Abstract
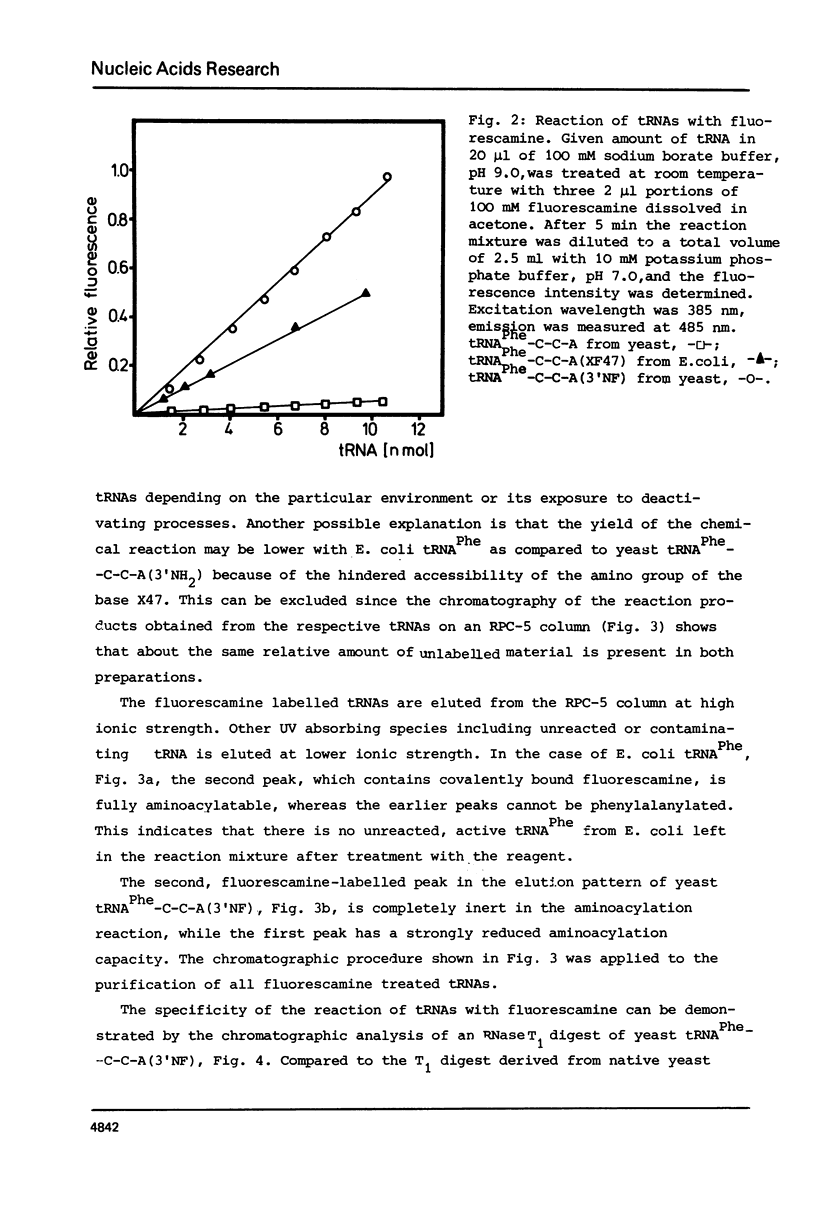
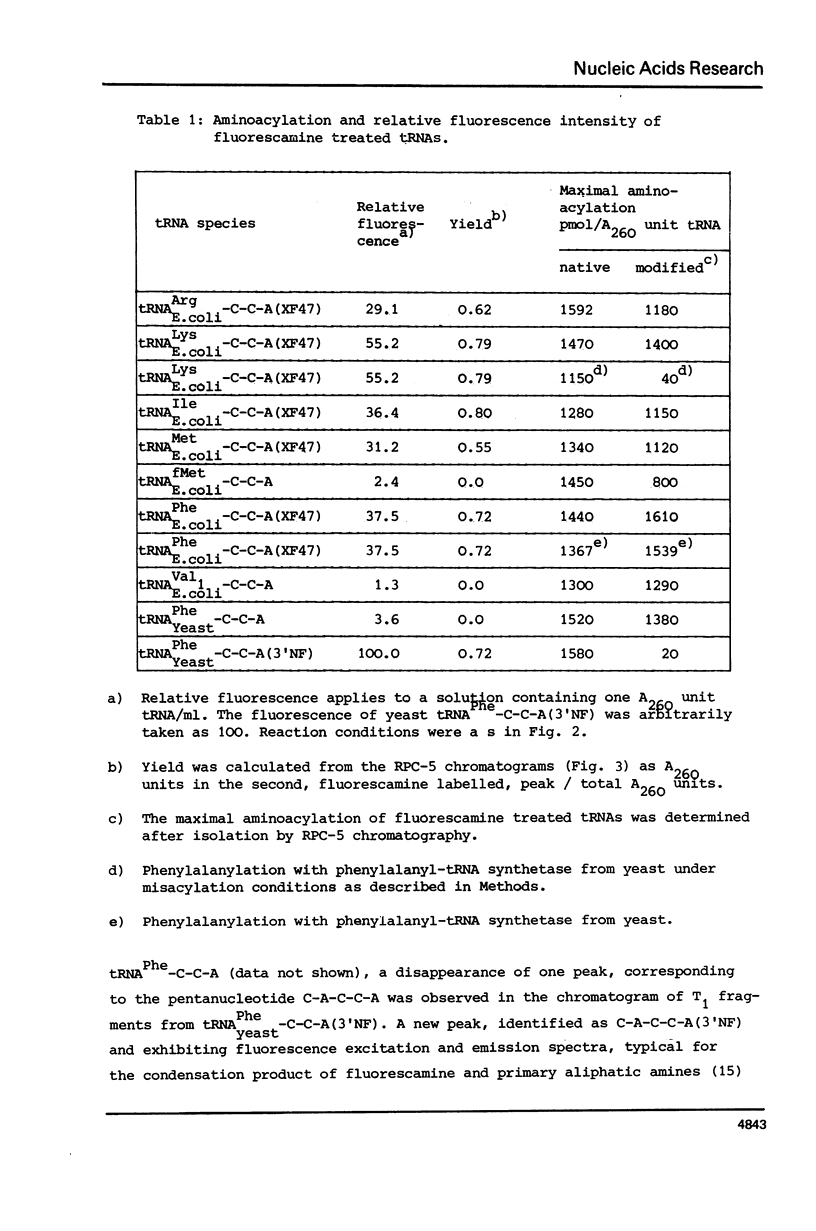
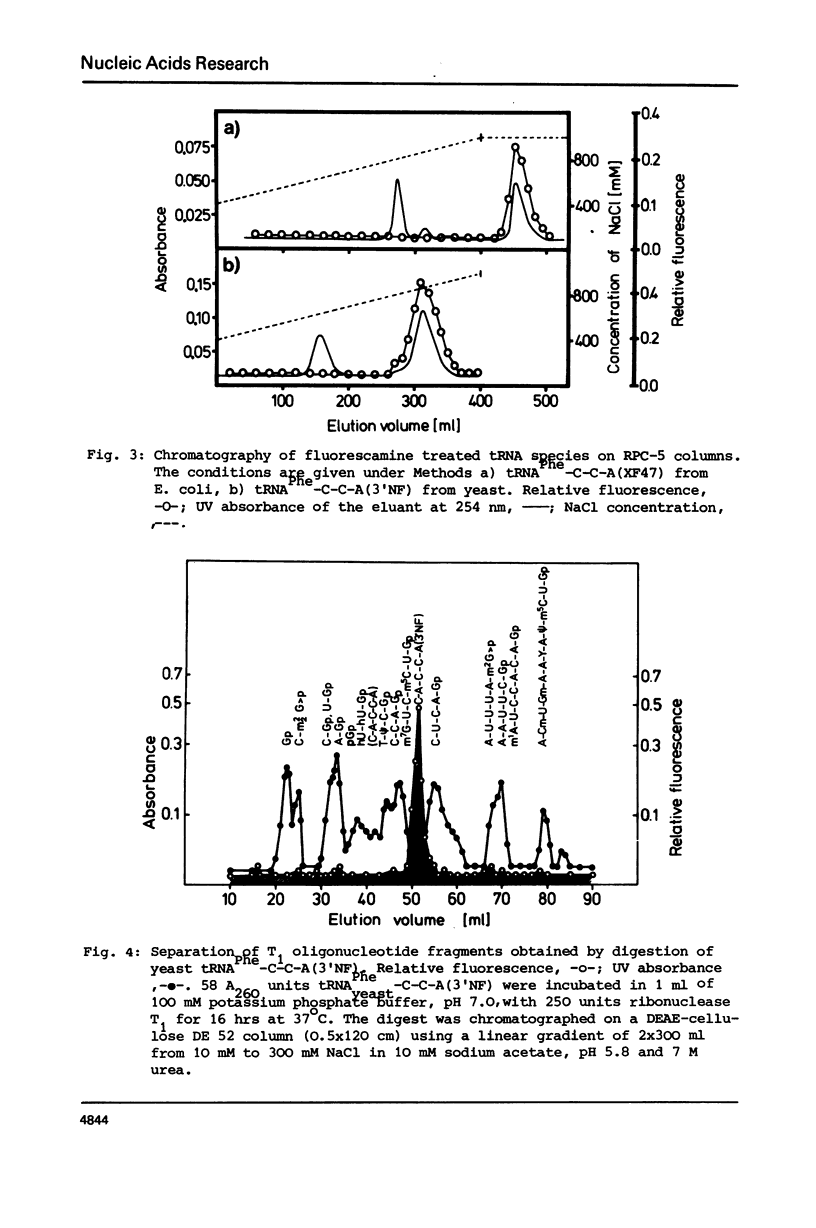
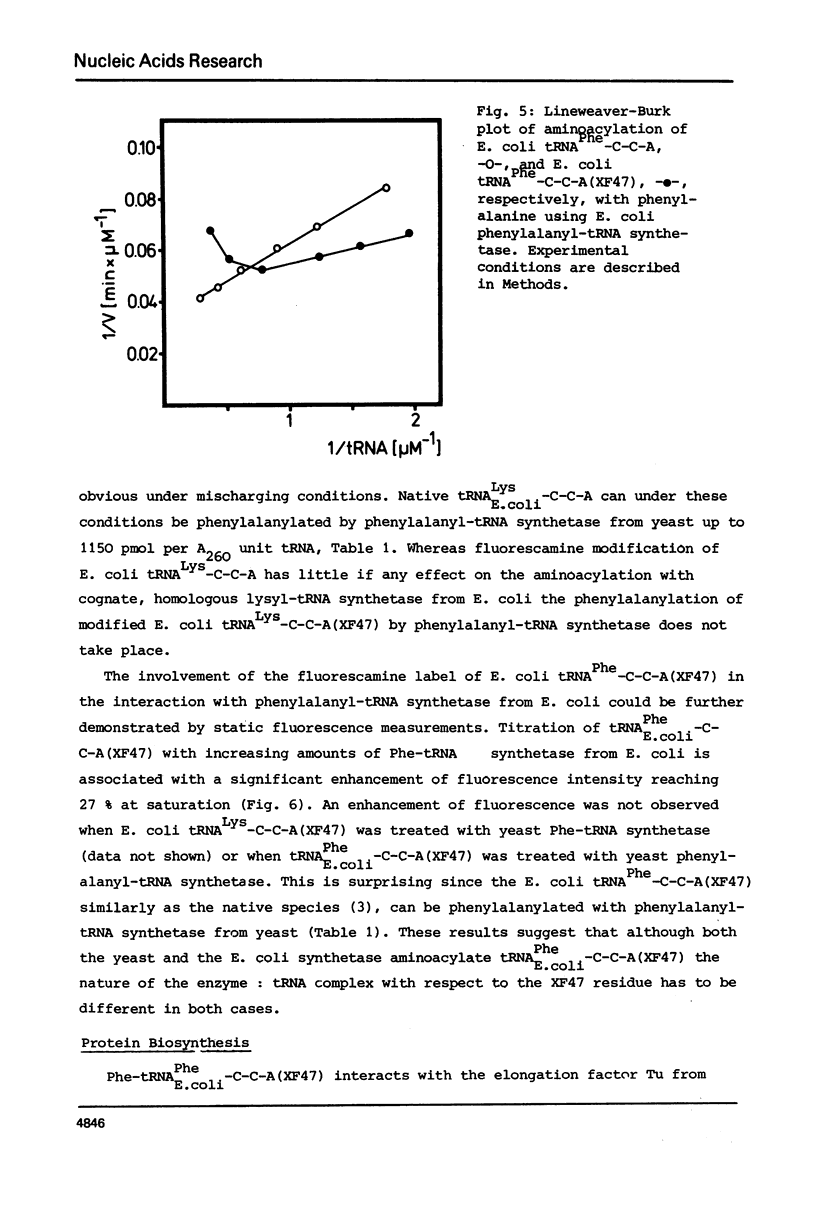
The reaction of fluorescamine with primary amino groups of tRNAs was investigated. The reagent was attached under mild conditions to the 3'-end of tRNAPhe-C-C-A(3'NH) from yeast and to the minor nucleoside x in E. coli tRNAArg, tRNALys, tRNAMet, tRNAIle and tRNAPhe. The primary aliphatic amino groups of these tRNAs react specifically so that the fluorescamine dye is not attached to the amino groups of the nucleobases. E. coli tRNA species modified on the minor nucleoside X47 can all be aminoacylated. An involvement of the minor modified nucleoside X47 in the tRNA: synthetase interaction is detected. Native tRNALys-C-C-A from E. coli can be phenylalanylated by phenylalanyl-tRNA synthetase from yeast, whereas this is not the case for fluorescamine treated tRNALys-C-C-A(XF47). Pre-tRNAPhe-C-C-A(XF47) forms a ternary complex with the elongation factor Tu:GTP from E. coli, binds enzymatically to the ribosomal A-site and is active in poly U dependent poly Phe synthesis. Fluorescamine-labelled E. coli tRNAs provide new substrates for the study of protein biosynthesis by spectroscopic methods.
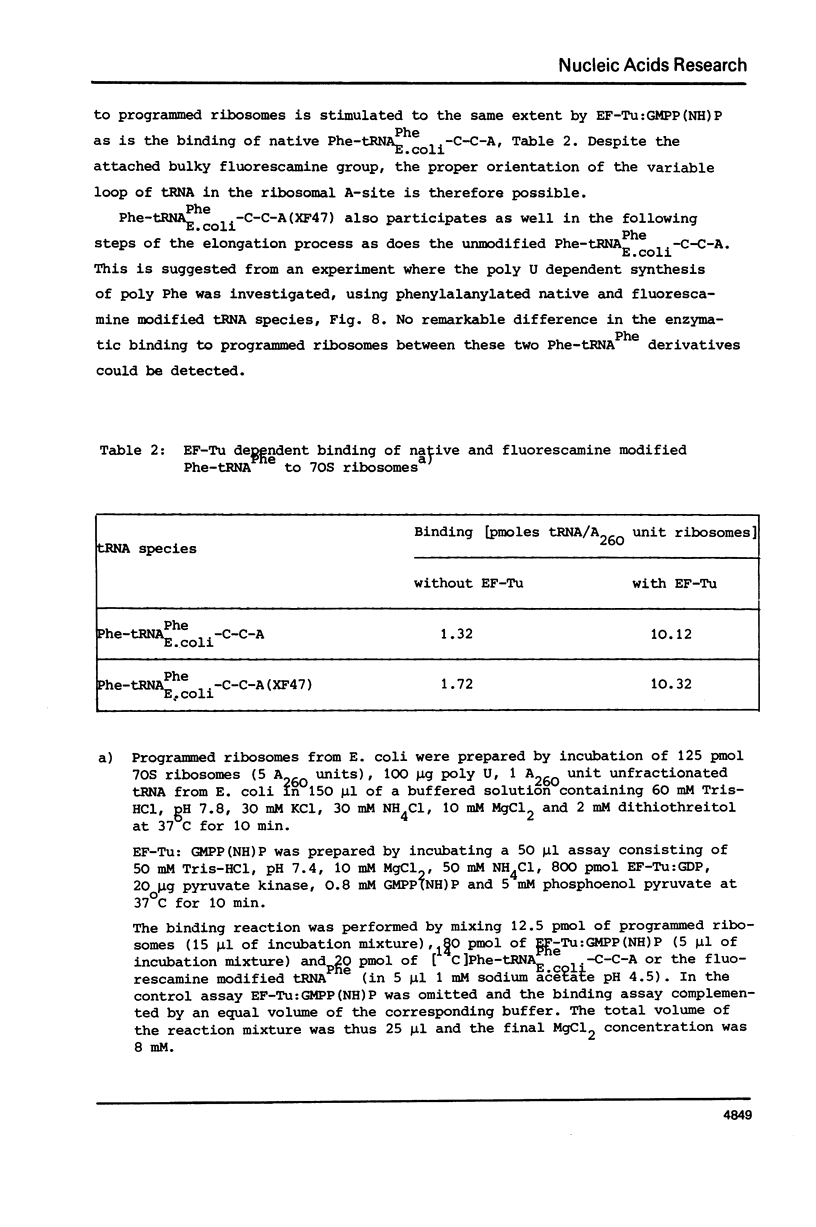
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
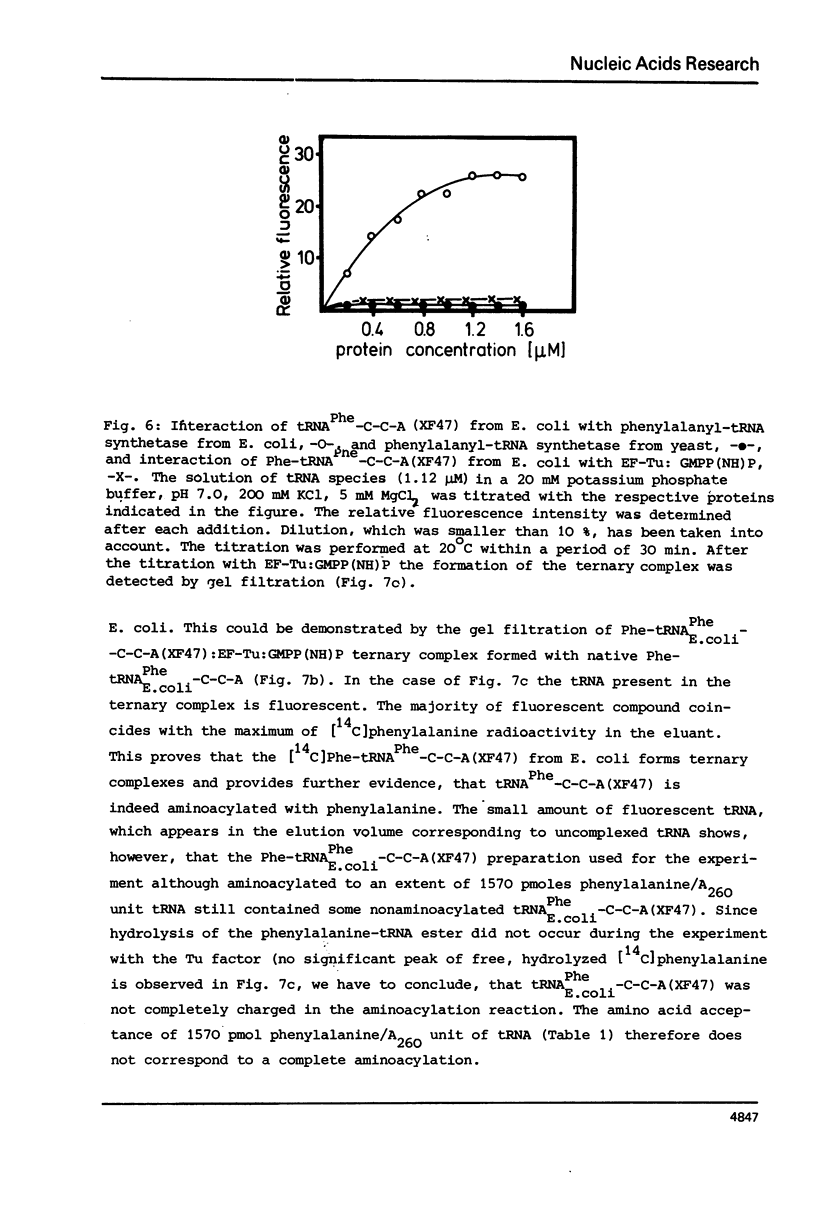
- Arai K. I., Kawakita M., Kaziro Y. Studies on polypeptide elongation factors from Escherichia coli. II. Purification of factors Tu-guanosine diphosphate, Ts, and Tu-Ts, and crystallization of Tu-guanosine diphosphate and Tu-Ts. J Biol Chem. 1972 Nov 10;247(21):7029–7037. [PubMed] [Google Scholar]
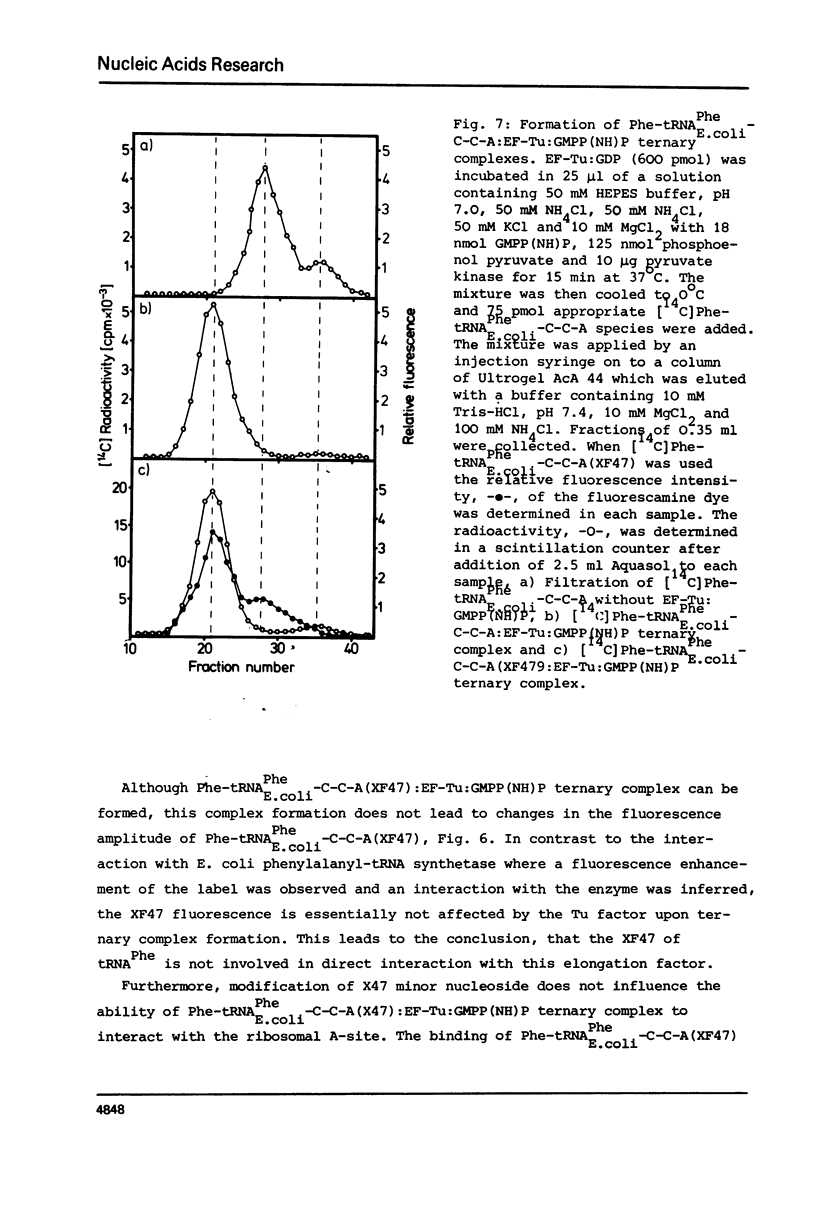
- Beres L., Lucas-Lenard J. Studies on the fluorescence of the Y base of yeast phenylalanine transfer ribonucleic acid. Effect of pH, aminoacylation, and interaction with elongation factor Tu. Biochemistry. 1973 Sep 25;12(20):3998–4002. doi: 10.1021/bi00744a033. [DOI] [PubMed] [Google Scholar]
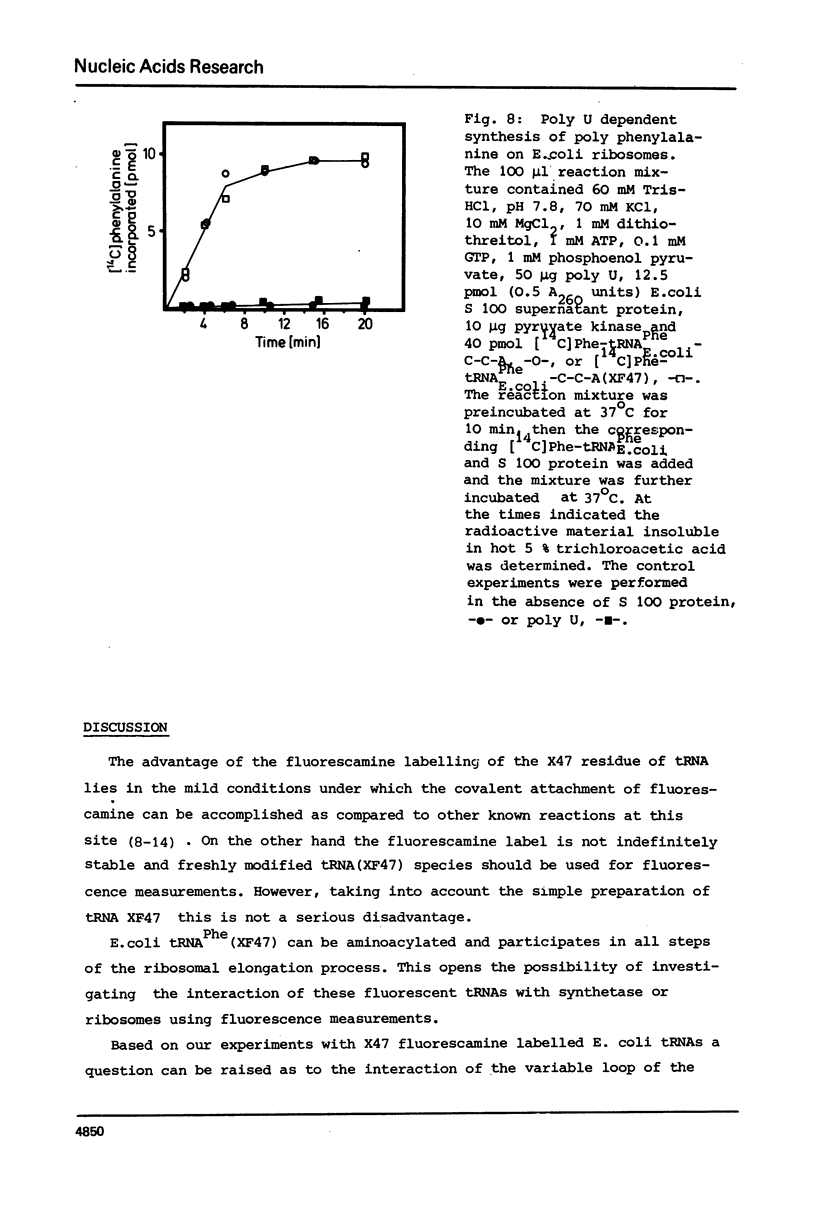
- De Bernardo S., Weigele M., Toome V., Manhart K., Leimgruber W., Böhlen P., Stein S., Udenfriend S. Studies on the reaction of fluorescamine with primary amines. Arch Biochem Biophys. 1974 Jul;163(1):390–399. doi: 10.1016/0003-9861(74)90490-1. [DOI] [PubMed] [Google Scholar]
- Egan B. Z., Kelmers A. D. Rapid chromatographic separation of ribosomal ribonucleic acids. Prep Biochem. 1972;2(3):265–274. doi: 10.1080/00327487208061476. [DOI] [PubMed] [Google Scholar]
- Fraser T. H., Rich A. Synthesis and aminoacylation of 3'-amino-3'-deoxy transfer RNA and its activity in ribosomal protein synthesis. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2671–2675. doi: 10.1073/pnas.70.9.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. Acylation of transfer ribonucleic acid with the N-hydroxysuccinimide ester of phenoxyacetic acid. Biochemistry. 1972 Aug 29;11(18):3435–3443. doi: 10.1021/bi00768a017. [DOI] [PubMed] [Google Scholar]
- Friedman S., Li H. J., Nakanishi K., Van Lear G. 3-(3-amino-3-carboxy-n-propyl)uridine. The structure of the nucleoside in Escherichia coli transfer ribonucleic acid that reacts with phenoxyacetoxysuccinimide. Biochemistry. 1974 Jul 2;13(14):2932–2937. doi: 10.1021/bi00711a024. [DOI] [PubMed] [Google Scholar]
- Gavrilova L. P., Spirin A. S. "Nonenzymatic" translation. Methods Enzymol. 1974;30:452–462. [PubMed] [Google Scholar]
- Holmes W. M., Hurd R. E., Reid B. R., Rimerman R. A., Hatfield G. W. Separation of transfer ribonucleic acid by sepharose chromatography using reverse salt gradients. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1068–1071. doi: 10.1073/pnas.72.3.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss G., Riesner D., Maass G. Mechanism of discrimination between cognate and non-cognate tRNAs by phenylalanyl-tRNA synthetase from yeast. Eur J Biochem. 1976 Sep;68(1):81–93. doi: 10.1111/j.1432-1033.1976.tb10766.x. [DOI] [PubMed] [Google Scholar]
- Maelicke A., Sprinzl M., von der Haar F., Khwaja T. A., Cramer F. Structural studies on phenylalanine transfer ribonucleic acid from yeast with the spectroscopic label formycin. Eur J Biochem. 1974 Apr 16;43(3):617–625. doi: 10.1111/j.1432-1033.1974.tb03449.x. [DOI] [PubMed] [Google Scholar]
- Nauheimer U., Hedgcoth C. Activation of several tRNAs of Escherichia coli by the phenoxyacetyl derivative of N-hydroxysuccinimide. Arch Biochem Biophys. 1974 Feb;160(2):631–642. doi: 10.1016/0003-9861(74)90440-8. [DOI] [PubMed] [Google Scholar]
- Ofengand J., Liou R. Ability of modified forms of phenylalanine tRNA to stimulate guanosine pentaphosphate synthesis by the stringent factor-ribosome complex of E. coli. Nucleic Acids Res. 1978 Apr;5(4):1325–1334. doi: 10.1093/nar/5.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi Z., Maeda M., McCloskey J. A., Nishimura S. 3-(3-Amino-3-carboxypropyl)uridine: a novel modified nucleoside isolated from Escherichia coli phenylalanine transfer ribonucleic acid. Biochemistry. 1974 Jun 4;13(12):2620–2625. doi: 10.1021/bi00709a023. [DOI] [PubMed] [Google Scholar]
- Pingoud A., Kownatzki R., Maass G. Fluoresceinylthiocarbamyl-tRNATyr: a useful derivative of tRNATyr (E.coli) for physicochemical studies. Nucleic Acids Res. 1977 Feb;4(2):327–338. doi: 10.1093/nar/4.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., Schimmel P. R. Structural organization of complexes of transfer RNAs with aminoacyl transfer RNA synthetases. Nucleic Acids Res. 1977;4(5):1649–1665. doi: 10.1093/nar/4.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller P. W., Schechter A. N. Covalent attachment of fluorescent probes to the X-base of Escherichia coli phenylalanine transfer ribonucleic acid. Nucleic Acids Res. 1977 Jul;4(7):2161–2167. doi: 10.1093/nar/4.7.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleich H. G., Wintermeyer W., Zachau H. G. Replacement of wybutine by hydrazines and its effect on the active conformation of yeast tRNAPhe. Nucleic Acids Res. 1978 May;5(5):1701–1713. doi: 10.1093/nar/5.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Grüter F., Gauss D. H. Collection of published tRNA sequences. Nucleic Acids Res. 1978 May;5(5):r15–r27. [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Kucharzewski M., Hobbs J. B., Cramer F. Specificity of elongation factor Tu from Escherichia coli with respect to attachment to the amino acid to the 2' or 3'-hydroxyl group of the terminal adenosine of tRNA. Eur J Biochem. 1977 Aug 15;78(1):55–61. doi: 10.1111/j.1432-1033.1977.tb11713.x. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Sternbach H., von der Haar F., Cramer F. Enzymatic incorporation of ATP and CTP analogues into the 3' end of tRNA. Eur J Biochem. 1977 Dec;81(3):579–589. doi: 10.1111/j.1432-1033.1977.tb11985.x. [DOI] [PubMed] [Google Scholar]


