Abstract
Background
Baked egg is tolerated by a majority of egg-allergic children.
Objective
To characterize immunologic changes associated with ingestion of baked egg and evaluate the role that baked egg diets plays in the development of tolerance to regular egg.
Methods
Egg-allergic subjects who tolerated baked egg challenge incorporated baked egg into their diet. Immunologic parameters were measured at follow-up visits. A comparison group strictly avoiding egg was used to evaluate the natural history of the development of tolerance.
Results
Of the 79 subjects in the intent-to-treat group followed for a median of 37.8 months, 89% now tolerate baked egg and 53% now tolerate regular egg. Of 23 initial baked egg-reactive subjects, 14 (61%) subsequently tolerated baked egg and 6 (26%) now tolerate regular egg. Within the initially baked egg-reactive group, subjects with persistent reactivity to baked egg had higher median baseline egg white (EW)-specific IgE levels (13.5 kUA/L) than those who subsequently tolerated baked egg (4.4 kUA/L; P=0.04) and regular egg (3.1 kUA/L, P=0.05). In subjects ingesting baked egg, EW-induced SPT wheal diameter and EW-, ovalbumin-, and ovomucoid-specific IgE levels decreased significantly, while ovalbumin- and ovomucoid-specific IgG4 levels increased significantly. Subjects in the per-protocol group were 14.6 times more likely to develop regular egg tolerance than subjects in the comparison group (P < 0.0001), and they developed tolerance earlier (median 50.0 versus 78.7 months; P<0.0001).
Conclusion
Initiation of a baked egg diet accelerates the development of regular egg tolerance compared to strict avoidance. Higher serum EW-specific IgE level is associated with persistent baked and regular egg reactivity, while initial baked egg reactivity is not.
Keywords: egg allergy, hen’s egg allergy, baked egg, heated egg, food allergy, egg tolerance, oral food challenge, egg allergy immunotherapy
Introduction
Egg allergy affects an estimated1.8-2% of children under the age of 5 years.1 A recent study from Australia reported 8.9% prevalence of challenge-proven allergy to uncooked egg in a large cohort of infants under 12 months of age; of those 80.3% tolerated cooked egg.2 While 80% of children eventually outgrow egg allergy, and most in the general population do so by school age, studies indicate that many children evaluated at referral centers are retaining egg allergy into their teenage years.3-5 It appears that the longer the egg allergy persists, the less likely tolerance will develop.4 Thus, it has become imperative to understand individualized prognosis of egg allergy and develop clinical management that will improve the quality of life of egg-allergic children and, ideally, promote earlier tolerance development.
Food processing alters protein structure and affects allergenicity. Previous studies indicated that some egg-allergic individuals tolerate baked egg.6-9 Heating may decrease allergenicity by destroying conformational epitopes or blocking epitope access through interactions with the food matrix, as seen in egg and milk.10 Children with IgE antibodies predominantly against conformational ovomucoid epitopes are more likely to have transient allergy as opposed to those with IgE antibodies against sequential epitopes not altered by heating.11, 12 Indeed, studies have shown that baked egg tolerance occurs prior to regular egg tolerance.7, 13
In the clinical trials conducted at our center, 70-75% of egg- and milk-allergic children tolerated baked egg or milk, respectively.14, 15 In a population-based study in Australia, 80.3% of children with challenge-proven egg allergy tolerated a baked egg challenge.2 We recently reported that inclusion of baked milk accelerates resolution of milk allergy.16 In the initial phase of the baked egg study, we confirmed that baked egg is well tolerated and associated with decreasing egg white (EW)-induced skin prick test (SPT) wheal diameter and serum ovalbumin (OVA)-specific IgE level, and increasing serum OVA- and ovomucoid (OVM)-specific IgG4 levels. These immunologic changes parallel those seen in the natural resolution of egg allergy and associated with food oral immunotherapy (OIT).5, 17-19 In this paper, we present long-term immunologic changes and clinical outcomes of egg-allergic children who included baked egg in their diet. We evaluate predictors of baked and regular egg tolerance and assess whether ingestion of baked egg reduces the time to development of regular egg tolerance.
Methods
Participants
Subjects between 0.5 and 25 years of age with documented IgE-mediated egg allergy were recruited from the pediatric allergy clinics at the Mount Sinai Medical Center in New York, NY. Documented IgE-mediated egg allergy was defined by a positive EW SPT and/or detectable (≥ 0.35 kUA/L) serum EW-specific IgE level, and a recent history (within the past 6 months) of a Type I hypersensitivity reaction to egg or a positive physician-supervised oral food challenge (OFC) to egg; or, a serum EW-specific IgE level greater than 2 kUA/L in children younger than 2 years of age or greater than 7 kUA/L in children older than 2 years of age.3, 20 Subjects were excluded from the study if they had a negative SPT and undetectable serum EW-specific IgE level, a recent (within the past 6 months) Type I hypersensitivity reaction to baked egg, already tolerated and were ingesting baked egg, or a history of eosinophilic esophagitis (EoE), unstable asthma, or pregnancy. The study was approved by the Mount Sinai Institutional Review Board, and informed consent was obtained before enrollment.
Design
Tolerance to baked egg (muffin and waffle) was determined by OFC as previously described.14 Subjects tolerant to baked egg were challenged with regular egg. Regular egg-tolerant subjects were instructed to incorporate all forms of egg into their diet, and were encouraged to do so at least twice weekly. Baked egg-tolerant subjects were instructed to incorporate baked egg products into their diets. Baked egg-reactive subjects were instructed to strictly avoid all forms of egg.
Active Group
Subjects in the active group were categorized as baked egg-tolerant or baked egg-reactive. Subjects tolerant to baked egg were advised to consume 1-3 servings of baked egg per day and avoid regular egg as previously described.14 Subjects ingesting baked egg were reevaluated every 3-12 months, and after 6 months or more were offered challenges to regular egg. Subjects reactive to baked egg were offered repeat challenges to baked egg after 12 months or more.
Immunologic evaluation
SPTs were performed as previously described.21 A serum sample was collected at each visit to measure EW-, OVA-, and OVM-specific IgE, and OVA- and OVM-specific IgG4 levels using UniCAP (Phadia, Uppsala, Sweden).
Oral food challenges
OFCs were performed openly under physician supervision in the Mount Sinai Clinical Research Center. During baked egg challenges, a muffin and a waffle each containing one third of an egg (2.2 g of egg protein) were administered.14 Baked egg-tolerant subjects were challenged to regular egg if their test results were less than the 95% positive predictive values for a positive OFC: EW-specific IgE level greater than 2 kUA/L in children younger than 2 years of age or greater than 7 kUA/L in children older than 2 years of age, or an EW SPT wheal diameter greater than 8 mm.3, 20, 22, 23 For regular egg challenges, scrambled egg or French toast was administered (1 egg or 6.5 g of egg protein) as per routine protocol.24
Comparison Group
We retrospectively identified comparison egg-allergic subjects that were age-, sex- and IgE-matched with active subjects at the time of enrollment. The same inclusion and exclusions criteria were used, and none of the control subjects had tolerated or were ingesting baked egg at time of enrollment. Subjects in the comparison group continued strict egg avoidance (current standard of care).25 If they added baked egg to their diet, it was due to accidental exposures. They were challenged to regular egg as per their allergist’s recommendation.
Statistics
All statistical analyses were performed with SAS Version 9.2 (SAS Institute, Inc, Cary, NC). A Wilcoxon rank-sum test was used to compare medians of continuous measures, whereas the chi-square test (and the Fisher exact test when the expected cell count was <5) was used to compare distributions of categorical measures between various subject groups. A Wilcoxon signed-rank test was used to compare medians of continuous measures at different time points. Logistic regression models with nominal (using a generalized logit link function) and ordinal (using a cumulative logit link function) outcomes were used to estimate odds ratios, corresponding 95% confidence intervals and p-values with adjustment for gender, age and initial IgE values. Probabilities of regular egg tolerance were estimated with the Kaplan-Meier product limit method with comparison among groups evaluated with the log-rank test statistic. The Cox proportional hazards model was used to estimate hazard ratios, corresponding 95% CIs, and p-values with adjustment for gender, age and initial IgE values. All statistical hypothesis testing was performed at the 0.05 level of significance.
Intent-to-treat versus per-protocol analysis
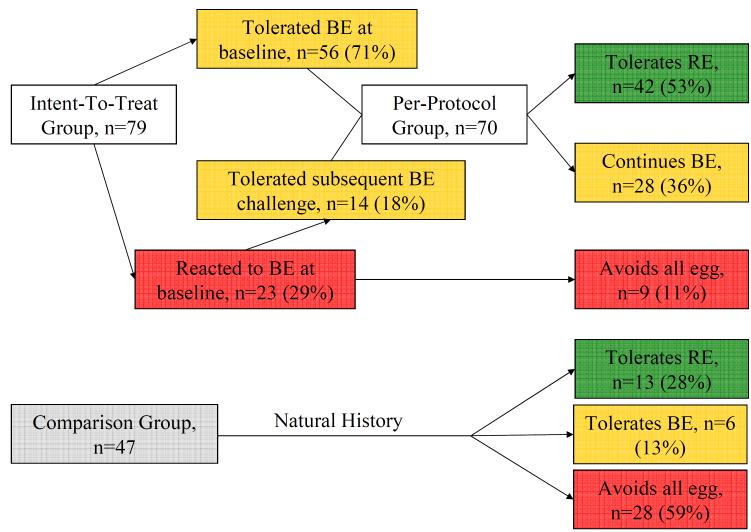
The intent-to-treat analysis includes 79 subjects who underwent the initial baked egg challenge, were available for follow-up, and either reacted to baked egg, regular egg, or tolerated baked egg but had immunologic indications of greater than 95% risk of reaction to regular egg.3, 20 The per-protocol analysis includes those subjects (n=70) who underwent treatment by adding baked egg to their diet. (Figure 1)
Figure 1.
Clinical outcomes of intent-to-treat and comparison groups.
Results
Baseline clinical characteristics
Between June 2004 and September 2007, 117 subjects were enrolled in the study. Detailed baseline characteristics at time of enrollment were previously described.14 Briefly, 79 subjects (71% male) were included in the intent-to-treat group with a median age of 5.8 years (range, 1.6-15.8) and a median initial serum egg white-specific IgE level of 2.5 (range, 0.2-101), and were followed for a median of 37.8 months (range, 7.6-69.7). At baseline challenge, 56 (71%) subjects in the intent-to-treat group were baked egg-tolerant and 23 (29%) were baked egg-reactive. (Figure 1) The remaining 38 of the 117 subjects initially enrolled and challenged were not included in the analysis because they: tolerated regular egg at baseline challenge (n=24), refused to ingest the entire baked egg serving resulting in an inconclusive baseline challenge (n=3), developed subsequent non-IgE-mediated intolerance to egg (n=1), were lost to follow-up after the baseline baked egg challenge (n=3), or passed the baseline baked egg challenge but regular egg allergy was not confirmed (n=7; of these, 6 subsequently passed a regular egg challenge).
Clinical outcomes
Overall, 70 (89%) subjects in the intent-to-treat group (n=79) tolerated baked egg over the length of the study, and 42 (53%) now tolerate regular egg with a median time to tolerance of 52.4 months (range, 7.6-67.5 months). (Figure 1) The remaining 9 (11%) continued to avoid egg strictly.
Progression to regular egg tolerance
Of the 56 subjects in the initial baked egg-tolerant group, 36 (64%) now tolerate regular egg. Of the 23 subjects in the initial baked egg-reactive group, 18 were re-challenged a second time and 4 were re-challenged a third time. Fourteen (61%) subsequently tolerated baked egg, and 6 (26%) now tolerate regular egg. Two of the five initial baked egg-reactive subjects who were not re-challenged had subsequent reactions to accidental exposures of egg in processed goods.
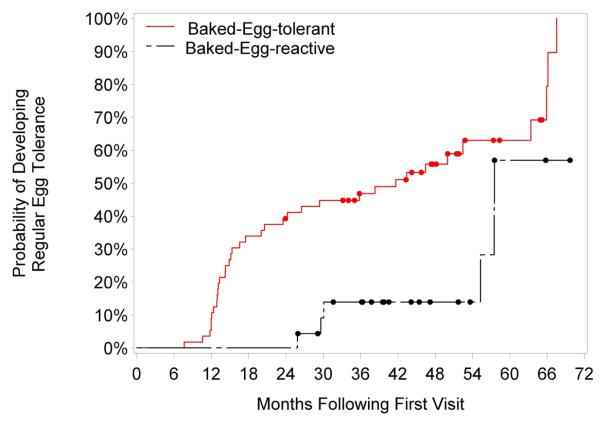
Subjects in the intent-to-treat group who initially tolerated baked egg were 12.2 times more likely to develop tolerance to regular egg than subjects in the intent-to-treat group who initially reacted to baked egg (95% CI, 3.7-40.3; P<0.001). (Table I) In contrast, subjects in the per-protocol group who initially tolerated baked egg were not significantly more likely to develop tolerance to regular egg as compared to subjects in the per-protocol group who initially reacted to baked egg. In other words, once initially baked-egg reactive subjects became baked egg tolerant, they were just as likely as the initially baked-egg tolerant subjects to develop tolerance to regular egg. Overall, subjects in the intent-to-treat group who initially tolerated baked egg were 3.3 times more likely to develop regular egg tolerance than subjects initially reactive to baked egg over the follow-up period (hazard ratio, 3.3; 95% CI, 1.2-8.9; P=0.017). (Figure 2)
Table I.
Odds ratios of clinical outcome comparing initially baked egg-tolerant versus initially baked egg-reactive groups, adjusted for sex, age at initial visit and baseline serum egg white-specific IgE.
| Clinical Outcome | BE tolerant vs. BE reactive intent-to-treat, OR (95% CI) |
P-value |
|---|---|---|
| Regular egg tolerant a | 12.2 (3.7-40.3) | <0.001 |
|
BE tolerant vs. BE reactive
per-protocol, OR (95% CI) |
||
| Regular egg tolerant b | 3.1 (0.8-11.2) | 0.092 |
The intent-to-treat group consists of all subjects enrolled in the active arm of the study, including baked egg–reactive subjects. The per-protocol group consists of children in the active group who were, or eventually became, tolerant to baked egg over the length of the study.
The reference group is comprised of patients avoiding regular egg or all egg products.
The reference group is comprised of patients avoiding regular egg as there were no patients avoiding all egg products in the per-protocol group.
OR, odds ratio
Figure 2.
Development of regular egg tolerance in the per-protocol group stratified by initial baked egg challenge: tolerant versus reactive. The log-rank P value comparing time to development of tolerance between the initial baked egg-tolerant versus initial baked egg-reactive groups is 0.004.
Time to regular egg tolerance
Initial baked egg-tolerant subjects developed regular egg tolerance significantly earlier than initial baked egg-reactive subjects. The median time to regular egg tolerance was 41.7 months in the initial baked egg-tolerant group versus 57.5 months in the initial baked egg-reactive group; P=0.004.
Immunologic parameters in subjects ingesting baked egg
Baked egg-tolerant subjects had lower baseline EW-specific IgE levels than baked egg-reactive subjects; median 1.9 kUA/L (IQR, 0.6-6.1; range, 0.0->100) versus 13.5 kUA/L (IQR, 5.9-18.9; range, 2.8-58.9) (P=0.002). Baked egg-tolerant subjects also had smaller baseline EW-induced SPT wheal diameters than baked egg-reactive subjects; median 6 mm (IQR, 5-8; range, 0-19) versus 8 mm (IQR, 8-9; range, 7-15) (P=0.005). EW-induced SPT wheal diameter, and EW-, OVA-, and OVM-specific IgE levels all decreased significantly from baseline in subjects ingesting baked egg (P<0.0001, P<0.0001, P<0.0001, and P=0.0002, respectively). (Table II) OVA- and OVM-specific IgG4 levels increased significantly (both P<0.0001) and the ratio of OVA and OVM IgE/IgG4 decreased significantly (P=0.0003 and P<0.0001, respectively) from baseline in subjects ingesting baked egg.
Table II.
Immunologic parameters of the per-protocol group and subgroup that eventually tolerated regular egg, expressed as median (25-75% interquartile range).
| Baseline per-protocol |
Last follow-up per-protocol |
P-value | |
|---|---|---|---|
| SPT (mm), n=46 | 6 (4.5-8) | 4 (2.3-5) | <0.0001 |
| EW IgE (kUA/L), n=64 | 2.1 (0.6-6.4) | 0.9 (0.0-2.3) | <0.0001 |
| OVA IgE (kUA/L), n=45 | 1.9 (0.6-6.1) | 0.9 (0.0-2.2) | <0.0001 |
| OVA IgG4 (kUA/L), n=46 | 0.5 (0.1-1.6) | 2.6 (0.6-9.2) | <0.0001 |
| OVA IgE/G4*, n=36 | 3.9 (1.1-20.3) | 0.3 (0.0-2.5) | 0.0003 |
| OVM IgE (kUA/L), n=45 | 1.0 (0.0-2.7) | 0.4 (0.0-1.3) | 0.0002 |
| OVM IgG4 (kUA/L), n=46 | 0.0 (0.0-0.4) | 0.4 (0.1-1.5) | <0.0001 |
| OVM IgE/G4*, n=20 | 5.6 (1.0-12.5) | 0.8 (0.0-1.9) | <0.0001 |
| Baseline of subgroup who tolerated RE |
Subgroup at time of RE tolerance |
P-value | |
| SPT (mm), n=41 | 6 (5-8) | 3 (2.5-5) | <0.0001 |
| EW IgE (kUA/L), n=40 | 1.3 (0.6-4.4) | 0.6 (0.0-1.5) | 0.0003 |
| OVA IgE (kUA/L), n=29 | 1.5 (0.5-3.1) | 0.5 (0.0-1.2) | <0.0001 |
| OVA IgG4 (kUA/L), n=29 | 0.5 (0.0-1.6) | 4.4 (1.9-10.0) | <0.0001 |
| OVA IgE/G4*, n=23 | 3.2 (0.5-6.6) | 0.0 (0.0-0.6) | <0.0001 |
| OVM IgE (kUA/L), n=29 | 0.9 (0.0-2.8) | 0.0 (0.0-0.6) | <0.0001 |
| OVM IgG4 (kUA/L), n=29 | 0.1 (0.0-0.5) | 0.6 (0.1-1.3) | <0.0001 |
| OVM IgE/G4*, n=16 | 5.3 (0.6-10.8) | 0.4 (0.0-1.6) | 0.0002 |
BE, baked egg; RE, regular egg; SPT, skin prick test; EW, egg white; OVA, ovalbumin; OVM, ovomucoid
denominator (IgG4) zero in many cases
Characteristics of children with persistent reactivity to baked egg
Within the group of initially baked egg-reactive subjects, those with persistent baked egg-reactivity had significantly higher median baseline EW-specific IgE levels (13.5 kUA/L) than those who subsequently tolerated baked egg (4.4 kUA/L; P=0.04) and regular egg (3.1 kUA/L; P=0.05). (Table III) Final EW-specific IgE levels were greater and final EW-induced SPT wheal diameters were larger in subjects with persistent baked egg-reactivity compared to subjects initially reactive to baked egg who subsequently tolerated baked egg (P=0.02 for both) and regular egg (P=0.01 and P=0.02, respectively). Two of the four initial baked egg-reactive subjects who were treated with epinephrine during the baseline baked egg challenge were still strictly avoiding egg at the end of the study, compared with 7/19 (37%) initial baked egg-reactive subjects who did not receive epinephrine during the baseline baked egg challenge (P=0.05, chi square test).
Table III.
Baseline and final egg white-induced SPT and egg white-specific IgE of the initial baked egg-reactive group based on clinical outcome, expressed as median (25-75% interquartile range).
| Avoids all egg (n=9) |
Subsequently tolerated BE (n=14) |
P-value | Subsequently tolerated RE (n=6) |
P-value | |
|---|---|---|---|---|---|
| SPT (mm) | |||||
| Baseline | 8 (8-9) | 7.5 (6-9) | 0.13 | 6.5 (6-9) | 0.19 |
| Final | 8 (6-10) | 4 (2.5-5.5) | 0.02 | 2.8 (1.5-4.5) | 0.02 |
| EW IgE (kUA/L) | |||||
| Baseline | 13.5 (5.9-18.9) | 4.4 (1.9-8.8) | 0.04 | 3.1 (1.2-4.4) | 0.05 |
| Final | 5.4 (3.3-25.4) | 1.8 (1.1-3.5) | 0.02 | 0.9 (0.6-1.1) | 0.01 |
BE, baked egg; RE, regular egg; SPT, skin prick test; EW, egg white
Comparison group
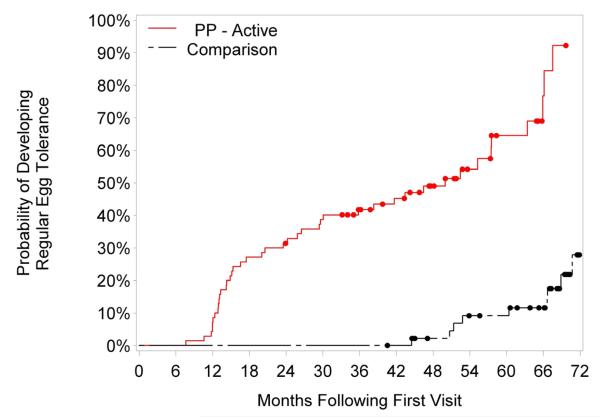
The comparison group consisted of 47 subjects (66% male) with a median age of 4.6 (range, 1.7-20.9 years) and a median initial serum egg white-specific IgE level of 4.8 (range, 0.2-58) who were evaluated in our clinic during the period of study enrollment and followed for a median of 67.3 months (range, 40.5-81.8). Baseline age, sex, and initial EW-specific IgE levels were not significantly different between the intent-to-treat and comparison groups. The comparison group was followed for a significantly longer time than the intent-to-treat group (median 67.3 versus 37.8 months; P<0.0001). A majority (59%) of the comparison group was strictly avoiding egg at the end of the study period, while 13% were baked egg-tolerant and 28% were tolerant to regular egg. (Figure 1) Subjects who underwent active treatment (the per-protocol group, which excludes those with persistent baked egg reactivity) developed regular egg tolerance significantly earlier than those in the comparison group. The median time to regular egg tolerance was 50.0 months in the per-protocol group versus 78.7 months in the comparison group; P<0.0001. Subjects who developed regular egg tolerance in the per-protocol group were slightly older at baseline compared to their counterparts in the comparison group (median 5.5 versus 4.5 years; P=0.048).
Subjects in the per-protocol group were 20.9 times more likely to tolerate baked egg (95% CI, 5.8-76.2; P<0.0001), and 18.3 times more likely to tolerate regular egg (95% CI, 5.5-60.9; P<0.0001) than the comparison group. (Table IV) This significance is maintained when comparing the intent-to-treat and comparison groups (OR 4.7; 95% CI, 1.9-11.5; P=0.0006). Overall, subjects in the per-protocol group were 14.6 times more likely to develop regular egg tolerance than subjects in the comparison group over the follow-up period (hazard ratio, 14.6; 95% CI, 5.8-36.4; P < 0.0001). (Figure 3)
Table IV.
Odds ratios of clinical outcome comparing per-protocol and intent-to-treat versus comparison groups, adjusted for sex, age at initial visit and egg white-specific IgE.
| Clinical Outcome | Versus | Per-protocol vs. Comparison, OR (95% CI) |
P-value |
|---|---|---|---|
| Regular egg tolerant | Avoiding all egg | 18.3 (5.5-60.9) | <0.0001 |
| Baked egg tolerant | 0.9 (0.3-2.8) | 0.817 | |
| Baked egg tolerant | Avoiding all egg | 20.9 (5.8-76.2) | <0.0001 |
|
Intent-to-treat vs.
Comparison, OR (95% CI) |
|||
| Regular egg tolerant |
Avoiding all egg or
BE tolerant |
4.7 (1.9-11.5) | 0.0006 |
OR, odds ratio
Figure 3.
Development of regular egg tolerance: per-protocol (PP) versus comparison groups. The log-rank P value comparing survival between the per-protocol versus comparison groups is less than 0.0001.
Tolerability of baked egg diet
Baked egg was well tolerated without reports of acute allergic reactions to baked egg at home or worsening of eczema or asthma. One subject initially reactive to baked egg passed a baked egg re-challenge, then subsequently developed vomiting and diarrhea hours after accidental exposures to regular egg (in icing and cookie dough ice cream). This reaction was consistent with atypical food protein induced enterocolitis syndrome and this child reverted to complete egg avoidance. None of the subjects developed EoE.
Withdrawals
Three subjects initially reactive to baked egg in the intent-to-treat group were lost to follow-up. Eighteen subjects initially tolerant to baked egg withdrew from the study by one year14, however, we were able to follow-up with these subjects by telephone and confirm that they were continuing to ingest baked egg or had become tolerant to regular egg.
Discussion
While avoidance continues to be the safest way to prevent symptoms of allergic food reactions, reports of food-sensitized eczema patients who developed systemic reactions after a period of avoidance, and the recurrence of peanut allergy in former peanut-allergic patients who ingested peanut infrequently or in limited amounts has begun to change our way of thinking about tolerance.26-30 There is an increasing interest in OIT with native (unmodified) protein for the treatment of food allergy, and several clinical trials have shown promising results as subjects were able to tolerate increased amounts of the offending food.19, 31-34 However, adherence to OIT suffers from the relatively high prevalence of adverse side effects.35 Baked egg may represent an alternative and safer method of introducing allergens into the diets of egg-allergic individuals with the goal of improving quality of life and accelerating the resolution of their allergy.
We report that 89% (70/79) of subjects tolerated baked egg and 53% (42/79) now tolerate regular egg over a median of 37.8 months of follow-up. In addition to the 70% of subjects who tolerated baked egg at the baseline OFC as we previously reported, we found that a majority of subjects initially reactive to baked egg subsequently developed tolerance to baked egg over the follow-up period and many of them now tolerate regular egg. This is in contrast to what we reported in the baked milk study, where initial baked milk reactivity was a predictor of persistent baked and unheated milk reactivity.16 Instead, higher baseline EW-specific IgE level in the initial baked egg-reactive group was associated with persistent baked and regular egg reactivity.
We previously reported a decrease in EW-induced SPT wheal diameter and OVA-specific IgE level, and an increase in OVA- and OVM-specific IgG4 levels after 3 months of ingesting baked egg.14 Here we report that long-term ingestion of baked egg is associated with significantly decreasing whole and component egg-specific IgE levels, and sustained changes in SPT wheal diameter and IgG4 levels.
We followed our subjects for up to 6 years and confirmed that continued ingestion of baked egg in the diet of egg-allergic children was well tolerated. Even those patients who withdrew from the study or reported an increase of eczema (not confirmed by exam) by one year continued to ingest baked egg regularly in their diet years later. Despite reports of EoE developing in children who had undergone OIT, none of our patients developed EoE.36, 37 We observed one subject with a history of immediate IgE-mediated symptoms to baked egg develop delayed gastrointestinal symptoms hours after accidental exposure to regular egg, which is consistent with atypical food protein-induced enterocolitis syndrome (FPIES).38 This occurred after the subject passed a baked egg challenge and began to ingest baked egg regularly. It is unknown whether a baked egg diet may have predisposed this subject to developing FPIES-like symptoms.
Regular egg tolerance was achieved in a greater proportion of the active study group and earlier than the comparison group (P<0.0001). This may be an underestimation of the difference considering the comparison group was followed for a significantly longer time, giving these subjects more of a chance to naturally outgrow their egg allergy. It appears that the approach of adding baked egg to the diets of egg-allergic children who can tolerate baked egg accelerates the induction of regular egg tolerance. Alternatively, the shorter time to tolerance might reflect close follow-up of the subjects within the active study group and a more proactive pursuit of diagnostic challenges to regular egg. Having a retrospective comparison group is one of the limitations of our study. However, we recently we reported that among 100 unselected open baked egg challenges done in our office in patients with median age 5.9 years (range 1.2-19.8 years), 66% tolerated baked egg [Lieberman J, et al JACI, in press]. Therefore, we feel reassured that the retrospective comparison group derived from our patient base was sufficiently comparable to the study subjects.
Clark et al recently reported in a longitudinal study of 95 young children that egg-allergic subjects were able to tolerate well-cooked egg at a median age of 5.6 years and uncooked egg at 10.3 years.39 Epinephrine was not administered during any OFC in the study, although nine subjects experienced respiratory symptoms during uncooked egg challenges and three received nebulized bronchodilators. In our cohort, 19% of baked egg-reactive subjects and 23% of baked egg-tolerant but regular egg-reactive subjects experienced mild anaphylaxis that was treated with intramuscular epinephrine.14 This argues against the notion that tolerance of baked egg products reliably predicts milder reactions to regular egg and highlights the difficulty of predicting baked egg tolerance based on history of reaction severity to regular egg.
In conclusion, the results of our study indicate that the majority of subjects with egg allergy tolerate baked egg and that long-term ingestion of these products is well tolerated and accelerates the development of tolerance to regular egg. Ingestion of baked egg is associated with immunologic changes, including decreasing EW-induced SPT wheal diameter and EW-specific IgE levels. Higher baseline EW-specific IgE levels are associated with baked and regular egg reactivity, while initial baked egg reactivity is not. We propose that for as many as 89% of egg allergic-children, ingestion of baked egg products is a safer, more convenient, less costly and less labor-intensive form of oral immunomodulation.
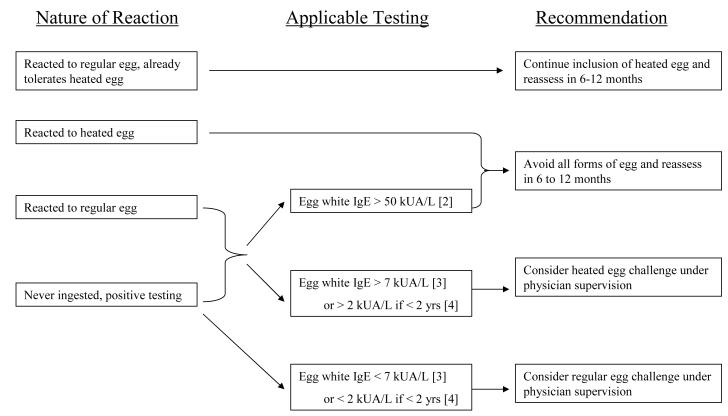
While our data are encouraging for improving the quality of life and hastening the tolerance of regular egg for a majority of egg-allergic children, oral challenges to baked egg must be undertaken under physician supervision with all precautions typically used for performing food challenges in children. Egg allergy phenotypes and markers of baked egg tolerance have not been fully defined, and the safety of home introduction of baked egg has not been validated.40 Our study shows that anaphylaxis to baked egg occurs and is not easily predicted. The British Society for Allergy and Clinical Immunology (BSACI) recently published recommendations for home reintroduction of well-cooked (baked) egg, however the new guidelines by the NIAID (National Institute of Allergy and Infectious Diseases)-sponsored expert panel in the United States did not incorporate the introduction of baked egg into the recommendations regarding the management of egg allergy.25, 41 Further studies are required to more clearly define which egg-allergic patients can safely tolerate and benefit from inclusion of baked egg in their diets. Until such studies are completed, the introduction of baked egg into the diet of those strictly avoiding egg should be undertaken with physician supervision. Our proposed guidelines for the introduction of baked egg into the diets of egg-allergic children can be found in Figure 4.
Figure 4.
Recommendations on whom to perform a baked egg challenge based on clinical status and testing.
Clinical implications.
Addition of dietary baked egg is safe, convenient, and well accepted by patients. Introducing baked egg to egg-allergic children presents an important shift in the treatment paradigm for egg allergy.
Acknowledgements
Ramon Bencharitiwong, PhD for technical assistance in the lab, Shideh Mofidi, RD for development of food challenge recipes, Joanna Lis, BA and Natasha Setia, MS for assistance with telephone follow-up.
Funding: This project has been supported by NIH NIAID AI 059318 to A. Nowak-Wȩgrzyn and Grant Number UL1-RR-029887 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health.
Abbreviations
- EoE
eosinophilic esophagitis
- EW
egg white
- FPIES
food protein-induced enterocolitis syndrome
- OFC
oral food challenge
- OIT
oral immunotherapy
- OVA
ovalbumin
- OVM
ovomucoid
- SPT
skin prick test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127:594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 2.Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127:668–76. e1–2. doi: 10.1016/j.jaci.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 3.Boyano-Martinez T, Garcia-Ara C, Diaz-Pena JM, Martin-Esteban M. Prediction of tolerance on the basis of quantification of egg white-specific IgE antibodies in children with egg allergy. J Allergy Clin Immunol. 2002;110:304–9. doi: 10.1067/mai.2002.126081. [DOI] [PubMed] [Google Scholar]
- 4.Wood RA. The natural history of food allergy. Pediatrics. 2003;111:1631–7. [PubMed] [Google Scholar]
- 5.Savage JH, Matsui EC, Skripak JM, Wood RA. The natural history of egg allergy. J Allergy Clin Immunol. 2007;120:1413–7. doi: 10.1016/j.jaci.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 6.Urisu A, Ando H, Morita Y, Wada E, Yasaki T, Yamada K, et al. Allergenic activity of heated and ovomucoid-depleted egg white. J Allergy Clin Immunol. 1997;100:171–6. doi: 10.1016/s0091-6749(97)70220-3. [DOI] [PubMed] [Google Scholar]
- 7.Eigenmann PA. Anaphylactic reactions to raw eggs after negative challenges with cooked eggs. J Allergy Clin Immunol. 2000;105:587–8. doi: 10.1067/mai.2000.104255. [DOI] [PubMed] [Google Scholar]
- 8.Des Roches A, Nguyen M, Paradis L, Primeau MN, Singer S. Tolerance to cooked egg in an egg allergic population. Allergy. 2006;61:900–1. doi: 10.1111/j.1398-9995.2006.01134.x. [DOI] [PubMed] [Google Scholar]
- 9.Konstantinou GN, Giavi S, Kalobatsou A, Vassilopoulou E, Douladiris N, Saxoni-Papageorgiou P, et al. Consumption of heat-treated egg by children allergic or sensitized to egg can affect the natural course of egg allergy: hypothesis-generating observations. J Allergy Clin Immunol. 2008;122:414–5. doi: 10.1016/j.jaci.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 10.Nowak-Wegrzyn A, Fiocchi A. Rare, medium, or well done? The effect of heating and food matrix on food protein allergenicity. Curr Opin Allergy Clin Immunol. 2009;9:234–7. doi: 10.1097/ACI.0b013e32832b88e7. [DOI] [PubMed] [Google Scholar]
- 11.Jarvinen KM, Beyer K, Vila L, Chatchatee P, Busse PJ, Sampson HA. B-cell epitopes as a screening instrument for persistent cow’s milk allergy. J Allergy Clin Immunol. 2002;110:293–7. doi: 10.1067/mai.2002.126080. [DOI] [PubMed] [Google Scholar]
- 12.Jarvinen KM, Beyer K, Vila L, Bardina L, Mishoe M, Sampson HA. Specificity of IgE antibodies to sequential epitopes of hen’s egg ovomucoid as a marker for persistence of egg allergy. Allergy. 2007;62:758–65. doi: 10.1111/j.1398-9995.2007.01332.x. [DOI] [PubMed] [Google Scholar]
- 13.Clark AT, Skypala I, Leech SC, Ewan PW, Dugue P, Brathwaite N, et al. British Society for Allergy and Clinical Immunology guidelines for the management of egg allergy. Clin Exp Allergy. 2010;40:1116–29. doi: 10.1111/j.1365-2222.2010.03557.x. [DOI] [PubMed] [Google Scholar]
- 14.Lemon-Mule H, Sampson HA, Sicherer SH, Shreffler WG, Noone S, Nowak-Wegrzyn A. Immunologic changes in children with egg allergy ingesting extensively heated egg. J Allergy Clin Immunol. 2008;122:977–83. e1. doi: 10.1016/j.jaci.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Nowak-Wegrzyn A, Bloom KA, Sicherer SH, Shreffler WG, Noone S, Wanich N, et al. Tolerance to extensively heated milk in children with cow’s milk allergy. J Allergy Clin Immunol. 2008;122:342–7. 7, e1–2. doi: 10.1016/j.jaci.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 16.Kim JS, Nowak-Wegrzyn A, Sicherer SH, Noone S, Moshier EL, Sampson HA. Dietary baked milk accelerates the resolution of cow’s milk allergy in children. J Allergy Clin Immunol. 2011;128:125–31. e2. doi: 10.1016/j.jaci.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomicic S, Norrman G, Falth-Magnusson K, Jenmalm MC, Devenney I, Bottcher MF. High levels of IgG4 antibodies to foods during infancy are associated with tolerance to corresponding foods later in life. Pediatr Allergy Immunol. 2009;20:35–41. doi: 10.1111/j.1399-3038.2008.00738.x. [DOI] [PubMed] [Google Scholar]
- 18.Ford RP, Taylor B. Natural history of egg hypersensitivity. Arch Dis Child. 1982;57:649–52. doi: 10.1136/adc.57.9.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchanan AD, Green TD, Jones SM, Scurlock AM, Christie L, Althage KA, et al. Egg oral immunotherapy in nonanaphylactic children with egg allergy. J Allergy Clin Immunol. 2007;119:199–205. doi: 10.1016/j.jaci.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. The Journal of Allergy and Clinical Immunology. 2001;107:891–6. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 21.Knight AK, Shreffler WG, Sampson HA, Sicherer SH, Noone S, Mofidi S, et al. Skin prick test to egg white provides additional diagnostic utility to serum egg white-specific IgE antibody concentration in children. J Allergy Clin Immunol. 2006;117:842–7. doi: 10.1016/j.jaci.2005.12.1304. [DOI] [PubMed] [Google Scholar]
- 22.Sporik R, Hill DJ, Hosking CS. Specificity of allergen skin testing in predicting positive open food challenges to milk, egg and peanut in children. Clin Exp Allergy. 2000;30:1540–6. doi: 10.1046/j.1365-2222.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- 23.Verstege A, Mehl A, Rolinck-Werninghaus C, Staden U, Nocon M, Beyer K, et al. The predictive value of the skin prick test weal size for the outcome of oral food challenges. Clin Exp Allergy. 2005;35:1220–6. doi: 10.1111/j.1365-2222.2005.2324.x. [DOI] [PubMed] [Google Scholar]
- 24.Nowak-Wegrzyn A, Assa’ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS. Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009;123:S365–83. doi: 10.1016/j.jaci.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 25.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larramendi CH, Martin Esteban M, Pascual Marcos C, Fiandor A, Diaz Pena JM. Possible consequences of elimination diets in asymptomatic immediate hypersensitivity to fish. Allergy. 1992;47:490–4. doi: 10.1111/j.1398-9995.1992.tb00670.x. [DOI] [PubMed] [Google Scholar]
- 27.Busse PJ, Nowak-Wegrzyn AH, Noone SA, Sampson HA, Sicherer SH. Recurrent peanut allergy. N Engl J Med. 2002;347:1535–6. doi: 10.1056/NEJM200211073471921. [DOI] [PubMed] [Google Scholar]
- 28.Fleischer DM, Conover-Walker MK, Christie L, Burks AW, Wood RA. The natural progression of peanut allergy: Resolution and the possibility of recurrence. J Allergy Clin Immunol. 2003;112:183–9. doi: 10.1067/mai.2003.1517. [DOI] [PubMed] [Google Scholar]
- 29.Fleischer DM, Conover-Walker MK, Christie L, Burks AW, Wood RA. Peanut allergy: recurrence and its management. J Allergy Clin Immunol. 2004;114:1195–201. doi: 10.1016/j.jaci.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 30.Flinterman AE, Knulst AC, Meijer Y, Bruijnzeel-Koomen CA, Pasmans SG. Acute allergic reactions in children with AEDS after prolonged cow’s milk elimination diets. Allergy. 2006;61:370–4. doi: 10.1111/j.1398-9995.2006.01018.x. [DOI] [PubMed] [Google Scholar]
- 31.Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy. 2007;62:1261–9. doi: 10.1111/j.1398-9995.2007.01501.x. [DOI] [PubMed] [Google Scholar]
- 32.Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, Hamilton RG, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol. 2008;122:1154–60. doi: 10.1016/j.jaci.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveira LC, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010;126:83–91. doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 34.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127:654–60. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thyagarajan A, Varshney P, Jones SM, Sicherer S, Wood R, Vickery BP, et al. Peanut oral immunotherapy is not ready for clinical use. J Allergy Clin Immunol. 2010;126:31–2. doi: 10.1016/j.jaci.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narisety SD, Skripak JM, Steele P, Hamilton RG, Matsui EC, Burks AW, et al. Open-label maintenance after milk oral immunotherapy for IgE-mediated cow’s milk allergy. J Allergy Clin Immunol. 2009;124:610–2. doi: 10.1016/j.jaci.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ridolo E, De Angelis GL, Dall’aglio P. Eosinophilic esophagitis after specific oral tolerance induction for egg protein. Ann Allergy Asthma Immunol. 2011;106:73–4. doi: 10.1016/j.anai.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Sicherer SH, Eigenmann PA, Sampson HA. Clinical features of food protein-induced enterocolitis syndrome. J Pediatr. 1998;133:214–9. doi: 10.1016/s0022-3476(98)70222-7. [DOI] [PubMed] [Google Scholar]
- 39.Clark A, Islam S, King Y, Deighton J, Szun S, Anagnostou K, et al. A longitudinal study of resolution of allergy to well-cooked and uncooked egg. Clin Exp Allergy. 2011;41:706–12. doi: 10.1111/j.1365-2222.2011.03697.x. [DOI] [PubMed] [Google Scholar]
- 40.Leonard SA, Nowak-Wegrzyn A. Re-defining food allergy phenotypes and management paradigm: is it time for individualized egg allergy management? Clin Exp Allergy. 2011;41:609–11. doi: 10.1111/j.1365-2222.2011.03732.x. [DOI] [PubMed] [Google Scholar]
- 41.Clark AT, Skypala I, Leech SC, Ewan PW, Dugue P, Brathwaite N, et al. British Society for Allergy and Clinical Immunology guidelines for the management of egg allergy. Clin Exp Allergy. 2010;40:1116–29. doi: 10.1111/j.1365-2222.2010.03557.x. [DOI] [PubMed] [Google Scholar]