2 | 203.8 | 520.9 | 167.6 | 484.1 | 252.9 | F2,85 = 0.32 | .73 |
| Post-error reaction time (msec) | 452.8 | 137.7 | 566.1 | 142.0 | 506.4 | 224.8 | F2,85 = 1.60 | .21 |
| Error-Related Potential Data | ||||||||
| Error-related negativity, FCz (μV) | −4.52 | 5.39 | −2.34 | 3.92 | −2.61 | 4.17 | F2,84 = 3.71 | .03b,c |
| Error-related negativity, Cz (μV) | −2.25 | 5.61 | 0.88 | 4.46 | 1.04 | 5.43 | F2,84 = 6.93 | .002d,e |
| Correct response negativity, FCz (μV) | 1.91 | 5.61 | 2.56 | 3.13 | 1.85 | 4.03 | F2,85 = 0.09 | .92 |
| Correct response negativity, Cz (μV) | 3.16 | 6.05 | 4.23 | 3.62 | 3.51 | 4.87 | F2,85 = 0.15 | .86 |
Note:
Patients with non-tic-related OCD significantly different from healthy controls, p = .02.
Patients with non-tic-related OCD significantly different from patients with tic-related OCD, p = .04;
Patients with non-tic-related OCD significantly different from healthy controls, p = .02.
Patients with non-tic-related OCD significantly different from patients with tic-related OCD, p = .01;
Patients with tic-related OCD significantly different from healthy controls, p = .001.
Event-Related Brain Potential Data in Obsessive-Compulsive Disorder Patients and Healthy Controls
ERN amplitudes at FCz and Cz were significantly correlated with error number in all subjects, becoming smaller (or less negative) with increasing errors (r = 0.24, p = .024 and r = 0.25, p = .021, respectively). ERN amplitudes at FCz and Cz were significantly correlated with error number in the OCD group (r = .44, p = .003 and r = .47, p = .001, respectively), but not the control group (r = .09, p = .57 and r = .15, p = .34, respectively). In particular, the ERN at FCz and Cz was highly correlated with error number in the patients with non-tic-related OCD (r = .46, p = .006 = and r = .49, p = .003). ERN amplitudes at FCz and Cz had no significant correlations with reaction time on either correct or error trials or with post-error slowing.
Although there was no significant correlation between ERN amplitude at FCz and age, ERN amplitude at Cz had a significant correlation with age in all subjects, becoming larger (or more negative) with increasing age (r = −.21, p = .049). The correlation of ERN amplitude at Cz with age was significant in the control group (r = −.36, p = .017), but not the OCD group (r = −.09, p = .55).
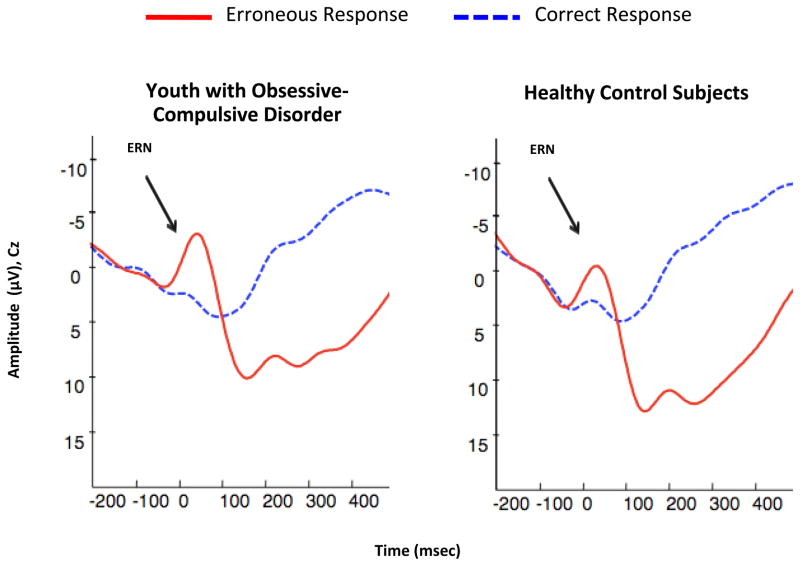
ERN amplitudes at FCz and Cz were significantly increased in patients with OCD compared to healthy controls (F1,85 = 4.21, p = .043 and F1,85 = 8.70, p = .004, respectively) with significant effects for error number (F1,85 = 7.33, p = .008 and F1,85 = 9.06, p = .003, respectively) (Figure 1). There were no significant interactions between the two groups and error number at either electrode. There were no significant differences between the OCD and control groups in CRN amplitudes at either electrode. There were no significant sex differences in any brain potentials.
FIGURE 1.
Grand Averages of Electroencephalogram Recordings in Youth with Obsessive-Compulsive Disorder (OCD) and Healthy Control Subjects. Note: The images depict response-locked grand average waveforms recorded at electrode Cz for correct and incorrect responses. Responses occurred at 0 msec. The mean amplitude of the error-related negativity (ERN) was computed in a window from 0 to 80 msec following incorrect response trials.
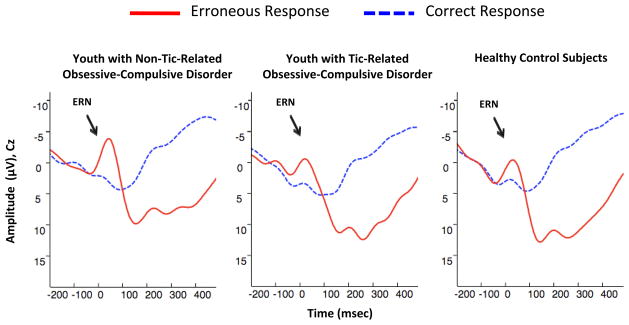
In a comparison of patients with tic-related OCD, patients with non-tic-related OCD, and healthy controls, there were significant effects on ERN amplitude at FCz and Cz for diagnosis (F2,84 = 3.71, p = .028 and F2,84 = 6.83, p = .002, respectively) and error number (F1,84 = 8.90, p = .004 and F1,84 = 11.36, p = .001, respectively) (Figure 2). There were no significant interactions between the three diagnostic groups and error number at either electrode.
FIGURE 2.
Grand Averages of Electroencephalogram Recordings in Youth with Non-Tic-Related Obsessive-Compulsive Disorder (OCD), Youth with Tic-Related OCD, and Healthy Control Subjects. Note: The images depict response-locked grand average waveforms recorded at electrode Cz for correct and incorrect responses. Responses occurred at 0 msec. The mean amplitude of the error-related negativity (ERN) was computed in a window from 0 to 80 msec following incorrect response trials.
In comparisons of the patients with non-tic-related OCD with healthy controls and patients with tic-related OCD, ERN amplitude at FCz was significantly larger in patients with non-tic-related OCD than healthy controls (F1,76 = 5.86, p = .018) with a significant effect for error number (F1,76 = 6.12, p = .016), and significantly larger in patients with non-tic-related OCD than patients with tic-related OCD (F1,41 = 4.38, p = .043) with a significant effect for total error number (F1,41 = 13.65, p = .0006) (Table 2). Moreover, ERN amplitude at Cz was significantly larger in patients with non-tic-related OCD than healthy controls (F1,76 = 11.32, p = .001) with a significant effect for error number (F1,76 = 7.62, p = .007), and significantly larger in patients with non-tic-related OCD than patients with tic-related OCD (F1,41 = 7.41, p = .010) with a significant effect for error number (F1,41 = 17.62, p = .0001). There were no significant differences between the patients with tic-related OCD and healthy controls in ERN amplitude at either electrode.
Clinical Correlations and Comparisons in Obsessive-Compulsive Disorder Patients
In all patients with OCD, ERN amplitude at FCz and Cz had no significant correlations with age at onset or duration of OCD symptoms. However, in the patients with non-tic-related OCD, ERN amplitude had a significant positive correlation with age at onset of OCD symptoms at Cz (r = .37, p = .029) but not FCz (r = .31, p = .07). Conversely, in the patients with non-tic-related OCD, ERN amplitude had significant negative correlations with duration of OCD symptoms at both Cz (r = −.41, p = .015) and FCz (r = −.34, p = .043), becoming larger (or more negative) with increasing duration.
In all patients with OCD and in each tic-related and non-tic-related group, there were no significant correlations between any evented-related brain potentials and either current or lifetime measures of OCD symptom severity. There were no significant differences in any brain potentials between patients with a current or past diagnosis of OCD. There were no significant differences in any brain potentials between patients with OCD receiving and not receiving a serotonin reuptake inhibitor, or between patients receiving and not receiving cognitive-behavioral therapy with exposure/response prevention.
Discussion
The finding of a larger ERN in youth with a lifetime diagnosis of OCD is consistent with previous reports of increased error-related brain activity in adults and children with OCD.14,25–32,34 However, the ERN increase appeared to be specific to patients with non-tic-related OCD, who made more mistakes than controls and had a strong positive correlation between the ERN and error number, suggesting that increased error-related brain activity in this OCD subtype may arise to compensate for cognitive deficits. The ERN had a negative correlation with age in healthy controls, as described in previous studies,23,34 but not patients with OCD. Instead, the ERN had a positive correlation with age at onset of OCD symptoms in patients with non-tic-related OCD, suggesting that development of error-related brain activity may be accelerated in this OCD subtype. Although patients with tic-related OCD had an earlier age at onset of OCD symptoms than patients with non-tic-related OCD,3 the patients with tic-related OCD had no evidence of increased error-related brain activity.
The observation of a larger ERN in patients with non-tic-related OCD compared to patients with tic-related OCD provides preliminary evidence of another neurobiological difference between these two OCD subtypes.10,11 The ERN difference between the two OCD subtypes is consistent with functional brain imaging studies showing increased metabolic activity in the ACC in OCD12 and decreased metabolic activity in the same region in Tourette’s disorder.39 Nonetheless, the results require replication with a larger sample of patients with OCD with and without chronic tics before concluding the ERN increase is specific to non-tic-related OCD.
The results provide further evidence that the ERN with tasks eliciting response conflict is a trait-like measure in OCD that appears independent of OCD symptom severity, current diagnostic status, or treatment effects.29–31,34 ERN results have been more variable, however, in studies of adults with OCD using probabilistic learning tasks or other tasks.49–52 The study showing enlarged ERN amplitudes in the unaffected first-degree relatives of OCD probands excluded probands with motor tics,30 so that the effect of proband tic history on ERN amplitude in unaffected relatives remains unknown. Although the ERN has been proposed as an endophenotype for OCD,30,34,36 it is often difficult to determine whether a putative endophenotype is associated with the causes rather than the effects of a disorder.53,54 Further research is necessary to demonstrate that increased error-related brain activity mediates the genetic risk for non-tic-related OCD rather than merely indicating risk through a pleiotropic effect.53,54 An alternative term such a biomarker may better describe the current status of an enlarged ERN as a possible genetic correlate of non-tic-related OCD.
Our study has several limitations requiring further consideration. The sample size for the group with tic-related OCD, in particular, was small. No corrections were made for multiple testing, although one-tailed tests may have been justified for the main comparisons. The assessment of lifetime obsessive-compulsive symptom severity and age at onset of OCD symptoms was done retrospectively rather than prospectively. The treatment of patients in this study was not controlled, albeit patients on medications other than the selective serotonin reuptake inhibitors were excluded.
In summary, our results provide further evidence of increased error-related brain activity in pediatric OCD34. Greater error-related brain activity was found in the non-tic-related than tic-related OCD subtype, with a significant correlation between the ERN and age at OCD symptom onset in non-tic-related OCD. If the results are replicated, they will provide another validation of the distinction between tic-related and non-tic-related OCD.2–11 A larger ERN was found in patients with non-tic-related OCD, who varied considerably in their current and lifetime symptom severity, indicating the ERN is a trait-like measure that may serve as a biomarker for this OCD subtype.30,34,36 Hence, the ERN may be a useful quantitative phenotype in genetic studies of tic-related and non-tic-related OCD.
Acknowledgments
This research was supported by the National Institute of Mental Health grants R01 MH086321 (G.L.H.), F31 MH086273 (M.C.), and K23 MH 082176 (K.D.F.), the International Obsessive-Compulsive Disorder (OCD) Foundation (G.L.H.), the Dana Foundation (K.D.F.), the National Alliance for Research on Schizophrenia and Depression Young Investigator Award (K.D.F.), the Undergraduate Research Opportunity Program (C.E.L.), and the Studying Abroad Scholarship from the Ministry of Education, Taiwan (P.C.).
Footnotes
Disclosure: Drs. Hanna, Carrasco, Chen, Fitzgerald, and Gehring, and Ms. Harbin, Ms. Neinhuis, and Ms. LaRosa report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Leckman JF, Denys D, Simpson HB, et al. Obsessive-compulsive disorder: a review of the diagnostic criteria and possible subtypes and dimensional specifiers for DSM-V. Depress Anxiety. 2010;27:507–527. doi: 10.1002/da.20669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leonard HL, Lenane MC, Swedo SE, Rettew DC, Gershon ES, Rapoport JL. Tics and Tourette’s disorder: a 2- to 7-year follow-up of 54 obsessive-compulsive children. Am J Psychiatry. 1992;149:1244–1251. doi: 10.1176/ajp.149.9.1244. [DOI] [PubMed] [Google Scholar]
- 4.Pauls DL, Alsobrook JP, Goodman W, Rasmussen S, Leckman JF. A family study of obsessive-compulsive disorder. Am J Psychiatry. 1995;152:76–84. doi: 10.1176/ajp.152.1.76. [DOI] [PubMed] [Google Scholar]
- 5.Do Rosario-Campos MC, Leckman JF, Curi M, et al. A family study of early-onset obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2005;136B:92–97. doi: 10.1002/ajmg.b.30149. [DOI] [PubMed] [Google Scholar]
- 6.March JS, Franklin ME, Leonard H, et al. Tics moderate treatment outcome with sertraline but not cognitive-behavior therapy in pediatric obsessive-compulsive disorder. Biol Psychiatry. 2007;67:344–347. doi: 10.1016/j.biopsych.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 7.McDougle CJ, Goodman WK, Leckman JF, Barr LC, Heninger GR, Price LH. The efficacy of fluvoxamine in obsessive-compulsive disorder: effects of comorbid chronic tic disorder. J Clin Psychopharmacol. 1993;13:354–358. [PubMed] [Google Scholar]
- 8.Bloch MH, Landeros-Weisenberg A, Kelmendi B, Coric V, Bracken MB, Leckman JF. A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Mol Psychiatry. 2006;11:622–632. doi: 10.1038/sj.mp.4001823. [DOI] [PubMed] [Google Scholar]
- 9.Bloch MH, Craiglow BG, Landeros-Weisenberger A, et al. Predictor of early adult outcomes in pediatric-onset obsessive-compulsive disorder. Pediatrics. 2009;124:1085–1093. doi: 10.1542/peds.2009-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanna GL, McCracken JT, Cantwell DP. Prolactin in childhood obsessive-compulsive disorder: clinical correlates and response to clomipramine. J Am Acad Child Adolesc Psychiatry. 1991;30:173–178. doi: 10.1097/00004583-199103000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Ahmari SE, Risbrogh VB, Geyer MA, Simpson HB. Impaired sensorimotor gating in unmedicated adults with obsessive-compulsive disorder. Neuropsychopharmacology. 2012;37:1216–1223. doi: 10.1038/npp.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saxena S, Gorbis E, O’Neill J, et al. Rapid effects of brief intensive cognitive-behavioral therapy on brain glucose metabolism in obsessive-compulsive disorder. Mol Psychiatry. 2009;14:197–205. doi: 10.1038/sj.mp.4002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends in Cogn Sci. 2012;16:43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzgerald KD, Welsh RC, Gehring WJ, et al. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biol Psychiatry. 2005;57:287–294. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 15.Pitman RK. A cybernetic model of obsessive-compulsive psychopathology. Compr Psychiatry. 1987;28:334–343. doi: 10.1016/0010-440x(87)90070-8. [DOI] [PubMed] [Google Scholar]
- 16.Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- 17.Gehring WJ, Liu Y, Orr JM, Carp J. The error-related negativity (ERN/Ne) In: Luck SK, Kappenman E, editors. Oxford Handbook of Event-Related Potential Components. New York: Oxford University Press; 2012. pp. 231–291. [Google Scholar]
- 18.Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components, II: error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- 19.O’Connell RG, Dockree PM, Bellgrove MA, et al. The role of cingulate cortex in the detection of errors with and without awareness: a high-density electrical mapping study. Eur J Neurosci. 2007;25:2571–2579. doi: 10.1111/j.1460-9568.2007.05477.x. [DOI] [PubMed] [Google Scholar]
- 20.Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate cortex: an event related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- 21.Keil J, Weisz N, Paul-Jordanov I, Wienbruch C. Localization of the magnetic equivalent of the ERN and induced oscillatory brain. NeuroImage. 2010;51:404–411. doi: 10.1016/j.neuroimage.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Vocat R, Pourtois G, Vuilleumier P. Unavoidable errors: a spatio-temporal analysis of time-course and neural sources of evoked potentials associated with error processing in a speeded task. Neuropsychologia. 2008;46:2545–2555. doi: 10.1016/j.neuropsychologia.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7- to 25-year-olds. Dev Neuropsychol. 2004;25:355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- 24.Anokhin AP, Golosheykin S, Heath AC. Heritability of frontal brain function related to action monitoring. Psychophysiology. 2008;45:524–534. doi: 10.1111/j.1469-8986.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- 25.Gehring WJ, Himle JA, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychol Sci. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- 26.Johannes S, Wieringa BM, Nager W, et al. Discrepant target detection and action monitoring in obsessive-compulsive disorder. Psychiatry Res. 2001;108:101–110. doi: 10.1016/s0925-4927(01)00117-2. [DOI] [PubMed] [Google Scholar]
- 27.Ruchsow M, Gron G, Reuter K, Spitzer M, Hermle L, Kiefer M. Error-related brain activity brain activity in patients with obsessive-compulsive disorder and in healthy controls. J Psychophysiol. 2005;19:298–304. [Google Scholar]
- 28.Endrass T, Klawohn J, Schuster F, Kathmann N. Overactive performance monitoring in obsessive-compulsive disorder: ERP evidence from correct and erroneous reactions. Neuropsychologia. 2008;46:1877–1887. doi: 10.1016/j.neuropsychologia.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Stern ER, Liu Y, Gehring WJ, et al. Chronic medication does not affect hyperactive error responses in obsessive-compulsive disorder. Psychophysiol. 2010;47:913–920. doi: 10.1111/j.1469-8986.2010.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riesel A, Endrass T, Kaufmann C, Kathmann N. Overactive error-related brain activity as a candidate endophenotype for obsessive-compulsive disorder: evidence from unaffected first-degree relatives. Am J Psychiatry. 2010;168:317–324. doi: 10.1176/appi.ajp.2010.10030416. [DOI] [PubMed] [Google Scholar]
- 31.Endrass T, Schuermann B, Kaufmann C, Spielberg R, Kniesche R, Kathmann N. Performance monitoring and error significance in patients with obsessive-compulsive disorder. Biol Psychol. 2010;84:257–263. doi: 10.1016/j.biopsycho.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Xiao Z, Wang J, Zhang M, et al. Error-related negativity abnormalities in generalized anxiety disorder and obsessive-compulsive disorder. Prog Neuro-Psychopharm Biol Psychiatry. 2011;35:265–272. doi: 10.1016/j.pnpbp.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 33.Hajcak G, Simons RF. Error-related brain activity in obsessive-compulsive undergraduates. Psychiatry Res. 2002;110:63–72. doi: 10.1016/s0165-1781(02)00034-3. [DOI] [PubMed] [Google Scholar]
- 34.Hajcak G, Franklin ME, Foa EB, Simons RF. Increased error-related brain activity in pediatric OCD before and after treatment. Am J Psychiatry. 2008;165:116–123. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- 35.Santesso DL, Segalowitz SJ, Schmidt LA. Error-related electrocortical responses are enhanced in children with obsessive-compulsive behaviors. Dev Neuropsychol. 2006;29:431–455. doi: 10.1207/s15326942dn2903_3. [DOI] [PubMed] [Google Scholar]
- 36.Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: toward an endophenotype. Clin Psychol Rev. 2008;28:1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johannes S, Wieringa BM, Nager W, Muller-Vahl KR, Dengler R, Munte TF. Excessive action monitoring in Tourette syndrome. J Neurol. 2002;249:961–966. doi: 10.1007/s00415-002-0657-9. [DOI] [PubMed] [Google Scholar]
- 38.Fahim C, Yoon U, Sandor P, Frey K, Evans AC. Thinning of the motor-cingulate-insular cortices in siblings concordant for Tourette syndrome. Brain Topogr. 2009;22:176–184. doi: 10.1007/s10548-009-0105-6. [DOI] [PubMed] [Google Scholar]
- 39.Pourfar M, Feigin A, Tang CC, Carbon-Correll M, Bussa M, Budman C, Dhawan V, Eidelberg D. Abnormal metabolic brain networks in Tourette syndrome. Neurology. 2011;76:944–952. doi: 10.1212/WNL.0b013e3182104106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. Br J Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- 41.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 42.Hanna GL. Schedule for Obsessive-Compulsive and Other Behavioral Syndromes. Ann Arbor, MI: University of Michigan; 2007. [Google Scholar]
- 43.Scahill L, Riddle MA, McSwiggin-Hardin M, et al. Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- 44.Achenbach TM, Rescorla LA. Manual for ASEBA School-Age Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- 45.March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J Am Acad Child Adolescent Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 46.Kovacs M. The Children’s Depression Inventory (CDI) Psychopharm Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- 47.Eriksen CW, Eriksen BA. Effects of noise letters upon the identification of a target letter in a non-search task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- 48.Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;54:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- 49.Grundler TOJ, Cavanagh JF, Figueroa CM, Frank MJ, Allen JJB. Task-related dissociation in ERN amplitude as a function of obsessive-compulsive symptoms. Neuropsychologia. 2009;47:1978–1987. doi: 10.1016/j.neuropsychologia.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nieuwenhuis S, Neilen MM, Mol N, Hajcak G, Veltman DJ. Performance monitoring in obsessive-compulsive disorder. Psychiatry Res. 2005;134:111–122. doi: 10.1016/j.psychres.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Hammer A, Kordon A, Helmann M, Zurowski B, Münte TF. Brain potentials of conflict and error-likelihood following errorful and errorless learning in obsessive-compulsive disorder. PLos One. 2009;4:e6553. doi: 10.1371/journal.pone.0006553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathews CA, Perez VB, Delucchi KL, Mathalon DH. Error-related negativity in individuals with obsessive-compulsive symptoms: toward an understanding of hoarding behaviors. Biol Psychol. 2012;89:487–494. doi: 10.1016/j.biopsycho.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walters JTR, Owen MJ. Endophenotypes in psychiatric genetics. Mol Psychiatry. 2007;12:886–890. doi: 10.1038/sj.mp.4002068. [DOI] [PubMed] [Google Scholar]
- 54.Kendler KS, Neale MC. Endophenotype: a conceptual analysis. Mol Psychiatry. 2010;15:789–797. doi: 10.1038/mp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]